Abstract
Background
With a national program initiated recently to reduce transmission of Schistosoma japonicum in the People's Republic of China (P.R. China), there is an urgent need for accessible, quality-assured diagnostics for case detection, surveillance, and program monitoring of chemotherapy efficacy and other control interventions in areas of low endemicity. We compared the performance of nine immunodiagnostic tests developed in P.R. China for detection of antibodies against S. japonicum and established their priority for further assessment in field settings.
Methodology/Principal Findings
Using the Kato-Katz technique as the reference standard, 240 well-characterized archived serum specimens (100 positive and 140 negative) were evaluated in nine immunological tests developed in P.R. China. The enzyme-linked immunoelectrotransfer blot assay (EITB), which uses an adult worm extract of S. japonicum, supplied by the Center of Disease Control and Prevention, USA, was also evaluated. The sensitivity and specificity of each test were determined and the reproducibility of each test was assessed by evaluating operator-to-operator and run-to-run variation. In addition the simplicity of use for the end-user was evaluated. All tests showed good sensitivities ranging from 92.0% (95% confidence interval (CI): 86.7–97.3%) to 98.0% (95% CI: 95.3–100.0%). The test specificities varied from 70.0% (95% CI: 62.4–77.6%) to 97.1% (95% CI: 94.4–99.9%). All tests showed excellent reproducibility with a discordant rate in the range of 0–10.0% for operator-to-operator variation and run-to-run variation. All tests, except one magnetic particle-based enzyme-linked immunosorbent assay, were found to be easy to use, especially the dot immunogold filtration assays.
Conclusions/Significance
Most evaluated tests had acceptable performance characteristics and could make an impact on the schistosomiasis control programs in P.R. China. Three tests with the highest sensitivity, specificity and greatest ease of use, were selected for further evaluation in field settings.
Author Summary
With the advantages of higher sensitivity and simpler ease of use over stool examination, antibody-detection methods have been integrated into programs for schistosomiasis control in the People's Republic of China after the notable decrease of prevalence and intensity of Schistosoma japonicum infection. We compared the performance of nine immunoassays for diagnosis of S. japonicum using well-characterized archived serum specimens and prioritized tests for future evaluation. Most tests had acceptable performance characteristics and could have an impact on the control of schistosomiasis. Three tests, including one indirect hemagglutination assay, one enzyme-linked immunosorbent assay and one dot immunogold filtration assay were selected for further assessment in field settings. Our final goal is to have appropriate tools for different stages of schistosomiasis control, such as screening targets for chemotherapy, evaluating the efficacy of schistosomiasis control programs, and monitoring the endemic status of schistosomiasis.
Introduction
Schistosomiasis, caused by infection with Schistosoma spp., remains a public health problem in tropical and subtropical areas of the world. It is currently estimated that 207 million people harbor the parasites and about 779 million people are at risk of being infected with schistosomes [1]. Among three main disease-causing species, namely Schistosoma mansoni, S. haematobium, and S. japonicum, the later is the only species endemic in the People's Republic of China (P.R. China). Through more than a half century of effort, the prevalence and intensity of S. japonicum infection have decreased significantly resulting in a decrease in the number of infected people, from 11.6 million in the 1950s to about 0.73 million in 2004 [2]–[4]. According to the latest national epidemiological sampling survey, the average prevalence rate was 2.5% in all surveyed endemic areas and 5.1% in the areas where control of schistosomiasis transmission had not been achieved [4]. With the ultimate goal of elimination, a national program was initiated in 2004, with specific objectives to decrease the prevalence of schistosome infection in all endemic counties below 5% in 2008 and 1% in 2015 [5]–[7]. To reach these goals, accurate, simple, and affordable diagnostic tests are needed urgently for case detection, surveillance, and program evaluation, including evaluation of control interventions and verification of elimination of schistosomiasis in areas with very low endemicity.
Demonstration of eggs or miracidia in the feces is considered to be the ‘gold’ standard for the identification of S. japonicum infections [8]. The Kato-Katz technique is frequently used in the field because it is quantitative, inexpensive, and easy to use [9], but it can be plagued by decreased sensitivity in areas of low endemicity and particularly in individuals with low worm burdens [10]–[12]. The sensitivity of the Kato-Katz technique can be improved by repeated stool collection and examination but this is more labor intensive and costly [10]–[13]. Nucleic acid amplification techniques based on polymerase chain reaction (PCR) have been reported for S. japonicum detection [14]–[17]. However, the methods are not standardized and their sensitivity needs to be verified further. Moreover, the equipment, personnel, and financial investments required are too costly for primary health-care settings in P.R. China.
With the advantages of higher sensitivity and ease of use over stool examination, immunological techniques and methods for antibody detection have attracted scientists' attention. Two national collaborative studies to evaluate the antigens used for detection of antibodies against S. japonicum were organized in the 1980's and the results showed crude soluble antigens extracted from parasite eggs performed with the highest sensitivity and specificity [18], [19]. For unknown reasons, no single test evaluated in these studies was further developed or widely adopted in P.R. China. Based on the findings of these previous studies using crude egg antigens, a variety of immunodiagnostic methods such as the circumoval precipitin test (COPT), indirect hemagglutination assay (IHA), enzyme-linked immunosorbent assay (ELISA), dipstick dye immunoassay (DDIA) and dot immunogold filtration assay (DIGFA) have been developed and integrated into national control programs in P.R. China [4,8,20.21]. Although a number of immunodiagnostic kits have been widely used in the field in P.R. China, none had been standardized and licensed by 2008. Furthermore, due to the lack of strict regulatory approval standards, several poorer performing diagnostics are also used. Because so many different tests with differing performance characteristics are being used, it is difficult to interpret the reported data on prevalence of schistosome infection accurately.
In order to pre-qualify available diagnostic tests for S. japonicum infection and identify their priorities for future field trials [22], a laboratory-based evaluation for test performance and operational characteristics using panels of well-characterized archived serum specimens from geographically diverse settings of P.R. China was carried out. The study was also extended to include an evaluation of the enzyme-linked immunoelectrotransfer blot assay (EITB) provided by the Center for Disease Control and Prevention (CDC), USA, to determine its utility as an independent reference method for confirmation of cases in low transmission areas.
Materials and Methods
Selection of tests for evaluation
Considering the urgent need for quality-assured and approved reagents for diagnosis of schistosome infection in P.R. China, letters of invitation and the study protocol were sent to companies and institutes, and only products that met the following criteria were included for evaluation: (1) tests which have been used in field or clinical settings with reported performance characteristics; (2) tests which could be performed with only minimal training; and (3) products which were in the licensing process. Tests were donated by companies or institutes who were interested in participation in the evaluation, and all test packages were stored according to manufacturers' instructions prior to, and during the evaluation. Well-characterized archived human serum specimens from the serum bank of the China National Reference Center for Schistosomiasis Diagnosis (based at the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, abbreviated as IPD, China CDC), which were collected in October 2007, were used to evaluate the validity and reproducibility of tests. The examinations were conducted by well trained technicians in a double blind way and operational characteristics of the tests were assessed in one week in May, 2008.
Collection and handling of specimens
Serum specimens from patients infected with S. japonicum were collected in endemic areas in Jiangxi, Anhui and Hubei provinces, P.R. China. S. japonicum infection was diagnosed on the basis of the ‘gold’ standard method, demonstration of schistosome eggs in feces with the Kato-Katz technique [9]. Nine slides (41.7 mg feces in each slide) were prepared from three separate stool specimens (three slides for each sample), resulting in evaluation of a total sample weight of 375.3 mg per person. Egg counts reflecting the infection intensity were expressed in eggs per gram of feces (EPG).
Sera were collected from healthy people living in Shandong province who had never traveled to schistosomiasis endemic areas when they underwent routine health check-ups. Serum specimens were also collected from patients with heterologous infections, i.e., hepatitis B, food-borne parasitic diseases (e.g. paragonimiasis, clonorchiasis, and trichinellosis) and soil-transmitted helminthiases (ascariasis, hookworm disease and trichuriasis) in areas free of schistosome infections.
Heterologous infections were diagnosed by stool examinations and/or specific serological tests. Blood samples of 5–10 ml from each donor were collected by venipuncture. Whole blood was kept at 4°C overnight for complete clot retraction. Whole blood was centrifuged and the serum was removed and divided among individual serum storage tubes. Aliquots of serum were frozen at −70°C and linked by a unique numerical code to detailed clinical and parasitological information.
Preparation of serum panels for evaluation
Assuming a sensitivity and specificity of 90% and 90%, respectively, against the ‘gold’ standard test, and allowing a 6% margin of error, a serum panel sample size of 240 was used for the evaluation. One hundred sera were collected from S. japonicum egg-positive persons, which were characterized as 73.0% (73/100) male, with a mean age of 47±13 years. The median infection intensity in patients was 9 EPG and 89.0% had infection intensities below 40 EPG. Fifty serum specimens from healthy persons and 90 specimens from patients with other heterologous diseases (20 cases with hepatitis B, 20 cases with paragonimiasis, and 10 cases each with trichinellosis, clonorchiasis, ascariasis, hookworm disease, and trichuriasis) were also included. Ten aliquots (0.1 ml per aliquot) were prepared per serum specimen and each aliquot was coded with a unique study ID.
For the assessment of reproducibility, panels were prepared with 19 specimens from patients with schistosomiasis and 11 specimens from healthy persons. Each specimen was divided by 40 aliquots (0.1 ml per aliquot) and labeled randomly with unique number.
Tests for evaluation
A total of nine immunodiagnostic kits developed in P.R. China met the inclusion criteria for evaluation. The antigen for all tests was the crude soluble egg extract of S. japonicum and the type of specimen tested in all kits was serum. According to assay type, these kits were classified into three categories: IHA (four tests coded as IHA_AJ, IHA_JX, IHA_XY, and IHA_HB), DIGFA (three tests coded as DIGFA_SH, DIGFA_XC and DIGFA_ZJ), and ELISA (two tests coded as ELISA_SZ and MELISA_BE). Major features of these tests are given in Table 1.
Table 1. Major features of the evaluated tests for the diagnosis of S. japonicum infection.
| Assay code | Assay type | Antigen* | Solid phase | No. samples per run (min. - max.) | Time required per run | Volumes of sample | Extra supplies** | References |
| IHA_AJ | Indirect hemagglutination | SEA | Red cell from sheep | 1–30 | <1 h | 25 µl | Yes | 23,24 |
| IHA_HB | Indirect hemagglutination | SEA | Red cell from sheep | 1–30 | <1 h | 25 µl | Yes | 25 |
| IHA_XY | Indirect hemagglutination | SEA | Red cell from sheep | 1–30 | <1 h | 25 µl | Yes | 26 |
| IHA_JX | Indirect hemagglutination | SEA | Red cell from humans with “O” type blood | 1–30 | <1 h | 25 µl | Yes | 27,28 |
| DIGFA_SH | Dot immunogold filtration assay | SEA | Nitrocellulose membrane | 1 | <5 min | 50 µl | No | 29 |
| DIGFA_XC | Dot immunogold filtration assay | SEA | Nitrocellulose membrane | 1 | <5 min | 40 µl | No | 30 |
| DIGFA_ZJ | Dot immunogold filtration assay | SEA | Nitrocellulose membrane | 1 | <5 min | 25 µl | No | 31 |
| ELISA_SZ | Indirect ELISA | SEA | Microtitre plate | 1–96 | <2 h | 5 µl | Yes | 4,32 |
| MELISA_BE | Magnetic particle-based ELISA | SEA | Magnetic particle | 1–30 | <2 h | 5 µl | Yes | 33 |
| EITB | Enzyme-linked immunoelectrotransfer blot assay | AWA | Nitrocellulose membrane | 1–10 | Overnight | 3 µl | Yes | 34 |
*SEA = soluble egg antigen, AWA = adult microsomal antigen.
**Additional equipment of all tests required was consisted of handheld micropipettes and micropipette tips.
The four IHA tests were based on an indicator system consisting of sheep red blood cell or human red blood cell with “O” blood type coated with soluble antigen extracted from S. japonicum eggs. The IHA tests were performed as described in previous studies [23]–[28]. The titer in the test sera was recorded as one dilution before that which yielded a clear, sharp dark spot similar to that in the negative control wells. Titers were expressed as reciprocal values. Titers of ≥10 indicated a positive result.
The three DIGFA tests were based on the immunofiltration technique, using anti-human antibody labeled with colloidal gold as the indicator system. The volumes of specimen and reaction liquid were added according to the instructions provided by their manufacturers. For the DIGFA_SH and DIGFA_XC, the appearance of two red dots in the well indicated a positive reaction, and the appearance of a single red dot indicated a negative reaction [29], [30]. For the DIGFA_ZJ, the color of one dot in the well equivalent to or deeper than that of positive control serum was considered a positive result, while only pale or pink background in the well was defined as a negative result [31].
The ELISA_SZ and MELISA_BE were based on the indirect ELISA method but differed significantly in their operational procedures. In the ELISA_SZ, all serum specimens were diluted 1∶100 and transferred into the kit supplied microtiter wells. The incubation procedure, washing steps and detection steps were carried out according to the manufacturer's instructions. Optical density (OD) values were read at 450 nm zeroed by the reagent blank wells. For each run, positive and negative control sera were measured simultaneously. A positive result was defined as an OD value greater than 2.1 times the OD value of the negative control serum provided by the kit, as specified by the manufacturer's instructions [32]. The MELISA_BE test combined indirect ELISA and magnetic particle isolation techniques to identify antibodies against S. japonicum [33]. Briefly, serum specimens diluted 1∶200 and fluorescein isothiocyanate (FITC) labeled S. japonicum SEA were added to each tube successively. All the washing steps and detection steps were performed as described previously [33]. OD values were read at 550 nm zeroed by the reagent blank wells and the positives were defined as OD values greater than 0.635.
Finally, an EITB supplied by CDC, USA, was also evaluated. The EITB was performed as described in previous reports [34]. A positive result was defined as the appearance of any of three distinct glycoproteins with molecular masses of 18KD, 23KD and 29KD.
Performance of evaluation
To assess assay performance, each assay was tested with a panel of 240 serum specimens. The testers were blinded to reference standard results and performed the tests independently to avoid comparison of results between kits. Test reproducibility was investigated using a panel of 30 serum specimens. Operator-to-operator reproducibility was compared with two technicians who ran tests with the same 30 sera. Run-to-run variability was investigated using 30 sera that were tested on two different days for each test by the same testers.
Each test was assessed for its operational characteristics by the testing technicians. Tests were scored for clarity of the kit instructions (very clear = 3, clear = 2, not clear = 1), technical complexity (<5 steps and short intervals between steps = 3; 5–10 steps and short intervals between steps = 2; ≥10 steps or long intervals between steps = 1) and ease of interpretation (by eye and easy = 3, by eye but not easy = 2, by machine = 1). The maximum possible score for each index was 3. In addition, a score of 1 was given to the tests that did not require any additional equipment, giving a maximum score of 10. The higher the score, the more suitable the test was considered for use in the field.
Statistics
All data were processed and analyzed with SPSS statistical software package 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Sensitivities and specificities were calculated relative to the ‘gold’ standard Kato-Katz results that correlated to each serum specimen. The 95% confidence intervals (CIs) were determined for the sensitivity and specificity of each test. Youden's index, expressed as sensitivity + specificity - 1, was used to assess the ability of the test to discriminate true positives and true negatives. Operator-to-operator and run-to-run variability were calculated as the number of tests for which different results were obtained by two independent operators, two different days, divided by the number of specimens tested. Categorical variables between groups were compared by χ2 test or Fisher's exact test as appropriate. Significance was assigned at P<0.05 for all parameters and was two-sided unless otherwise indicated.
Ethics statement
The study was approved by the Ethics Committee of IPD, China CDC. The study procedures, potential risks, and benefits were explained to the village leaders. After their consent to perform the study, field workers visited the homes of the selected families where detailed information was provided to all potential participants, and questions were answered. All adult participants and parents/guardians of child participants in a given household provided informed consent. Written confirmation that full information had been provided and individual participation was voluntary (informed consent) was obtained from the head of each participating household or a literary substitute (adult or relative), and this procedure was approved by the aforementioned ethical committee.
Collection of serum specimens was conducted with approval from the Ethics Committee and Academic Board of IPD, China CDC. All personal identifiers and patient information were delinked from the serum specimens.
Results
The sensitivity of each immunological test using 100 serum specimens collected from S. japonicum-infected individuals is detailed in Table 2. The IHA_HB, MELISA_BE and EITB tests performed with the highest sensitivities, 98.0% (98/100; 95% CI: 95.3–100.0%), while the IHA_JX and DIGFA_SH tests showed the lowest sensitivities (92.0%; 92/100; 95% CI: 86.7–97.3%). The nine evaluated tests did not differ from EITB in sensitivity (P>0.05). The 47 false negative specimens were mostly from patients with 20 or less EPG.
Table 2. Detection of antibodies in S. japonicum egg-positive patients (n = 100).
| Assay code | No. of true positives | No. of false negatives | No. of EPG* for misdiagnosed | Sensitivity (%) [95% CI] | P value against EITB |
| IHA_AJ | 93 | 7 | 3, 3, 5, 5, 16, 21, 139 | 93.0 [88.0–98.0] | 0.17 |
| IHA_HB | 98 | 2 | 5, 21 | 98.0 [95.3–100.0] | 1 |
| IHA_XY | 96 | 4 | 3, 5, 11, 139 | 96.0 [92.2–99.8] | 0.68 |
| IHA_JX | 92 | 8 | 3, 3, 5, 11, 19, 21, 27, 139 | 92.0 [86.7–97.3] | 0.1 |
| DIGFA_SH | 92 | 8 | 3, 5, 5, 5, 8, 11, 11, 37 | 92.0 [86.7–97.3] | 0.1 |
| DIGFA_XC | 97 | 3 | 11, 24, 37 | 97.0 [93.7–100.0] | 1 |
| DIGFA_ZJ | 94 | 6 | 3, 5, 5, 5, 11, 37 | 94.0 [89.3–98.7] | 0.28 |
| MELISA_BE | 98 | 2 | 5, 21 | 98.0 [95.3–100.0] | 1 |
| ELISA_SZ | 95 | 5 | 3, 3, 5, 8, 37 | 95.0 [90.7–99.3] | 0.45 |
| EITB | 98 | 2 | 11, 35 | 98.0 [95.3–100.0] | - |
*EPG: egg per gram feces.
The cross-reactivity with heterologous serum specimens and specificity of each evaluated test is listed in Table 3. Cross-reactivity mainly resulted with sera from patients with paragonimiasis (false positive rates: 40.0–60.0%) for all tests except the EITB. Otherwise, false positive rates of each test were very low (0–20%) for patients infected with soil-transmitted helminths, including ascariasis, hookworm disease, and trichuriasis. The highest specificity was observed with the EITB, 97.1% (136/140; 95% CI: 94.4–99.9%). The DIGFA_SH showed the highest specificity (95.1%; 116/122; 95% CI: 91.2–98.9%) among the other nine tests developed in P.R. China, while IHA_XY performed with the lowest specificity (70.0%; 98/140; 95% CI: 62.4–77.6%).
Table 3. False positive results of the evaluated immunoassays.
| Assay code | No. false positives | Specificity (%) [95% CI**] | ||||||||
| Healthy persons n = 50 | Hepatitis B n = 20* | Clonorchiasis n = 20 | Paragonimiasis n = 10* | Trichinellosis n = 10 | Ascariasis n = 10 | Hookworm disease n = 10 | Trichuriasis n = 10 | Total n = 40* | ||
| IHA_AJ | 3 | 1 | 2 | 5 | 2 | 0 | 0 | 1 | 14 | 90.0 [85.0–95.0] |
| IHA_HB | 4 | 1 | 2 | 6 | 3 | 0 | 0 | 2 | 18 | 87.1 [81.6–92.7] |
| IHA_XY | 15 | 4 | 10 | 6 | 6 | 0 | 1 | 0 | 42 | 70.0 [62.4–77.6] |
| IHA_JX | 1 | 0 | 1 | 5 | 2 | 0 | 0 | 1 | 10 | 92.9 [88.6–97.1] |
| DIGFA_SH | 1 | 0 (7) | 1 | 2 (5) | 2 | 0 | 0 | 0 | 6 (122) | 95.1 [91.2–98.9] |
| DIGFA_XC | 0 | 0 (11) | 1 | 4 (9) | 3 | 1 | 0 | 0 | 9 (130) | 93.1 [88.7–97.4] |
| DIGFA_ZJ | 1 | 1 (13) | 0 | 6 (9) | 3 | 0 | 1 | 0 | 12 (132) | 90.9 [86.0–95.8] |
| MELISA_BE | 1 | 0 | 1 | 5 | 1 | 0 | 0 | 0 | 8 | 94.3 [90.5–98.1] |
| ELISA_SZ | 2 | 1 | 0 | 5 | 1 | 0 | 0 | 0 | 9 | 93.6 [89.5–97.6] |
| EITB | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 4 | 97.1 [94.4–99.9] |
*() indicates number of tests that gave valid results;
**CI: confidence interval.
The specificity of each two tests was compared (Table 4). The specificity of IHA_XY was significantly lower than those of other evaluated tests. Compared to the EITB, which had the highest specificity, four tests (IHA_XY, IHA_AJ, IHA_HB, and DIGFA_ZJ) performed with significantly lower specificities (P <0.05). The other five tests, which did not differ from the EITB in specificity, also showed no difference between any two tests (P>0.05).
Table 4. Comparative differences in testing specificity (P values).
| Assay code | IHA_AJ | IHA_HB | IHA_XY | IHA_JX | DIGFA_SH | DIGFA_XC | DIGFA_ZJ | MELISA_BE | ELISA_SZ | EITB |
| IHA_AJ | ||||||||||
| IHA_HB | 0.452 | |||||||||
| IHA_XY | <0.001** | <0.001** | ||||||||
| IHA_JX | 0.393 | 0.111 | <0.001** | |||||||
| DIGFA_SH | 0.122 | 0.026* | <0.001** | 0.453 | ||||||
| DIGFA_XC | 0.365 | 0.104 | <0.001** | 0.944 | 0.501 | |||||
| DIGFA_ZJ | 0.272 | 0.727 | <0.001** | 0.053 | 0.011* | 0.051 | ||||
| MELISA_BE | 0.276 | 0.068* | <0.001** | 0.812 | 0.600 | 0.871 | 0.031* | |||
| ELISA_SZ | 0.183 | 0.039* | <0.001** | 0.626 | 0.775 | 0.683 | 0.017* | 0.802 | ||
| EITB | 0.026* | 0.003* | <0.001** | 0.168 | 0.522 | 0.157 | 0.001* | 0.255 | 0.377 |
*indicate significant difference;
**indicate highly significant difference.
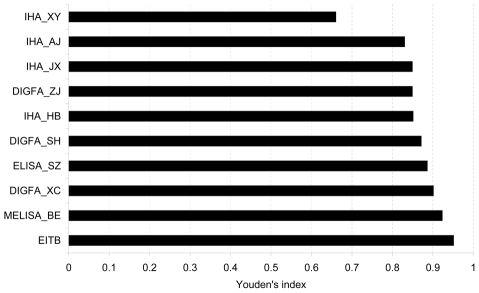
Overall assay performance was evaluated using Youden's indices (Fig. 1). Except for the IHA_XY, all tests demonstrated good performance as indicated by Youden's indices higher than 0.80, especially the MELISA_BE and the EITB (0.92 and 0.95, respectively).
Figure 1. Validity of evaluated tests for the diagnosis of S. japonicum infection (Youden's index).
For all tests, reproducibility was measured by determining operator-to-operator and run-to-run variation. The results are summarized in Fig. 2. Overall, variability was low. The maximum variability observed was 10.0% (6/60) for DIGFA_ZJ test for operator-to-operator and run-to-run variation, respectively, while no variance was detected in the IHA_AJ, IHA_HB, and ELISA_SZ assays. The scores for operational characteristics are summarized in Table 5. Three DIGFA assays obtained the best scores on the questionnaire (10/10) but all presented technical problems with membrane permeability, as evidenced by several serum specimens that could not penetrate. The MELISA_BE received the lowest score (5/10). All tests scored 3 for clarity of kit instructions. Given the similar test principles, four IHA assays and three DIGFA assays were scored equally for technical complexity (2/3, 3/3, accordingly), while MELISA_BE and EITB scored the lowest (1/3). The three DIGFAs scored the highest for ease of interpretation (3/3) and no extra equipment except handheld micropipettes and micropipette tips was needed. The MELISA_BE and ELISA_SZ scored the lowest on ease of interpretation because results were generated using an enzyme-linked analyzer.
Figure 2. Test reproducibility results (discordance %).
A: Operator-to-operator variability (n = 60). B: Run-to-run variability (n = 60).
Table 5. Operational characteristics of the evaluated tests.
| Assay code | Clarity of instructions | Technical complexity | Ease of interpretation of results | Equipment required but not supplied | Total score | Technical problem |
| IHA_AJ | 3 | 2 | 2 | 0 | 7 | No |
| IHA_HB | 3 | 2 | 2 | 0 | 7 | No |
| IHA_XY | 3 | 2 | 2 | 0 | 7 | No |
| IHA_JX | 3 | 2 | 2 | 0 | 7 | No |
| DIGFA_SH | 3 | 3 | 3 | 1 | 10 | Yes |
| DIGFA_XC | 3 | 3 | 3 | 1 | 10 | Yes |
| DIGFA_ZJ | 3 | 3 | 3 | 1 | 10 | Yes |
| MELISA_BE | 3 | 1 | 1 | 0 | 5 | No |
| ELISA_SZ | 3 | 2 | 1 | 0 | 6 | No |
| EITB | 3 | 1 | 2 | 0 | 6 | No |
For each test, a score of 3 for the first three characteristics was given with 3 being the best, and then a score of 1 was given if the test does not require any additional equipment.
Discussion
Parasitological techniques, the definitive methods for determining S. japonicum infection, are relatively insensitive in populations with light infections, as is the case in P.R. China after years of integrated schistosomiasis control [20], [24], [35]. Other diagnostic alternatives include immunologic methods for detection of parasitic specific antibodies or circulating antigens. Although a number of monoclonal antibodies developed for detection of circulating antigens have been developed in P.R. China, these assays showed unsatisfactory sensitivity, especially in patients with light infections [36]–[38]. Antibody-detection has been shown to be more sensitive than stool examination and is needed in areas characterized by low level of transmission, low prevalence and particularly low intensity [39]–[41]. To identify quality-assured diagnostic assays for use in schistosomiasis control areas with low endemicity, we compared diagnostic tests developed in P.R. China in a controlled laboratory setting using well-characterized archived serum specimens (Figure S1), and prioritized the methods for further assessment in field settings.
Once transmission of the disease drops to very low levels, the positive predictive value for any test is decreased. Therefore a confirmatory method may be needed to verify results obtained in the field, especially in areas of low prevalence. The ideal confirmatory method should be independent of the first test method to increase the statistical reliability of the results. The EITB methods using S. mansoni and S. haematobium adult worm microsomal fractions have been demonstrated to be a highly sensitive and specific for detection of schistosomiasis caused by these species [34], [42]. The EITB using S. japonicum adult worm microsomal fractions was evaluated here to determine if this test would be a suitable reference method to confirm S. japonicum infections in especially low transmission settings. The EITB was also chosen for evaluation here because the assay format is independent of the other methods evaluated in this study, all of which detect antibodies to egg antigens. Instead the EITB detects antibodies to adult worm antigens presented in the microsomal fraction and thereby provides an independent means of confirming positives identified using the field tests. In this study, the EITB performed with a high sensitivity for detection of patients with light S. japonicum infections and also had a low cross-reaction rate with normal or heterologous sera. Considering these data and other studies, the EITB has been demonstrated to be effective and specific for detection of schistosome infections. For these reasons, we have identified the EITB as a possible method for definitive serodiagnosis of S. japonicum infections.
The nine other diagnostic tests evaluated demonstrated a good ability to identify schistosomiasis patients with light infections, with sensitivities in the range of 92.0% (95% CI: 86.7–97.3%) to 98.0% (95% CI: 95.3–100.0%). This observation is consistent with a previous study performed by our laboratory with archived serum specimens, in which most immunoassays had sensitivities above 90% [43]. We also found that the serum specimens that generated the most false negative results were collected from patients with very low infection intensities (generally less than 20 EPG). Thus, more sensitive assays need to be developed to avoid misidentifying those individuals with low level infections, but who pose a tremendous risk for continuing transmission. There are quite a few reports of PCR-based methods for detecting S. japonicum infection [14]–[17]. Although the application of molecular techniques in field studies needs further evaluation, these techniques could be improved and standardized as laboratory reference or confirmatory assays because of their excellent sensitivity and specificity.
The specificities of the nine assessed tests in P.R. China varied greatly and ranged from 70.0% (95% CI: 62.4–77.6%) to 95.1% (95% CI: 91.2–98.9%). Four tests, including the IHA_AJ, IHA_HB, IHA_XY, and DIGFA_ZJ, with specificities lower than 91%, differed significantly in specificity from that of the EITB (specificity: 97.1%, 95% CI: 94.4–99.9%). The remaining five assays, IHA_JX, DIGFA_SH, DIGFA_XC, MELISA_BE and ELISA_SZ, did not differ between any two tests in specificity. The specificities of the evaluated tests were mainly influenced by the cross-reactivity with specimens from paragonimiasis or trichinellosis patients. These observations suggested that when a positive result is obtained for a patient from an area co-endemic with schistosomiasis and paragonimiasis or trichinellosis, the history of infection with, or exposure to, the other two diseases should be considered also.
In addition to high sensitivity and specificity, an ideal diagnostic test to be considered for use in developing countries should be user-friendly, rapid and robust, require simple or no equipment, be deliverable to end-users, and be manufactured according to quality standards [44], [45]. First, reproducibility, which is a measure of the closeness of agreement between test results when the conditions for testing or measurement change [46], is an intrinsic factor that may affect performance especially when used in the field for a large-scale evaluation. Our study measured the reproducibility of assays by determining operator-to-operator and run-to-run variation. The discordant rate in the range of 0-10.0% indicates that all tests performed with good reproducibility. Second, the operational characteristics of each test were assessed based on a questionnaire administered to technicians performing the assays. Results from the questionnaire indicated that three DIGFA tests had the greatest advantage for field use, because of their short time to test completion, no extra equipment requirements and ease of interpreting results. We did note that membrane permeability needs to be improved in the DIGFA assays. Further modification for the DIGFA_SH and DIGFA_XC methods is necessary because the specimen volumes needed requires collection of whole blood via venipuncture. This is a limitation because well trained personnel are required and the practice is not always widely accepted in all populations. A later assessment of modified DIGFA tests, which only required a 25 µl serum specimen showed improved membrane permeability; however, only the DIGFA_SH still had high sensitivity and specificity, while the sensitivity of the other two DIGFA tests had decreased below 90% (data not shown). The IHA tests are the most widely used assays in P.R. China and no technical problems with these were identified except the need for an incubator. The ELISA_SZ, which had a high sensitivity and specificity and could detect specimens in panels and give quantitative results based on OD values, is more suitable for a large-scale community survey. However, the needs for a 37°C incubator and a microplate reader for results interpretation limit its use to settings with some moderate amount of laboratory resources. The MELISA_SE, which performed with highest sensitivity and specificity, similar to the EITB, is more appropriate for use as a reference laboratory method or a clinical diagnostic assay because of its complicated procedures and instrument requirements. If it is to be used in field trials, its operational procedures still need simplifying.
The specimen type for all of the tests evaluated here was serum, which means the whole blood collected from donors should be centrifuged or kept at a low temperature for a long time to promote clot formation. The use of whole blood or dried whole blood could negate the difficulties of processing and transporting blood samples for serologic surveys in rural areas of P.R. China.
In conclusion, most immunodiagnostic tests evaluated had an acceptable level of performance relative to the ‘gold’ standard Kato-Katz technique. After considering all the requirements of field diagnostics, i.e., sensitivity, specificity, validity, and ease of use, the IHA_JX, DIGFA_SH, and ELISA_SZ were selected for further field trials. With the caveat that the diagnostic approaches may need to be adjusted to the stage of control and the objective of the control activity [43], we expect to prioritize those tools depending on the specific need [47], [48], such as screening chemotherapy targets, assessing the efficacy of schistosomiasis control programs or monitoring the endemic status of schistosomiasis.
Supporting Information
Translation of the Abstract into Chinese by Jing Xu.
(0.02 MB DOC)
Flowchart used for studies of diagnostic tests.
(2.18 MB TIF)
Acknowledgments
We express our special thanks to Yeuk-Mui Lee and Patricia P. Wilkins from the Center for Disease Control and Prevention, USA, for their kind help in providing the EITB assay. We also would like to thank Professor Ming-gang Chen, National Institute of Parasitic Disease, China CDC, and Patricia P. Wilkins for their valuable comments on the manuscript.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by UNICEF/UNDP/World Bank/WHO Special Programme on Research and Training in Tropical Diseases (No. 70350) and Chinese National Science and Technology Major Project (No. 2008ZX10004-11). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta- analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Zhou XN, Wang TP, Wang LY, Guo JG, Yu Q, et al. The current status of schistosomiasis epidemics in China. Chin J Epidemiol. 2004;25:555–558. [PubMed] [Google Scholar]
- 3.Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, et al. The public health significance and control of schistosomiasis in China-then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Zhou XN, Guo JG, Wu XH, Jiang QW, Zheng J, et al. Epidemiology of schistosomiasis in the People's Republic of China, 2004. Emerg Infect Dis. 2007;13:1470–1476. doi: 10.3201/eid1310.061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang LD, Utzinger J, Zhou XN. Schistosomiasis control: experiences and lessons from China. Lancet. 2008;372:1793–1795. doi: 10.1016/S0140-6736(08)61358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 7.Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, et al. China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14:1475–1483. doi: 10.1111/j.1365-3156.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhu YC. Immunodiagnosis and its role in schistosomiasis control in China: a review. Acta Trop. 2005;96:130–136. doi: 10.1016/j.actatropica.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 10.Yu JM, de Vlas SJ, Yuan HC, Gryseels B. Variations in faecal Schistosoma japonicum egg counts. Am J Trop Med Hyg. 1998;59:370–375. doi: 10.4269/ajtmh.1998.59.370. [DOI] [PubMed] [Google Scholar]
- 11.Zhu HQ, Cao CL, Gao FH, Guo JG, Bao ZP, et al. Evaluation of effectiveness of modified Kato-Katz technique for diagnosis of schistosomiasis japonica. Chin J Schisto Control. 2005;17:273–277. [Google Scholar]
- 12.Lin DD, Liu JX, Liu YM, Hu F, Zhang YY, et al. Routine Kato-Katz technique underestimates the prevalence of Schistosoma japonicum: a case study in an endemic area of the People's Republic of China. Parasitol Int. 2008;57:281–286. doi: 10.1016/j.parint.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard A, Liang S, Maszle D, Qiu D, Gu X, et al. Estimating the distribution of worm burden and egg excretion of Schistosoma japonicum by risk group in Sichuan province, China. Parasitology. 2003;125:221–231. doi: 10.1017/s003118200200207x. [DOI] [PubMed] [Google Scholar]
- 14.Lier T, Simonsen GS, Haaheim H, Hjelmevoll SO, Vennervald BJ, et al. Novel real-time PCR for detection of Schistosoma japonicum in stool. Southeast Asian J Trop Med Public Health. 2006;37:257–264. [PubMed] [Google Scholar]
- 15.Lier T, Johansen MV, Hjelmevoll SO, Vennervald BJ, Simonsen GS. PCR for detection of low intensity Schistosoma japonicum infections in a pig model. Acta Trop. 2008;105:74–80. doi: 10.1016/j.actatropica.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Xia CM, Rong R, Lu ZX, Shi CJ, Xu J, et al. Schistosoma japonicum: a PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp Parasitol. 2009;121:175–179. doi: 10.1016/j.exppara.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, et al. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int J Parasitol. 2010;40:327–331. doi: 10.1016/j.ijpara.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Mott KE, Dixon H. Collaborative study on antigens for immunodiagnosis of schistosomiasis. Bull World Health Organ. 1982;60:729–753. [PMC free article] [PubMed] [Google Scholar]
- 19.Mott KE, Dixon H, Carter CE, Garcia E, Ishii A, et al. Collaborative study on antigens for immunodiagnosis of Schistosoma japonicum infection. Bull World Health Organ. 1987;65:233–244. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu GL. A historical perspective on the immunodiagnosis of schistosomiasis in China. Acta Trop. 2002;82:193–198. doi: 10.1016/s0001-706x(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhu R, Dang H, Zhang LJ, Li HZ, Zheng CJ, et al. National surveillance of schistosomiasis in China, 2005-2008. Chin J Schisto Control. 2009;21:358–362. [Google Scholar]
- 22.World Health Organization. Accessible quality-assured diagnostics. 2009 Available: http://dosei.who.int/uhtbin/cgisirsi/vMCqTyBvfI/307430055/5/0. Accessed 2010 May 17. [Google Scholar]
- 23.Wang EM, Xu Q, Zhang SQ, Zhang LS, Xie JF, et al. Advance development of standardized indirect hemagglutination assay kit for detection of Schistosoma japonicum antibody. J Pathogen Biol. 2007;2:421–423, 403. [Google Scholar]
- 24.Zhou YB, Yang MX, Wang QZ, Zhao GM, Wei JG, et al. Field comparison of immunodiagnostic and parasitological techniques for the detection of schistosomiasis japonica in the people's republic of China. Am J Trop Med Hyg. 2007;76:1138–1143. [PubMed] [Google Scholar]
- 25.Yuan MZ, Wang JS, Peng XW, Chen LC, He J, et al. Effect of IHA and NP30 for screening schistosomiasis. Chin J Schisto Control. 2008;2:76–77. [Google Scholar]
- 26.Zhu L, Zhu JJ, Wang J. Clinical application of two diagnostic kits for schistosomiasis japonica. Shi Yong Yu Fang Yi Xue. 2007;14:1912–1913. [Google Scholar]
- 27.Wu FD, Xie ZM, Yuan SJ, Zhao QH, Guo JG, et al. Study on the diagnosis of schistosomiasis with IHA. Chin J Schisto Control. 1991;3:138–141. [Google Scholar]
- 28.Yu JM, Yuan HC, Yang QJ, Vlas SD, Gryseels B. Comparison of common methods in the field diagnosis of Schistosoma japonicum infection. Tongji Da Xue Xue Bao. 2001;22:1–4. [Google Scholar]
- 29.Jiang SF, Qiu JW, Liu Jing, Zhang XP, He YY, et al. Development of dot immunogold filtration assay kit for rapid detection of antibody to schistosome in human sera. Chin J Schisto Control. 2009;21:500–502. [Google Scholar]
- 30.Yu XL, Liu ZW, Liu Y, Hu PC, He YK, et al. Application of DIGFA for detection of antibodies to schistosomiasis japonica. Shi Yong Yu Fang Yi Xue. 2009;16:333–334. [Google Scholar]
- 31.Wen LY, Chen JH, Ding JZ, Zhang JF, Lu SH, et al. Evaluation on the applied value of the dot immunogold filtration assay (DIGFA) for rapid detection of anti-Schistosoma japonicum antibody. Acta Trop. 2005;96:142–147. doi: 10.1016/j.actatropica.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Lin DD, Xu JM, Zhang YY, Liu YM, Hu F, et al. Evaluation of IgG-ELISA for the diagnosis of Schistosoma japonicum in a high prevalence, low endemicity endemic area of China. Acta Trop. 2008;107:128–133. doi: 10.1016/j.actatropica.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Liu ZS, Yao L, Dong SJ, He Qi, et al. Application of magnetic particle antibody immunoassay in detection of anti-Schistosoma japonicum egg antibody. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27:223–226. [PubMed] [Google Scholar]
- 34.Tsang VC, Wilkins PP. Immunodiagnosis of schistosomiais. Immunol Invest. 1997;26:175–188. doi: 10.3109/08820139709048925. [DOI] [PubMed] [Google Scholar]
- 35.Wang XH, Wu XH, Zhou XN. Bayesian estimation of community prevalences of Schistosoma japonicum infection in China. Int J Parasitol. 2006;36:895–902. doi: 10.1016/j.ijpara.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Guan XH, Shi Y. Collaborative study on evaluation of immunodiagnostic assays in schistosomiasis japonica by treatment efficacy assessment. Chin Med J. 1996;109:659–664. [PubMed] [Google Scholar]
- 37.Qiu LS, Feng Z, Zhang YH, Li H. Circulating antigen detection in schistosomiasis japonica patients by sandwich ELISA using polyclonal and monoclonal antibodies. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2000;18:92–93. [PubMed] [Google Scholar]
- 38.Chen HG, Zeng XJ, Ge J, Jiang WS, Kikuchi M, et al. Study of the efficacy of a monoclonal antibody biotin-avidin system for the diagnosis of schistosomiasis japonica. Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40:244–247. [PubMed] [Google Scholar]
- 39.Doenhoff MJ, Chiodini PL, Hamilton JV. Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends Parasitol. 2004;20:35–39. doi: 10.1016/j.pt.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Bergquist R, Johansen MV, Utzinger J. Diagnostic dilemmas in helminthology: what tools to use and when? Trends Parasitol. 2009;25:151–156. doi: 10.1016/j.pt.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Tsang VC, Hancock K, Kelly MA, Wilson BC, Madison SE. Schistosoma mansoni adult microsomal antigens, a serologic reagent. II. Specificity of antibody responses to the S. mansoni microsomal antigen (MAMA). J Immunol. 1983;130:1366–1370. [PubMed] [Google Scholar]
- 43.Xu J, Feng T, Guo JG, Zheng H, Wang Q, et al. Comprehensive evaluation of several diagnosis agents of schistosomiasis japonica in China. Chin J Schisto Control. 2005;17:116–119. [Google Scholar]
- 44.Mabey D, Peeling RW, Ustianowski Diagnostics for the developing world. Nat Rev Microbio. 2004;l2:231–240. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 45.Johansen MV, Sithithaworn P, Bergquist R, Utzinger J. Towards improved diagnosis of zoonotic trematode infections in Southeast Asia. Adv Parasitol. 2010;73:171–195. doi: 10.1016/S0065-308X(10)73007-4. [DOI] [PubMed] [Google Scholar]
- 46.Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2006;4:20–32. doi: 10.1038/nrmicro1570. [DOI] [PubMed] [Google Scholar]
- 47.Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sodomo M, Olveda R. Schistosomiasis japonica: control and research needs. Adv Parasitol. 2010;72:145–178. doi: 10.1016/S0065-308X(10)72006-6. [DOI] [PubMed] [Google Scholar]
- 48.Utzinger J, Bergquist R, Olveda R, Zhou XN. Important helminth infections in Southeast Asia: diversity, potential for control and prospects for elimination. Adv Parasitol. 2010;72:1–30. doi: 10.1016/S0065-308X(10)72001-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Translation of the Abstract into Chinese by Jing Xu.
(0.02 MB DOC)
Flowchart used for studies of diagnostic tests.
(2.18 MB TIF)