Abstract
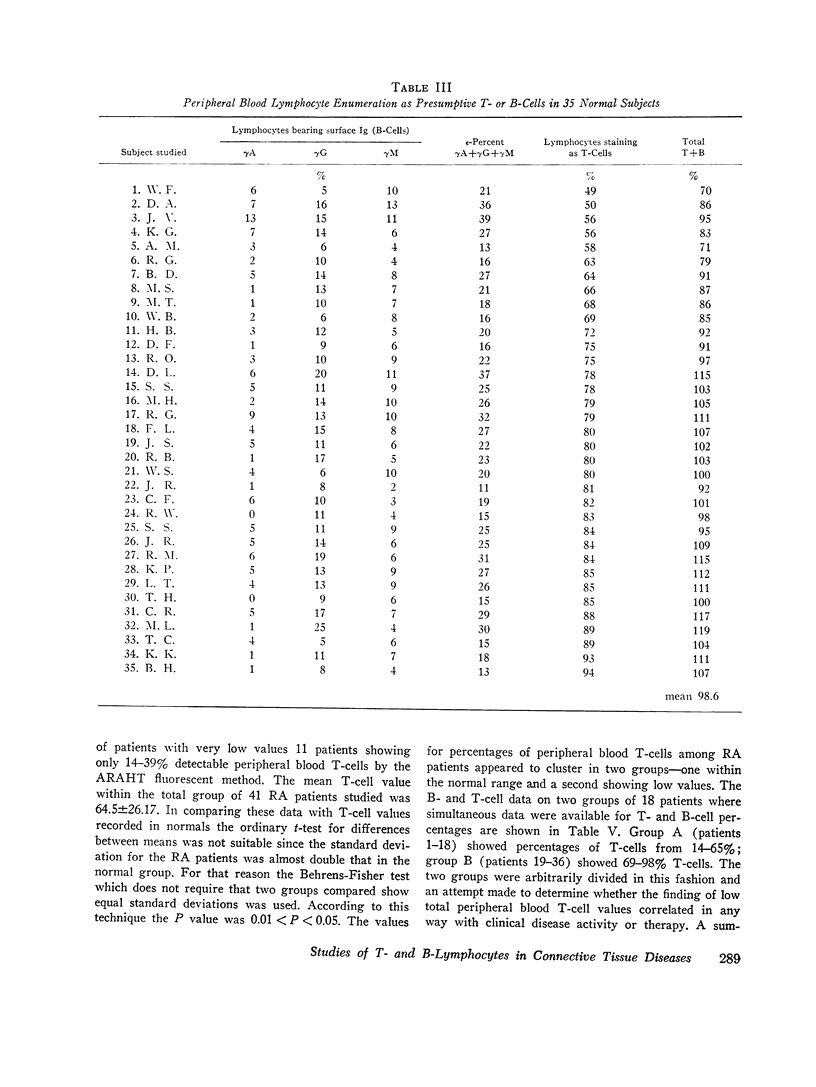
Peripheral blood lymphocytes from normal subjects as well as patients with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and active tuberculosis were studied for the relative distribution of bone marrow-derived lymphocytes (B-cells) and thymic-derived T-cells. B-cells were identified by direct immunofluorescence of surface Ig markers; T-cells were studied using rabbit antisera to pooled human fetal thymocytes absorbed with chronic lymphatic leukemia lymphocytes as a source of B-cells. In normal subjects, the sum of percentages of peripheral blood lymphocytes staining for surface Ig (B-cells) plus the percentage of cells staining with the absorbed antithymocyte antiserum closely approximated 100%. The mean value for percent B-cells among 51 normals tested was 22.9%±7.1; mean T-cells value was 75.3±13.95%. T-cell-specific antiserum stained 18% of normal human bone marrow lymphocytes, 42.5% of lymphocytes from normal spleens, and 98% of cells obtained from thoracic duct drainage of patients with RA. Specificity of antihuman thymocyte antiserum appeared to depend on the use of living cells.
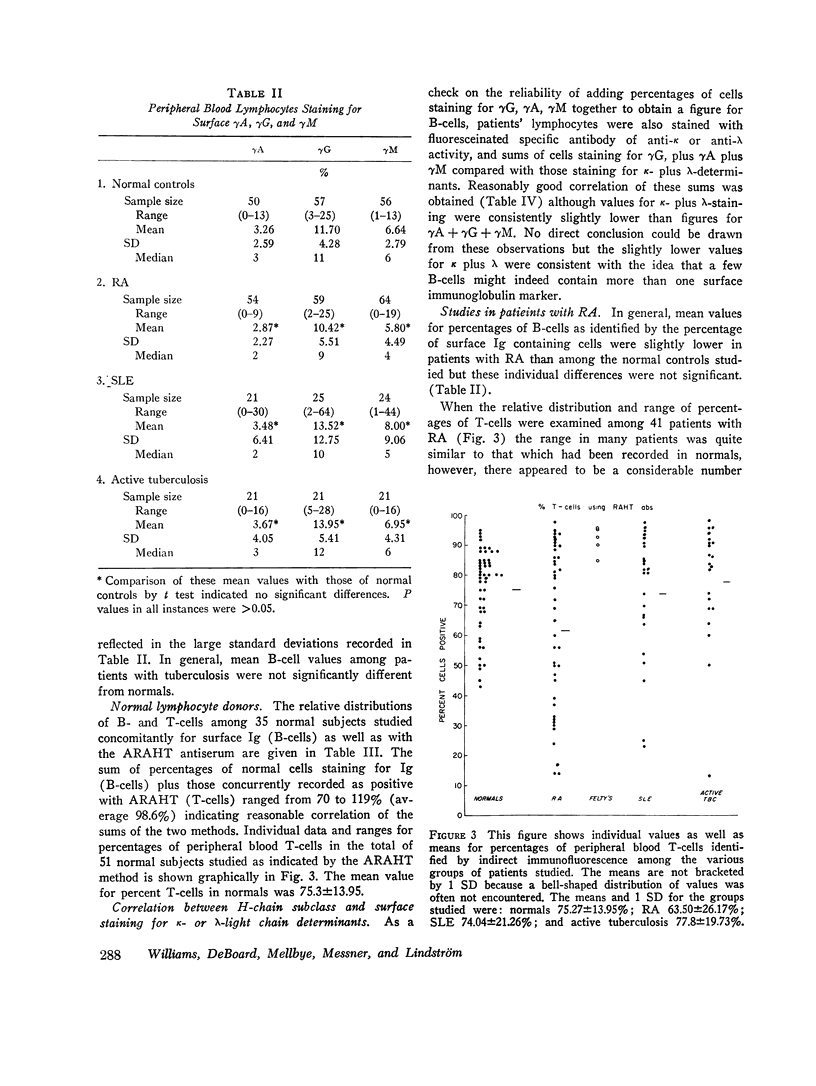
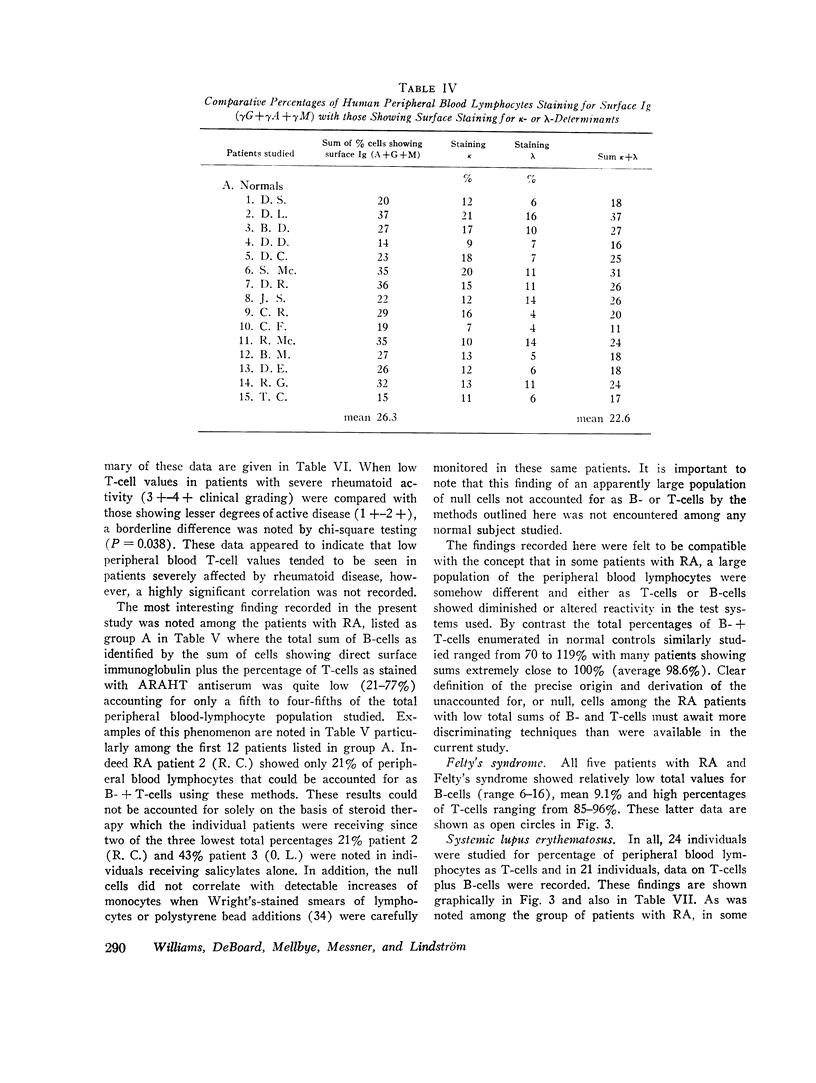
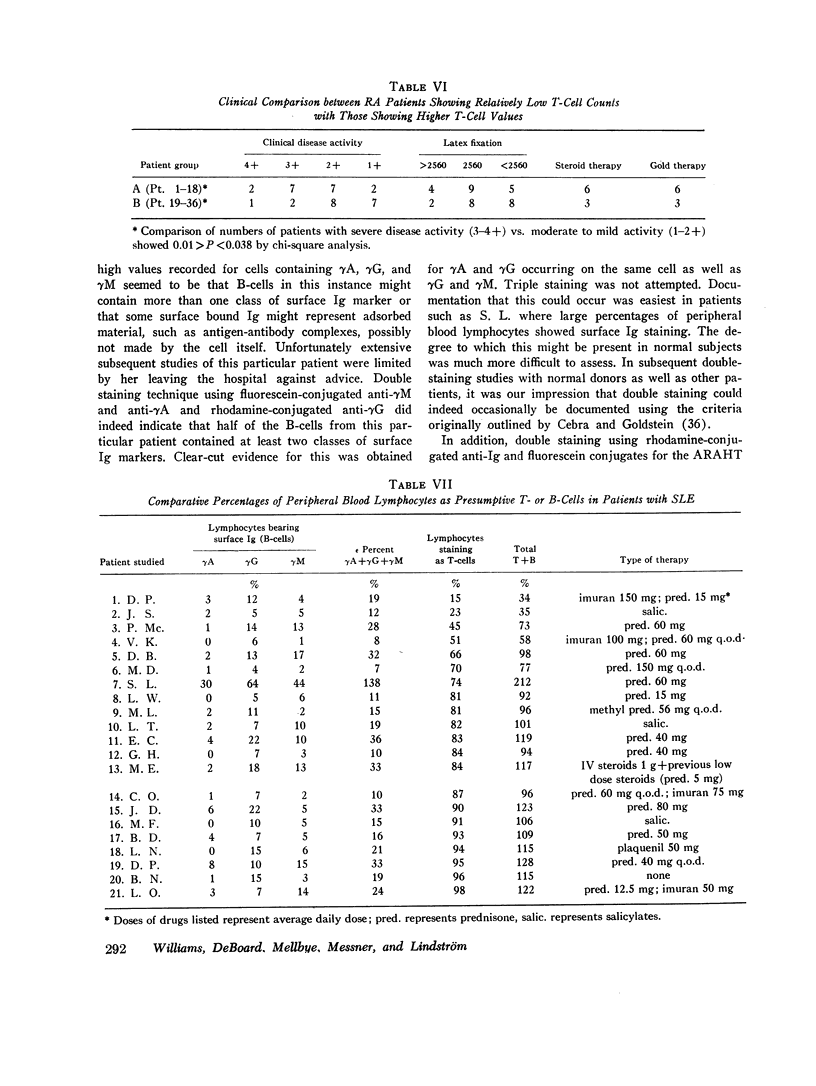
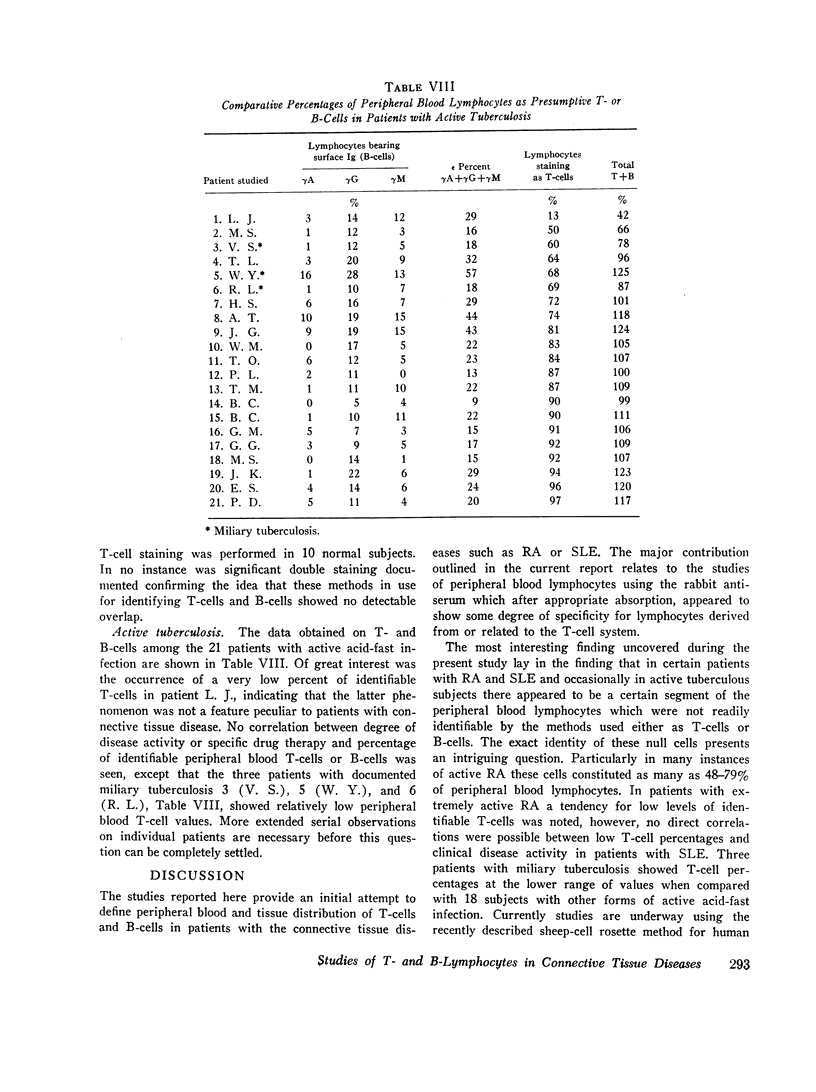
When patients with RA were examined, a wide range (14-98%) of peripheral blood T-cell values was found. Values for low percentages of peripheral blood T-cells appeared to correlate to some extent with severe clinical disease. In 11 of 36 RA patients, the sum of identifiable B- plus T-cells accounted for only 34-55% of peripheral blood lymphocytes. The identity of the remaining “null” cells could not be identified.
3 of 24 SLE patients studied showed low percentages of peripheral blood T-cells, but no correlation could be drawn between T- to B-cell ratios and clinical disease activity. Among 21 patients with active tuberculosis, one had a low value for identifiable T-cells. No significant differences from normals in range or proportion of B-cells was identified in patients with active tuberculous infection.
Full text
PDF


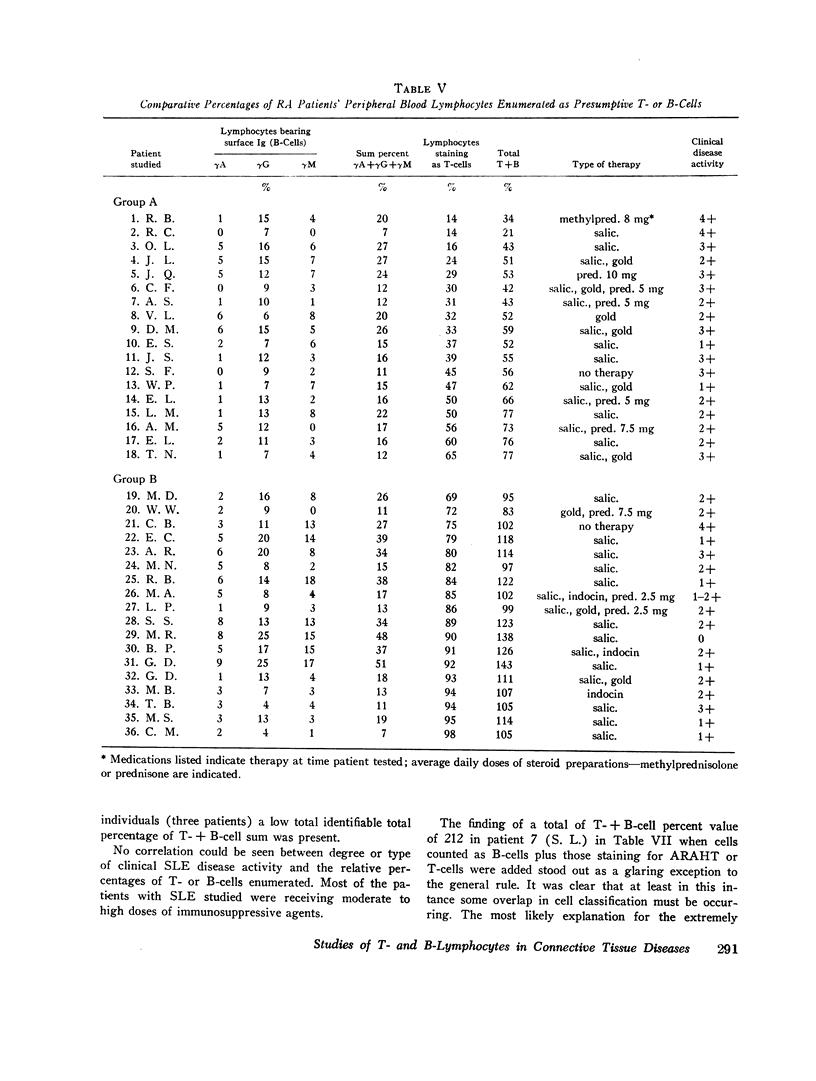


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I. Immunoglobulin (Ig) receptors on human peripheral leukocytes. II. Class restriction Ig receptors. J Immunol. 1971 Dec;107(6):1637–1642. [PubMed] [Google Scholar]
- Astorga G. P., Williams R. C., Jr Altered reactivity in mixed lymphocyte culture of lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1969 Dec;12(6):547–554. doi: 10.1002/art.1780120602. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Goldstein G., Tomasi T. B., Jr Urinary gammaA rheumatoid factor. J Lab Clin Med. 1969 Mar;73(3):389–398. [PubMed] [Google Scholar]
- Bonomo L., Tursi A., Trizio D., Gillardi U., Dammacco F. Immune complexes in rheumatoid synovitis: a mixed staining immunofluorescence study. Immunology. 1970 Apr;18(4):557–563. [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Wilson A. B., Holm G., Lindgren B. Rosette-formation between human lymphocytes and sheep red cells not involving immunoglobulin receptors. Int Arch Allergy Appl Immunol. 1970;39(5-6):658–663. doi: 10.1159/000230390. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C., HOLMAN H. R., MULLER-EBERHARD H. J., KUNKEL H. G. An unusual protein component of high molecular weight in the serum of certain patients with rheumatoid arthritis. J Exp Med. 1957 May 1;105(5):425–438. doi: 10.1084/jem.105.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUDENBERG H. H., KUNKEL H. G. Specificity of the reaction between rheumatoid factors and gamma globulin. J Exp Med. 1961 Aug 1;114:257–278. doi: 10.1084/jem.114.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröland S., Natvig J. B., Berdal P. Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol. 1971 Dec 22;234(51):251–252. doi: 10.1038/newbio234251a0. [DOI] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated stem cell antigen: an antigen shared by brain and hemopoietic stem cells. J Exp Med. 1972 Aug 1;136(2):369–374. doi: 10.1084/jem.136.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNKEL H. G., TAN E. M. AUTOANTIBODIES AND DISEASE. Adv Immunol. 1964;27:351–395. doi: 10.1016/s0065-2776(08)60711-7. [DOI] [PubMed] [Google Scholar]
- Klein E., Eskeland T., Inoue M., Strom R., Johansson B. Surface immunoglobulin-moieties on lymphoid cells. Exp Cell Res. 1970 Sep;62(1):133–148. doi: 10.1016/0014-4827(79)90515-9. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Glassock R. J., Dixon F. J. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967 Dec 1;126(6):989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal B. G., Waldorf D. S., Talal N. Impaired Lymphocyte Transformation and Delayed Hypersensitivity in Sjögren's Syndrome. J Clin Invest. 1967 Aug;46(8):1338–1345. doi: 10.1172/JCI105626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellbye O. J., Messner R. P., DeBord J. R., Williams R. C., Jr Immunoglobulin and receptors for C3 on lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1972 Jul-Aug;15(4):371–380. doi: 10.1002/art.1780150408. [DOI] [PubMed] [Google Scholar]
- Meltzer M., Franklin E. C. Cryoglobulinemia--a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966 Jun;40(6):828–836. doi: 10.1016/0002-9343(66)90199-9. [DOI] [PubMed] [Google Scholar]
- Ohayon E., Ducos J., Goret P., Salmon H., Toma B. Cytotoxicity of A.L.S. and anti-HL-A antibodies in human leukaemia. Lancet. 1972 Jan 1;1(7740):39–40. doi: 10.1016/s0140-6736(72)90030-x. [DOI] [PubMed] [Google Scholar]
- Pachman L. M., Esterly N. B., Peterson R. D. The effect of salicylate on the metabolism of normal and stimulated human lymphocytes in vitro. J Clin Invest. 1971 Jan;50(1):226–230. doi: 10.1172/JCI106478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Chemistry and mechanism of action of complement. Prog Hematol. 1966;5:2–25. [PubMed] [Google Scholar]
- Preud'homme J. L., Klein M., Verroust P., Seligmann M. Immunoglobulines monoclonales de membrane dans les leucémies lymphoïdes chroniques. Rev Eur Etud Clin Biol. 1971 Dec;16(10):1025–1031. [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Grey H. M. Immunoglobulins on the surface of lymphocytes. 3. Bursal origin of surface immunoglobulins on chicken lymphocytes. J Immunol. 1971 May;106(5):1418–1420. [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- STOLLAR D., LEVINE L., LEHRER H. I., VAN VUNAKIS H. The antigenic determinants of denatured DNA reactive with lupus erythematosus serum. Proc Natl Acad Sci U S A. 1962 May 15;48:874–880. doi: 10.1073/pnas.48.5.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegelius O., Laine V., Lindström B., Klockars M. Fistula of the thoracic duct as immunosuppressive treatment in rheumatoid arthritis. Acta Med Scand. 1970 Jun;187(6):539–544. doi: 10.1111/j.0954-6820.1970.tb02982.x. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Emmons J. D., Astorga G. P. Studies of antilymphocyte antisera produced against normal and rheumatoid arthritis lymphocytes. Arthritis Rheum. 1971 Nov-Dec;14(6):697–705. doi: 10.1002/art.1780140604. [DOI] [PubMed] [Google Scholar]
- Yata J., Klein G., Kobayashi N., Furukawa T., Yanagisawa M. Human thymus-lymphoid tissue antigen and its presence in leukaemia and lymphoma. Clin Exp Immunol. 1970 Dec;7(6):781–792. [PMC free article] [PubMed] [Google Scholar]