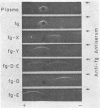
Abstract
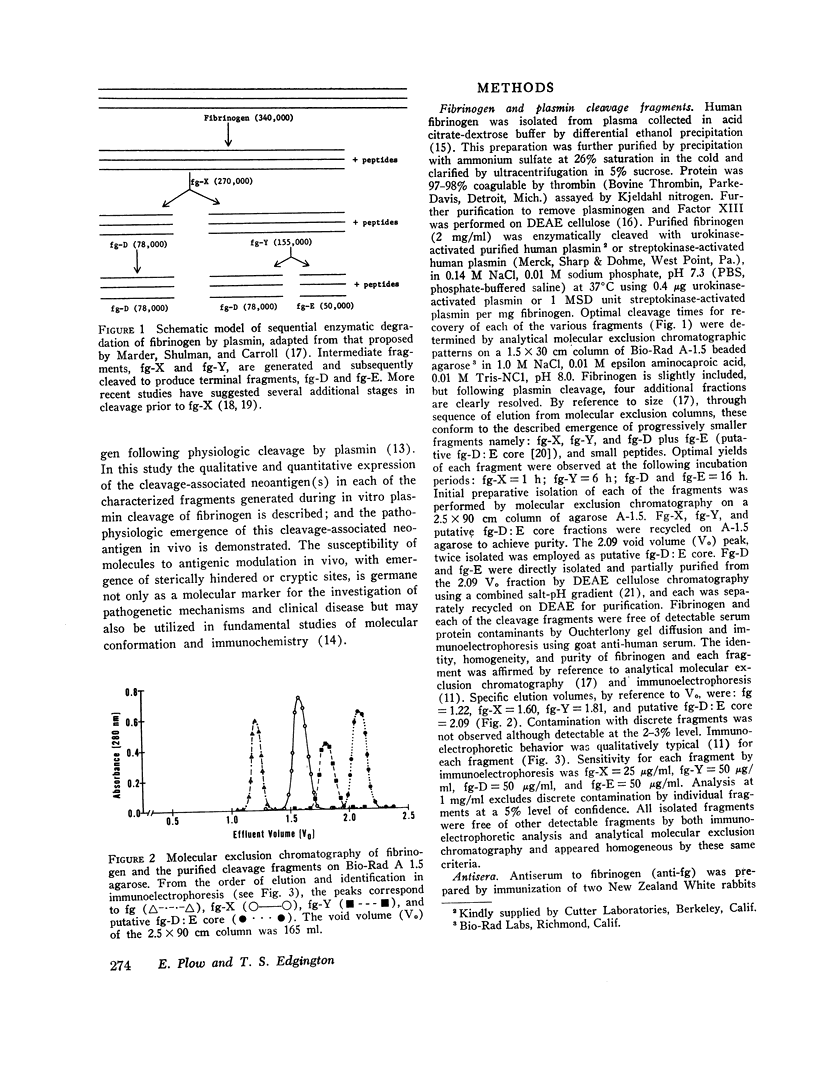
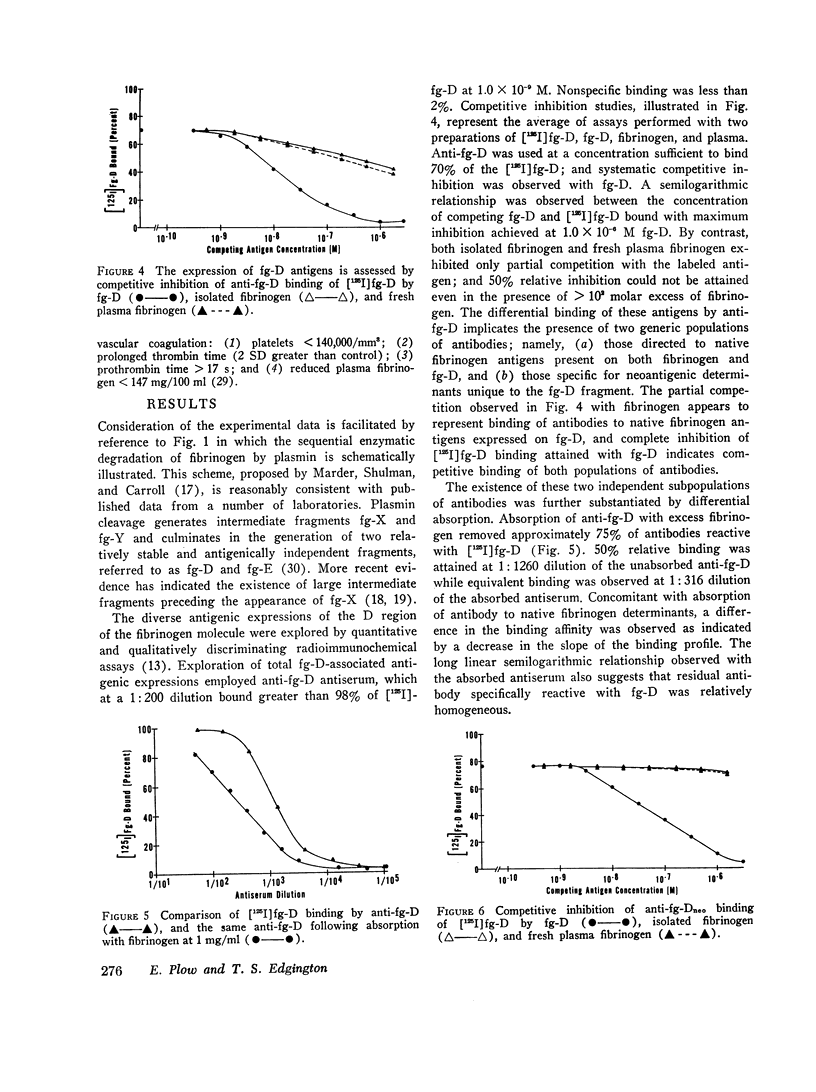
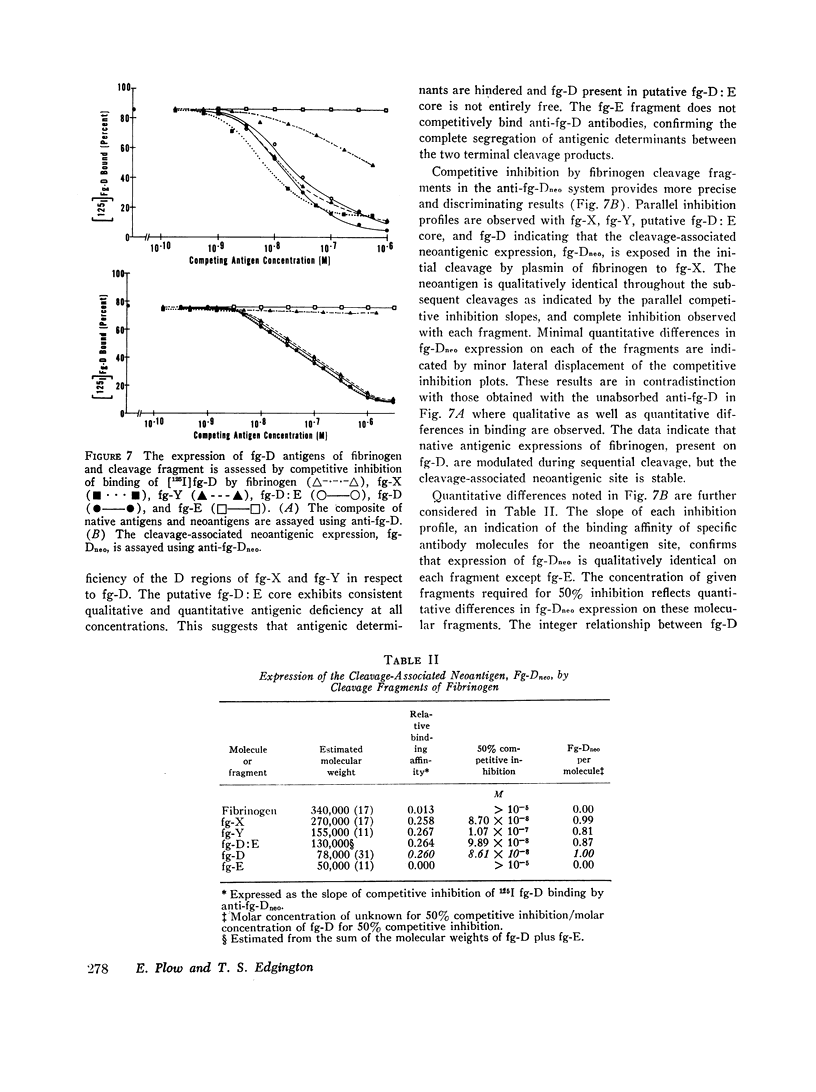
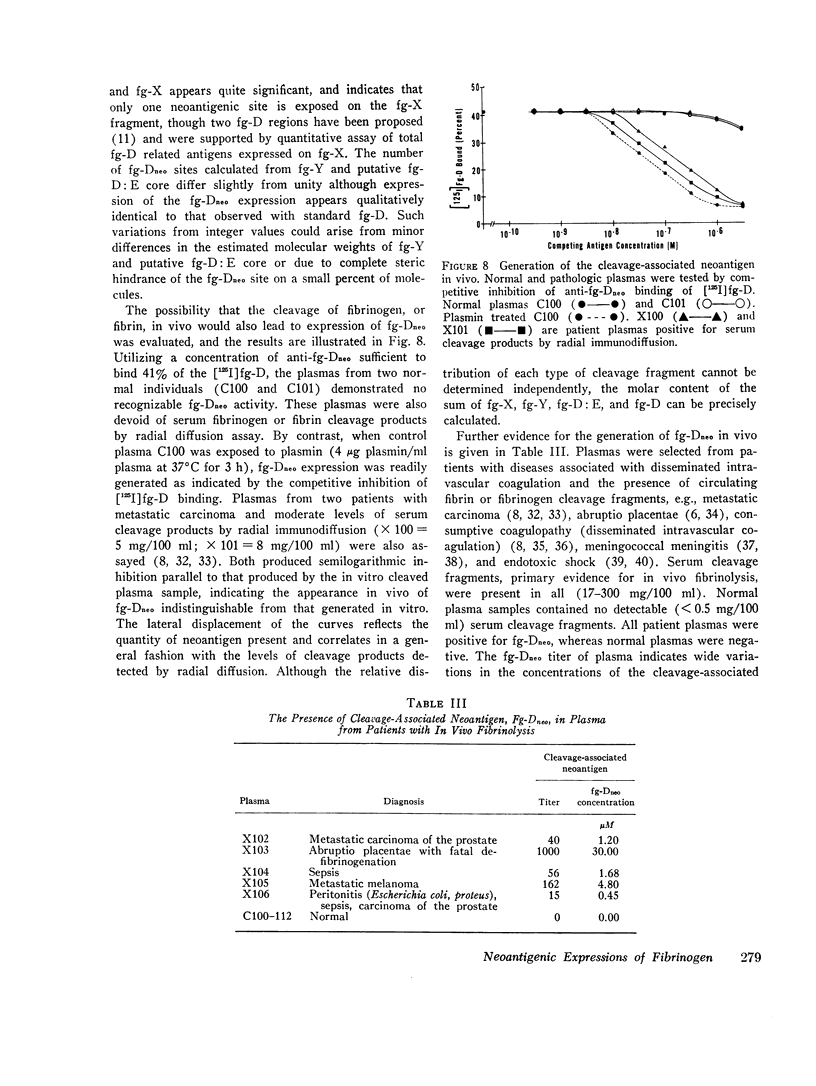
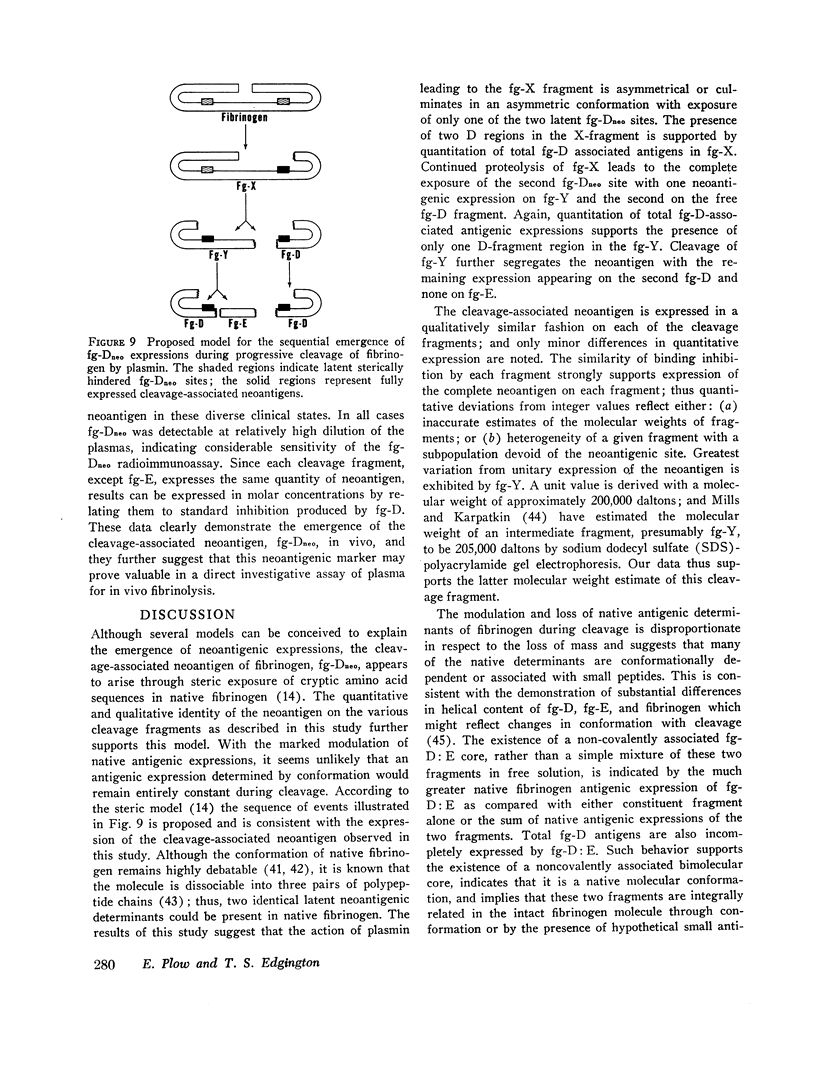
Physiological degradation of fibrinogen by plasmin leads to a recognized series of intermediate and stable terminal cleavage fragments and is associated with complex modulation and progressive loss of native antigenic expressions. Early in association with progressive plasmin cleavage, a stable cleavage-associated neoantigen, present in the D-fragment region of the molecule, is exposed in vitro and can be recognized by competitive inhibition radioimmunoassay with specific antiserum. It is demonstrated that there is an approximate equimolar expression of the cleavage-associated neoantigen. fg-Dneo, on the X-, Y-, and D-fragments and no recognizable (< 10-3) expression by fibrinogen or by the E-fragment. The X-fragment contains two D regions in respect to total D-fragment-associated antigenic expressions but unitary expression of fg-Dneo is observed. The Y-fragment appears to contain one D-fragment region in respect to total D-fragment-associated antigens and exhibits close to unitary expression of fg-Dneo. Terminal cleavage digests containing the D- and E-fragments exhibit more than 10-fold greater native fibrinogen antigenic expression than the sum of the constituent fragments. This suggests the presence of a non-covalently associated native complex of the D- and E-fragments, and implies contiguity of the D- and E-fragments in the native fibrinogen molecule. The cleavage-associated neoantigen, fg-Dneo, is also generated in vivo and is generically demonstrable in the plasma of patients with various forms of in vivo fibrinolysis.
These studies offer a precise immunochemical system, based upon defined molecular events, for the investigation of physiological and pathophysiological cleavage of fibrinogen. By contrast with other approaches to the assay of in vitro or in vivo cleavage of fibrinogen, assay of the cleavage-associated neoantigen fg-Dneo is specific, sensitive, directly yields the molar concentration of all cleavage fragments except E, and is directly applicable to plasma.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attar S., Mansberger A. R., Jr, Irani B., Kirby W., Jr, Masaitis C., Cowley R. A. Coagulation changes in clinical shock. II. Effect of septic shock on clotting times and fibrinogen in humans. Ann Surg. 1966 Jul;164(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- Bonnar J., McNicol G. P. Douglas AS: The behaviour of the coagulation and fibrinolytic mechanisms in abruptio placentae. J Obstet Gynaecol Br Commonw. 1969 Sep;76(9):799–805. doi: 10.1111/j.1471-0528.1969.tb06181.x. [DOI] [PubMed] [Google Scholar]
- Brittin G. M., Rafinia H., Raval D., Werner M., Brown B. Evaluation of single radial immunodiffusion for quantitation of plasma fibrinogen. Am J Clin Pathol. 1972 Jan;57(1):89–94. doi: 10.1093/ajcp/57.1.89. [DOI] [PubMed] [Google Scholar]
- Budzynski A. Z. Difference in conformation of fibrinogen degradation products as revealed by hydrogen exchange and spectropolarimetry. Biochim Biophys Acta. 1971 Mar 23;229(3):663–671. doi: 10.1016/0005-2795(71)90282-0. [DOI] [PubMed] [Google Scholar]
- Budzyński A. Z., Stahl M., Kopeć M., Latallo Z. S., Wegrzynowicz Z., Kowalski E. High molecular weight products of the late stage of fibrinogen proteolysis by plasmin and their structural relation to the fibrinogen molecule. Biochim Biophys Acta. 1967 Oct 23;147(2):313–323. doi: 10.1016/0005-2795(67)90409-6. [DOI] [PubMed] [Google Scholar]
- Catanzaro A., Hathaway G., Strathern J., Edgington T., Weigle W. O. Structure and in vivo behavior of human fibrinogen fragment-D. Proc Soc Exp Biol Med. 1972 Apr;139(4):1401–1406. doi: 10.3181/00379727-139-36372. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Hirsh J., Castelan D. J., Niall H. D., Tregear G. W. Radioimmunoassay of fibrinogen and its proteolysis products. Thromb Diath Haemorrh. 1968 Nov 15;20(1):1–6. [PubMed] [Google Scholar]
- Clarkson A. R., Sage R. E., Lawrence J. R. Consumption coagulopathy and acute renal failure due to gram-negative septicemia after abortion. Complete recovery with heparin therapy. Ann Intern Med. 1969 Jun;70(6):1191–1199. doi: 10.7326/0003-4819-70-6-1191. [DOI] [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Ray W. L., May N. Changes in the blood coagulation system associated with septicemia. N Engl J Med. 1968 Oct 17;279(16):851–856. doi: 10.1056/NEJM196810172791603. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Schubert D., Schwartz S. A. Amino acid sequence studies on artiodactyl fibrinopeptides. I. Dromedary camel, mule deer, and cape buffalo. Arch Biochem Biophys. 1967 Feb;118(2):456–467. doi: 10.1016/0003-9861(67)90374-8. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Glick B., Kimball F., Bobell M. Fatal intravascular consumption coagulopathy in meningococcal sepsis. Am J Med. 1969 Jun;46(6):910–918. doi: 10.1016/0002-9343(69)90093-x. [DOI] [PubMed] [Google Scholar]
- Fletcher A. P., Alkjaersig N., Fisher S., Sherry S. The proteolysis of fibrinogen by plasmin: the identification of thrombin-clottable fibrinogen derivatives which polymerize abnormally. J Lab Clin Med. 1966 Nov;68(5):780–802. [PubMed] [Google Scholar]
- Gormsen J., Andersen R. B., Feddersen C. Fibrinogen-fibrin breakdown products in pathologic synovial fluids. An immunologic study. Arthritis Rheum. 1971 Jul-Aug;14(4):503–512. doi: 10.1002/art.1780140410. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Gaffney P. J., Jr Nature of the high molecular weight fraction of fibrinolytic digests of human fibrinogen. Biochim Biophys Acta. 1968 Jan 22;154(1):96–109. doi: 10.1016/0005-2795(68)90263-8. [DOI] [PubMed] [Google Scholar]
- LATALLO Z. S., BUDZYNSKI A. Z., LIPINSKI B., KOWALSKI E. INHIBITION OF THROMBIN AND OF FIBRIN POLYMERIZATION, TWO ACTIVITIES DERIVED FROM PLASMIN-DIGESTED FIBRINOGEN. Nature. 1964 Sep 12;203:1184–1185. doi: 10.1038/2031184a0. [DOI] [PubMed] [Google Scholar]
- LATALLO Z. S., FLETCHER A. P., ALKJAERSIG N., SHERRY S. Inhibition of fibrin polymerization by fibrinogen proteolysis products. Am J Physiol. 1962 Apr;202:681–686. doi: 10.1152/ajplegacy.1962.202.4.681. [DOI] [PubMed] [Google Scholar]
- Lederer K., Finkelstein A. Hydrodynamic study of fibrinogen molecular models to test their compatibility with data from the ultracentrifuge and viscosity measurements. Biopolymers. 1970;9(12):1553–1556. doi: 10.1002/bip.1970.360091216. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Matchett M. O., Sherry S. Detection of serum fibrinogen and fibrin degradation products. Comparison of six technics using purified products and application in clinical studies. Am J Med. 1971 Jul;51(1):71–82. doi: 10.1016/0002-9343(71)90325-1. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. The importance of intermediate degradation products of fibrinogen in fibrinolytic hemorrhage. Trans Assoc Am Physicians. 1967;80:156–167. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R. High molecular weight derivatives of human fibrinogen produced by plasmin. II. Mechanism of their anticoagulant activity. J Biol Chem. 1969 Apr 25;244(8):2120–2124. [PubMed] [Google Scholar]
- McKee P. A., Rogers L. A., Marler E., Hill R. L. The subunit polypeptides of human fibrinogen. Arch Biochem Biophys. 1966 Sep 26;116(1):271–279. doi: 10.1016/0003-9861(66)90033-6. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Finlayson J. S., Umfleet R. A., Galanakis D. Human fibrinogen heterogeneities. I. Structural and related studies of plasma fibrinogens which are high solubility catabolic intermediates. J Biol Chem. 1972 Aug 25;247(16):5210–5219. [PubMed] [Google Scholar]
- NUSSENZWEIG V., SELIGMANN M., PELMONT J., GRABAR P. [The products of degradation of human fibrinogen by plasmin. I. Separation and physicochemical properties]. Ann Inst Pasteur (Paris) 1961 Mar;100:377–389. [PubMed] [Google Scholar]
- Nanninga L. B., Guest M. M. Antifibrinolytic action of the anticoagulant split product of fibrinogen. Thromb Diath Haemorrh. 1968 Jul 31;19(3):526–532. [PubMed] [Google Scholar]
- Niewiarowski S., Stewart G. J., Marder V. J. Formation of highly ordered polymers from fibrinogen and fibrin degradation products. Biochim Biophys Acta. 1970 Nov 17;221(2):326–341. doi: 10.1016/0005-2795(70)90273-4. [DOI] [PubMed] [Google Scholar]
- Niléhn J. E. Split products of fibrinogen after prolonged interaction with plasmin. Thromb Diath Haemorrh. 1967 Aug 15;18(1-2):89–100. [PubMed] [Google Scholar]
- Pitney W. R. Disseminated intravascular coagulation. Semin Hematol. 1971 Jan;8(1):65–83. [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrinogen. J Biol Chem. 1972 Feb 10;247(3):636–645. [PubMed] [Google Scholar]
- Plow E. F., Hougie C., Edgington T. S. Neoantigenic expressions engendered by plasmin cleavage of fibrinogen. J Immunol. 1971 Nov;107(5):1496–1500. [PubMed] [Google Scholar]
- Plow E., Edgington T. S. Molecular events responsible for modulation of neoantigenic expression: the cleavage-associated neoantigen of fibrinogen (blood coagulation-fibrinogen cleavage products-fibrinolysis-molecular conformation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):208–212. doi: 10.1073/pnas.69.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., MENZIE C. A new method for the determination of fibrinogen in small samples of plasma. J Lab Clin Med. 1951 Feb;37(2):316–320. [PubMed] [Google Scholar]
- Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965 Dec 16;273(25):1370–1378. doi: 10.1056/NEJM196512162732506. [DOI] [PubMed] [Google Scholar]
- Sack E. S., Buraschi J. Fibrinogen degradation products. N Engl J Med. 1971 Jun 24;284(25):1441–1441. doi: 10.1056/nejm197106242842519. [DOI] [PubMed] [Google Scholar]
- Straub P. W., Riedler G., Frick P. G. Hypofibrinogenaemia in metastatic carcinoma of the prostate: suppression of systemic fibrinolysis by haparin. J Clin Pathol. 1967 Mar;20(2):152–157. doi: 10.1136/jcp.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGNON H. J., WHITMORE W. F., Jr, SHULMAN N. R. Fibrinolysis in metastatic cancer of the prostate. Cancer. 1952 Jan;5(1):9–12. doi: 10.1002/1097-0142(195201)5:1<9::aid-cncr2820050104>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Thomas D. P., Niewiarowski S., Myers A. R., Bloch K. J., Colman R. W. A comparative study of four methods for detecting fibrinogen degradation products in patients with various diseases. N Engl J Med. 1970 Sep 24;283(13):663–668. doi: 10.1056/NEJM197009242831301. [DOI] [PubMed] [Google Scholar]
- Winkelstein A., Songster C. L., Caras T. S., Berman H. H., West W. L. Fulminant meningococcemia and disseminated intravascular coagulation. Arch Intern Med. 1969 Jul;124(1):55–59. [PubMed] [Google Scholar]
- Yoshikawa T., Tanaka K. R., Guze L. B. Infection and disseminated intravascular coagulation. Medicine (Baltimore) 1971 Jul;50(4):237–258. doi: 10.1097/00005792-197107000-00001. [DOI] [PubMed] [Google Scholar]