Abstract
Background
The high incidence of ductal carcinoma in situ (DCIS) and variations in its treatment motivate inquiry into the comparative effectiveness of treatment options. Few such comparative effectiveness studies of DCIS, however, have been performed with detailed information on clinical and treatment attributes.
Methods
We collected detailed clinical, nonclinical, pathological, treatment, and long-term outcomes data from multiple medical records of 994 women who were diagnosed with DCIS from 1985 through 2000 in Monroe County (New York) and the Henry Ford Health System (Detroit, MI). We used ipsilateral disease-free survival models to characterize the role of treatments (surgery and radiation therapy) and margin status (positive, close [<2 mm], or negative [≥2 mm]) and logistic regression models to characterize the determinants of treatments and margin status, including the role of surgeons. All statistical tests were two-sided.
Results
Treatments and margin status were statistically significant and strong predictors of long-term disease-free survival, but results varied substantially by surgeon. This variation by surgeon accounted for 15%–35% of subsequent ipsilateral 5-year recurrence rates and for 13%–30% of 10-year recurrence rates. The overall differences in predicted 5-year disease-free survival rates for mastectomy (0.993), breast-conserving surgery with radiation therapy (0.945), and breast-conserving surgery without radiation therapy (0.824) were statistically significant (Pdiff < .001 for each of the differences). Similarly, each of the differences at 10 years was statistically significant (P < .001).
Conclusions
Our work demonstrates the contributions of treatments and margin status to long-term ipsilateral disease-free survival and the link between surgeons and these key measures of care. Although variation by surgeon could be generated by patients’ preferences, the extent of variation and its contribution to long-term health outcomes are troubling. Further work is required to determine why women with positive margins receive no additional treatment and why margin status and receipt of radiation therapy vary by surgeon.
CONTEXTS AND CAVEATS
Prior knowledge
Treatment strategies for ductal carcinoma in situ include breast-conserving surgery, with and without radiation therapy, and mastectomy, but the important factors influencing treatment outcomes, including extent of disease, choice of treatment, choice of physician, and margin of disease-free tissue from a resected tumor are unknown.
Study design
Clinical, demographic, and long-term outcomes data of 994 women diagnosed with ductal carcinoma in situ were used in survival, margin status, and logistic regression models to investigate the comparative effectiveness of treatment strategies and to characterize the roles of margin status and surgeon on treatment outcomes.
Contribution
There was substantial variation by surgeon in surgical treatment, receipt of radiation therapy, and margin status, all of which were statistically significantly associated with ipsilateral disease-free survival.
Implications
Treatment choices are made by both patients and surgeons, but the quality of care could be improved with more effective and standardized management of ductal carcinoma in situ by surgeons.
Limitations
The study was retrospective, and the data did not include detailed pathological characteristics of margins, which could influence treatment choices by surgeons. The roles of patient preferences in choice of treatment and surgeons and the beliefs of surgeons regarding breast conservation vs mastectomy were unknown.
From the Editors
The high incidence of ductal carcinoma in situ (DCIS) and variations in its treatment have motivated studies of the comparative effectiveness of the available treatment options. In fact, the Institute of Medicine has identified the management of DCIS as a priority for comparative effectiveness research (1). DCIS itself is nonlethal. The goal of treatment is to reduce the likelihood of developing invasive breast cancer while respecting patient preferences for treatment options, which include breast-conserving surgery (BCS) alone, BCS followed by radiation, and mastectomy (2). Tamoxifen is offered to some women to reduce the risk of subsequent ipsilateral breast events, both invasive and in situ (3). The purpose of this study was to investigate the comparative effectiveness of the treatment strategies in the management of DCIS and key factors associated with variations in treatments and outcomes.
Surgical decision making in patients with DCIS is influenced by the extent of disease within the breast and the presence of multifocality, which, in turn, affect the ability to achieve negative margins (areas bordering a resected tumor that are free of cancer cells) (2,4). In those who are candidates for breast conservation, patient preferences regarding breast preservation and receipt of radiation therapy also influence treatment selection (5,6). Some patients who initially attempt breast preservation ultimately have a mastectomy because of the presence of close (<2 mm) or positive surgical margins (7,8). The proportion of women who have breast preservation as their final surgery in the treatment of DCIS and have positive or close margins is unknown. Moreover, the clinical and nonclinical characteristics that predict receipt of breast preservation with positive or close final margins are unknown.
Radiation therapy is part of the breast conservation strategy for most patients with DCIS (9–12). Consensus guidelines, however, have for years suggested that omission of radiation therapy after BCS can be considered in women with small volume disease and wide negative margins (eg, >10 mm) after resection (13,14). Omission of radiation therapy was recently shown to be associated with a 6% ipsilateral breast event rate in women with low- or intermediate-grade DCIS, with margins of 3 mm or more compared with a 15% ipsilateral breast event rate in women with high-grade DCIS (15). Receipt of radiation therapy after BCS in patients treated in nontrial settings has been shown to be associated with clinical factors such as age (4,16,17), features of the disease such as tumor size and grade (4,5,17), and nonclinical factors such as treatment site (5,16,18), marital status (16), and educational attainment (16). Little work has been done to examine the relationship between receipt of radiation and margin status in DCIS patients, although one population-based study in Ontario found that half of 727 patients with margins smaller than 1 cm did not receive radiation therapy (4).
Rates of ipsilateral breast tumor recurrence after surgical treatment for DCIS are influenced by attributes of the surgical treatment (BCS vs mastectomy and margin status in women having BCS) (3), receipt of postoperative radiation therapy (17,19,20), extent of disease (3), nuclear grade (21), method of detection (palpation vs screening mammography) (3), younger age (3,22), comedo subtype (3), mammographic tumor size (23), and comorbidity (17).
Because DCIS is nonlethal, physicians’ attitudes regarding optimal management and patient preference may have an increased role in treatment decisions. Even in the treatment of invasive cancer, which affords less discretion in decision making because of effects on survival, treatment choice and receipt of adjuvant therapy vary by physician (5,24). The extent to which the type of surgery, surgical margins, and receipt of radiation therapy vary by surgeon in the management of DCIS is unknown.
We conducted a retrospective study to examine patterns of care and outcomes in women with DCIS diagnosed between 1985 and 2000. We used a large clinically detailed dataset with long-term outcomes of patients treated in diverse care settings to investigate the following: 1) the comparative effectiveness of the treatment strategies in the management of DCIS; 2) the factors associated with unfavorable outcomes; 3) the role of margin status as an intermediate outcome; and 4) the role of the treating surgeon in treatment, margin status, and outcomes.
Methods
Study Sample
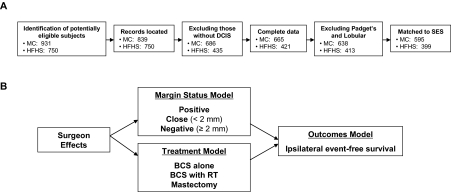
Women diagnosed with DCIS between the years 1985 and 2000 were identified from two tumor registries—the population-based Monroe County (MC) (New York) tumor registry and the tumor registry of the Henry Ford Health System (HFHS, Detroit, MI) (Figure 1, A). Patients with a history of cancer before the study period were excluded, as were those with microinvasive disease. We identified 931 and 750 potentially eligible subjects in MC and in the HFHS, respectively. We excluded observations for which records could not be found (92 in MC), subjects that did not have DCIS (153 in MC; 315 in HFHS) or complete data (21 in MC; 14 in HFHS), subjects found to have Padget disease or lobular cancer (27 in MC; eight in HFHS), and observations that could not be matched with census data containing socioeconomic status measures (43 in MC; 14 in HFHS), resulting in a final analytic sample that included 595 subjects in MC and 399 in the HFHS (Figure 1, A). Our estimated completion rates for eligible subjects were 81.5% (MC) and 93.5% (HFHS). There were 27 treating surgeons for the MC sample (a mean of 22.04 subjects per surgeon), 19 of whom treated more than 10 subjects. There were 23 surgeons for the HFHS sample (a mean of 17.35 subjects per surgeon), 12 of whom treated more than 10 subjects. The institutional review boards at the University of Rochester, the HFHS, and the RAND Corporation approved the study procedures.
Figure 1.
Sample selection and analytic flow diagrams. A) Sample selection flow diagram for Monroe County (MC) and Henry Ford Health System (HFHS) samples. B) Analytic plan diagram. BCS = breast-conserving surgery; RT = radiation therapy.
Data Collection
Trained medical record abstractors completed the data collection instrument via an exhaustive review of surgical, hospital, radiation oncology, and medical oncology records; pathology and operative reports; and, if necessary, primary care and gynecology records. The data collection instrument included clinical factors (age, comorbidity, menopausal status, and family history), nonclinical factors (date of diagnosis, race, ethnicity, insurance status and type of insurance, and address for census block group assignment), disease characteristics (grade, tumor size, width of the surgical margins, presence of a palpable mass at diagnosis, presence of calcifications, presence of “extensive” DCIS on pathology report, and mammographic extent of disease), treatment characteristics (type of surgery; number and type of surgical procedures; treating surgeon; receipt of tamoxifen; and receipt, extent, and dates of radiation therapy), and outcomes (date and laterality of recurrence, death, and date of last follow-up). Because we extracted data directly from the medical charts, the radiation therapy data were complete.
All pathology and treatment information was collected for each diagnostic and therapeutic procedure performed, including core biopsies, BCS, re-excisions, and mastectomy. Margin status, when noted in the pathology report, was categorized into one of three mutually exclusive categories—“positive,” “close” (within 2 mm), or “negative” (≥2 mm). A margin threshold of 2 mm or more has been identified as being associated with an important reduction in the risk of ipsilateral breast tumor recurrence in a recent meta-analysis (25). The number of margins and the extent of margin involvement were not available in the pathology records for most of the patients with close or positive margins. Every histologic subtype identified in the pathology reports was recorded, allowing for multiple subtypes per subject. The 2000 Census was used to assign a census block group for each patient; socioeconomic variables for each census block group included the percentage of people living below poverty and percentage of adults who were black. A medical oncologist (J. J. Griggs), surgical oncologist (G. M. Ahrendt), and breast pathologist (L. Schiffhauer) performed continuous quality checks of the data.
A strength of our dataset is that it contains detailed pathology information for every procedure performed on each subject. Thus, we were able to define measures that accumulated pathology information from biopsies, BCS, re-excisions, and mastectomy to characterize what was known at the time of the treatment decision (surgical or radiation therapy decision) and following therapy (outcomes). For treatment models (eg, BCS vs mastectomy), we accumulated pathology information, referred to here as treatment pathology, through the point at which the final decision was made: following the last diagnostic or breast-conserving procedure but before mastectomy (among those having mastectomy). At that point, and with all of the pathology information accumulated until then, the patient decided either to undergo mastectomy or to stop surgical treatment rather than to have an additional attempt at breast preservation (re-excision). Pathology information following mastectomy could not be used to inform this decision. For outcomes models, however, the accumulation of all pathology (referred to here as outcomes pathology) through the last surgical procedure (including mastectomy) provided the best information set to predict posttreatment ipsilateral event-free survival.
The main outcome of the study was ipsilateral recurrence. Our data included the date and laterality of breast events, the type of recurrence (invasive vs in situ), and the last date of follow-up for each subject after treatment. We calculated the time from the final surgical treatment until the first ipsilateral event, death, or the last date of follow-up.
Statistical Analyses
Our goals were 1) to examine the comparative effectiveness of treatment strategies, 2) to identify the factors associated with unfavorable outcomes, 3) to quantify the role of margin status, and 4) to quantify surgeon effects on treatments and outcomes. We developed three related models (Figure 1, B): an outcomes model (ipsilateral event-free survival), a treatment model (mastectomy, BCS alone, or BCS with radiation therapy), and a margins model (positive, close, or negative) (Figure 1, B). We addressed goals 1–3 by examining results of the outcomes model. We combined the estimates from each of the models to address goal 4, first assessing the effects of surgeons on treatments and margin status and then the surgeon effects through treatments and margins on outcomes (Figure 1, B).
We used bivariate analyses to assess the relationship between dichotomous indicators of outcomes (ipsilateral event vs no event), treatments (BCS alone, BCS with radiation therapy, mastectomy, and tamoxifen use), margin status, and each of the other clinical and nonclinical independent measures described above. Pearson χ2 tests of independence were used for categorical variables and for each dichotomous covariate defined for the multivariable model.
Outcomes (ipsilateral event-free survival) Model.
We specified standard discrete-time duration models to estimate the relationship between time to ipsilateral breast tumor recurrence and the clinical and nonclinical factors described above. The basic element of the duration model is the transition probability, which characterizes the probability of having an ipsilateral recurrence from 1 year to the next. We specified these transition probabilities as standard logistic regression models with the log of the odds ratio given by
for the ith subject τ years after surgery, where f (τ) is a flexible function of duration (time since treatment, τ); XC, XN, and XD are, respectively, vectors of clinical, nonclinical, and disease factors; the β are estimated parameters; and ϵ is an error term. We included outcomes pathology measures in XD. We estimated Huber–White SEs to account for the fact that each study subject can contribute multiple observations of annual transitions. We specified f(τ) with flexible functions to characterize duration dependence, alternatively specifying f(τ) nonparametrically (entering a vector of indicator variables to characterize time since treatment) and as second- and third-order polynomials inτ. Without interactions between τ and the other independent variables, the model is a proportional hazards model (each of the independent variables shift the hazard function proportionally over all τ). We estimated alternative specifications to test for interactions between τ and the clinical and nonclinical factors to determine if the proportional hazards assumption was valid. These tests failed to reject the proportional hazards assumption. Ultimately, we determined that quadratic specifications (second-order polynomials in τ) of the hazard function produced the best combination of parsimony and fit, failing to reject the implied restrictions against the nonparametric hazard specification.
Treatment Models.
We estimated multivariable logistic regression models to characterize treatment. We specified a model of mastectomy vs BCS as a function of XC, XN, and XD, where, this time, XD contained treatment pathology measures; that is, we modeled the decision to stop with BCS or to continue on to mastectomy using all the information available at the time of that key decision, including the margin status following the last BCS. We then specified a model of radiation therapy (vs no radiation therapy), given BCS, as a function of XC, XN, and XD. Because we estimated the radiation therapy equation only for those who received BCS, the treatment and outcomes pathology measures are equivalent. By estimating this sequential model (first surgical treatment, then radiation therapy conditional on BCS), we imposed a limited amount of structure on the relationships between treatments (mastectomy, BCS alone, and BCS with radiation therapy) and the independent variables. We estimated alternative specifications that included and excluded surgeon fixed effects.
Margins Models.
We estimated two multivariable logistic regression models to characterize margin outcomes for those who received BCS. First, we estimated whether a woman had positive margins as a function of XC, XN, and XD (where XD included outcomes pathology and excluded surgical margins). Then, conditional on a woman not having positive margins, we estimated a multivariable logistic regression model of close margins as a function of XC, XN, and XD. As above, the use of this sequential model allowed us to impose minimal structure on the relationships between margin status and the covariates. We limited these models to the sample of women who had BCS (n = 611) because non-negative margins were very rare following mastectomy in our sample. In each model, we estimated alternative specifications that included and excluded surgeon fixed effects.
To assess the substantive importance of our estimates after adjusting for clinical, nonclinical, and disease characteristics, we calculated predicted probabilities from our multivariable model estimates. We “standardized” the predictions by assigning each subject in our sample to each treatment (alternately BCS without radiation therapy, BCS with radiation therapy, and mastectomy) and predicting the probability of each margin status [the three probabilities given by P(M = m) for m = positive, close, and negative], given BCS, from our margins models. To determine margin status when we assigned a subject to mastectomy, we carried forward the actual margin status for those subjects who actually underwent mastectomy, and we assumed negative margins for those who actually underwent BCS. We then used the outcomes model to generate the patient's predicted ipsilateral event-free survival function conditional on her assigned treatment, indexed by k, and for each margin status, indexed by m [S(Tx = k,M = m, where S is the survival function, and Tx is treatment for k = BCS with radiation therapy, BCS without radiation therapy, and mastectomy, and for m = positive, close, and negative]. Finally, for each treatment, we generated the subject's unconditional survival function as
with the sum over m = positive, close, and negative for each treatment k. We then computed the population standardized survival function as the mean of S(Tx = k) over all subjects. These standardized predictions eliminated the consequences of population differences among treatments in the comparisons of effects.
Quantifying the Role of Surgeons.
To assess the importance of variation across surgeons in margin status and treatments (the surgeon fixed effects), we calculated predicted probabilities from the multivariable models under three alternative scenarios. For the receipt of radiation therapy and margins models, we set surgeon performance to be no worse than a standard (alternately the sample mean rate, the rate of the median surgeon, or the rate of the 75th percentile surgeon). Thus, we limited the variation across surgeons by systematically raising the performance (to a standard) of those performing poorly. We did not alter the performance of those who were outperforming the standard. All models were estimated using Stata version 9.0 (StataCorp, 2005, Stata Statistical Software: Release 9, College Station, TX, StataCorp LP).
Results
Most of the subjects in our study (53.9%) had more than one surgical procedure, but few (4.3%) had more than two procedures. The first procedure was almost always BCS (93.8%). Mastectomy was more common on the second (56.1%) and third procedures (83%). We classified surgical treatment based on the last procedure performed, but as described above, we accumulated pathology information from all surgical procedures. Most of the patients (58.4%) were between 40 and 64 years of age. Only 5.3% were younger than 40 years, and 36.3% were older than 64 years (Table 1). Many women had positive (4.6%) or close (20.0%) margins following their last surgery. Nearly 86% of the sample had DCIS detected with mammography. Approximately 61% had BCS, and approximately 60% of those had radiation therapy. The median follow-up was 5 years with a maximum of 18 years. Among women with ipsilateral breast events, 52.5% of the recurrences were in situ and 47.5% were invasive disease (Table 1).
Table 1.
Patient, treatment, and disease characteristics*
| No. (%) | ||
| Socioeconomic and demographic characteristics | ||
| Age group, y | ||
| <40 | 53 (5.3) | |
| 40–49 | 230 (23.1) | |
| 50–64 | 350 (35.2) | |
| ≥65 | 361 (36.3) | |
| Insurance | ||
| Private | 395 (39.7) | |
| Medicare and private | 73 (7.3) | |
| Medicare | 328 (33.0) | |
| Medicaid/other/unknown | 198 (19.9) | |
| Race | ||
| White | 789 (79.4) | |
| Black | 152 (15.3) | |
| Asian | 10 (1.0) | |
| Other | 43 (4.3) | |
| Clinical characteristic | No. (%) | |
| No. of comorbid conditions | ||
| 0 | 339 (34.1) | |
| 1 | 330 (33.2) | |
| ≥2 | 325 (32.7) | |
| Family history of breast cancer | ||
| Yes | 201(20.2) | |
| No | 672 (67.6) | |
| Unknown | 121 (12.2) | |
| Menopausal status | ||
| Premenopausal | 241 (24.2) | |
| Perimenopausal | 38 (3.8) | |
| Postmenopausal | 637 (64.1) | |
| Unknown | 78 (7.8) | |
| Detected with mammogram | 853 (85.8) | |
| Mammographic tumor size unknown† | 785 (79.0) | |
| Calcifications | ||
| Yes | 807 (81.2) | |
| No | 142 (14.3) | |
| Unknown | 45 (4.5) | |
| Continuous variables | Mean (SD) | |
| Census block group variables (N = 994) | ||
| Percent living below poverty | 9.7 (11.2) | |
| Percent black | 17.53 (1.1) | |
| Mammographic tumor size (cm) | ||
| If known (N = 199)† | 1.8 (1.4) | |
| Pathology characteristic | Treatment pathology,‡ No. (%) | Outcome pathology,§ No. (%) |
| Histology║ | ||
| Comedo | 318 (32.0) | 335 (33.7) |
| Cribriform | 371 (37.3) | 388 (39.0) |
| Apocrine | 12 (1.2) | 12 (1.2) |
| Solid | 217 (20.8) | 217 (21.8) |
| Papillary | 80 (7.5) | 80 (8.0) |
| Micropapillary | 129 (12.8) | 129 (13.0) |
| Other | 24 (2.4) | 27 (2.7) |
| Unknown | 48 (4.8) | 55 (5.5) |
| Multifocal disease | ||
| No | 146 (14.7) | 140 (14.1) |
| Yes | 345 (34.7) | 391 (39.3) |
| Unknown | 503 (50.6) | 463 (46.6) |
| Extensive DCIS | 99 (10.0) | 59 (5.9) |
| Margin status | ||
| Negative (≥2 mm) | 550 (55.3) | 749 (75.4) |
| Close (<2 mm) | 250 (25.2) | 199 (20.0) |
| Positive | 194 (19.5) | 46 (4.6) |
| Nuclear grade | ||
| High | 133 (13.4) | 134 (13.5) |
| Medium | 193 (19.4) | 193 (19.4) |
| Low | 425 (42.8) | 437 (44.0) |
| Unknown | 243 (24.4) | 230 (23.1) |
| Necrosis | ||
| Present | 553 (55.6) | 565 (56.8) |
| Absent | 386 (38.8) | 320 (32.2) |
| Punctate | 55 (5.5) | 57 (5.7) |
| Central | 449 (45.2) | 463 (46.6) |
| Treatments, margins, and ipsilateral breast tumor recurrences | ||
| Treatments and margins, No. (% of total)* | Recurrences, No. (% of women with specified treatment)* | |
| Mastectomy | 383 (38.5) | 7 (1.8) |
| With RT¶ | 9 (0.9) | 2 (22.2) |
| Without RT | 374 (97.6) | 5 (1.4) |
| BCS | 611 (61.5) | 52 (8.5) |
| With RT# | 357 (36.8) | 19 (5.3) |
| Without RT | 254 (25.5) | 33 (13.0) |
| Negative margins | 749 (75.3) | 31 (4.1) |
| Close margins | 199 (20.0) | 19 (9.5) |
| Positive margins | 46 (4.6) | 9 (19.6) |
Total N = 994; number of women with recurrences = 59; BCS = breast-conserving surgery; NA = not applicable; RT = radiation therapy.
Mammographic tumor size was often not applicable and therefore not recorded (unknown).
Refers to pathology information through the point at which the final surgical decision was made.
Refers to the final pathology information accumulated through the final surgical procedure.
Histology categories are not mutually exclusive and therefore percents sum to more than 100%.
2.3% of those having mastectomy.
59.9% of those having BCS.
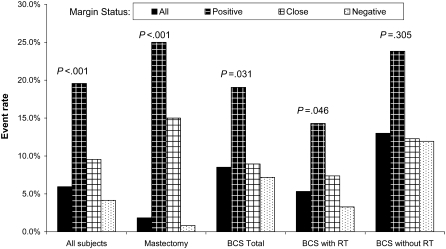
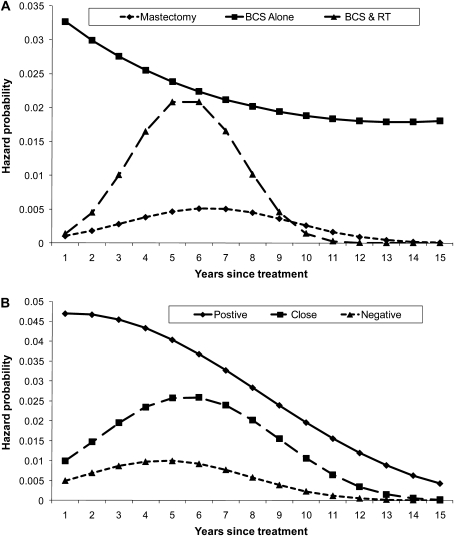
We calculated unadjusted ipsilateral breast tumor recurrence rates that do not account for censoring or the timing of recurrences (Figure 2). In all treatment groups except BCS without radiation therapy, the rates of recurrence were statistically significantly different by margin status (P values reflect Pearson χ2 tests of independence), with positive margins associated with substantially higher recurrence rates. Unadjusted hazard functions for ipsilateral event-free survival account for censoring and the timing of recurrences by admitting a quadratic function in duration but are not adjusted for other factors (Figure 3). A comparison by treatment shows that the hazard function for BCS without radiation therapy was always higher than either of the other two functions and was particularly high in the first few years after surgery (Figure 3, A). The hazard function for BCS with radiation therapy was higher than that of mastectomy for most of the follow-up period (Figure 3, A). A comparison of the hazard functions by margin status shows that the hazard function for those with positive margins was high over the first few years after surgery and, although it decreased over time, it was always substantially higher than the functions for either close margins or negative margins (Figure 3, B). The hazard function for those with negative margins was low throughout the follow-up period (Figure 3, B).
Figure 2.
Unadjusted ipsilateral event rates by treatment and margin status. Comparisons were made using Pearson χ2 test of independence (two-sided). BCS = breast-conserving surgery; RT = radiation therapy.
Figure 3.
Unadjusted hazard functions for ipsilateral event-free survival, accounting for censoring and admitting quadratic duration dependence. A) Hazard functions by treatments, including breast-conserving surgery (BCS) alone, BCS with radiation therapy, and mastectomy. B) Hazard functions by margin status, including positive, close (<2 mm), and negative (≥2 mm) margins.
Multivariable Outcomes Models
In the multivariable, discrete-time ipsilateral event-free survival models, we found that a quadratic specification of time since treatment (duration) was sufficient to characterize duration dependence (Table 2). In addition, more flexible specifications produced similar results for other parameter estimates. All of the pathology measures were defined following the last surgery (outcomes pathology) to provide the best information set for determining health outcomes. Treatments, age, margin status, comedo histology, multiple comorbidities, and mammographic tumor size were all statistically significant and substantively important predictors of outcomes; Table 2). Younger age was strongly associated with increased rates of recurrence (age <40 years compared with age ≥65 years, relative risk [RR] = 8.41, 95% confidence interval [CI] = 2.68 to 26.42, P < .001). Comedo histologic subtype was associated with substantially higher relative risk of ipsilateral breast tumor recurrence (RR = 2.05, 95% CI = 1.12 to 3.76, P = .02) as was having two or more comorbid illnesses (RR = 2.33, 95% CI = 1.22 to 4.45, P = .01).
Table 2.
Hazard model for ipsilateral event-free survival*
| Covariate† | RR (95% CI) | P |
| Duration | 1.25 (0.90 to 1.73) | .18 |
| Duration2‡ | 0.99 (0.96 to 1.02) | .35 |
| Age, y | ||
| <40 | 8.41 (2.68 to 26.42) | <.001 |
| 40–49 | 3.20 (1.42 to 7.19) | .01 |
| 50–64 | 1.29 (0.61 to 2.70) | .51 |
| ≥65 | 1.00 (referent) | |
| Race | ||
| Non-black race | 1.00 (referent) | |
| Black race | 1.01 (0.49 to 2.12) | .97 |
| No. of comorbid conditions | ||
| <2 | 1.00 (referent) | |
| ≥2 | 2.33 (1.22 to 4.45) | .01 |
| Histologic subtype | ||
| Non-comedo histologic subtype | 1.00 (referent) | |
| Comedo histologic subtype | 2.05 (1.12 to 3.76) | .02 |
| Multifocality | ||
| Absent | 1.00 (referent) | |
| Present | 2.19 (0.92 to 5.23) | .08 |
| Treatment | ||
| Mastectomy | ||
| Negative margins (≥2 mm) | 0.03 (0.01 to 0.09) | <.001 |
| Close margins (<2mm) | 0.36 (0.07 to 1.81) | .22 |
| Positive margins | 1.98 (0.19 to 20.13) | .56 |
| BCS | ||
| Negative margins | 1.00 (referent) | |
| Close margins | 1.39 (0.71 to 2.73) | .33 |
| Positive margins | 3.38 (1.22 to 9.34) | .02 |
| Radiation therapy | 0.25 (0.14 to 0.46) | <.001 |
| Annual transitions (person-years) | 5445.00 | |
| Log pseudo-likelihood | −274.91 | |
| Pseudo R2 | 0.1558 | |
| Test of equivalence of BCS + RT and mastectomy§ | χ2 = 13.63 | <.001 |
N = 994 women; BCS = breast-conserving surgery; CI = confidence interval; RR = relative risk; RT = radiotherapy.
Other covariates not shown include year, census level percent black, census level percent below poverty, insurance status and type, histologic subtype, mammographic tumor size, presence of extensive ductal carcinoma in situ, nuclear grade, menopausal status, calcifications, tamoxifen use, and method of detection.
Duration is time since treatment and duration2 is time since treatment squared.
Two-sided χ2 test with 1 df of BCS + RT = 0.
The treatment received was independently associated with the risk of ipsilateral breast events. For women who had mastectomy and achieved negative margins, the relative risk of recurrence was 0.03 (95% CI = 0.01 to 0.09, P < .001) compared with women who had BCS and negative margins. Those who had BCS with positive margins had a relative risk of recurrence of 3.38 (95% CI = 1.22 to 9.34, P = .02). Radiation therapy was independently associated with a reduced relative risk of recurrence (RR = 0.25, 95% CI = 0.14 to 0.46, P < .001). Among women who had mastectomy, margin status was also important, but few women had positive (n = 4) or close (n = 20) margins. An alternative model that excluded margin status showed that, although margin status is highly predictive of recurrence rates, the exclusion of these measures had very little effect on the other covariate estimates.
Multivariable Treatment Models
Estimates of the logistic regression treatment models with and without physician fixed effects were similar; thus, we present only the results from the models with fixed effects (Table 3). The results indicate that many of the clinical and nonclinical measures were statistically significant and important predictors of BCS. Use of BCS increased over calendar time (year) but at a decreasing rate (year2). Disease characteristics and risk factors played a strong role in the determination of surgical treatment. In particular, family history (odds ratio [OR] = 0.48, 95% CI = 0.32 to 0.71, P < .001), multifocal disease (OR = 0.38, 95% CI = 0.22 to 0.67, P = .001), and extensive DCIS (OR = 0.27, 95% CI = 0.15 to 0.52, P < .001) were all associated with lower rates of BCS. Detection of disease with mammography (OR = 1.85, 95% CI = 1.08 to 3.16, P = .03) and cribiform histologic subtype (OR = 1.56, 95% CI = 1.07 to 2.28, P = .02) were associated with higher rates of BCS. There is little evidence that surgical treatment was associated with the comedo subtype of DCIS. As expected, the margin status following the last breast-conserving procedure (treatment pathology) was strongly predictive of the final surgical treatment. That is, positive margins following BCS were strongly associated with subsequent mastectomy (OR = 0.07, 95% CI = 0.04 to 0.12, P < .001). Close margins, however, were unrelated to subsequent mastectomy (OR = 0.74, 95% CI = 0.49 to 1.12, P = .16). Positive margins were fairly common in the treatment pathology (19.5% following the last breast-preserving surgery and before a possible mastectomy, Table 1), and although positive margins were highly predictive of subsequent mastectomy, nearly 5% of the patients in our sample completed surgical treatment with BCS and positive margins and 20% with BCS and close margins (Table 1). The physician fixed effects were jointly statistically significant (P = .03), revealing unexplained surgical treatment variation by surgeon. These differences across physicians occurred even when controlling for detailed clinical and pathological characteristics.
Table 3.
Treatment equation models*
| Covariate† | BCS (N = 994) |
Radiation therapy among those having BCS (N = 611) |
||
| OR (95% CI) | P | OR (95% CI) | P | |
| Year | 1.61 (1.26 to 2.06) | <.001 | 0.94 (0.66 to 1.35) | .74 |
| Year2 | 0.98 (0.97 to 0.99) | .004 | 1.01 (0.99 to 1.03) | .29 |
| Age, y | ||||
| <40 | 0.50 (0.18 to 1.42) | .19 | 1.08 (0.27 to 4.43) | .91 |
| 40–49 | 0.55 (0.26 to 1.16) | .11 | 4.49 (1.65 to 2.23) | .003 |
| 50–64 | 0.63 (0.37 to 1.06) | .08 | 2.71 (1.44 to 5.12) | .002 |
| ≥65 | 1.00 (referent) | 1.00 (referent) | ||
| Race | ||||
| Black | 0.92 (0.41 to 2.06) | .84 | 2.24 (0.82 to 6.09) | .12 |
| Non-black | 1.00 (referent) | 1.00 (referent) | ||
| No. of comorbid conditions | ||||
| <2 | 1.00 (referent) | 1.00 (referent) | ||
| ≥2 | 0.79 (0.55 to 1.14) | .21 | 0.91 (0.58 to 1.45) | .71 |
| Histologic subtype | ||||
| Comedo | 0.77 (0.45 to 1.33) | .36 | 1.88 (0.91 to 3.92) | .09 |
| Cribriform | 1.56 (1.07 to 2.28) | .02 | 1.03 (0.65 to 1.65) | .90 |
| Multifocality | ||||
| Yes | 0.38 (0.22 to 0.67) | .001 | 2.33 (1.25 to 4.35) | .01 |
| Unknown | 0.46 (0.27 to 0.80) | .01 | 1.59 (0.91 to 2.79) | .10 |
| No | 1.00 (referent) | 1.00 (referent) | ||
| Extensive DCIS | ||||
| Yes | 0.27 (0.15 to 0.52) | <.001 | 0.74 (0.21 to 2.59) | .64 |
| No | 1.00 (referent) | 1.00 (referent) | ||
| Nuclear grade | ||||
| Low | 1.00 (referent) | 1.00 (referent) | ||
| Medium | 0.88 (0.45 to 1.72) | .72 | 2.73 (1.33 to 5.62) | .01 |
| High | 0.66 (0.32 to 1.35) | .25 | 6.18 (2.63 to 14.53) | <.001 |
| Unknown | 0.57 (0.30 to 1.07) | .08 | 3.59 (1.71 to 7.53) | .001 |
| Margins | ||||
| Negative (≥2 mm) | 1.00 (referent) | 1.00 (referent) | ||
| Close (<2 mm) | 0.74 (0.49 to 1.12) | .16 | 1.62 (0.98 to 2.70) | .06 |
| Positive | 0.07 (0.04 to 0.12) | <.001 | 0.46 (0.19 to 1.13) | .09 |
| Family history of breast cancer | ||||
| No | 1.00 (referent) | 1.00 (referent) | ||
| Yes | 0.48 (0.32 to 0.71) | <.001 | 0.63 (0.37 to 1.08) | .09 |
| Unknown | 1.03 (0.59 to 1.79) | .92 | 0.32 (0.16 to 0.63) | .001 |
| Method of detection | ||||
| Detected with mammogram | 1.85 (1.08 to 3.16) | .03 | 1.21 (0.60 to 2.46) | .60 |
| Palpable disease | 1.00 (referent) | 1.00 (referent) | ||
| Mammographic tumor size | ||||
| Unknown | 0.52 (0.26 to 1.05) | .07 | 0.47 (0.19 to 1.12) | .09 |
| Mammographic tumor size | 0.73 (0.55 to 0.97) | .03 | 0.80 (0.53 to 1.23) | .31 |
| Log pseudo-likelihood | 474.889 | −301.667 | ||
| Pseudo R2 | 0.283 | 0.273 | ||
| Test of physician fixed effects‡ | χ2 = 32.530 | .03 | χ2 = 51.340 | <.001 |
Multivariable logistic regression models: the breast-conserving surgery (BCS) model is a logistic regression of BCS vs mastectomy; the radiation therapy (RT) model is a logistic regression of RT vs no RT among women who had BCS. CI = confidence intervals; DCIS = ductal carcinoma in situ; OR = odds ratio.
Other covariates not shown include census level percent black, census level percent below poverty, insurance status and type, menopausal status, and calcifications.
The test of the joint significance of the physician fixed effects: two-sided χ2 with 19 df.
The multivariable logistic regression model for radiation therapy was estimated on the sample of women who had BCS (n = 611) (Table 3). Few sociodemographic characteristics were associated with the use of radiation therapy. Subjects aged 40–49 years (OR = 4.49, 95% CI = 1.65 to 12.23, P = .003) and 50–64 years (OR = 2.71, 95% CI = 1.44 to 5.12, P = .002) were more likely to receive radiation therapy than older women. Younger women (<40 years) had similar rates of radiation therapy than older women, but the estimates are not precise because of small sample size. Clinical characteristics that were important predictors of radiation therapy included multifocal disease (OR = 2.33, 95% CI = 1.25 to 4.35, P = .008) and nuclear grade (high relative to low: OR = 6.18, 95% CI = 2.63 to 14.53, P < .001; medium relative to low: OR = 2.73, 95% CI = 1.33 to 5.62, P = .01). Margin status was not statistically significantly associated with radiation therapy. Positive margins, however, were weakly associated with lower rates of radiation therapy (OR = 0.46, 95% CI = 0.19 to 1.13, P = .09), suggesting that the women who were at increased risk of recurrence were less likely to get radiation therapy. Histologic subtype or sociodemographic characteristics (other than age) were not associated with the use of radiation therapy. The surgeon fixed effects were jointly statistically significant (P < .001), again suggesting that even after controlling for detailed clinical and pathological characteristics, there was substantial unexplained variation in rates of radiation therapy use across surgeons. When we characterized the importance of variation across surgeons by alternative adjusted prediction scenarios—in which we assigned surgeons with low radiation therapy rates alternately to mean, median, and 75th percentile values—we found that surgeon effects were large. The alternative scenarios increased the overall rate of radiation receipt by 13.7%, 14.9%, and 30.6%, respectively.
Multivariable Margin Models
The multivariable models of margin status identified characteristics predictive of positive or close margins for women who had BCS (Table 4). The positive margins model (relative to close or negative margins) showed that multifocal disease (OR = 16.40, 95% CI = 2.26 to 118.87, P = .01) and extensive DCIS (OR = 8.93, 95% CI = 1.48 to 53.82, P = .02) were predictive of positive margins. Nuclear grade (medium relative to low: OR = 11.13, 95% CI = 2.69 to 46.07, P = .001; high relative to low: OR = 5.65, 95% CI = 1.48 to 21.52, P = .01) was also associated with margin status. No other characteristics were statistically significantly associated with positive margins. Surgeon fixed effects were jointly statistically significant (P = .025), indicating that after controlling for detailed clinical and nonclinical characteristics, there was still unexplained variation across surgeons in margin status following the last surgical treatment. Quantifying the role of surgeon variation with alternative adjusted prediction scenarios, we found that the surgeon effects were large, that is, positive margins would be reduced by 45.5% (from 7% to 3.8%) if each surgeon with positive margin rates above the overall mean were assigned the mean rate.
Table 4.
Margin models in patients having breast-conserving surgery (BCS)*
| Covariate† | Positive margins (N = 611) |
Close margins (N = 569) |
||
| OR (95% CI) | P | OR (95% CI) | P | |
| Age, y | ||||
| ≤40 | 0.43 (0.07 to 2.76) | .37 | 0.15 (0.03 to 0.78) | .03 |
| 40–49‡ | 0.60 (0.23 to 1.54) | .29 | ||
| 50–64 | 0.54 (0.16 to 1.90) | .34 | 0.58 (0.31 to 1.09) | .09 |
| ≥65 | 1.00 (referent) | 1.00 (referent) | ||
| Race | ||||
| Black | 3.76 (0.76 to 18.62) | .10 | 1.17 (0.44 to 3.09) | .75 |
| Non-black | 1.00 (referent) | 1.00 (referent) | ||
| Histologic subtype | ||||
| Comedo | 2.50 (0.64 to 9.77) | .19 | 1.50 (0.76 to 2.98) | .25 |
| Cribriform | 1.43 (0.49 to 4.18) | .52 | 0.98 (0.61 to 1.60) | .95 |
| Multifocality | ||||
| Yes | 16.40 (2.26 to 118.87) | .01 | 0.93 (0.50 to 1.75) | .83 |
| Unknown | 9.58 (1.35 to 68.00) | .02 | 0.85 (0.49 to 1.49) | .57 |
| No | 1.00 (referent) | 1.00 (referent) | ||
| Extensive DCIS | ||||
| Present | 8.93 (1.48 to 53.82) | .02 | 6.70 (1.39 to 32.34) | .02 |
| Absent | 1.00 (referent) | 1.00 (referent) | ||
| Nuclear grade | ||||
| Low | 1.00 (referent) | 1.00 (referent) | ||
| Unknown | 3.52 (0.63 to 19.64) | .15 | 1.04 (0.50 to 2.16) | .92 |
| Medium | 11.13 (2.69 to 46.07) | .001 | 2.48 (1.26 to 4.87) | .009 |
| High | 5.65 (1.48 to 21.52) | .01 | 4.18 (2.26 to 7.72) | <.001 |
| Log pseudo-likelihood | −93.97 | −281.07 | ||
| Pseudo R2 | 0.386 | 0.207 | ||
| Test of physician fixed effects§ | χ2 = 27.530 | .02 | χ2 = 29.710 | .04 |
Multivariable logistic regression models: the positive margins model is a logistic regression of positive margins vs close (<2 mm) or negative (≥2 mm) margins among women who had BCS; the close margins model is a logistic regression of close margins vs negative margins among women who had BCS and did not have positive margins. CI = confidence interval; DCIS = ductal carcinoma in situ; OR = odds ratio.
Other covariates not shown include calendar year, insurance, census level percent black, census level percent below poverty, family history, menopausal status, method of detection, mammographic tumor size, and calcifications.
Age groups 40 years or younger and 40–49 years are combined because of the small number of observations with positive margins in these categories.
The test of the joint significance of the physician fixed effects in the margins positive model is two-sided χ2 with 15 df; in the margins close model it is two-sided χ2 with 18 df.
The close margins model (relative to negative margins), conditional on patients receiving BCS and not having positive margins, showed similar results to the positive margins model for extensive DCIS and nuclear grade (Table 4). Multifocal DCIS, however, was not a statistically significant predictor. The surgeon fixed effects were jointly statistically significant (P = .04). Alternative scenarios that set surgeons’ rates to no higher than the overall mean rate for positive margins over all surgeons reduced the probability of close margins by 25.5%.
The results from the outcomes and margin models were used to generate adjusted or “standardized” predictions from the models to show the substantive importance of margin status and treatment differences in ipsilateral event-free survival (Table 5). The estimated adjusted probabilities of margin status given BCS indicate that 7% of patients were predicted to have positive margins and 31.2% close margins. Compared with mastectomy, in which the observed rates were 1% for positive margins and 5% for close margins (data not shown), the results indicated that margin status differed dramatically by treatment. The estimated adjusted probabilities of 5- and 10-year ipsilateral event-free survival rates given treatment (mastectomy, BCS alone, and BCS with radiation therapy) and margin status revealed importance differences in event-free survival. Positive margins had large effects on outcomes, even for mastectomy and BCS with radiation therapy. There were substantial reductions in ipsilateral event-free survival for BCS alone vs either BCS with radiation therapy or mastectomy, regardless of margin status. The overall differences in predicted 5-year disease-free survival rates for mastectomy (0.993), BCS with radiation therapy (0.945), and BCS without radiation therapy (0.824) were statistically significant (differences in 5-year event-free survival: mastectomy vs BCS with RT = 0.047, P < .001; mastectomy vs BCS without = 0.169, P < .001; and BCS with vs without RT = 0.121, P < .001). At 10 years, each of the differences was also statistically significant (P < .001 in each case).
Table 5.
Adjusted probabilities of margin status and ipsilateral breast tumor recurrence*
| Adjusted probabilities of margin status given BCS |
Adjusted probability of being event-free given treatments and margins |
|||||||
| Margin status | Adjusted probability (SE) | Treatment | Positive margins adjusted probability (SE) | Close margins adjusted probability (SE) | Negative margins adjusted probability (SE) | Total adjusted probability (SE) | Difference | Pdifference |
| 5-y ipsilateral event-free rate | ||||||||
| Negative (≥2 mm) | .618 (0.018) | Mastectomy | .750 (0.280) | .939 (0.053) | .995 (0.003) | .993 (0.004) | ||
| Close (<2 mm) | .312 (0.020) | BCS | .641 (0.106) | .808 (0.053) | .852 (0.030) | .824 (0.033) | ||
| Positive | .070 (0.014) | BCS and RT | .871 (0.051) | .940 (0.017) | .956 (0.011) | .945 (0.012) | ||
| Mastectomy—BCS | .169 | <.001 | ||||||
| Mastectomy—BCS and RT | .047 | <.001 | ||||||
| BCS—BCS and RT | −.121 | <.001 | ||||||
| 10-y ipsilateral event-free rate | ||||||||
| Mastectomy | .558 (0.319) | .870 (0.099) | .988 (0.008) | .984 (0.008) | ||||
| BCS | .419 (0.130) | .642 (0.085) | .714 (0.056) | .671 (0.057) | ||||
| BCS and RT | .744 (0.088) | .873 (0.036) | .905 (0.023) | .884 (0.024) | ||||
| Mastectomy—BCS | .313 | <.001 | ||||||
| Mastectomy—BCS and RT | .100 | <.001 | ||||||
| BCS—BCS and RT | −.213 | <.001 | ||||||
The Role of Variation by Surgeon in Outcomes
After quantifying the role of surgeons in treatments and margin status with the three alternative scenarios (setting surgeon effects to be no worse than the mean, median, and 75th percentile), and applying these treatment and margin effects to the outcome model estimates, we found that the effect on ipsilateral events was substantial. Setting surgeon effects to be no worse than the sample mean, median, and 75th percentile, we found reductions of 14.8%, 21.7%, and 35.0% in 5-year event rates, respectively, and 13.2%, 19.0%, and 31.4% in 10-year event rates, respectively.
Discussion
In this sample of 994 women with DCIS, for whom we had rich clinical and nonclinical data, ipsilateral event-free survival was predicted by surgical treatment, margin status, and receipt of radiation therapy. BCS in the absence of radiation therapy resulted in substantially lower ipsilateral event-free survival than either BCS followed by radiation therapy or mastectomy, regardless of margins, confirming the role of radiation therapy in the treatment of DCIS demonstrated in randomized controlled trials (9–12). BCS with radiation therapy also resulted in lower disease-free survival rates than mastectomy. Regardless of treatments, positive or close margins following the last surgical treatment substantially compromised ipsilateral event-free survival. Because close or positive margins were far more common following BCS than mastectomy, margin status contributed to the superior outcomes of mastectomy relative to BCS. Indeed, an important finding of our work is the large difference in subsequent breast event rates following BCS with positive margins, pointing to the value of additional surgery to achieve negative margins in these cases. Nevertheless, even among subjects with negative margins, we found that ipsilateral event-free survival following mastectomy was substantially greater than following BCS with radiation therapy. This increased risk of subsequent ipsilateral events may be offset, however, by patient preferences for breast preservation.
We found that there were substantial differences by surgeons in surgical treatment, receipt of radiation therapy, and margin status, the three most important predictors of ipsilateral event-free survival in our outcomes model. Other investigators have similarly identified a role of surgeons in the selection of surgical procedure (26) and in the use of radiation therapy after BCS in the management of DCIS (5). Our findings suggest that this variation across surgeons is substantively important. Simply by increasing surgeons’ rates of radiation therapy to no less than the sample mean, the overall receipt of radiation therapy could be increased by nearly 15%.
Similar models that predicted margin status, controlling for the detailed clinical and nonclinical factors, found very large unexplained differences by surgeon. We found that positive margins and close margins could be reduced by, respectively, 46% and 25% if all surgeons had positive and close margin rates no greater than the sample means.
Because of the importance of treatment choice and margin status in predicting outcomes, these unexplained differences by surgeon could have profound implications for health outcomes. With reductions in variation by surgeon, based only on changes among those surgeons with low rates of radiation therapy and high rates of positive or close margins, we found that ipsilateral 5- and 10-year event rates could be reduced by 15%–30%. These results have important implications about processes of care, decision making, and ultimately the quality of care for DCIS.
This study had several limitations. Although we found surgeon effects to be robust to the inclusion of the rich set of clinical and nonclinical measures and to the choice of model specifications, these differences by surgeon could be explained by other unobserved clinical or nonclinical factors. Our study was retrospective and our data did not include either the extent of the margin involvement or the number or location of margins involved, either of which could contribute to treatment variations by surgeon. Patient preferences about care (also unobservable to us) could be related to the choice of treating surgeon, consequently generating unexplained differences by surgeon. For example, a surgeon could develop a reputation for aggressively pursuing breast conservation, attracting patients who would, ceteris paribus, prefer to avoid mastectomy. If unrelated to any of our observable measures, this sort of patient-surgeon matching could generate variation in treatments across surgeons that is not strictly attributable to surgeon recommendations. Not only could this matching affect initial surgical choice and the decision to pursue subsequent breast-conserving procedures (re-excisions), but it could also explain the decision to decline additional surgery with close or positive margins. Depending on a woman's preferences, each of the decisions could be optimal, particularly if made with full information about the consequences.
Variation in decision making by surgeons could also, however, account for the large differences by surgeon. The variation could reflect differences in surgeons’ knowledge, attitudes, and beliefs, resulting in differences in treatments or margins for women who appear similar and who have equivalent preferences. For example, surgeons’ beliefs regarding the importance of breast conservation or the threat of DCIS, given lack of natural history data, could generate different recommendations for care that are not consistent with differences in women's preferences. Similarly, surgeons’ recommendations might vary based on their assessments of patients’ ability to adhere to adequate follow-up care.
Whether through differences in surgical technique or differences in beliefs about the importance of negative margins after surgery for DCIS (or both), the independent effect of surgeon on margin status, after controlling for extensive clinical and nonclinical factors, is striking. There is currently no consensus on what constitutes a negative margin (25,27,28). The width of the surgical margin—and the decision to stop surgical treatment with persistently close or positive margins—may be related to surgeons’ aggressiveness with respect to the size of the excision specimen and the location of the lumpectomy cavity combined with their willingness to offer or recommend re-excision, particularly if the surgeons believe that patients will consider the need for additional surgery as a medical error. Failure to achieve negative margins could indicate an a priori need for mastectomy, implying a failure of the breast conservation therapy strategy in a given patient. Lack of knowledge about the importance of margins, and differences in beliefs about the role of radiation therapy in local control, together with differences in physician–patient communication during the decision-making process could explain the substantial variation in the acceptance of positive margins and the determination not to proceed to mastectomy.
Differences in financial incentives, such as relative reimbursement rates for BCS and mastectomy, could induce differences in surgical treatment (29). Similarly, insufficient reimbursements for performing re-excision could result in too few efforts to achieve negative margins. We controlled for patients’ insurance status and other socioeconomic status measures in our models, however, and found very little evidence that treatment choices were influenced by insurance.
The substantial variation in receipt of radiation therapy by surgeon is also a concern. The strength of referral relationships between treating surgeons and radiation oncologists could explain differences in rates of radiation therapy by surgeon in that such relationships may affect referral rates or the type of discussions held with patients about the role of radiation therapy. Surgeons may also vary in the degree of logistic support available in their practices to track referrals and follow-up after referral (30). In addition, surgeons’ beliefs about the importance of radiation therapy in improving the short- and long-term outcomes after surgery for DCIS have been shown to vary (5,31). Although we have shown that radiation therapy substantially increases ipsilateral event-free survival following BCS regardless of margin status, it is particularly alarming that there is not a strong relationship between margin status and receipt of radiation therapy.
Our work quantifies the important relationships among treatment choice, margin status, and ipsilateral event-free survival and identifies substantial unexplained variation in treatment choice and margin status across treating surgeons. Our results raise questions that go beyond our data. Why are patients willing to complete surgical treatment with close or positive margins? Is it because they are unwilling to undergo re-excision or mastectomy or because they are uninformed about the presence of or consequences of positive margins? How are the consequences of close or positive margins presented to patients by their surgeons, and is the presentation influenced by the surgeons’ confidence that radiation therapy can adequately provide local control? The latter is particularly interesting in light of the weak relationship we found between margin status and the receipt of radiation therapy among women who received BCS.
Because these decisions have important health outcomes consequences, it would be unsettling if the variation in surgeon effects were not a reflection of differences in women's preferences about the relative merits of risk and breast conservation. Our work, however, provides no evidence regarding patient–surgeon interactions and, more specifically, whether surgeons or patients are ultimately responsible for the variation in treatments. Nevertheless, an important implication of our work is that surgeons may play a critical role both in the surgical treatment choices made by patients (the initial choice to pursue BCS and subsequent re-excisions) and in the receipt of radiation therapy. Because these are the most important factors in predicting outcomes (particularly margin status and receipt of radiation therapy), the substantial variation by surgeon suggests that the quality of DCIS care could be improved.
Funding
National Cancer Institute at the National Institutes of Health (R01 CA922444-01A1 to A.W.D., M.S.S., G.M.A., J.A.H., H.T.G., L.S., A.Z., and J.J.G.).
Footnotes
The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit the article for publication. We gratefully acknowledge the contributions of Susanne Heininger and Julie Mallinger for their work in collecting and assembling the data.
References
- 1.Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 2.Morrow M, Harris J. Ductal carcinoma in situ and microinvasive carcinoma. In: Harris J, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 4th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams and Wilkins; 2010. pp. 349–362. [Google Scholar]
- 3.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 4.Rakovitch E, Pignol JP, Chartier C, et al. The management of ductal carcinoma in situ of the breast: a screened population-based analysis. Breast Cancer Res Treat. 2007;101(3):335–347. doi: 10.1007/s10549-006-9302-0. [DOI] [PubMed] [Google Scholar]
- 5.Katz SJ, Lantz PM, Janz NK, et al. Patterns and correlates of local therapy for women with ductal carcinoma-in-situ. J Clin Oncol. 2005;23(13):3001–3007. doi: 10.1200/JCO.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101(19):1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15(5):1297–1303. doi: 10.1245/s10434-007-9777-x. [DOI] [PubMed] [Google Scholar]
- 9.Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 11.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853–a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26(8):1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 13.Carlson RW, Goldstein LJ, Gradishar WJ, et al. NCCN Breast Cancer Practice Guidelines. The National Comprehensive Cancer Network. Oncology. 1996;10(11 suppl):47–75. [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology. Breast Cancer V.1.2009. Ft Washington, PA: National Comprehensive Cancer Network; 2009. [DOI] [PubMed] [Google Scholar]
- 15.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(32):5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold HT, Dick AW. Variations in treatment for ductal carcinoma in situ in elderly women. Med Care. 2004;42(3):267–275. doi: 10.1097/01.mlr.0000114915.98256.b4. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Weaver DL, Bocklage T, et al. The frequency of ipsilateral second tumors after breast-conserving surgery for DCIS: a population based analysis. Cancer. 2005;104(9):1840–1848. doi: 10.1002/cncr.21406. [DOI] [PubMed] [Google Scholar]
- 18.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96(6):443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 19.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86(3):429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Rakovitch E, Pignol JP, Hanna W, et al. Significance of multifocality in ductal carcinoma in situ: outcomes of women treated with breast-conserving therapy. J Clin Oncol. 2007;25(35):5591–5596. doi: 10.1200/JCO.2007.11.4686. [DOI] [PubMed] [Google Scholar]
- 21.Kerlikowske K, Molinaro A, Cha I, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95(22):1692–1702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 22.Vargas C, Kestin L, Go N, et al. Factors associated with local recurrence and cause-specific survival in patients with ductal carcinoma in situ of the breast treated with breast-conserving therapy or mastectomy. Int J Radiat Oncol Biol Phys. 2005;63(5):1514–1521. doi: 10.1016/j.ijrobp.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald HR, Silverstein MJ, Mabry H, et al. Local control in ductal carcinoma in situ treated by excision alone: incremental benefit of larger margins. Am J Surg. 2005;190(4):521–525. doi: 10.1016/j.amjsurg.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Hiotis K, Ye W, Sposto R, Skinner KA. Predictors of breast conservation therapy: size is not all that matters. Cancer. 2005;103(5):892–899. doi: 10.1002/cncr.20853. [DOI] [PubMed] [Google Scholar]
- 25.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27(10):1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 26.Hawley ST, Hofer TP, Janz NK, et al. Correlates of between-surgeon variation in breast cancer treatments. Med Care. 2006;44(7):609–616. doi: 10.1097/01.mlr.0000215893.01968.f1. [DOI] [PubMed] [Google Scholar]
- 27.Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005;241(4):629–639. doi: 10.1097/01.sla.0000157272.04803.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diagnosis and Management of Ductal Carcinoma in Situ (DCIS) Bethesda, MD: National Institutes of Health State-of-the-Science Conference Statement; 2009. [Google Scholar]
- 29.Hadley J, Mandelblatt JS, Mitchell JM, Weeks JC, Guadagnoli E, Hwang YT. Medicare breast surgery fees and treatment received by older women with localized breast cancer. Health Serv Res. 2003;38(2):553–573. doi: 10.1111/1475-6773.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25(18):2516–2521. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 31.Ceilley E, Jagsi R, Goldberg S, et al. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61(2):365–373. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]