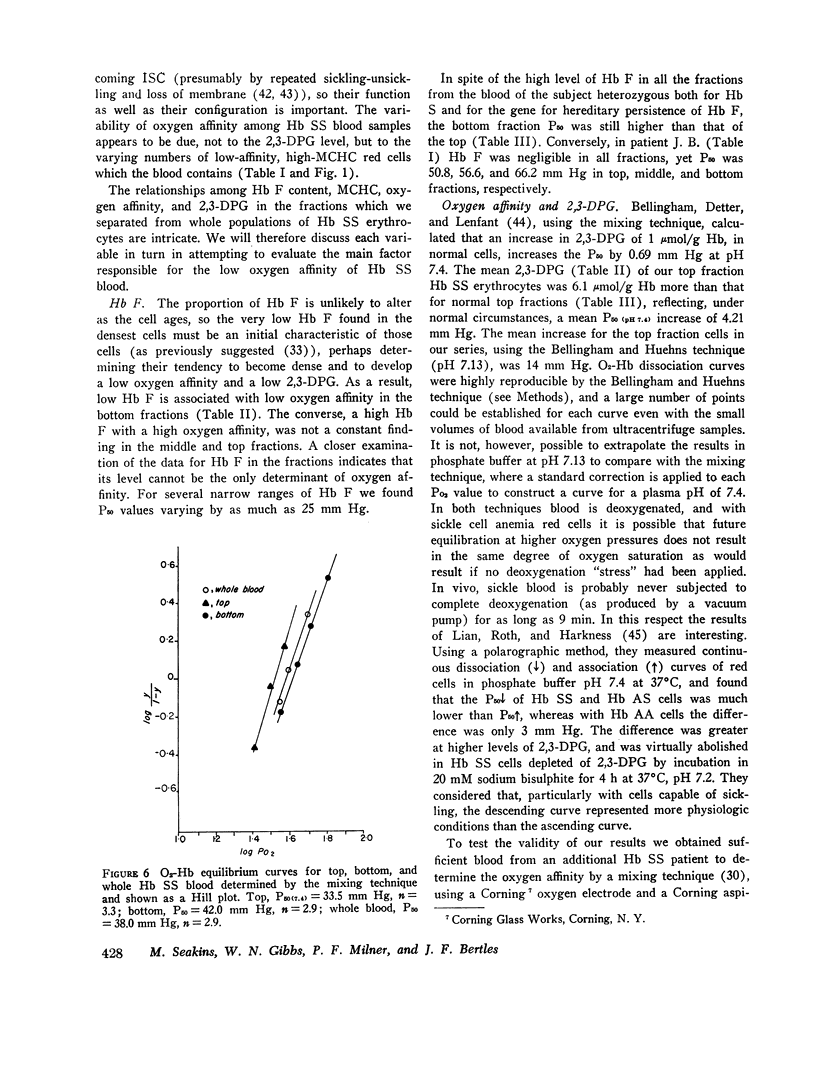
Abstract
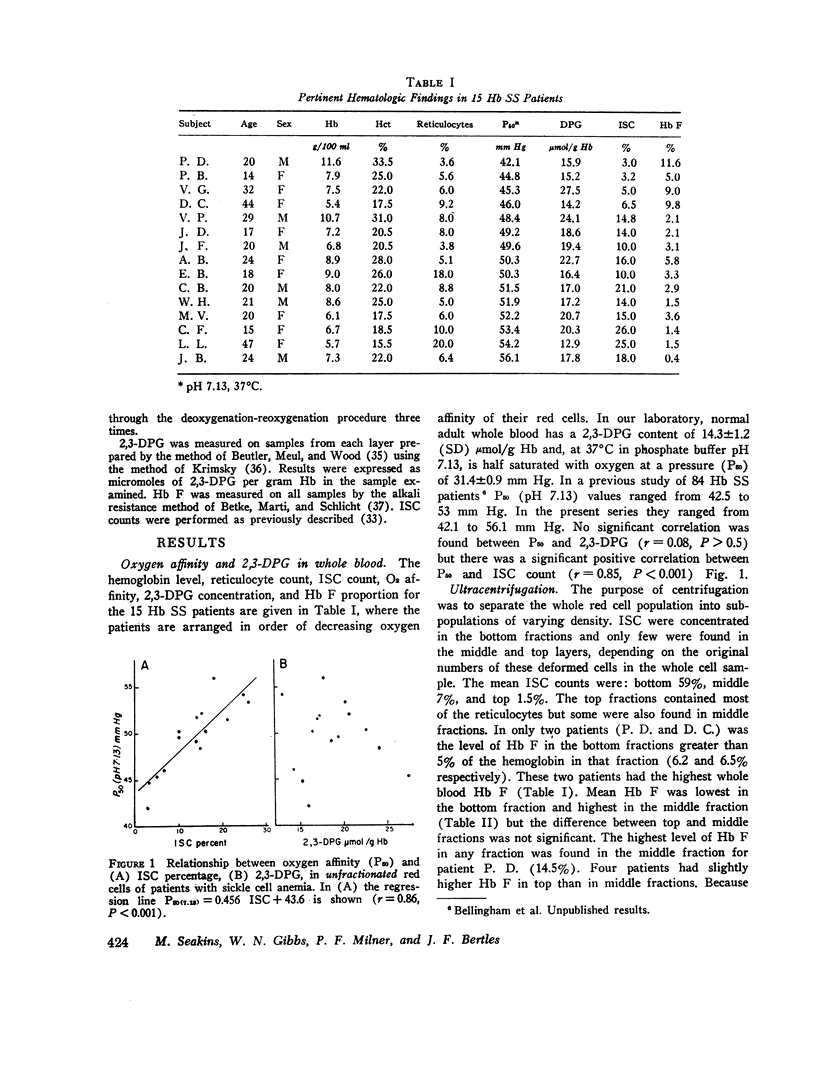
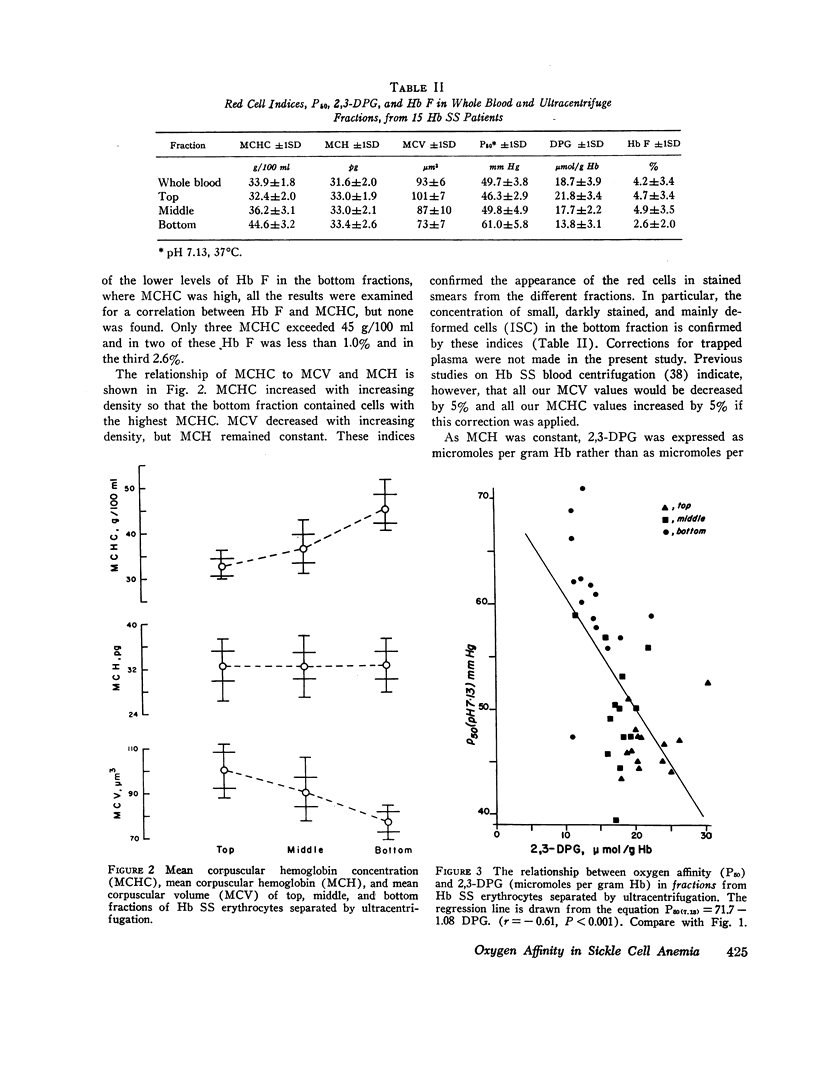
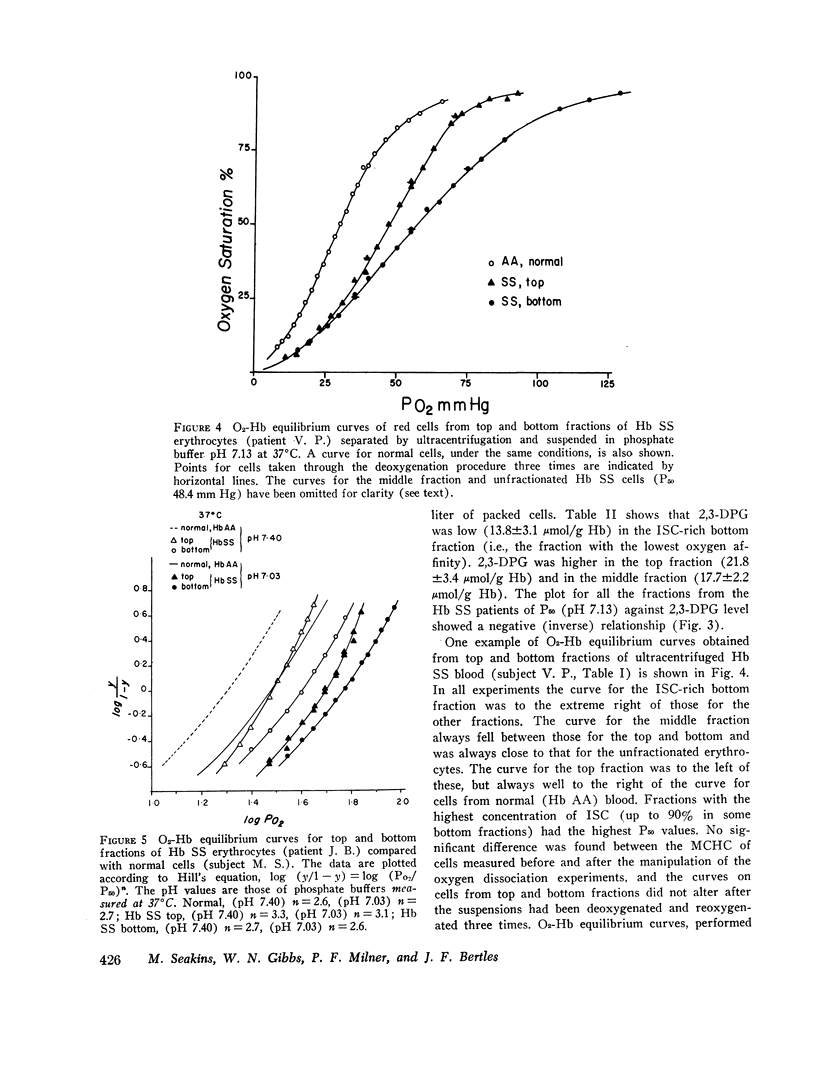
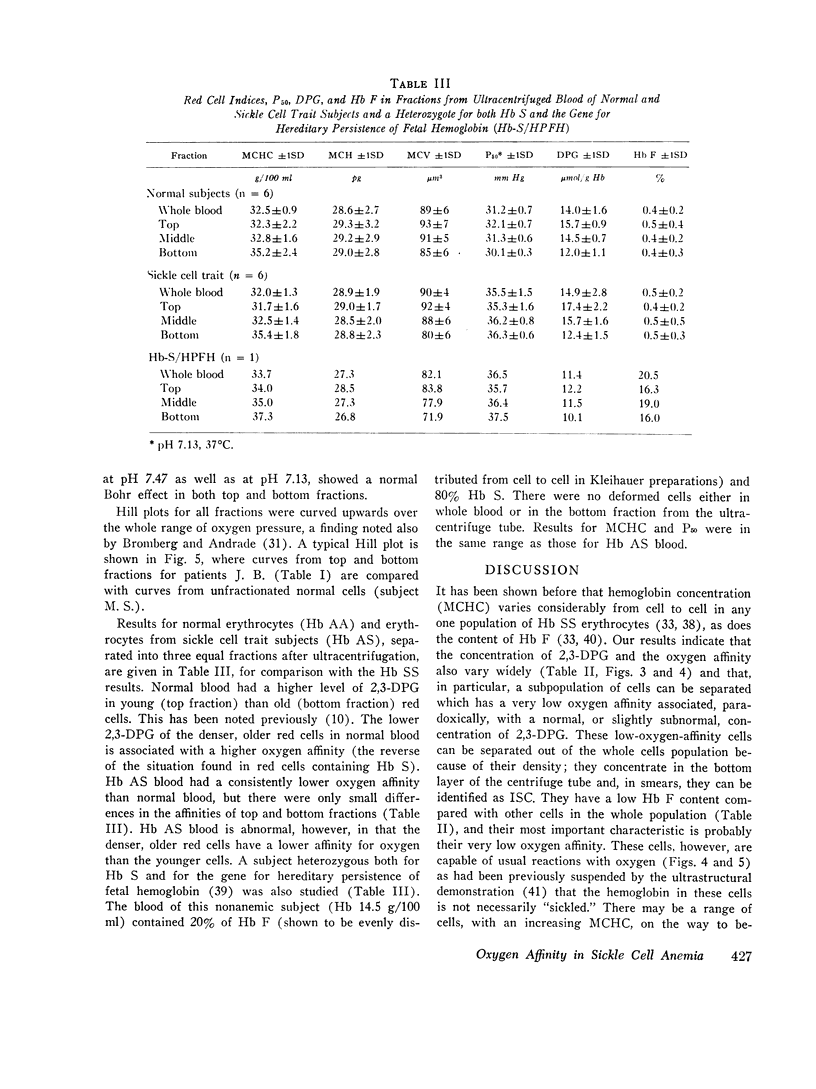
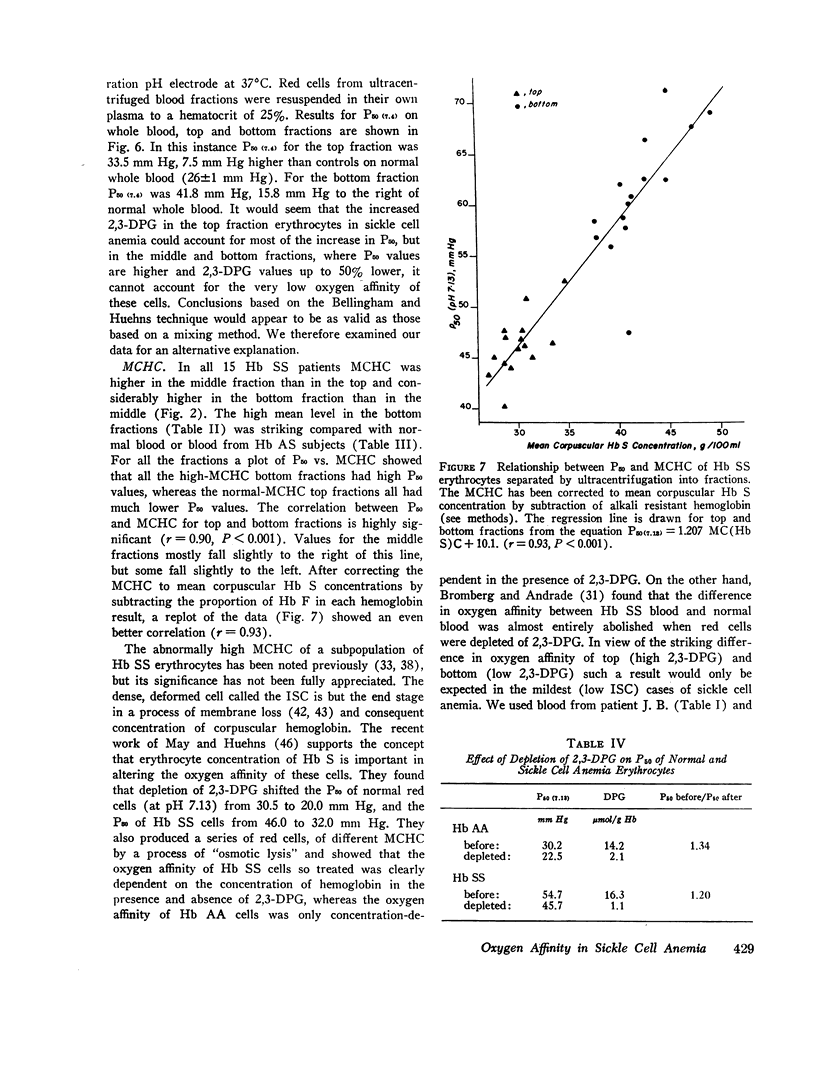
The blood in sickle cell anemia has a very low oxygen affinity and, although 2,3-diphosphoglycerate (2,3-DPG) is increased, there is doubt as to whether this is the only factor responsible. In this study of 15 patients with sickle cell anemia (Hb SS) no correlation was found between oxygen affinity (P50 at pH 7.13) and 2,3-DPG in fresh venous blood. Whole populations of Hb SS erythrocytes were therefore separated, by an ultracentrifuge technique, into fractions of varying density. The packed red cell column was divided into three fractions; a bottom fraction rich in deformed cells or irreversibly sickled cells (ISC), with a very high mean corpuscular hemoglobin concentration (MCHC); a middle fraction containing cells with the highest content of fetal hemoglobin; and a top fraction containing reticulocytes and discoid cells but free of deformed cells. Oxygen affinity was shifted to the right in all layers (mean P50 (pH 7.13)±1SD: top 46.3±2.9 mm Hg: middle 49.8±4.9 mm Hg; bottom 61.0±5.8 mm Hg) compared with normal blood (top 32.1±0.7 mm Hg: bottom 30.1±0.5 mm Hg). 2.3-DPG was increased in the top fraction, but was low or normal in the bottom fraction (top 21.8±3.4 μmol/g Hb: middle 17.7±2.2 μmol/g Hb; bottom 13.8±3.1 μmol/g Hb; normal whole blood 14.3±1.2 μmol/g Hb). The level of 2,3-DPG in top fractions could not account for the degree of right shift of P50, and in the middle and bottom fractions the even greater right shifts were associated with lower levels of 2,3-DPG. Top fraction cells depleted of 2,3-DPG had a higher, but still abnormally low, oxygen affinity. A strong relationship was found between oxygen affinity and MCHC. The fractions with the greatest right shift in P50 had the highest MCHC (top 32.4±2.0; middle 36.2±3.1; bottom 44.6±3.2 g/100 ml, respectively) and the plot of P50 vs. MCHC showed a positive correlation (r = 0.90, P < 0.001).
The red cell population in sickle cell anemia is not homogeneous but contains cells of widely varying Hb F content, 2,3-DPG, and hemoglobin concentration. Paradoxically, the cells with the lowest O2 affinity have the lowest 2,3-DPG, but they also have the highest concentration of Hb S. The dense, deformed cell called the ISC is but the end stage in a process of membrane loss and consequent increase in hemoglobin concentration. The P50 of Hb SS blood is, to a large extent, determined by the presence of these cells (r = 0.85, P < 0.001). Increased concentration of Hb S in the cell favors deoxygenation and crystallization even at relatively high Po2. Lowered affinity for oxygen appears to be closely associated with Hb S concentration and not with 2,3-DPG content.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., WYMAN J., Jr Equilibre de l'hémoglobine de drépanocytose avec l'oxygène. Rev Hematol. 1954;9(2):155–157. [PubMed] [Google Scholar]
- Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972 May 19;237(5351):146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- BECKLAKE M. R., GRIFFITHS S. B., McGREGOR M., GOLDMAN H. I., SCHREVE J. P. Oxygen dissociation curves in sickle cell anemia and in subjects with the sickle cell trait. J Clin Invest. 1955 May;34(5):751–755. doi: 10.1172/JCI103129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETKE K., MARTI H. R., SCHLICHT I. Estimation of small percentages of foetal haemoglobin. Nature. 1959 Dec 12;184(Suppl 24):1877–1878. doi: 10.1038/1841877a0. [DOI] [PubMed] [Google Scholar]
- Bellingham A. J., Detter J. C., Lenfant C. Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis. J Clin Invest. 1971 Mar;50(3):700–706. doi: 10.1172/JCI106540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham A. J., Huehns E. R. Compensation in haemolytic anaemias caused by abnormal haemoglobins. Nature. 1968 Jun 8;218(5145):924–926. doi: 10.1038/218924a0. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Intracellular organic phosphates as regulators of oxygen release by haemoglobin. Nature. 1969 Feb 15;221(5181):618–622. doi: 10.1038/221618a0. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertles J. F., Döbler J. Reversible and irreversible sickling: a distinction by electron microscopy. Blood. 1969 Jun;33(6):884–898. [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertles J. F., Rabinowitz R., Döbler J. Hemoglobin interaction: modification of solid phase composition in the sickling phenomenon. Science. 1970 Jul 24;169(3943):375–377. doi: 10.1126/science.169.3943.375. [DOI] [PubMed] [Google Scholar]
- Beutler E., Meul A., Wood L. A. Depletion and regeneration of 2,3-diphosphoglyceric acid in stored red blood cells. Transfusion. 1969 May-Jun;9(3):109–115. doi: 10.1111/j.1537-2995.1969.tb05527.x. [DOI] [PubMed] [Google Scholar]
- Beutler E., Paniker N. V., West C. The effect of 2,3-DPG on the sickling phenomenon. Blood. 1971 Feb;37(2):184–186. [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L. Ligand-induced conformational dependence of hemoglobin in sickling interactios. J Mol Biol. 1971 Sep 14;60(2):263–270. doi: 10.1016/0022-2836(71)90292-0. [DOI] [PubMed] [Google Scholar]
- Bromberg P. A., Jensen W. N. Arterial oxygen unsaturation in sickle cell disease. Am Rev Respir Dis. 1967 Sep;96(3):400–407. doi: 10.1164/arrd.1967.96.3.400. [DOI] [PubMed] [Google Scholar]
- Bromberg P. A., Jensen W. N. Blood oxygen dissociation curves in sickle cell disease. J Lab Clin Med. 1967 Sep;70(3):480–488. [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Control of hemoglobin function within the red cell. N Engl J Med. 1970 Jun 18;282(25):1414–1421. doi: 10.1056/NEJM197006182822507. [DOI] [PubMed] [Google Scholar]
- CONLEY C. L., WEATHERALL D. J., RICHARDSON S. N., SHEPARD M. K., CHARACHE S. Hereditary persistence of fetal hemoglobin: a study of 79 affected persons in 15 Negro families in Baltimore. Blood. 1963 Mar;21:261–281. [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- Charache S., Grisolia S., Fiedler A. J., Hellegers A. E. Effect of 2,3-diphosphoglycerate on oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):806–812. doi: 10.1172/JCI106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Furia F. G., Miller D. R., Cerami A., Manning J. M. The effects of cyanate in vitro on red blood cell metabolism and function in sickle cell anemia. J Clin Invest. 1972 Mar;51(3):566–574. doi: 10.1172/JCI106845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. J., Novy M. J., Walters C. L., Metcalfe J. Improved oxygen release: an adaptation of mature red cells to hypoxia. J Clin Invest. 1968 Aug;47(8):1851–1857. doi: 10.1172/JCI105875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAIMOW W., RODMAN T., CLOSE H. P., CATHCART R., PURCELL M. K. The oxyhemoglobin dissociation curve in sickle cell anemia. Am J Med Sci. 1958 Aug;236(2):225–232. doi: 10.1097/00000441-195808000-00011. [DOI] [PubMed] [Google Scholar]
- HILPERT P., FLEISCHMANN R. G., KEMPE D., BARTELS H. THE BOHR EFFECT RELATED TO BLOOD AND ERYTHROCYTE PH. Am J Physiol. 1963 Aug;205:337–340. doi: 10.1152/ajplegacy.1963.205.2.337. [DOI] [PubMed] [Google Scholar]
- JENSEN W. N., RUCKNAGEL D. L., TAYLOR W. J. In vivo study of the sickle cell phenomenon. J Lab Clin Med. 1960 Dec;56:854–865. [PubMed] [Google Scholar]
- Jensen W. N. Fragmentation and the "freakish poikilocyte". Am J Med Sci. 1969 Jun;257(6):355–364. doi: 10.1097/00000441-196906000-00001. [DOI] [PubMed] [Google Scholar]
- KENNEDY A. C., VALTIS D. J. The oxygen dissociation curve in anemia of various types. J Clin Invest. 1954 Oct;33(10):1372–1381. doi: 10.1172/JCI103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C., Torrance J., English E., Finch C. A., Reynafarje C., Ramos J., Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest. 1968 Dec;47(12):2652–2656. doi: 10.1172/JCI105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C., Ways P., Aucutt C., Cruz J. Effect of chronic hypoxic hypoxia on the O2-Hb dissociation curve and respiratory gas transport in man. Respir Physiol. 1969 Jun;7(1):7–29. doi: 10.1016/0034-5687(69)90066-8. [DOI] [PubMed] [Google Scholar]
- Lian C. Y., Roth S., Harkness D. R. The effect of alteration of intracellular 2,3-DPG concentration upon oxygen binding of intact erythrocytes containing normal and mutant hemoglobins. Biochem Biophys Res Commun. 1971 Oct 1;45(1):151–158. doi: 10.1016/0006-291x(71)90063-5. [DOI] [PubMed] [Google Scholar]
- May A., Bellingham A. J., Huehns E. R., Beaven G. H. Effect of cyanate on sickling. Lancet. 1972 Mar 25;1(7752):658–661. doi: 10.1016/s0140-6736(72)90462-x. [DOI] [PubMed] [Google Scholar]
- May A., Huehns E. R. The control of oxygen affinity of red cells with Hb-Shepherds Bush. Br J Haematol. 1972 May;22(5):599–607. doi: 10.1111/j.1365-2141.1972.tb05706.x. [DOI] [PubMed] [Google Scholar]
- Messer M. J., Harris J. W. Filtration characteristics of sickle cells: rates of alteration of filterability after deoxygenation and reoxygenation, and correlations with sickling and unsickling. J Lab Clin Med. 1970 Oct;76(4):537–547. [PubMed] [Google Scholar]
- Novy M. J., Edwards M. J., Metcalfe J. Hemoglobin Yakina. II. High blood oxygen affinity associated with compensatory erythrocytosis and normal hemodynamics. J Clin Invest. 1967 Nov;46(11):1848–1854. doi: 10.1172/JCI105675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oski F. A., Gottlieb A. J., Delivoria-Papadopoulos M., Miller W. W. Red-cell 2,3-diphosphoglycerate levels in subjects with chronic hypoxemia. N Engl J Med. 1969 May 22;280(21):1165–1166. doi: 10.1056/NEJM196905222802108. [DOI] [PubMed] [Google Scholar]
- Parer J. T. Oxygen transport in human subjects with hemoglobin variants having altered oxygen affinity. Respir Physiol. 1970 Apr;9(1):43–49. doi: 10.1016/0034-5687(70)90004-6. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- RODMAN T., CLOSE H. P., CATHCART R., PURCELL M. K. The oxyhemoglobin dissociation curve in the common hemoglo-binopathies. Am J Med. 1959 Oct;27:558–566. doi: 10.1016/0002-9343(59)90041-5. [DOI] [PubMed] [Google Scholar]
- RODMAN T., CLOSE H. P., PURCELL M. K. The oxyhemoglobin dissociation curve in anemia. Ann Intern Med. 1960 Feb;52:295–309. doi: 10.7326/0003-4819-52-2-295. [DOI] [PubMed] [Google Scholar]
- Richards D. W., Strauss M. L. OXY-HEMOGLOBIN DISSOCIATION CURVES OF WHOLE BLOOD IN ANEMIA. J Clin Invest. 1927 Apr;4(1):105–126. doi: 10.1172/JCI100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD M. K., WEATHERALL D. J., CONLEY C. L. Semi-quantitative estimation of the distribution of fetal hemoglobin in red cell populations. Bull Johns Hopkins Hosp. 1962 Jun;110:293–310. [PubMed] [Google Scholar]
- Torrance J., Jacobs P., Restrepo A., Eschbach J., Lenfant C., Finch C. A. Intraerythrocytic adaptation to anemia. N Engl J Med. 1970 Jul 23;283(4):165–169. doi: 10.1056/NEJM197007232830402. [DOI] [PubMed] [Google Scholar]
- Tyuma I., Shimizu K. Different response to organic phosphates of human fetal and adult hemoglobins. Arch Biochem Biophys. 1969 Jan;129(1):404–405. doi: 10.1016/0003-9861(69)90192-1. [DOI] [PubMed] [Google Scholar]
- Tyuma I., Shimizu K. Effect of organic phosphates on the difference in oxygen affinity between fetal and adult human hemoglobin. Fed Proc. 1970 May-Jun;29(3):1112–1114. [PubMed] [Google Scholar]
- Woodson R. D., Torrance J. D., Shappell S. D., Lenfant C. The effect of cardiac disease on hemoglobin-oxygen binding. J Clin Invest. 1970 Jul;49(7):1349–1356. doi: 10.1172/JCI106351. [DOI] [PMC free article] [PubMed] [Google Scholar]


