Abstract
Background
Corynebacterium glutamicum is able to grow with lactate as sole or combined carbon and energy source. Quinone-dependent L-lactate dehydrogenase LldD is known to be essential for utilization of L-lactate by C. glutamicum. D-lactate also serves as sole carbon source for C. glutamicum ATCC 13032.
Results
Here, the gene cg1027 was shown to encode the quinone-dependent D-lactate dehydrogenase (Dld) by enzymatic analysis of the protein purified from recombinant E. coli. The absorption spectrum of purified Dld indicated the presence of FAD as bound cofactor. Inactivation of dld resulted in the loss of the ability to grow with D-lactate, which could be restored by plasmid-borne expression of dld. Heterologous expression of dld from C. glutamicum ATCC 13032 in C. efficiens enabled this species to grow with D-lactate as sole carbon source. Homologs of dld of C. glutamicum ATCC 13032 are not encoded in the sequenced genomes of other corynebacteria and mycobacteria. However, the dld locus of C. glutamicum ATCC 13032 shares 2367 bp of 2372 bp identical nucleotides with the dld locus of Propionibacterium freudenreichii subsp. shermanii, a bacterium used in Swiss-type cheese making. Both loci are flanked by insertion sequences of the same family suggesting a possible event of horizontal gene transfer.
Conclusions
Cg1067 encodes quinone-dependent D-lactate dehydrogenase Dld of Corynebacterium glutamicum. Dld is essential for growth with D-lactate as sole carbon source. The genomic region of dld likely has been acquired by horizontal gene transfer.
Background
Lactate is a major product of anaerobic metabolism. D-, L, and DL-lactic acid can be utilized by anaerobic and aerobic microorganisms as a carbon and energy source. Propionibacteria preferentially ferment L-lactate to propionate, acetate and carbon dioxide [1], Eubacterium hallii ferments both lactate isomers to butyrate in the human colon [2], while D-lactate is fermented to acetate by sulfate-reducing bacteria such as Desulfovibrio vulgaris [3], or to butyrate by e.g. Clostridium indolis-related strains isolated from human feces [2]. D-lactic acidosis in humans, which can lead to neurotoxicity and cardiac arythmia, is associated with an imbalance of production and degradation of D-lactate by the colonic microbiome [4]. D-lactate oxidizing enzymes have been described in eukaryotes and bacteria [5-8]. In Escherichia coli two membrane associated oxidizing lactate dehydrogenases are known. LldD is specific for L-lactate and is not able to oxidize D-lactate as substrate, meanwhile the second Lactate dehydrogenase Dld shows high affinity to D-lactate but also low affinity activity with L-lactate. Both enzymes convert lactate to pyruvate by removing electrons from lactate to enter the electron transport chain.
Peptidoglycan precursors may contain D-lactate as the C-terminal D-alanine residue of the muramyl pentapeptide is replaced by D-lactate, known as a pentadepsipeptide. This pentadepsipeptide is the cause of the acquired resistance of pathogenic enterococci to vancomycin and of the natural resistance of several lactobacilli to this glycopeptide antibiotic [9]. In L. plantarum, D-lactate for peptidoglycan precursor synthesis can be provided by the NAD-dependent fermentative D-lactate dehydrogenase or by a lactate racemase, which is encoded by an L-lactate-inducible operon, or by addition of D-lactate to the medium [10]. In E. coli, D-lactate can be generated during cell wall recycling and during growth on N-acetylmuramic acid as the etherase MurQ cleaves N-acetylmuramic acid 6-phosphate to yield N-acetylglucosamin 6-phoshate and D-lactate [11,12].
The uptake of lactate can be mediated by different kinds of transporters. The uptake systems LldP and GlcA, members of the lactate permease LctP family, are responsible for the uptake of DL-lactate and glycolate in E. coli [13]. In Rhizobium leguminosarum uptake of lactate and pyruvate, respectively, is mediated by MctP [14]. MctP belongs to the family of solute:sodium symporter (SSS).
C. glutamicum, a gram-positive facultative anaerobic bacterium is used for the biotechnological amino acid production in the million-ton-scale [15]. This bacterium can use a variety of carbon sources for growth, e.g. sugars like glucose, fructose and sucrose, organic acids like citrate, gluconate, pyruvate, acetate and propionate, but also ethanol, glutamate, vanillate or 4-hydroxybenzoate [16-23]. With two exceptions, namely glutamate and ethanol, carbon sources are utilized simultaneously by C. glutamicum. L-lactate and D-lactate are also known as sole or combined carbon sources of C. glutamicum [24]. MctC, a member of the solute:sodium symporter family recently identified and characterized, catalyzes the uptake of the monocarboxylates acetate, pyruvate and propionate, but there is no indication of a MctC dependent uptake of lactate in C. glutamicum [25].
Utilization of L-lactate by C. glutamicum has been studied to some detail and requires quinone-dependent L-lactate dehydrogenase LldD (EC 1.1.2.3) which is encoded by the cg3226-lldD operon [24]. Although cg3226 encodes a putative lactate permease, it is not required for growth in L-lactate minimal medium [20]. Expression of the cg3226-lldD operon is maximal when L-lactate is present in the medium. The cg3226-lldD operon is repressed by the FadR-type transcriptional regulator LldR in the absence of its effector L-lactate [20]. LldR is also known to repress the fructose utilization operon fruR-fruK-ptsF [26] and the gene for the fermentative NAD-dependent L-lactate dehydrogenase ldhA [27].
Relatively little is known about utilization of D-lactate by C. glutamicum. Only the production of D-lactate has been demonstrated with C. glutamicum R by heterologous expression of fermentative D-lactate dehydrogenase (D-LDH)-encoding genes from Lactobacillus delbrueckii and Escherichia coli [28]. The analysis of D-lactate utilization by C. glutamicum is important with respect to biotechnological D-lactate production, to further understanding of its physiology and with respect to the so-called flexible feedstock concept. Therefore, this study aimed to identify and characterize gene(s) and enzyme(s) for D-lactate utilization by this bacterium.
Methods
Bacterial strains, plasmids, oligonucleotides, and culture conditions
Used Bacterial strains, plasmids and oligonucleotides are listed in Table 1. E. coli and Corynebacterium strains were grown on Luria-Bertani (LB) medium as complex medium [29]. For growth experiments with C. glutamicum and C. efficiens, in the first preculture, 50 ml LB medium was inoculated from a fresh LB agar plate and incubated at 30°C and 120 rpm. After washing the cells in 0.9% NaCl (w/v), the second preculture and the main culture were inoculated to an optical density at 600 nm (OD600) of 0.5 to 1.0 in 50 ml CgXII minimal medium [30], which contained 0.03 g/l protocatechuic acid. As carbon and energy sources, 100 mM glucose, 100 mM sodium L-lactate, 100 mM sodium D-lactate or 50 mM sodium L-lactate and 50 mM sodium D-lactate were used. Precultures and main cultures were incubated at 30°C and 120 rpm on a rotary shaker in 500 ml-baffled shake flasks. When appropriate, 1 mM isopropyl-β-D-thiogalactopyranosid (IPTG), kanamycin (25 μg/ml) or spectinomycin (100 μg/ml) was added to the media. Growth of C. glutamicum and C. efficiens was followed by measuring the OD600. For all cloning purposes, Escherichia coli DH5α was used as host.
Table 1.
List of bacterial strains, plasmids and oligonucleotides
| strain, plasmid or oligonucleotide | relevant characteristics or sequence | source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F- thi-1 endA1 hsdR17(r- m-) supE44 ΔlacU169 (ϕ80lacZΔM15) recA1 gyrA96 relA1 | [32] |
| Corynebacterium strains | ||
|
C. glutamicum ATCC 130302 |
ATCC [61] | |
| ::dld | dld inactivation mutant of ATCC 13032 | This work |
|
C. efficiens DSM44547 |
DSM | |
| Plasmids | ||
| pEKEx3 | SpecR; Ptac, lacIq | [24] |
| pEKEx3-dld | pEKEx3 containing dld from C. glutamicum and an artificial ribosome binding site | this work |
| pVWEx1 | KanR; Ptac, lacIq | [34] |
| pVWEx1-dld | pEKEx3 containing dld from C. glutamicum and an artificial ribosome binding site | This work |
| pK18mob | KanR; integration vector for C. glutamicum | [62] |
| pK18mob-dld | pK18mob carrying an internal fragment of dld | This work |
| Oligonucleotides | sequence |
underlined nucleotide (position in NC003450) |
| rbs-ndld | GTCGACAAGGAGATATAGATATGACGCAACCAGGACAGAC SalI | 955683 |
| cdld | GGAGCTCTTAGGCCCAGTCCTTGTGC SacI | 957398 |
| Ex-dld-fw | GCCCTGCAGGAAGGAGATATAGATATGACGCAACCAGGACAGAC SbfI | 955683 |
| Ex-dld-bw | GCGGTACCTTAGGCCCAGTCCTTGTGC KpnI | 957398 |
| dld1 | AATCATATGAGACGCAACCAGGACAGACCACC NdeI | 955683 |
| dld2 | AATGGATCCGGCCCAGTCCTTGTGCGGCGACGTGC BamHI | 957398 |
| Cg-dld-SalI-N498 | AAGTCGACAGCCAGATTCCAGATTCGCAAGGGT SalI | 956907 |
| Cg-dld-C1716-SalI | GGTCGACTTAGGCCCAGTCCTTGTGC SalI | 955683 |
Determination of glucose and D/L-lactate concentrations
During cultivation, samples (1 ml) were collected to determine biomass, glucose and D/L-lactate concentrations. After determinations of the OD600 and centrifugation of the sample (13,000 g, 5 min) aliquots of the supernatant were used to determine concentrations of glucose and D/L-lactate by reverse-phase high-pressure liquid chromatography (HPLC) as described by Engels et al. 2008. To discriminate between the D- and L- isomers of lactate enzymatic determinations were performed as described by the manufacturer (R-Biopharm, Darmstadt, Germany).
D-lactate dehydrogenase assay
For determination of enzyme activities, exponentially growing cells were harvested by centrifugation (4,500 g, 5 min, 4°C) and washed twice with 50 mM ice-cold KH2PO4, pH 7.0. Cell pellets were resuspended in 1 ml of 50 mM KH2PO4, pH 7.0, directly or after storage at -70°C. After disruption by ultrasonic treatment at 4°C (UP 200S; Dr. Hielscher GmbH, Teltow, Germany) at an amplitude of 55% and a duty cycle of 0.5 for 6 min and centrifugation at 4°C for 60 min at 13,000 g, enzyme activity was determined immediately in the cell-free supernatant. D-Lactate dehydrogenase activity was determined by a modified assay according to [31]. Reaction mixtures of 1 ml contained 100 mM KH2PO4 (pH 7.5), 50 μM 2,6-dichloroindophenol (DCPIP) and 20 μl crude extract. The reaction was started by addition of 10 mM D-lactate and quinone-dependent D-lactate dehydrogenase was assayed spectrophotometrically at 30°C by determining the decrease in absorbance of DCPIP (ε600 = 20 mM-1 cm-1).
Construction of plasmids and strains
The oligonucleotides listed in Table 1 were obtained from Operon (Cologne, Germany). Standard methods such as PCR, restriction, and ligation were carried out as described previously [29]. Plasmids were constructed in Escherichia coli DH5α from PCR-generated fragments (KOD, Novagen) and isolated with the QIAprep spin miniprep kit (QIAGEN, Hilden, Germany). E. coli was transformed by the RbCl2 method [32], while C. glutamicum was transformed via electroporation [33]. All cloned DNA fragments were shown to be correct by sequencing (BigDye Terminator v3.1 Cycle Sequencing Kit and ABI Prism Capillary Sequencer Model 3730, Applied Biosystems, Forster-City, USA).
Disruption of dld
To construct a C. glutamicum dld inactivation mutant, an internal 1224-bp fragment of dld was amplified by using primer pair Cg-dld-SalI-N498 and Cg-dld-C1716-SalI which was subsequently cloned into pT7-blue T-vector (Novagen). The SalI restricted PCR fragment was ligated into the SalI site of pK18mob. Gene inactivation with pk18mobN498dld was carried out as described previously [24]. The correct genotype of the insertion mutant was verified by PCR analysis and determination of enzyme activity.
Homologous overexpression of dld
For homologous overexpression dld was amplified from genomic DNA of C. glutamicum WT by using primers rbs-ndld and cdld and was cloned into the expression vector pEKEx3 [24]. The amplified PCR fragment was ligated to a SmaI bluntend restriction site of pEKEx3. The constructed vector pEKEx3-dld allows the IPTG-inducible expression of dld in C. glutamicum. Because C. efficiens could not be transformed with pEKEx3-dld, dld was amplified using the primer Ex-dld-fw and Ex-dld-bw. The PCR fragment was cloned into the expression vector pVWEx1 [34] via SbfI and KpnI restriction sites. The vector pVWEx1-dld was transformed into C. effiens by electroporation and allowed IPTG-inducible expression of dld in this species.
Expression of dld from C. glutamicum ATCC 13032 in Escherichia coli BL21 (DE3)
Based on the 5'- and 3'- sequences of dld (accession no. YP_225194) in the genomic DNA of Corynebacterium glutamicum ATCC 13032, the oligonucleotides dld1 and dld2 were designed, and dld was amplified by PCR from the genomic DNA of C. glutamicum ATCC 13032 (1 ng) with dld1and dld2 (0.2 pmol). The thermal profiles for PCR involved the denaturation (94°C for 5 min), 5 cycles of annealing1 (98°C for 10 sec, 58°C for 30 sec, and 72°C for 90 sec) and subsequently 20 cycles of annealing 2 (98°C for 10 sec, 60°C for 30 sec, 72°C for 90 sec), and the extension (72°C for 7 min). A PCR amplification was carried out with a Blend Taq polymerase in a Gene Amp PCR system 9700 (PE Applied Biosystems, Piscataway, NJ, USA). The resulting 1,020-bp fragment with NdeI and BamHI restriction sites was sequenced with a DNA sequencing system, SQ5500 (Hitachi, Tokyo,). The obtained dld was ligated into an NdeI and BamHI-digested pT7 Blue-2 T-vector (50 ng/μl) and transformed into E. coli NovaBlue. After cultivation in an LB medium containing ampicillin, the plasmid was extracted with the alkaline mini-prep method and precipitated with polyethylene glycol 6,000. The purified DNA obtained was digested with NdeI and BamHI, and ligated into an NdeI and BamHI-restricted pET14b vector to form pET14b-dld. pET14b-dld was transformed into E. coli BL21 (DE3).
Expression of dld in E. coli BL21 (DE3) and protein purification
After the E. coli BL21 (DE3) cells harboring pET14b-dld were selected on an LB agar medium containing ampicillin (100 μg/ml), two clones were inoculated into a LB medium (5 ml) containing ampicillin (100 μg/ml) and cultivated at 30°C until the turbidity at 600 nm reached to 0.4-0.8. The culture was inoculated into the same medium (1 l) and cultivated at 30°C for 14 h. The cells were collected by centrifugation (7,100 × g, 10 min), suspended in 0.85% (w/v) NaCl, and centrifuged again. The cells were resuspended in a 20 mM sodium phosphate buffer (pH 8) containing 300 mM NaCl (Buffer A) and stored at -20°C. The cells were disrupted by ultrasonication (model UD-201, Tomy Seiko CO., Tokyo). The disruption conditions used were as follows: output 6; duty cycle 30; and operation time 5 min × 10 times. The suspension was ultracentrifuged (161,000 × g, 1 h), and the supernatant was dialyzed against Buffer A. The pellet obtained was suspended in Buffer A plus 0.5% Triton X-100 (Buffer B) at room temperature. After 1 h, the suspension was ultracentrifuged (161,000 × g, 1 h), and the supernatant obtained was stored at 4°C. The cell-free extract solubilized (about 120 mg) was applied to a column of TALON metal affinity resin (TaKaRa Bio, Inc. (Shiga, Japan); 10 × 15 cm). The column was equilibrated with Buffer B at a flow rate of 0.5 ml/min, and washed successively with Buffer B (90 ml), Buffer B plus 10 mM Imidazole (16 ml), Buffer B plus 20 mM Imidazole (16 ml), and Buffer B plus 50 m M Imidazole (4 ml). The adsorbed protein was eluted with Buffer B plus 250 mM imidazole (20 ml). The elution was collected with a Bio-collector (ATTO, Tokyo. Japan, 2 ml/tube), and the protein concentration was measured with a RC DC Protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The fractions containing the D-lactate dehydrogenase were dialyzed against two 1-l portions of Buffer A for 4 and 12 h, and stored at 4°C.
Comparative transcriptome analysis using DNA microarrays
Generation of C. glutamicum whole-genome DNA microarrays, total RNA preparation, synthesis of fluorescently labelled cDNA, microarray hybridization, washing, and statistical data analysis were performed as described previously [35-38]. Genes exhibiting mRNA levels that were significantly changed (P ≤ 0.05 in Student's t test) by at least a factor of 2.0 were determined in three DNA microarray experiments performed with RNA isolated from three independent cultures. The processed and normalized data have been deposited in the NCBI's Gene Expression Omnibus and are accessible under the accession number GSE25704.
Results
Cg1027 encodes D-lactate dehydrogenase
The C. glutamicum ATCC 13032 gene cg1027 was annotated to code for D-lactate dehydrogenase [39] as the deduced protein shows similarities to FAD/FMN-containing dehydrogenases encoded by the cluster of orthologous genes COG0277. The deduced protein contains the conserved domain PRK11183, and the domain (aa 279-570) was similar to membrane-binding D-lactate dehydrogenases belonging to the protein family pfam09330.
In order to determine whether the gene product of cg1027 is indeed active as D-lactate dehydrogenase, the gene was cloned into pET14b, and the hexahistidine-tagged protein was purified from E. coli BL21 (DE3) harboring pET14b-dld. Quinone-dependent D-lactate dehydrogenase activity was detected by using 2,6-dichloroindophenol as an electron acceptor. The optimum assay conditions were observed in a 100 mM potassium phosphate buffer at a pH of 7.0 and a temperature of 45°C. Subsequently, Dld activity was assayed at 30°C, the optimal temperature for growth of C. glutamicum. The enzyme showed Michaelis-Menten kinetics with D-lactate as the substrate and it was determined that 0.61 mM of D-lactate resulted in half maximal enzyme activity. The observed Vmax was 73.5 μmol mg-1. min-1. When D-lactate was replaced as substrate, the enzyme did not significantly act with D-malate, L-malate, D-tartrate and L-tartrate, but some oxidation of L-lactate and DL-2-hydroxybutyrate was observed ( < 5% of the activity observed for D-lactate). The comparison of the absorption spectra of the purified protein from C. glutamicum with those of the NAD-dependent D-lactate dehydrogenase from Leuconostoc mesenteroides revealed that the absorption maxima at 375 nm and 445 nm were observed only for the protein from C. glutamicum. These spectral features agree well with those for FAD. Moreover, the primary structure of the D-lactate dehydrogenase from C. glutamicum contains a domain (aa 50-187) similar to the FAD binding domain 4 found in the members of the protein family pfam01565. These results suggest that D-lactate dehydrogenase from C. glutamicum contains FAD as a bound cofactor. Taken together, it is concluded that cg1027 encodes quinone-dependent D-lactate dehydrogenase (EC 1.1.2.4) from C. glutamicum and, thus, was named dld.
Dld is required for utilization of D-lactate
In order to determine the role of quinone-dependent D-lactate dehydrogenase Dld for growth of C. glutamicum on D-lactate and racemic DL-lactate, a defined dld disruption mutant and dld overexpression plasmids for complementation were constructed. The constructed strains were assayed for Dld activity in crude extracts obtained after growth in LB medium containing kanamycin and IPTG when appropriate. Crude extracts of C. glutamicum WT and WT(pEKEx3) contained about 0.10 U mg-1 Dld activity (Figure 1), while no Dld activity was detectable in C. glutamicum ::dld (pEKEx3). Overexpression of dld resulted in about three fold higher Dld activity in WT(pEKEx3-dld) than in the empty vector control. Growth experiments with C. glutamicum strains WT(pEKEx3), WT(pEKEx3-dld), ::dld(pEKEx3), and ::dld(pEKEx3-dld) in CgXII mineral medium containing 100 mM D-lactate and 1 mM IPTG revealed that dld is required for growth of C. glutamicum on D-lactate as sole carbon and energy source as only strains with intact dld either on the chromosome or on plasmid could grow (Figure 1).
Figure 1.
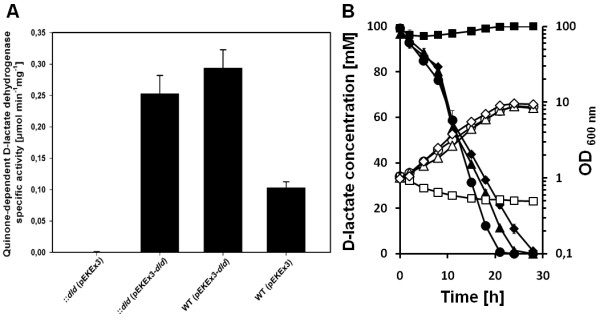
Specific activities of the quinone-dependent D-lactate dehydrogenase Dld (A) and growth (B) of various C. glutamicum strains. Specific Dld activities (A) were determined after growth in LB complex medium containing 1 mM IPTG. The values represent means and standard deviations of at least three independent cultivations. Growth (B) of C. glutamicum WT(pEKEx3) (diamonds), WT(pEKEx3-dld) (circles), ::dld(pEKEx3) (squares), and ::dld(pEKEx3-dld) (triangles) in CgXII mineral medium containing 100 mM D-lactate and 1 mM IPTG was monitored as OD600nm (open symbols). The concentration of D-lactate in the supernatant was measured by HPLC (closed symbols). Averages and experimental errors from at least three independent growth experiments are shown.
In media containing 100 mM racemic DL-lactate as carbon and energy source, C. glutamicum::dld(pEKEx3) formed about half as much biomass as strains WT(pEKEx3), WT(pEKEx3-dld), and ::dld(pEKEx3-dld) indicating that only L-lactate is utilized in the absence of Dld while strains possessing Dld utilized both L- and D-lactate for growth (data not shown).
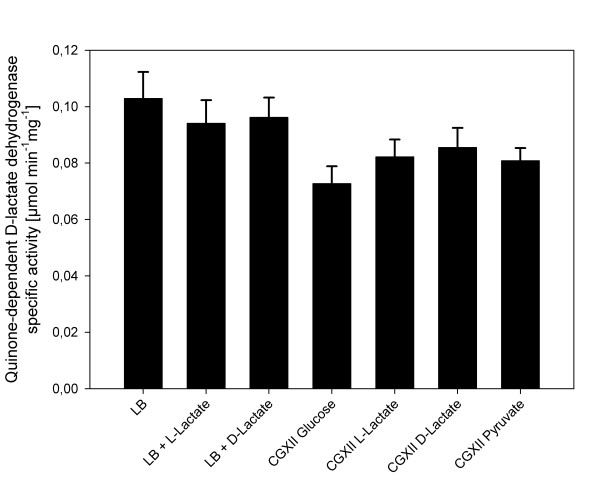
Dld activities under various growth conditions
The specific quinone-dependent D-lactate dehydrogenase activity was determined in crude extracts of C. glutamicum ATCC 13032 grown under different conditions. Neither the addition of L-lactate nor of D-lactate to complex medium affected the specific activity of Dld (Figure 2). Dld activities were also similar after growth in CgXII minimal medium with various carbon sources (Figure 2). Thus, the comparable Dld activities in C. glutamicum cells grown in different media suggested that dld is expressed constitutively.
Figure 2.
Specific activities of the quinone-dependent D-lactate dehydrogenase Dld in crude extracts of C. glutamicum WT grown in different media. The values represent means and standard deviations of at least three independent cultivations in LB complex medium without or with 100 mM L-lactate or 100 mM D-lactate or in CgXII mineral medium containing either 100 mM glucose, 100 mM L-lactate, 100 mM D-lactate or 100 mM pyruvate as carbon source.
DNA microarray analysis of D-lactate specific gene expression changes
Comparative transcriptome analysis was performed for C. glutamicum cells grown in LB with/without added D-lactate as well as in CgXII minimal medium with DL-lactate or L-lactate as sole carbon sources. These carbon source combinations were chosen to avoid secondary effects in comparisons with non-gluconeogenic carbon sources such as glucose and because L-lactate specific gene expression patterns were known [24]. Neither the addition of D-lactate to LB nor the presence of D-lactate in minimal medium affected dld expression. However, upon addition of D-lactate to LB medium eight genes showed altered expression levels as compared to the absence of D-lactate. Of these, five genes showed higher and three genes lower RNA levels in the presence of D-lactate. Growth in DL-lactate minimal medium was characterized by lower expression of fourteen genes as compared to growth in L-lactate. As most of these genes encoded ATPase subunits or ribosomal proteins this expression pattern likely reflects the lower growth rate in DL-lactate than in L-lactate minimal medium.
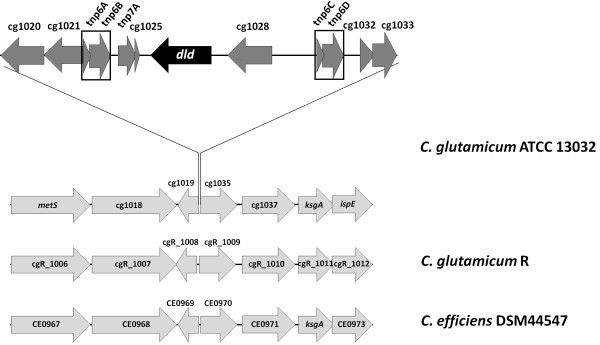
Heterologous expression of dld from C. glutamicum ATCC 13032 in C. Efficiens
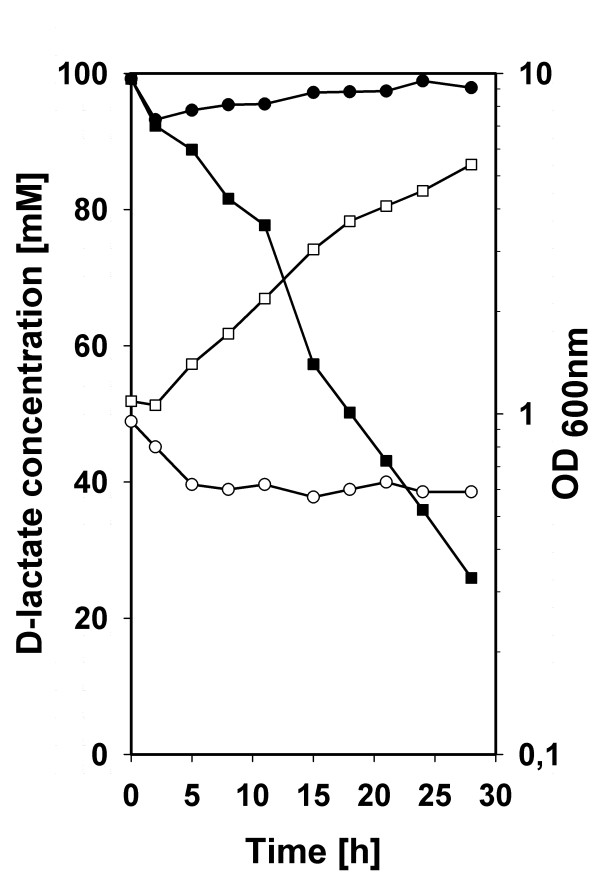
Comparison of the genome of C. glutamicum ATCC 13032 with the genomes of closely related species revealed that C. glutamicum R, C. efficiens, C. jeikeium and C. urealytikum do not possess a protein homologous to Dld (Figure 3). C. efficiens has been described to be unable to assimilate D-lactate [40]. To test whether the absence of a gene homologous to dld resulted in the inability of C. efficiens to grow in D-lactate minimal medium, C. efficiens DSM44547 was transformed either with the empty vector pVWEx1 or the dld expression vector pVWEx1-dld and growth of C. efficiens strains DSM44547, DSM44547(pVWEx1) and DSM44547(pVWEx1-dld) was analysed in CgXII mineral medium containing 100 mM D-lactate and 1 mM IPTG. As expected [40], C. efficiens strains DSM44547 and DSM44547(pVWEx1) could not grow with D-lactate as sole carbon source (data not shown and Figure 4), while C. efficiens ATCC DSM44547(pVWEx1-dld) utilized D-lactate for biomass formation and grew with a growth rate of 0.08 h-1 (Figure 4). Thus, heterologous expression of dld from C. glutamicum enabled C. efficiens to utilize D-lactate as sole source of carbon and energy.
Figure 3.
Comparison of the genomic context of dld in C. glutamicum ATCC13032 with the closely related C. glutamicum R and C. efficiens DSM44547. An insertion of twelve genes (including dld) is present only in the genome of C. glutamicum ATCC 13032. The regions flanking this genomic island are homologous to those in C. glutamicum R and C. efficiens. Direct repeats are located close to dld and are marked with boxes. The data were obtained from the open source bioinformatics tools CoryneRegNet [63] and PRODORIC Database [64].
Figure 4.
Growth of C. efficiens DSM44547 carrying either the empty vector pVWEx1 (squares) or the vector pVWEx1-dld (circles) in CgXII mineral medium containing 100 mM D-lactate and 1 mM IPTG. A representative growth curve is shown. The growth was monitored as OD600nm (closed symbols); the concentration of D-lactate in the supernatant was measured by HPLC (open symbols).
Discussion
In this study dld (cg1027) was demonstrated to encode the only D-lactate dehydrogenase essential for the growth with D-lactate as sole carbon source in C. glutamicum. The dld inactivation mutant was unable to grow and to utilize D-lactate, unless dld was restored by plasmid-borne expression. The enzyme Dld is a quinone-dependent D-lactate dehydrogenase (EC 1.1.2.4). Dld is specific for D-lactate reduction, while D-malate, L-malate, D-tartrate and L-tartrate were not significant substrates. The determined Km of 0.62 mM for D-lactate is similar to D-lactate dehydrogenase from Neisseria meningitidis (0.7 mM [7]) and E. coli (0.49 mM [41]).
Dld accepts L-lactate and DL-2-hydroxybuytrate with minor activities confirming earlier observations obtained with strain DL4, a classically obtained mutant of C. glutamicum ATCC 14310 with increased D-lactate dehydrogenase activity and an increased rate of DL-hydroxybutyrate utilization [42]. Unpublished data on D-lactate dehydrogenase from strain DL4 (Scheer et al. as referred to in Bott & Niebisch [43]) revealed a pH optimum of 7.0, a Km for D-lactate of 0.15 mM and Vmax 0.26 U per mg of solubilized protein. This protein preparation contained non-covalently bound FAD as it was confirmed here for Dld from C. glutamicum ATCC 13032. As deduced from Dld of E. coli Dld of C. glutamicum also contains residues relevant for non-covalent FAD binding as well as those of the proposed active site (Ile-142 and Ser-144). Dld appears to be membrane-associated as Dld from E. coli, which does not contain transmembrane helices, but is firmly attached to the membrane by electrostatic interactions between an electropositive surface composed of several arginine and lysine residues in the membrane-binding domain and the electronegative phospholipid head groups of the membrane [44]. Dld from C. glutamicum contains several of these basic residues and was identified as a membrane associated protein in membrane proteome analyses [45]. Thus, it is tempting to speculate that membrane association of Dld could facilitate oxidation of D-lactate immediately after its uptake. As an uptake system for D- and/or L-lactate is currently unknown it cannot be tested whether Dld associates to the membrane and interacts with the uptake system.
Expression of dld is constitutive and independent of the carbon source as revealed by transcriptome analysis (Table 2) and specific D-lactate dehydrogenase activity measurements (Figure 2) confirming earlier observations [42]. Constitutive expression of dld as opposed to L-lactate inducible expression of the L-lactate dehydrogenase gene lldD [20] is also found in E. coli [46], while synthesis of L- and D-lactate dehydrogenases is regulated in a coordinated manner in Acinetobacter calcoaceticus [47].
Table 2.
Comparative gene expression analysis of C. glutamicum ATCC 13032 grown in LB + D-lactate and LB or minimal media CgXII DL-lactate and CgXII L-lactate respectively.
| Genea | Annotationa | mRNA levelb | |
|---|---|---|---|
| LB | CgXII | ||
| cg0045 | ABC-type transporter, permease component | 0,1 | n.d. |
| cg0594 | ribosomal protein L3 | 1,3 | 0,2 |
| cg0598 | ribosomal protein L2 | 1,7 | 0,2 |
| cg0652 | ribosomal protein S13 | 0,9 | 0,2 |
| cg0653 | ribosomal protein S11 | 1,6 | 0,2 |
| cg0769 | ABC-type transporter, permease component | 0,2 | 0,7 |
| cg0771 | ABC-type transporter, periplasmic component | 0,3 | 0,7 |
| cg0921 | Siderophore-interacting protein | 0,2 | n.d. |
| cg1215 | nicotinate-nucleotide pyrophosphorylase | 1,0 | 0,2 |
| cg1218 | ADP-ribose pyrophosphatase | 0,7 | 0,2 |
| cg1351 | molybdopterin biosynthesis enzyme | 0,8 | 0,2 |
| cg1362 | F0F1-type ATP synthase a subunit | 1,1 | 0,2 |
| cg1366 | F0F1-type ATP synthase alpha subunit | 1,1 | 0,2 |
| cg1447 | Co/Zn/Cd efflux system component | 7,7 | 0,7 |
| cg1884 | hypothetical protein | 1,3 | 0,2 |
| cg2402 | cell wall-associated hydrolase | 0,8 | 0,2 |
| cg2931 | putative dihydrodipicolinate synthase | 4,4 | 1,0 |
| cg2937 | ABC-type transporter, periplasmic component | 4,6 | 0,9 |
| cg2938 | ABC-type transporter, permease component | 4,1 | 1,5 |
| cg3114 | sulfate adenylate transferase subunit 1 | 2,2 | 0,2 |
| cg3116 | phosphoadenosine phosphosulfate reductase | 2,2 | 0,1 |
| cg3118 | putative nitrite reductase | 2,3 | 0,2 |
| cg3303 | hypothetical protein | 4,0 | 1,5 |
a Gene identifiers and annotations are given according to BX927147.
b Statistically significant changes of at least fourfold in gene expression determined in at least two independent experiments from independent cultivations (P < 0.05 by Student's test) are listed.
While C. glutamicum ATCC 13032 and ATCC 14310 contain D-lactate dehydrogenase Dld, the genome of C. glutamicum strain R does not encode Dld. Thus, dld is one of only 60 and 189 genes, respectively, that are strain-specific [48]. In addition, the gene dld is absent from the genomes of other corynebacterial species (C. efficiens, C. jeikeium, C. urealytikum, C. diphtheriae, C. kroppenstedtii and C. aurimucosum) as well as from the sequenced genomes of Mycobacteriaceae and of the sequenced genomes of other members of the suborder Corynebacterineae (Dietziaceae, Gordoniaceae, Nocaridaceae and Tsukmurellaceae). The genomic locus of dld (Figure 3) indicates that dld is flanked by the insertion elements ISCg6a and ISCg6b [49] and, thus, dld might have been acquired by horizontal gene transfer. The closest homolog of Dld from C. glutamicum is D-lactate dehydrogenase from Propionibacterium freudenreichii subsp. shermanii, which is encoded by PFREUD_16710 and shares 370 of 371 identical amino acids with Dld from C. glutamicum. Moreover, on the DNA level the genes and flanking sequences differ only by five nucleotides in 2372 bp region (bp 956767-959138 in GI 62388892/C. glutamicum and bp 1833090-1830719 in GI 297625198/P. freudenreichii subsp. shermanii). Insertion sequences with transposase genes belonging to the same family (family IS3) as those in the insertion sequences flanking dld in C. glutamicum can also be found adjacent to PFREUD_16710 in the genome of P. freudenreichii supporting the hypothesis of horizontal gene transfer between the two species. The G+C content of dld from C. glutamicum and PFREUD_16710 from P. freudenreichii is 62.2% and, thus, between the G+C content of the genomes of C. glutamicum (53.8%) and P. freudenreichii (67%; NC_014215). Meanwhile a horizontal transfer of dld from E. coli is likely excluded. The G+C-content of dld from E. coli is 51% which is close to G+C content of the E. coli genome (50%; NC_000913). Also the genomic context does not show any insertion sequences with transposase genes close to dld.
P. freudenreichii belongs to the suborder of Propionibacterineae, which along with other suborders such as the Corynebacterineae belongs to the order of Actinomycetales. Propionibacteria such as P. freudenreichii subsp. shermanii and corynebacteria such as C. casei are used in the dairy industry in cheese making and occur in the secondary flora of cheeses. In swiss-type cheese making, P. freudenreichii subsp. shermanii converts lactate anaerobically to propionate, acetate and carbon dioxide [1], while corynebacteria are involved in surface-ripening of red smear cheeses [50]. There is evidence for horizontal gene transfer between lactic acid bacteria fermenting milk (Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus; [51]. However, it is unclear under which conditions the horizontal transfer of dld between C. glutamicum and P. freudenreichii occurred although propionibacteria and corynebacteria are known to co-exist on the human skin [52].
Here we showed a functional heterologous expression of dld from C. glutamicum in C. efficiens. While the pBL1-based expression vector pEKEx3 [24] did not work in C. efficiens in our hands, the pHM1519-based expression vector pVWEx1 [34] may be used as a tool to extend the genetic repertoire of C. efficiens e.g. for a broader usage of different carbon sources.
The biotechnological production of lactic acid is observed with special interest due to its use for poly lactic acid production, an alternative to petroleum based plastic. Poly D-lactic acid (PDLA) is more advantageous than poly L-lactic acid (PLLA) because of its higher melting point [53]. While, poly lactic acid could be synthesized within recombinant E. coli cells [54], poly lactic acid is typically produced in a two step process. After fermentative production of lactic acid, poly lactic acid is synthesized chemically by ring-opening polymerisation of lactide, the cyclic diester of lactic acid [53]. Lactic acid fermentation employs lactic acid bacteria, but also S. cerevisiae has been engineered for production of high purity L-lactate [55] or D-lactate [56]. In addition, E. coli has been engineered for lactate production [57-59]. To improve D-lactate production by recombinant E. coli, dld was deleted to avoid re-utilization of the product [60]. As C. glutamicum strains other than ATCC 13032 lack dld, C. glutamicum might be a useful host for D-lactate production. Indeed, C. glutamcium R, which lacks dld, was engineered for D-lactate production under oxygen limiting conditions employing fermentative NAD-dependent D-lactate dehydrogenase from E. coli [28].
Conclusion
Cg1067 encodes quinone-dependent D-lactate dehydrogenase Dld of Corynebacterium glutamicum. Dld is essential for growth with D-lactate as sole carbon source. The genomic region of dld likely has been acquired by horizontal gene transfer.
Authors' contributions
OK and DM purified and characterized the enzyme, OK and KCS carried out the transcriptional studies, OK, KCS and JWY constructed the recombinant strains and JWY performed the growth experiments and determined the enzyme activities. TO supervised the enzymatic analyses, participated in the interpretation of the data and critical revision of the manuscript. VFW supervised the experiments and was responsible for the draft and final version of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Osamu Kato, Email: sper036@yahoo.co.jp.
Jung-Won Youn, Email: jyoun@cebitec.uni-bielefeld.de.
K Corinna Stansen, Email: K.C.Stansen@web.de.
Daisuke Matsui, Email: matsui@ipcku.kansai-u.ac.jp.
Tadao Oikawa, Email: oikawa@ipcku.kansai-u.ac.jp.
Volker F Wendisch, Email: volker.wendisch@uni-bielefeld.de.
Acknowledgements
This work was supported by the research grant strategic project to support the formation of research bases at private universities, Japan.
References
- Crow VL. Utilization of lactate isomers by Propionibacterium freudenreichii subsp. shermanii: regulatory role for intracellular pyruvate. Appl Environ Microbiol. 1986;52(2):352–358. doi: 10.1128/aem.52.2.352-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70(10):5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Arihara K, Yagi T. D-lactate dehydrogenase of Desulfovibrio vulgaris. J Biochem. 1981;89(5):1423–1431. doi: 10.1093/oxfordjournals.jbchem.a133334. [DOI] [PubMed] [Google Scholar]
- Vella A, Farrugia G. D-lactic acidosis: pathologic consequence of saprophytism. Mayo Clin Proc. 1998;73(5):451–456. doi: 10.4065/73.5.451. [DOI] [PubMed] [Google Scholar]
- Ho C, Pratt EA, Rule GS. Membrane-bound D-lactate dehydrogenase of Escherichia coli: a model for protein interactions in membranes. Biochim Biophys Acta. 1989;988(2):173–184. doi: 10.1016/0304-4157(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Pallotta ML, Valenti D, Iacovino M, Passarella S. Two separate pathways for d-lactate oxidation by Saccharomyces cerevisiae mitochondria which differ in energy production and carrier involvement. Biochim Biophys Acta. 2004;1608(2-3):104–113. doi: 10.1016/j.bbabio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Erwin AL, Gotschlich EC. Oxidation of D-lactate and L-lactate by Neisseria meningitidis: purification and cloning of meningococcal D-lactate dehydrogenase. J Bacteriol. 1993;175(20):6382–6391. doi: 10.1128/jb.175.20.6382-6391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison N, O'Donnell MJ, Fewson CA. Membrane-bound lactate dehydrogenases and mandelate dehydrogenases of Acinetobacter calcoaceticus. Purification and properties. Biochem J. 1985;231(2):407–416. doi: 10.1042/bj2310407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour J, Ferain T, Deghorain M, Palumbo E, Hols P. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76(1-4):159–184. doi: 10.1023/A:1002089722581. [DOI] [PubMed] [Google Scholar]
- Goffin P, Deghorain M, Mainardi JL, Tytgat I, Champomier-Verges MC, Kleerebezem M, Hols P. Lactate racemization as a rescue pathway for supplying D-lactate to the cell wall biosynthesis machinery in Lactobacillus plantarum. J Bacteriol. 2005;187(19):6750–6761. doi: 10.1128/JB.187.19.6750-6761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger T, Arsic M, Mayer C. Scission of the lactyl ether bond of N-acetylmuramic acid by Escherichia coli "etherase". J Biol Chem. 2005;280(34):30100–30106. doi: 10.1074/jbc.M502208200. [DOI] [PubMed] [Google Scholar]
- Uehara T, Suefuji K, Jaeger T, Mayer C, Park JT. MurQ Etherase is required by Escherichia coli in order to metabolize anhydro-N-acetylmuramic acid obtained either from the environment or from its own cell wall. J Bacteriol. 2006;188(4):1660–1662. doi: 10.1128/JB.188.4.1660-1662.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez MF, Kwon O, Wilson TH, Aguilar J, Baldoma L, Lin EC. Transport of L-lactate, D-lactate, and glycolate by the LldP and GlcA membrane carriers of Escherichia coli. Biochem Biophys Res Commun. 2002;290(2):824–829. doi: 10.1006/bbrc.2001.6255. [DOI] [PubMed] [Google Scholar]
- Hosie AH, Allaway D, Poole PS. A monocarboxylate permease of Rhizobium leguminosarum is the first member of a new subfamily of transporters. J Bacteriol. 2002;184(19):5436–5448. doi: 10.1128/JB.184.19.5436-5448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C, Becker J. In: Amino Acid Biosynthesis - Pathways, Regulation and Metabolic Engineering. Wendisch VF, editor. Heidelberg: Springer; 2007. The L-lysine Story: From metabolic pathways to industrial production; pp. 39–70. full_text. [Google Scholar]
- Arndt A, Auchter M, Ishige T, Wendisch VF, Eikmanns BJ. Ethanol catabolism in Corynebacterium glutamicum. J Mol Microbiol Biotechnol. 2008;15(4):222–233. doi: 10.1159/000107370. [DOI] [PubMed] [Google Scholar]
- Chaudhry MT, Huang Y, Shen XH, Poetsch A, Jiang CY, Liu SJ. Genome-wide investigation of aromatic acid transporters in Corynebacterium glutamicum. Microbiology. 2007;153(Pt 3):857–865. doi: 10.1099/mic.0.2006/002501-0. [DOI] [PubMed] [Google Scholar]
- Claes WA, Puhler A, Kalinowski J. Identification of two prpDBC gene clusters in Corynebacterium glutamicum and their involvement in propionate degradation via the 2-methylcitrate cycle. J Bacteriol. 2002;184(10):2728–2739. doi: 10.1128/JB.184.10.2728-2739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frunzke J, Engels V, Hasenbein S, Gatgens C, Bott M. Co-ordinated regulation of gluconate catabolism and glucose uptake in Corynebacterium glutamicum by two functionally equivalent transcriptional regulators, GntR1 and GntR2. Mol Microbiol. 2008;67(2):305–322. doi: 10.1111/j.1365-2958.2007.06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi T, Engels V, Wendisch VF. Regulation of L-lactate utilization by the FadR-type regulator LldR of Corynebacterium glutamicum. J Bacteriol. 2008;190(3):963–971. doi: 10.1128/JB.01147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstmeir R, Wendisch VF, Schnicke S, Ruan H, Farwick M, Reinscheid D, Eikmanns BJ. Acetate metabolism and its regulation in Corynebacterium glutamicum. J Biotechnol. 2003;104(1-3):99–122. doi: 10.1016/S0168-1656(03)00167-6. [DOI] [PubMed] [Google Scholar]
- Merkens H, Beckers G, Wirtz A, Burkovski A. Vanillate metabolism in Corynebacterium glutamicum. Curr Microbiol. 2005;51(1):59–65. doi: 10.1007/s00284-005-4531-8. [DOI] [PubMed] [Google Scholar]
- Polen T, Schluesener D, Poetsch A, Bott M, Wendisch VF. Characterization of citrate utilization in Corynebacterium glutamicum by transcriptome and proteome analysis. FEMS Microbiol Lett. 2007;273(1):109–119. doi: 10.1111/j.1574-6968.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- Stansen C, Uy D, Delaunay S, Eggeling L, Goergen JL, Wendisch VF. Characterization of a Corynebacterium glutamicum lactate utilization operon induced during temperature-triggered glutamate production. Appl Environ Microbiol. 2005;71(10):5920–5928. doi: 10.1128/AEM.71.10.5920-5928.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkver E, Emer D, Ballan S, Kramer R, Eikmanns BJ, Marin K. Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J Bacteriol. 2009;191(3):940–948. doi: 10.1128/JB.01155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YG, Suzuki H, Itou H, Zhou Y, Tanaka Y, Wachi M, Watanabe N, Tanaka I, Yao M. Structural and functional characterization of the LldR from Corynebacterium glutamicum: a transcriptional repressor involved in L-lactate and sugar utilization. Nucleic Acids Res. 2008;36(22):7110–7123. doi: 10.1093/nar/gkn827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda K, Teramoto H, Inui M, Yukawa H. The ldhA gene, encoding fermentative L-lactate dehydrogenase of Corynebacterium glutamicum, is under the control of positive feedback regulation mediated by LldR. J Bacteriol. 2009;191(13):4251–4258. doi: 10.1128/JB.00303-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okino S, Suda M, Fujikura K, Inui M, Yukawa H. Production of D-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol. 2008;78(3):449–454. doi: 10.1007/s00253-007-1336-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. A Labortory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Labortory Press; 1989. Molecular Cloning. [Google Scholar]
- Keilhauer C, Eggeling L, Sahm H. Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol. 1993;175(17):5595–5603. doi: 10.1128/jb.175.17.5595-5603.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari R, Lara FJ. The lactic dehydrogenase of Propionibacterium pentosaceum. Biochem J. 1960;75:57–65. doi: 10.1042/bj0750057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166(4):557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Tauch A, Kirchner O, Loffler B, Gotker S, Puhler A, Kalinowski J. Efficient electrotransformation of Corynebacterium diphtheriae with a mini-replicon derived from the Corynebacterium glutamicum plasmid pGA1. Curr Microbiol. 2002;45(5):362–367. doi: 10.1007/s00284-002-3728-3. [DOI] [PubMed] [Google Scholar]
- Peters-Wendisch PG, Schiel B, Wendisch VF, Katsoulidis E, Mockel B, Sahm H, Eikmanns BJ. Pyruvate carboxylase is a major bottleneck for glutamate and lysine production by Corynebacterium glutamicum. J Mol Microbiol Biotechnol. 2001;3(2):295–300. [PubMed] [Google Scholar]
- Ishige T, Krause M, Bott M, Wendisch VF, Sahm H. The phosphate starvation stimulon of Corynebacterium glutamicum determined by DNA microarray analyses. J Bacteriol. 2003;185(15):4519–4529. doi: 10.1128/JB.185.15.4519-4529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Rittmann D, Wendisch VF, Bott M, Sahm H. Global expression profiling and physiological characterization of Corynebacterium glutamicum grown in the presence of L-valine. Appl Environ Microbiol. 2003;69(5):2521–2532. doi: 10.1128/AEM.69.5.2521-2532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polen T, Wendisch VF. Genomewide expression analysis in amino acid-producing bacteria using DNA microarrays. Appl Biochem Biotechnol. 2004;118(1-3):215–232. doi: 10.1385/ABAB:118:1-3:215. [DOI] [PubMed] [Google Scholar]
- Wendisch VF. Genome-wide expression analysis in Corynebacterium glutamicum using DNA microarrays. J Biotechnol. 2003;104(1-3):273–285. doi: 10.1016/S0168-1656(03)00147-0. [DOI] [PubMed] [Google Scholar]
- Bott M, Niebisch A. The respiratory chain of Corynebacterium glutamicum. J Biotechnol. 2003;104(1-3):129–153. doi: 10.1016/S0168-1656(03)00144-5. [DOI] [PubMed] [Google Scholar]
- Fudou R, Jojima Y, Seto A, Yamada K, Kimura E, Nakamatsu T, Hiraishi A, Yamanaka S. Corynebacterium efficiens sp. nov., a glutamic-acid-producing species from soil and vegetables. Int J Syst Evol Microbiol. 2002;52(Pt 4):1127–1131. doi: 10.1099/ijs.0.02086-0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Anraku Y, Futai M. Escherichia coli membrane D-lactate dehydrogenase. Isolation of the enzyme in aggregated from and its activation by Triton X-100 and phospholipids. J Biochem. 1976;80(4):821–830. doi: 10.1093/oxfordjournals.jbchem.a131343. [DOI] [PubMed] [Google Scholar]
- Scheer E, Cordes C, Eggeling L, Sahm H. Regulation of acetohydroxy acid synthase in Corynebacterium glutamicum during isoleucine formation from α-hydroxybutyric acid. Arch Microbiol. 1987;149(2):173–174. doi: 10.1007/BF00425085. [DOI] [Google Scholar]
- Bott M, Niebisch A. The respiratory chain of Corynebacterium glutamicum. J Biotechnol. 2003;104:129–153. doi: 10.1016/S0168-1656(03)00144-5. [DOI] [PubMed] [Google Scholar]
- Dym O, Pratt EA, Ho C, Eisenberg D. The crystal structure of D-lactate dehydrogenase, a peripheral membrane respiratory enzyme. Proc Natl Acad Sci USA. 2000;97(17):9413–9418. doi: 10.1073/pnas.97.17.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluesener D, Fischer F, Kruip J, Rogner M, Poetsch A. Mapping the membrane proteome of Corynebacterium glutamicum. Proteomics. 2005;5(5):1317–1330. doi: 10.1002/pmic.200400993. [DOI] [PubMed] [Google Scholar]
- Lin ECC. In: Escherichia coli and Salmonella: cellular and molecular biology. 2. Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editor. 0000. ASM Press, Washington, DC; 1996. Dissimilatory pathways for sugars, polyols and carboxylates; pp. 307–342. [Google Scholar]
- Allison N, O'Donnell MJ, Hoey ME, Fewson CA. Membrane-bound lactate dehydrogenases and mandelate dehydrogenases of Acinetobacter calcoaceticus. Location and regulation of expression. Biochem J. 1985;227(3):753–757. doi: 10.1042/bj2270753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa H, Omumasaba CA, Nonaka H, Kos P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y. et al. Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology. 2007;153(Pt 4):1042–1058. doi: 10.1099/mic.0.2006/003657-0. [DOI] [PubMed] [Google Scholar]
- Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L. et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol. 2003;104(1-3):5–25. doi: 10.1016/S0168-1656(03)00154-8. [DOI] [PubMed] [Google Scholar]
- Irlinger F, Mounier J. Microbial interactions in cheese: implications for cheese quality and safety. Curr Opin Biotechnol. 2009;20(2):142–148. doi: 10.1016/j.copbio.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Liu M, Siezen RJ, Nauta A. In silico prediction of horizontal gene transfer events in Lactobacillus bulgaricus and Streptococcus thermophilus reveals protocooperation in yogurt manufacturing. Appl Environ Microbiol. 2009;75(12):4120–4129. doi: 10.1128/AEM.02898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Tseng CH, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA. 2007;104(8):2927–2932. doi: 10.1073/pnas.0607077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H. Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci. 2005;5(7):569–597. doi: 10.1002/mabi.200500062. [DOI] [PubMed] [Google Scholar]
- Taguchi S, Yamada M, Matsumoto K, Tajima K, Satoh Y, Munekata M, Ohno K, Kohda K, Shimamura T, Kambe H. et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc Natl Acad Sci USA. 2008;105(45):17323–17327. doi: 10.1073/pnas.0805653105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Ishida N, Onishi T, Tokuhiro K, Nagamori E, Kitamoto K, Takahashi H. Genetically engineered wine yeast produces a high concentration of L-lactic acid of extremely high optical purity. Appl Environ Microbiol. 2005;71(5):2789–2792. doi: 10.1128/AEM.71.5.2789-2792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Suzuki T, Tokuhiro K, Nagamori E, Onishi T, Saitoh S, Kitamoto K, Takahashi H. D-lactic acid production by metabolically engineered Saccharomyces cerevisiae. J Biosci Bioeng. 2006;101(2):172–177. doi: 10.1263/jbb.101.172. [DOI] [PubMed] [Google Scholar]
- Chang DE, Jung HC, Rhee JS, Pan JG. Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl Environ Microbiol. 1999;65(4):1384–1389. doi: 10.1128/aem.65.4.1384-1389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO. Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol. 2003;69(1):399–407. doi: 10.1128/AEM.69.1.399-407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Lee YY, Elander RT. Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and cofermentation. Appl Biochem Biotechnol. 2007;137-140(1-12):721–738. doi: 10.1007/s12010-007-9092-9. [DOI] [PubMed] [Google Scholar]
- Mazumdar S, Clomburg JM, Gonzalez R. Escherichia coli strains engineered for homofermentative production of D-lactic acid from glycerol. Appl Environ Microbiol. 2010;76(13):4327–4336. doi: 10.1128/AEM.00664-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe S, Takayarna K, Kinoshita S. Taxonomical studies on glutamic acid producing bacteria. J Gen Appl Microbiol. 1967;13:279–301. doi: 10.2323/jgam.13.279. [DOI] [Google Scholar]
- Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145(1):69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Baumbach J, Wittkop T, Kleindt CK, Tauch A. Integrated analysis and reconstruction of microbial transcriptional gene regulatory networks using CoryneRegNet. Nat Protoc. 2009;4(6):992–1005. doi: 10.1038/nprot.2009.81. [DOI] [PubMed] [Google Scholar]
- Munch R, Hiller K, Barg H, Heldt D, Linz S, Wingender E, Jahn D. PRODORIC: prokaryotic database of gene regulation. Nucleic Acids Res. 2003;31(1):266–269. doi: 10.1093/nar/gkg037. [DOI] [PMC free article] [PubMed] [Google Scholar]