Abstract
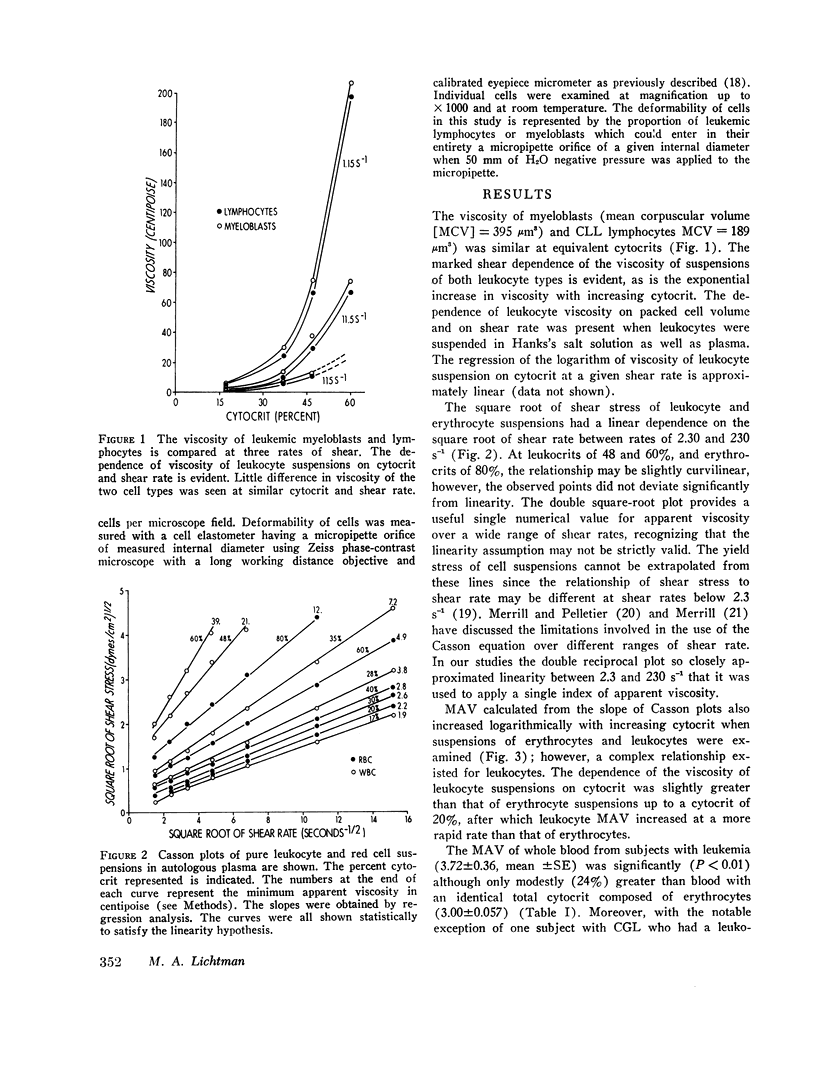
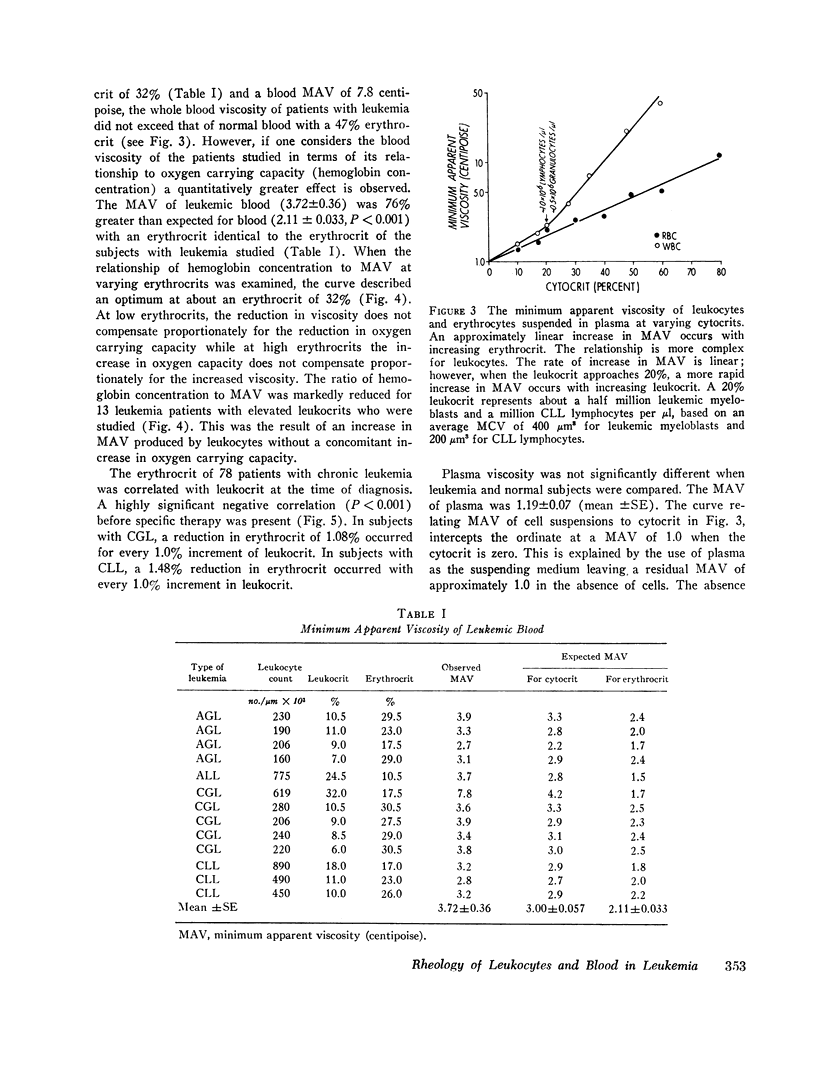
Suspensions of leukemic lymphocytes and myeloblasts and blood of leukemic patients were studied to examine (a) the effect of leukemic cells on blood viscosity and (b) the ability of leukemic cells to traverse channels of capillary diameter. The viscosity of suspensions of leukemic cells was dependent logarithmically on (a) shear strain rate and (b) cytocrit, although, suspensions of small lymphocytes and of myeloblasts had a similar viscosity at equivalent shear rates and cytocrit. The minimum apparent viscosity (MAV) of leukemic cells and red blood cells, measured over shear rates of 2.3-230 s-1 was dependent logarithmically on cytocrit. However, MAV was slightly greater for leukemic cells than for red cells at cytocrits up to 20%. At cytocrits above 20%. MAV of leukemic cells increased more rapidly than that of erythrocytes. For example, at a 15% cytocrit MAVWBC (1.85 centipoise) was only slightly greater than MAVRBC (1.59); whereas, at 45% cytocrit MAVWBC (14.9) was markedly greater than MAVRBC (3.81).
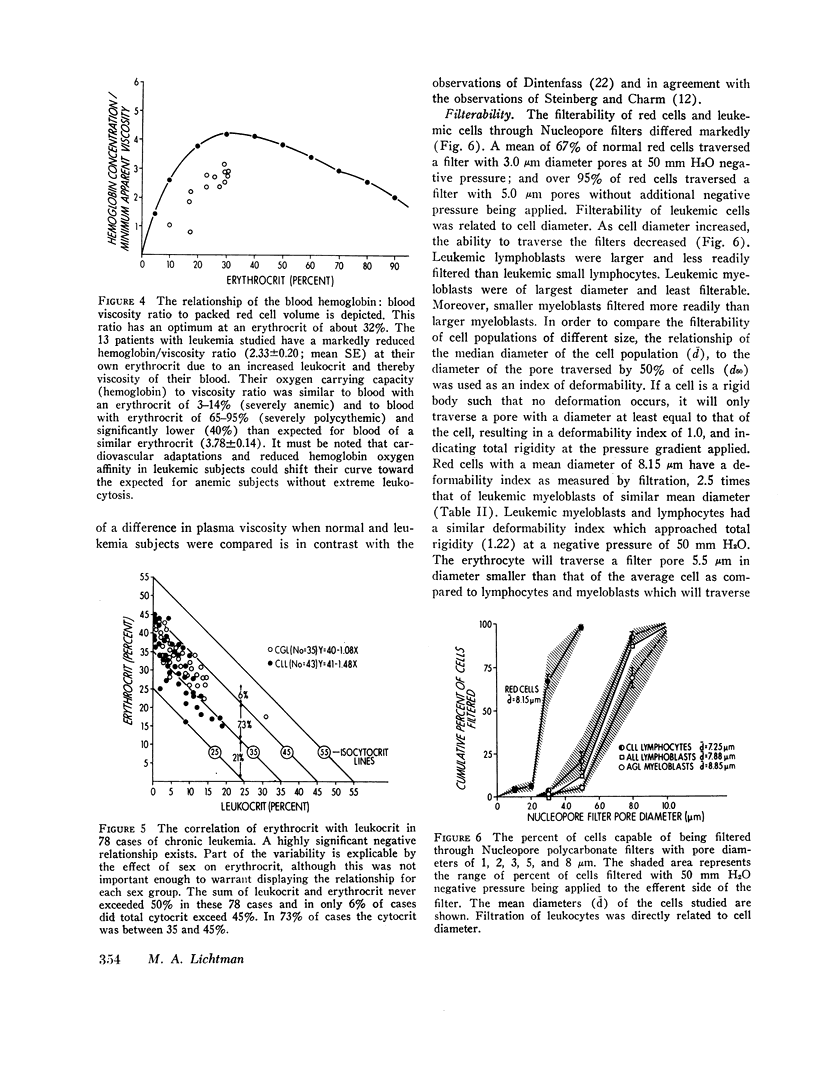
The blood of subjects with leukemia with marked elevation of leukocyte concentration (leukocrits of 6-32%) had 24% higher mean MAV (3.72) than blood with a similar total cytocrit composed of red cells (3.00). A negative correlation was present between leukocrit and erythrocrit in chronic lymphocytic (r = - 0.82) and chronic granulocytic (r = - 0.81) leukemia. Therefore, the modest increase in whole blood MAV in leukemia can be explained by (a) the negative association of leukocrit and erythrocrit and (b) the rarity of leukocrits over 20% and total cytocrits over 45%. However, the MAV of blood of leukemic patients was 71% greater than expected on the basis of their packed red cell volume. Hence, the ratio of hemoglobin concentration (O2 carrying capacity) to MAV was abnormally low in the subjects with leukemia studied.
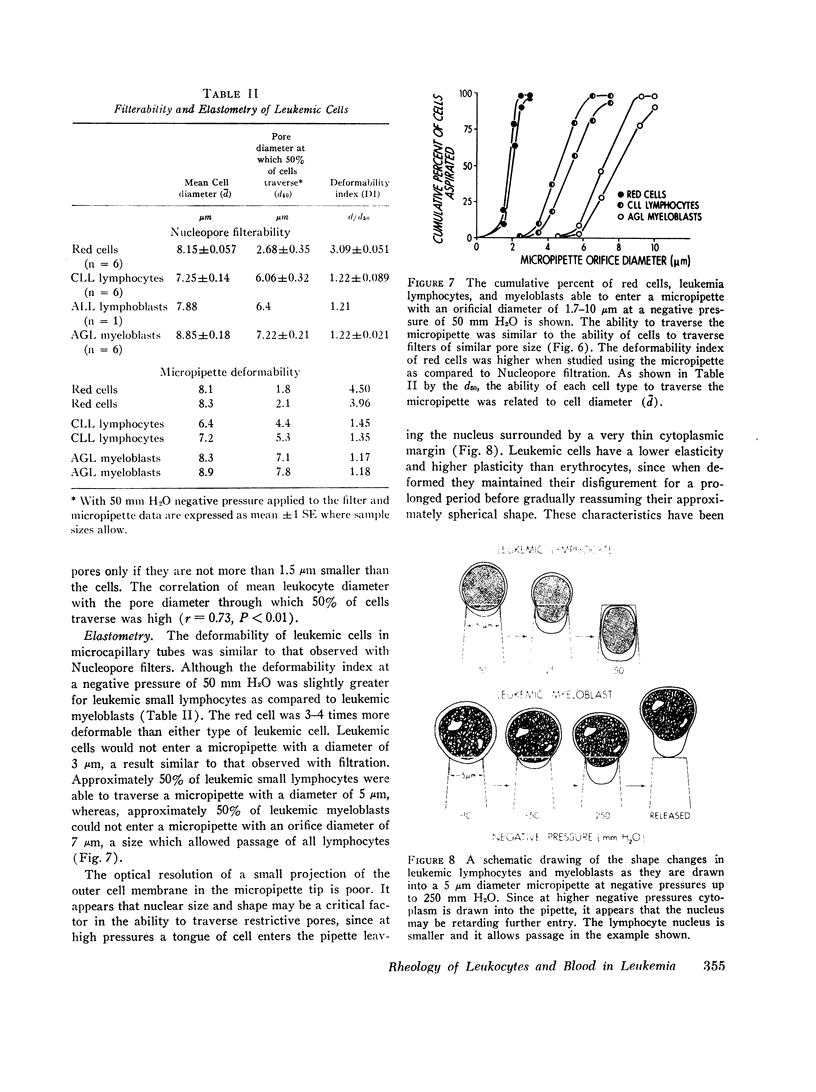
Individual leukemic leukocytes were nearly rigid. The mean deformability index (DI) of leukemic myeloblasts (1.22; 1.18) and lymphocytes (1.22; 1.40) as measured by filtration and elastometry, respectively, at 50 mm H2O negative pressure, approached that of a rigid body (1.0) as compared to red cells studied by filtration (3.09) or elastometry (4.23). The ability of leukemic cells to traverse nucleopore filter or micropipette channels was related to cell diameter. The relevance of the rheology of leukemic cells to the interruption of blood flow and of tissue oxygen delivery and thereby to clinical manifestations of leukemia is considered.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adell R., Skalak R., Branemark P. I. A preliminary study of rheology of granulocytes. Blut. 1970 Aug;21(2):91–105. doi: 10.1007/BF01632661. [DOI] [PubMed] [Google Scholar]
- BOGGS D. R. The cellular composition of inflammatory exudates in human leukemias. Blood. 1960 Apr;15:466–475. [PubMed] [Google Scholar]
- Bollinger A., Lüthy E. Blood viscosity and blood flow in the human forearm. Helv Med Acta. 1968 Jul;34(3):255–264. [PubMed] [Google Scholar]
- Chien S., Usami S., Taylor H. M., Lundberg J. L., Gregersen M. I. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J Appl Physiol. 1966 Jan;21(1):81–87. doi: 10.1152/jappl.1966.21.1.81. [DOI] [PubMed] [Google Scholar]
- Chisolm G. M., Gainer J. L. Tube viscometry of blood: effects of wall material. J Appl Physiol. 1971 Aug;31(2):313–317. doi: 10.1152/jappl.1971.31.2.313. [DOI] [PubMed] [Google Scholar]
- DINTENFASS L. SOME OBSERVATIONS ON THE VISCOSITY OF PATHOLOGICAL HUMAN BLOOD PLASMA. Thromb Diath Haemorrh. 1965 Jun 15;13:492–499. [PubMed] [Google Scholar]
- Dintenfass L. Viscosity of the packed red and white blood cells. Exp Mol Pathol. 1965 Dec;4(6):597–605. doi: 10.1016/0014-4800(65)90040-7. [DOI] [PubMed] [Google Scholar]
- Dormandy J. A. Influence of blood viscosity on blood flow and the effect of low molecular weight dextran. Br Med J. 1971 Dec 18;4(5789):716–719. doi: 10.1136/bmj.4.5789.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errill E. W. Rheology of blood. Physiol Rev. 1969 Oct;49(4):863–888. doi: 10.1152/physrev.1969.49.4.863. [DOI] [PubMed] [Google Scholar]
- FULTON G. P., LUTZ B. R. The use of the hamster cheek pouch and cinephotomicrography for research on the microcirculation and tumor growth, and for teaching purposes. BMQ. 1957 Mar;8(1):13–19. [PubMed] [Google Scholar]
- GUYTON A. C., RICHARDSON T. Q. Effect of hematocrit on venous return. Circ Res. 1961 Jan;9:157–164. doi: 10.1161/01.res.9.1.157. [DOI] [PubMed] [Google Scholar]
- Larcan A., Steiff F., Peters A., Genetet B. Les composantes de la viscosité sanguine. Pathol Biol. 1965 Jun-Jul;13(11):648–659. [PubMed] [Google Scholar]
- Lichtman M. A. Cellular deformability during maturation of the myeloblast. Possible role in marrow egress. N Engl J Med. 1970 Oct 29;283(18):943–948. doi: 10.1056/NEJM197010292831801. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Peripheral cytoplasmic characteristics of leukocytes in monocytic leukemia: relationship to clinical manifestations. Blood. 1972 Jul;40(1):52–61. [PubMed] [Google Scholar]
- Merrill E. W., Cheng C. S., Pelletier G. A. Yield stress of normal human blood as a function of endogenous fibrinogen. J Appl Physiol. 1969 Jan;26(1):1–3. doi: 10.1152/jappl.1969.26.1.1. [DOI] [PubMed] [Google Scholar]
- Merrill E. W., Pelletier G. A. Viscosity of human blood: transition from Newtonian to non-Newtonian. J Appl Physiol. 1967 Aug;23(2):178–182. doi: 10.1152/jappl.1967.23.2.178. [DOI] [PubMed] [Google Scholar]
- PHAIR J. P., ANDERSON R. E., NAMIKI H. THE CENTRAL NERVOUS SYSTEM IN LEUKEMIA. Ann Intern Med. 1964 Nov;61:863–875. doi: 10.7326/0003-4819-61-5-863. [DOI] [PubMed] [Google Scholar]
- Replogle R. L., Meiselman H. J., Merrill E. W. Clinical implications of blood rheology studies. Circulation. 1967 Jul;36(1):148–160. doi: 10.1161/01.cir.36.1.148. [DOI] [PubMed] [Google Scholar]
- Replogle R. L., Merrill E. W. Experimental polycythemia and hemodilution. Physiologic and rheologic effects. J Thorac Cardiovasc Surg. 1970 Oct;60(4):582–588. [PubMed] [Google Scholar]
- Steinberg M. H., Charm S. E. Effect of high concentrations of leukocytes on whole blood viscosity. Blood. 1971 Sep;38(3):299–301. [PubMed] [Google Scholar]
- Thorling E. B., Erslev A. J. The "tissue" tension of oxygen and its relation to hematocrit and erythropoiesis. Blood. 1968 Mar;31(3):332–343. [PubMed] [Google Scholar]
- Usami S., Magazinovic V., Chien S., Gregersen M. I. Viscosity of turkey blood: rheology of nucleated erythrocytes. Microvasc Res. 1970 Oct;2(4):489–499. doi: 10.1016/0026-2862(70)90041-5. [DOI] [PubMed] [Google Scholar]
- WELLS R. E., Jr, DENTON R., MERRILL E. W. Measurement of viscosity of biologic fluids by cone plate viscometer. J Lab Clin Med. 1961 Apr;57:646–656. [PubMed] [Google Scholar]
- WELLS R. E., Jr RHEOLOGY OF BLOOD IN THE MICROVASCULATURE. N Engl J Med. 1964 Apr 16;270:832–CONTD. doi: 10.1056/NEJM196404162701608. [DOI] [PubMed] [Google Scholar]




