Abstract
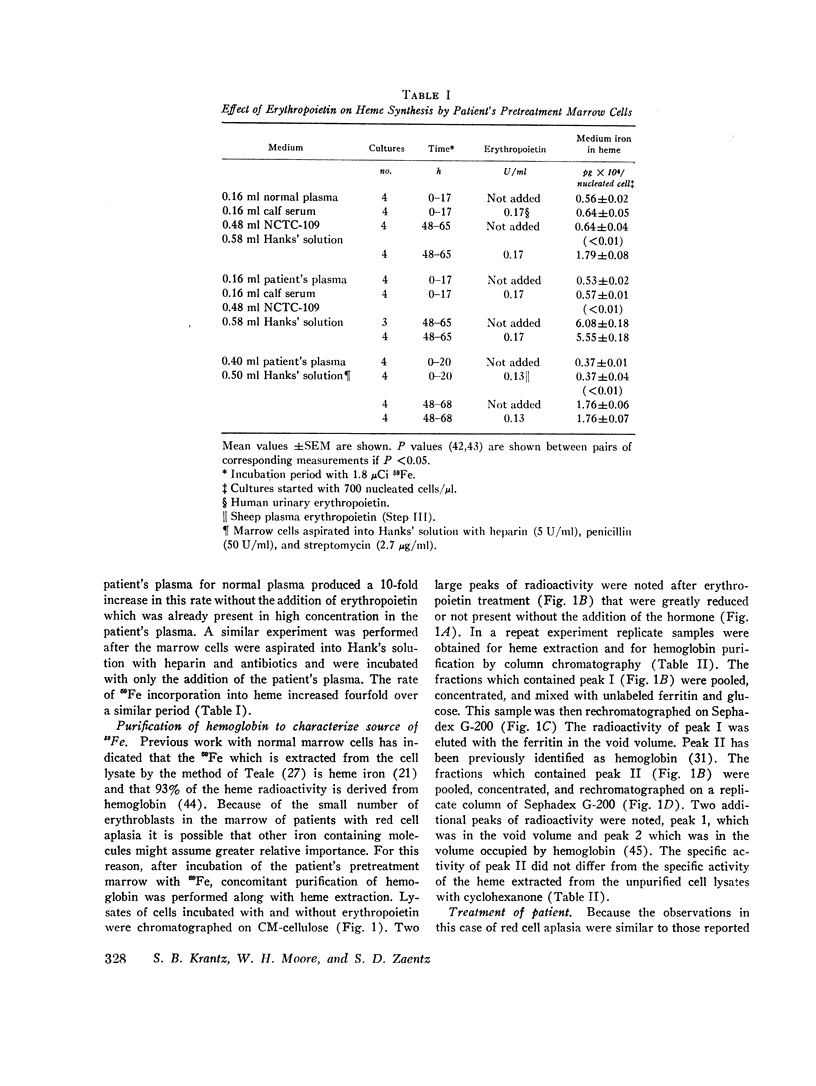
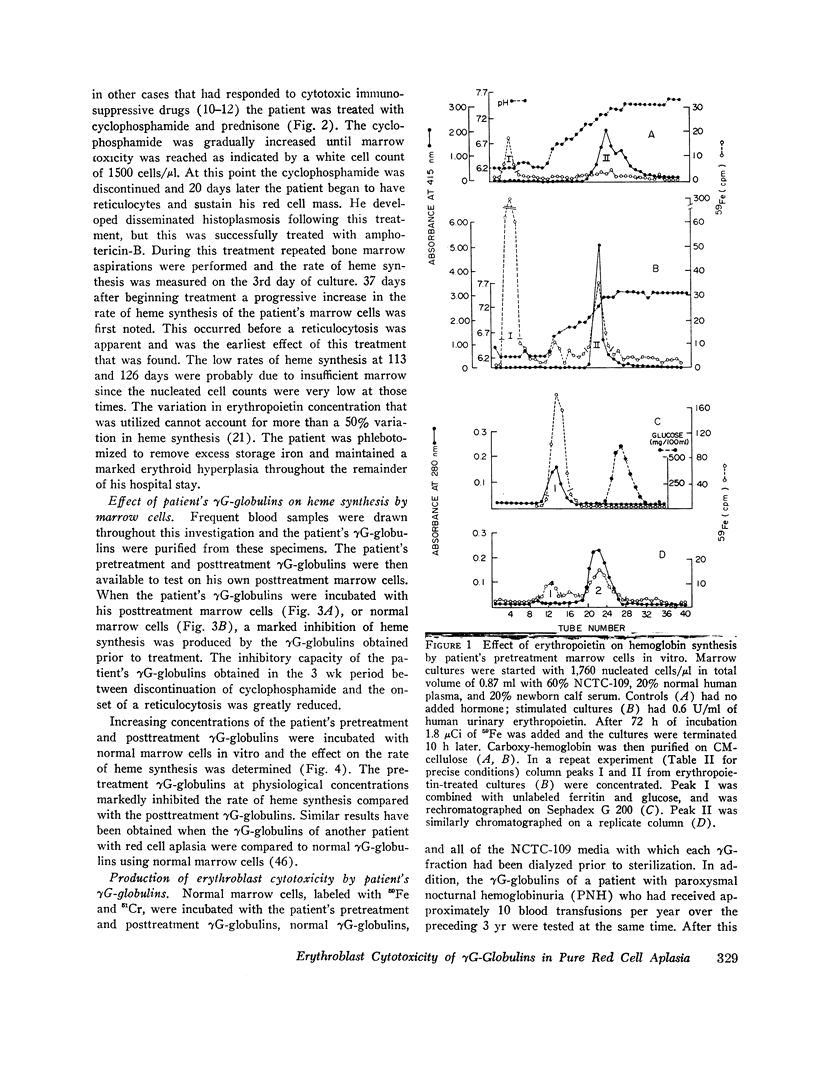
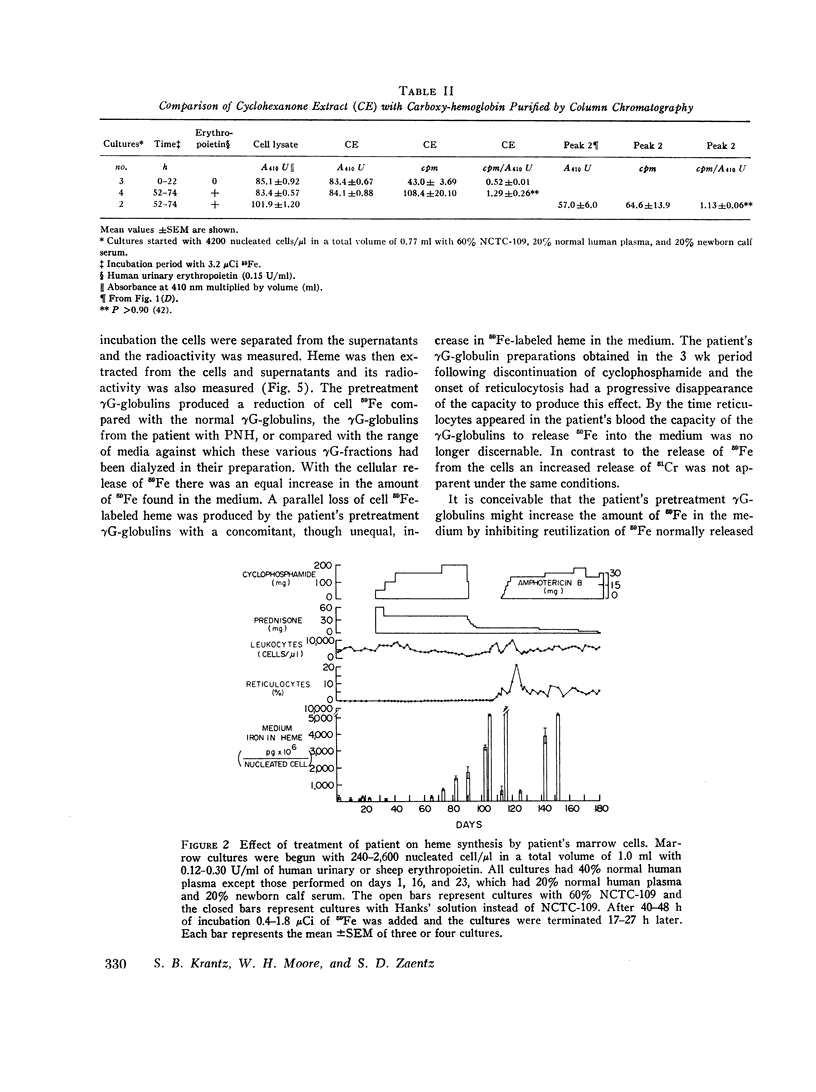
The marrow cells of a patient with pure red cell aplasia markedly increased their rate of heme synthesis when they were freed from the host environment and were incubated in vitro. When the red cell aplasia was treated with cyclophosphamide and prednisone, marrow cell incorporation of 59Fe into heme in vitro increased several weeks before a reticulocytosis was apparent, and was the earliest effect noted. The plasma γG-globulins of this patient inhibited heme synthesis by normal marrow cells or the patient's own marrow cells obtained after remission of the disease.
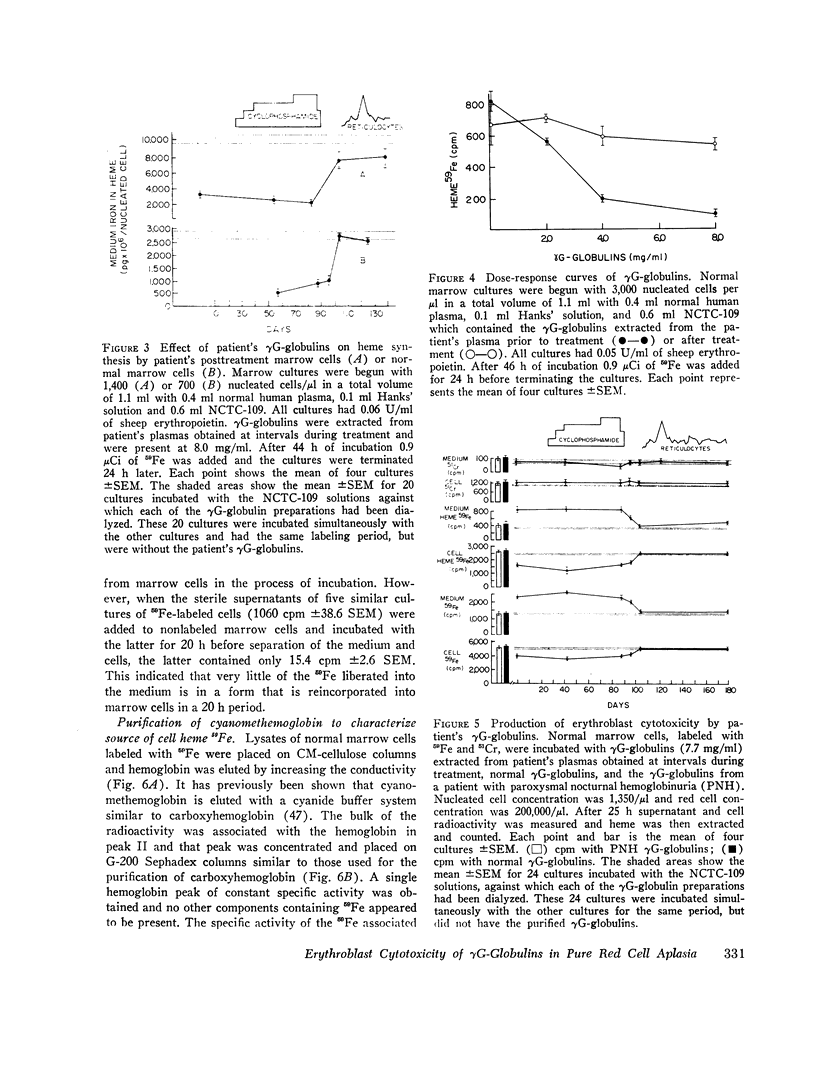
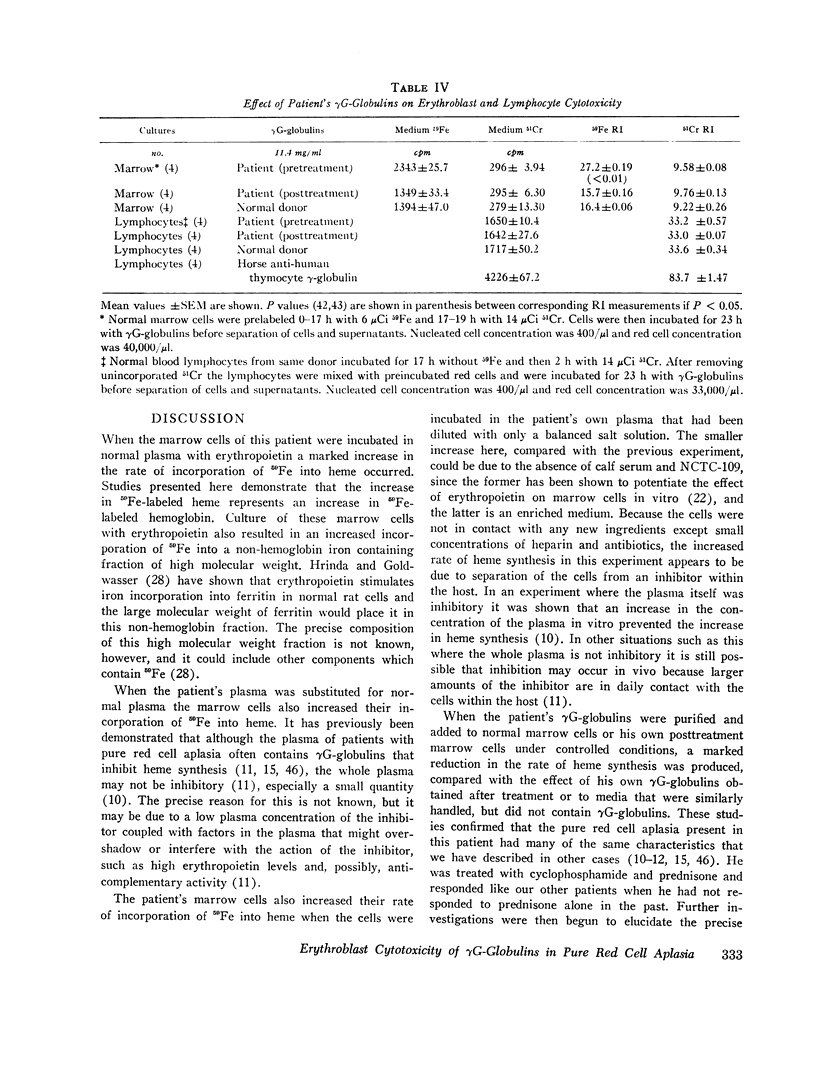
Since the inhibition of heme synthesis could be the result of damage to erythroblasts, the patient's posttreatment marrow cells or normal marrow cells were labeled with 59Fe and were then incubated with the patient's pretreatment, treatment, and posttreatment γG-globulins as well as normal γG-globulins. At the end of this incubation the supernatant and cells were separated and counted. Heme was extracted and also was counted. Treatment of the cells with the patient's pretreatment γG-globulins resulted in a release of 40% of the radioactive heme from the cells. This represented the loss of radioactive hemoglobin and was an index of erythroblast cytotoxicity. A progressive disappearance of the cytotoxic factor in the γG-globulins occurred in the 3 wk period preceding the onset of reticulocytes in the patient's blood. Posttreatment and normal γG-globulins did not produce this effect and increased injury of red cells and lymphocytes was not produced by the patient's pretreatment γG-globulins. These studies demonstrate a method for measuring erythroblast cytoxicity and show that red cell aplasia is associated with γG-globulins that specifically damage erythroblasts. Whether interference with new erythroblast development also occurs and contributes to the inhibition of heme synthesis has not yet been ascertained.
Full text
PDF



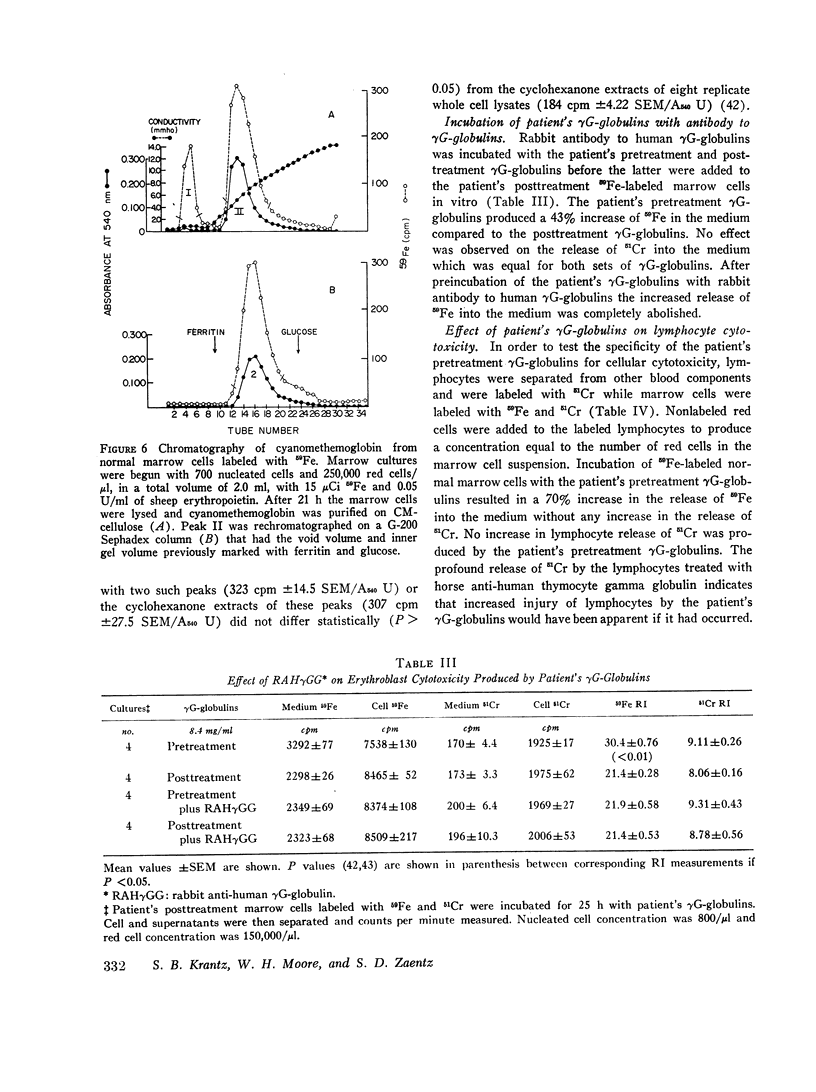



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AAS K. A., GARDNER F. H. Survival of blood platelets labeled with chromium. J Clin Invest. 1958 Sep;37(9):1257–1268. doi: 10.1172/JCI103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford R. H. Metal cation requirements for phytohemagglutinin-induced transformation of human peripheral blood lymphocytes. J Immunol. 1970 Mar;104(3):698–703. [PubMed] [Google Scholar]
- BARNES R. D. THYMIC NEOPLASMS ASSOCIATED WITH REFRACTORY ANAEMIA. Guys Hosp Rep. 1965;114:73–82. [PubMed] [Google Scholar]
- BURWELL E. L., BRICKLEY B. A., FINCH C. A. Erythrocyte life span in small animals; comparison of two methods employing radioiron. Am J Physiol. 1953 Mar;172(3):718–724. doi: 10.1152/ajplegacy.1953.172.3.718. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Böttiger L. E., Rausing A. Pure red cell anemia: immunosuppressive treatment. Ann Intern Med. 1972 Apr;76(4):593–597. doi: 10.7326/0003-4819-76-4-593. [DOI] [PubMed] [Google Scholar]
- CHALMERS J. N. M., BOHEIMER K. Pure red-cell anaemia in patients with thymic tumours. Br Med J. 1954 Dec 25;2(4903):1514–1518. doi: 10.1136/bmj.2.4903.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEGOWIN R. L., HOFSTRA D., GURNEY C. W. The mouse with hypoxia-induced erythremia, an erythropoietin bioassay animal. J Lab Clin Med. 1962 Nov;60:846–852. [PubMed] [Google Scholar]
- Dameshek W., Brown S. M., Rubin A. D. "Pure" red cell anemia (erythroblastic hypoplasia) and thymoma. Semin Hematol. 1967 Jul;4(3):222–232. [PubMed] [Google Scholar]
- DiGiacomo J., Furst S. W., Nixon D. D. Primary acquired red cell aplasia in the adult. J Mt Sinai Hosp N Y. 1966 Sep-Oct;33(5):382–395. [PubMed] [Google Scholar]
- Dresch C., Najean Y., Beauchet J. In vitro 51Cr and 32P-DFP labeling of granulocytes in man. J Nucl Med. 1971 Dec;12(12):774–784. [PubMed] [Google Scholar]
- EISEMANN G., DAMESHEK W. Splenectomy for pure red-cell hypoplastic (aregenerative) anemia associated with autoimmune hemolytic disease; report of a case. N Engl J Med. 1954 Dec 23;251(26):1044–1048. doi: 10.1056/NEJM195412232512603. [DOI] [PubMed] [Google Scholar]
- ENTWISTLE C. C., FENTEM P. H., JACOBS A. RED-CELL APLASIA WITH CARCINOMA OF THE BRONCHUS. Br Med J. 1964 Dec 12;2(5423):1504–1506. doi: 10.1136/bmj.2.5423.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantoni A., Bank A., Marks P. A. Globin composition and synthesis of hemoglobins in developing fetal mice erythroid cells. Science. 1967 Sep 15;157(3794):1327–1329. doi: 10.1126/science.157.3794.1327. [DOI] [PubMed] [Google Scholar]
- GALLIEN-LARTIQUE O., GOLDWASSER E. HEMOGLOBIN SYNTHESIS IN MARROW CELL CULTURE: THE EFFECT OF RAT PLASMA ON RAT CELLS. Science. 1964 Jul 17;145(3629):277–279. doi: 10.1126/science.145.3629.277. [DOI] [PubMed] [Google Scholar]
- Golstein P., Erik M. D., Svedmyr A. J., Wigzell H. Cells mediating specific in vitro cytotoxicity. I. Detection of receptor-bearing lymphocytes. J Exp Med. 1971 Dec 1;134(6):1385–1402. doi: 10.1084/jem.134.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLBOROW E. J., ASHERSON G. L., JOHNSON G. D., BARNES R. D., CARMICHAEL D. S. Antinuclear factor and other antibodies in blood and liver diseases. Br Med J. 1963 Mar 9;1(5331):656–658. doi: 10.1136/bmj.1.5331.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUISMAN T. H., MARTIS E. A., DOZY A. Chromatography of hemoglobin types on carboxymethylcellulose. J Lab Clin Med. 1958 Aug;52(2):312–327. [PubMed] [Google Scholar]
- HUISMAN T. H., MEYERING C. A. Studies on the heterogeneity of hemoglobin. I. The heterogeneity of different human hemoglobin types in carboxymethylcellulose and in amberlite IRC-50 chromatography qualitative aspects. Clin Chim Acta. 1960 Jan;5:103–123. doi: 10.1016/0009-8981(60)90098-x. [DOI] [PubMed] [Google Scholar]
- Harris R., Zervas J. D. Reticulocyte HL-A antigens. Nature. 1969 Mar 15;221(5185):1062–1063. doi: 10.1038/2211062a0. [DOI] [PubMed] [Google Scholar]
- Hrinda M. E., Goldwasser E. On the mechanism of erythropoietin-induced differentiation. VI. Induced accumulation of iron by marrow cells. Biochim Biophys Acta. 1969 Nov 19;195(1):165–175. doi: 10.1016/0005-2787(69)90613-3. [DOI] [PubMed] [Google Scholar]
- JACOBS E. M., HUTTER R. V., POOL J. L., LEY A. B. Benign thymoma and selective erythroid aplasia of the bone marrow. Cancer. 1959 Jan-Feb;12(1):47–57. doi: 10.1002/1097-0142(195901/02)12:1<47::aid-cncr2820120110>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Jepson J. H., Lowenstein L. Inhibition of erythropoiesis by a factor present in the plasma of patients with erythroblastopenia. Blood. 1966 Apr;27(4):425–434. [PubMed] [Google Scholar]
- KILLANDER J. SEPARATION OF HUMAN HEME- AND HEMOGLOBIN-BINDING PLASMA PROTEINS, CERULOPLASMIN AND ALBUMIN BY GEL FILTRATION. Biochim Biophys Acta. 1964 Oct 9;93:1–14. doi: 10.1016/0304-4165(64)90254-5. [DOI] [PubMed] [Google Scholar]
- KRANTZ S. B., GALLIEN-LARTIGUE O., GOLDWASSER E. THE EFFECT OF ERYTHROPOIETIN UPON HEME SYNTHESIS BY MARROW CELLS IN VITRO. J Biol Chem. 1963 Dec;238:4085–4090. [PubMed] [Google Scholar]
- Krantz S. B., Kao V. Studies on red cell aplasia. I. Demonstration of a plasma inhibitor to heme synthesis and an antibody to erythroblast nuclei. Proc Natl Acad Sci U S A. 1967 Aug;58(2):493–500. doi: 10.1073/pnas.58.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz S. B., Kao V. Studies on red cell aplasia. II. Report of a second patient with an antibody to erythroblast nuclei and a remission after immunosuppressive therapy. Blood. 1969 Jul;34(1):1–13. [PubMed] [Google Scholar]
- Krantz S. B. Studies on red cell aplasia. 3. Treatment with horse antihuman thymocyte gamma globulin. Blood. 1972 Mar;39(3):347–360. [PubMed] [Google Scholar]
- Krantz S. B. The effect of erythropoietin on human bone marrow cells in vitro. Life Sci. 1965 Dec;4(24):2393–2397. doi: 10.1016/0024-3205(65)90294-8. [DOI] [PubMed] [Google Scholar]
- LAJTHA L. G., SUIT H. D. Uptake of radioactive iron (59Fe) by nucleated red cells in vitro. Br J Haematol. 1955 Jan;1(1):55–61. doi: 10.1111/j.1365-2141.1955.tb05488.x. [DOI] [PubMed] [Google Scholar]
- Lau P., Cornwell G. G., 3rd, Williams W. J. Mucopolysaccharide synthesis by human bone marrow in short-term suspension cultures. J Lab Clin Med. 1970 Nov;76(5):739–746. [PubMed] [Google Scholar]
- MOLLISON P. L., VEALL N. The use of the isotope 51Cr as a label for red cells. Br J Haematol. 1955 Jan;1(1):62–74. doi: 10.1111/j.1365-2141.1955.tb05489.x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- RAMSAY W. N. The determination of iron in blood plasma or serum. Clin Chim Acta. 1957 Jun;2(3):214–220. doi: 10.1016/0009-8981(57)90105-5. [DOI] [PubMed] [Google Scholar]
- REIF A. E. IMMUNE CYTOLYSIS OF MOUSE THYMIC LYMPHOCYTES. J Immunol. 1963 Oct;91:557–567. [PubMed] [Google Scholar]
- ROLAND A. S. THE SYNDROME OF BENIGN THYMOMA AND PRIMARY AREGENERATIVE ANEMIA; AN ANALYSIS OF FORTY-THREE CASES. Am J Med Sci. 1964 Jun;247:719–731. doi: 10.1097/00000441-196406000-00013. [DOI] [PubMed] [Google Scholar]
- SANDERSON A. R. APPLICATIONS OF ISO-IMMUNE CYTOLYSIS USING RADIOLABELLED TARGET CELLS. Nature. 1964 Oct 17;204:250–253. doi: 10.1038/204250a0. [DOI] [PubMed] [Google Scholar]
- SCHILLING R. F. Intrinsic factor studies II. The effect of gastric juice on the urinary excretion of radioactivity after the oral administration of radioactive vitamin B12. J Lab Clin Med. 1953 Dec;42(6):860–866. [PubMed] [Google Scholar]
- SCHMID J. R., KIELY J. M., HARRISON E. G., Jr, BAYRD E. D., PEASE G. L. THYMOMA ASSOCIATED WITH PURE RED-CELL AGENESIS. REVIEW OF LITERATURE AND REPORT OF 4 CASES. Cancer. 1965 Feb;18:216–230. doi: 10.1002/1097-0142(196502)18:2<216::aid-cncr2820180214>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Safdar S. H., Krantz S. B., Brown E. B. Successful immunosuppressive treatment of erythroid aplasia appearing after thymectomy. Br J Haematol. 1970 Oct;19(4):435–443. doi: 10.1111/j.1365-2141.1970.tb06971.x. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- TSAI S. Y., LEVIN W. C. Chronic erythrocytic hypoplasia in adults; review of literature and report of a case. Am J Med. 1957 Feb;22(2):322–330. doi: 10.1016/0002-9343(57)90017-7. [DOI] [PubMed] [Google Scholar]
- Vilan J., Rhyner K., Ganzoni A. Immunosuppressive treatment of pure red-cell aplasia. Lancet. 1971 Jul 3;2(7714):51–51. doi: 10.1016/s0140-6736(71)90048-1. [DOI] [PubMed] [Google Scholar]



