Abstract
Specific IgE anti-ragweed antibodies (IgEAR) were measured over two years in two groups of highly sensitive patients treated (immunized) with either ragweed extract or placebo and in a third group of placebo-treated, relatively insensitive patients. The IgEAR on the patients' basophils were assessed by ragweed antigen E (AgE)-induced histamine release; blocking (IgG) antibodies were measured by their ability to inhibit AgE induced histamine release. These data were evaluated against the clinical severity of ragweed hay fever in each patient.
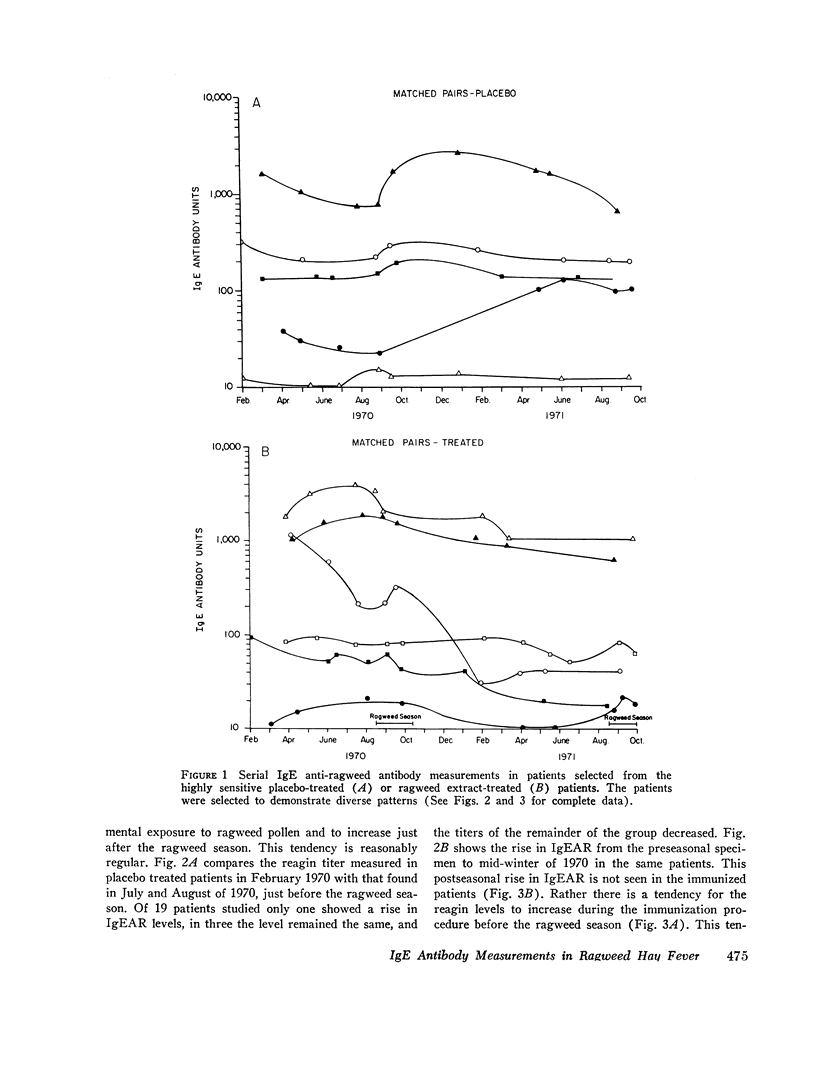
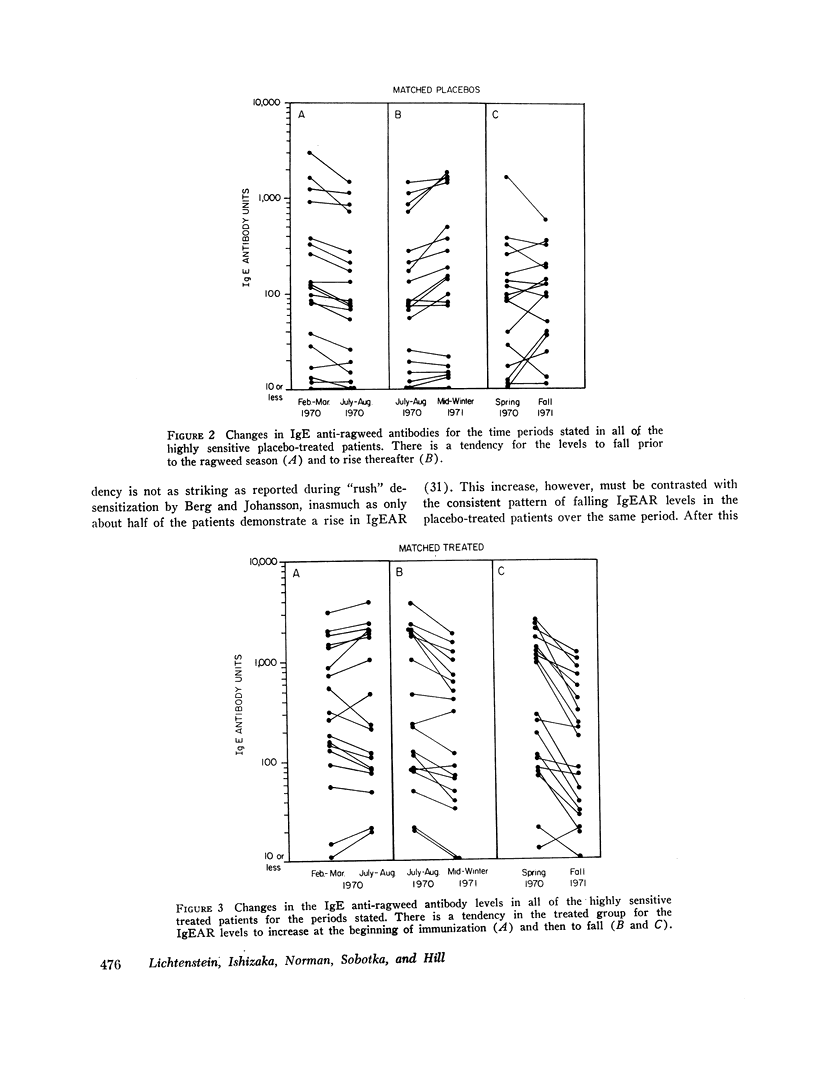
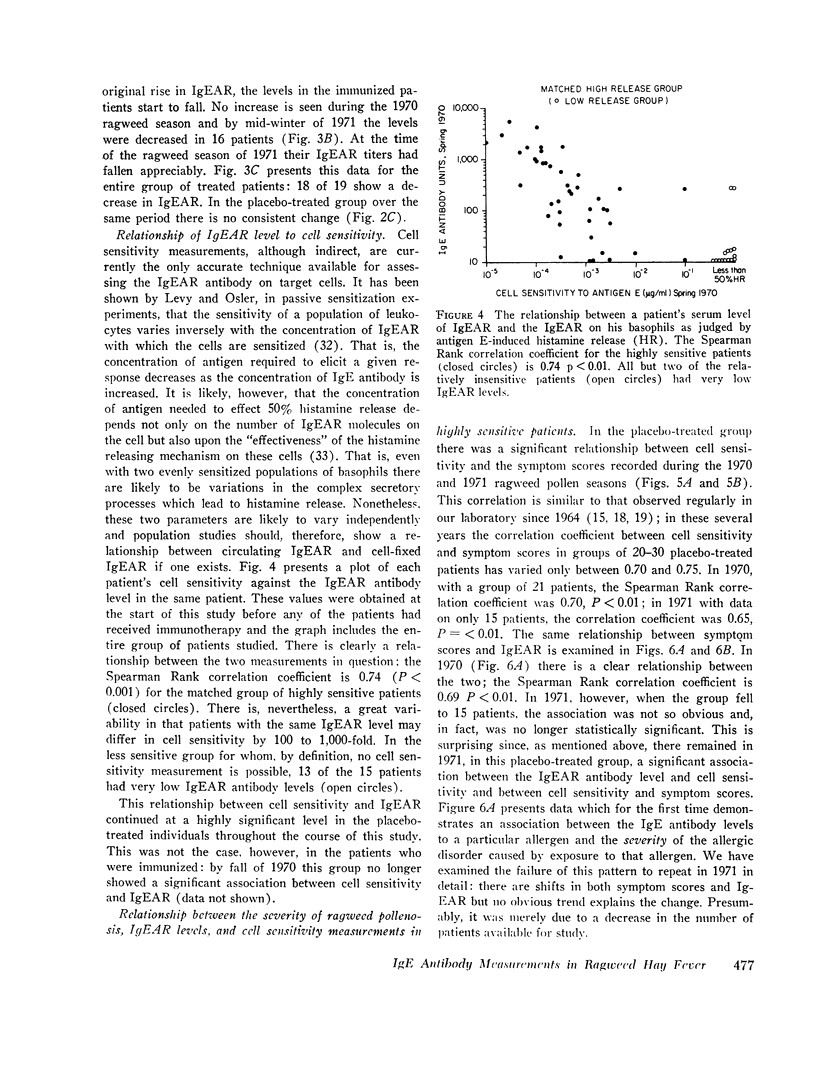
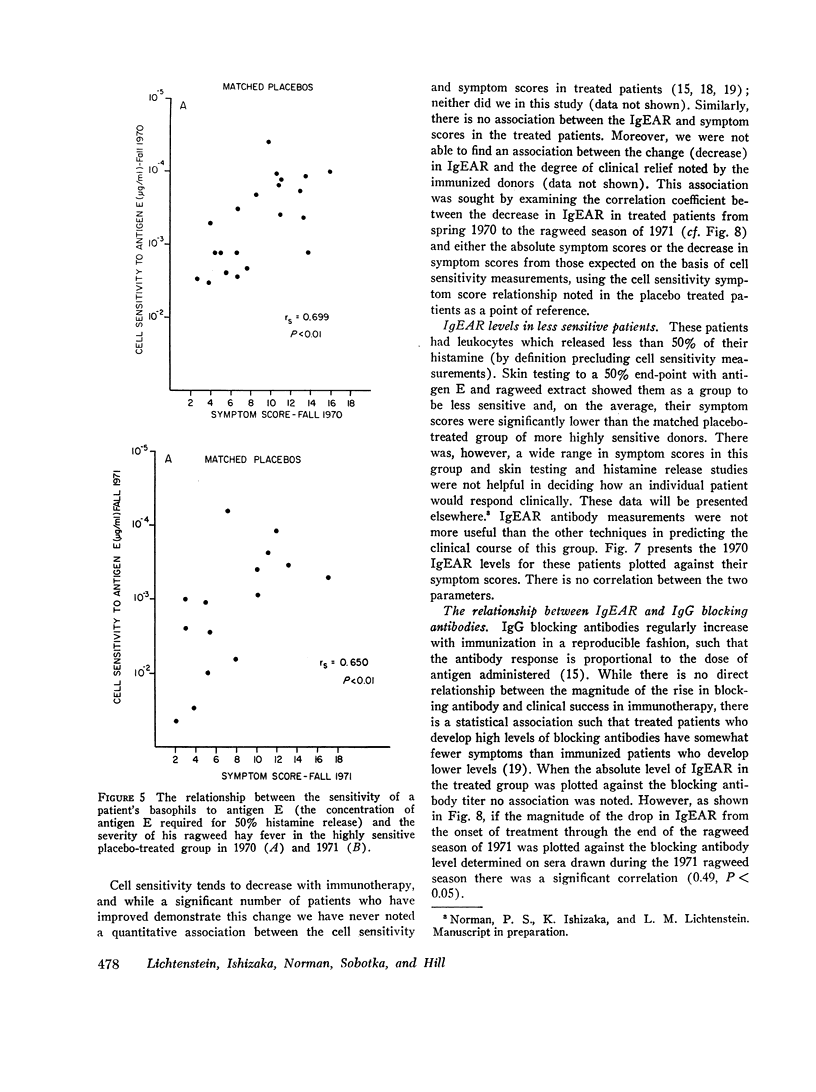
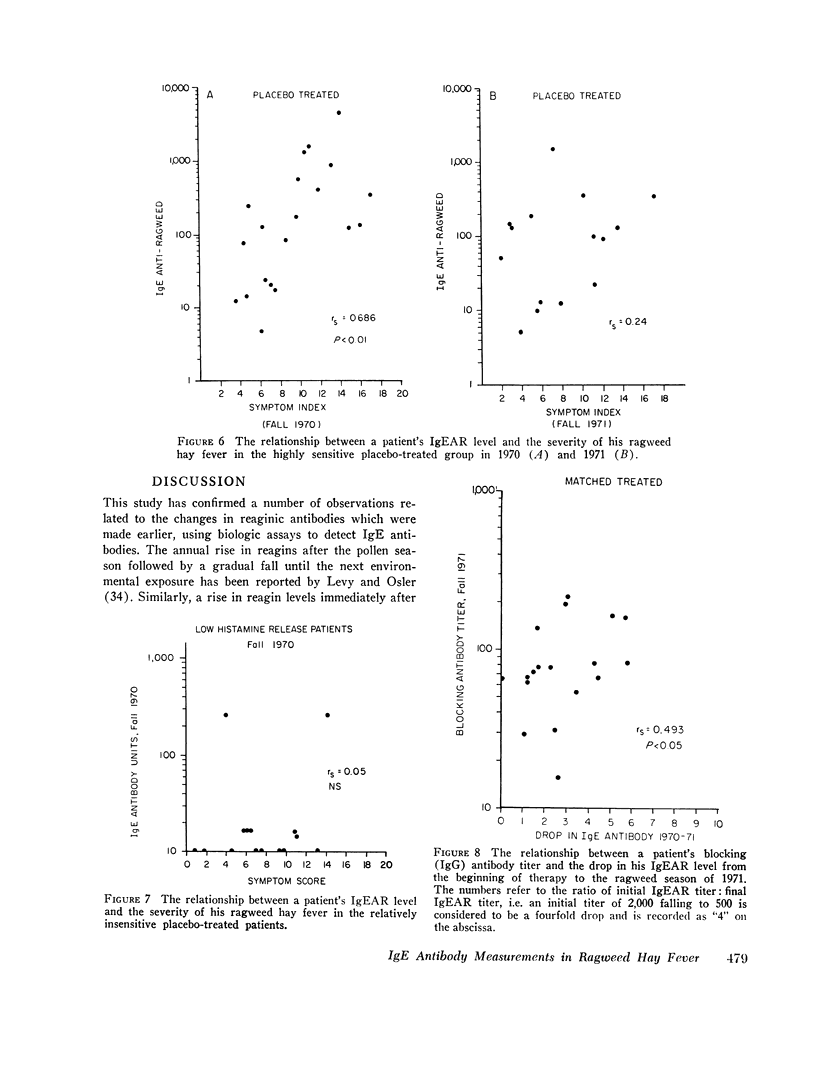
We found that: (a) In placebo treated patients IgEAR usually declined gradually prior to the ragweed season and were boosted by environmental exposure to ragweed pollen. (b) In immunized patients the IgEAR rose at the beginning of treatment, but fell as immunotherapy proceeded; by the end of the second year the levels had decreased in 18/19 patients. (c) The increase in blocking antibody during immunotherapy correlated significantly (P < 0.05) with the decrease in serum IgEAR. (d) Judged by their sensitivity to AgE induced histamine release, IgEAR on basophils correlated significantly with IgEAR in the serum of untreated patients (P < 0.01). (e) The highly sensitive placebo treated patients' symptom scores were significantly correlated with their IgEAR in serum (P < 0.01) and with the sensitivity of their basophils to AgE-induced histamine release (P < 0.01). Neither correlation was observed in the relatively insensitive patients. (f) In the treated group the IgEAR measurements predicted neither the degree of their illness nor their clinical improvement We conclude tha IgE antibody measurements may be useful in the assessment of the severity of reaginic allergy in highly sensitive patients. Its use in modestly sensitive patients requires patients requires further study, as does the inverse association between IgE and IgG antiragweed antibodies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg T., Bennich H., Johansson S. G. In vitro diagnosis of atopic allergy. I. A comparison between provocation tests and the radioallergosorbent test. Int Arch Allergy Appl Immunol. 1971;40(6):770–778. [PubMed] [Google Scholar]
- Berg T., Johansson S. G. In vitro diagnosis of atopic allergy. II. IgE and reaginic antibodies during and after rush desensitization. Int Arch Allergy Appl Immunol. 1971;41(2):434–442. [PubMed] [Google Scholar]
- Gleich G. J., Averbeck A. K., Swedlund H. A. Measurement of IgE in normal and allergic serum by radioimmunoassay. J Lab Clin Med. 1971 Apr;77(4):690–698. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. Identification of gamma-E-antibodies as a carrier of reaginic activity. J Immunol. 1967 Dec;99(6):1187–1198. [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Terry W. D. Antigenic structure of gamma-E-globulin and reaginic antibody. J Immunol. 1967 Nov;99(5):849–858. [PubMed] [Google Scholar]
- Ishizaka K., Okudaira H. Reaginic antibody formation in the mouse. I. Antibody-mediated suppression of reaginic antibody formation. J Immunol. 1972 Jul;109(1):84–89. [PubMed] [Google Scholar]
- Ishizaka T., De Bernardo R., Tomioka H., Lichtenstein L. M., Ishizaka K. Identification of basophil granulocytes as a site of allergic histamine release. J Immunol. 1972 Apr;108(4):1000–1008. [PubMed] [Google Scholar]
- Johansson S. G., Bennich H., Berg T., Högman C. Some factors influencing the serum IgE levels in atopic diseases. Clin Exp Immunol. 1970 Jan;6(1):43–47. [PMC free article] [PubMed] [Google Scholar]
- Johansson S. G., Bennich H., Berg T. In vitro diagnosis of atopic allergy. 3. Quantitative estimation of circulating IgE antibodies by the radioallergosorbent test. Int Arch Allergy Appl Immunol. 1971;41(2):443–451. [PubMed] [Google Scholar]
- Johansson S. G., Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967 Oct;13(4):381–394. [PMC free article] [PubMed] [Google Scholar]
- Johansson S. G., Bennich H., Wide L. A new class of immunoglobulin in human serum. Immunology. 1968 Feb;14(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- Johansson S. G., Mellbin T., Vahlquist B. Immunoglobulin levels in Ethiopian preschool children with special reference to high concentrations of immunoglobulin E (IgND). Lancet. 1968 May 25;1(7552):1118–1121. doi: 10.1016/s0140-6736(68)90187-6. [DOI] [PubMed] [Google Scholar]
- Johansson S. G. Serum IgND levels in healthy children and adults. Int Arch Allergy Appl Immunol. 1968;34(1):1–8. doi: 10.1159/000230089. [DOI] [PubMed] [Google Scholar]
- KING T. P., NORMAN P. S. Isolation studies of allergens from regweed pollen. Biochemistry. 1962 Jul;1:709–720. doi: 10.1021/bi00910a027. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. A., Lichtenstein L. M., Goldstein E. O., Ishizaka K. Immunologic and cellular changes accompanying the therapy of pollen allergy. J Clin Invest. 1971 Feb;50(2):360–369. doi: 10.1172/JCI106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XIV. Passive sensitization in vitro of human leukocytes to ragweed pollen antigen. J Immunol. 1966 Aug;97(2):203–212. [PubMed] [Google Scholar]
- Levy D. A., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XVI. In vitro assays of reaginic activity in human sera: effect of therapeutic immunization on seasonal titer changes. J Immunol. 1967 Dec;99(6):1068–1077. [PubMed] [Google Scholar]
- Lichtenstein L. M., Holtzman N. A., Burnett L. S. A quantitative in vitro study of the chromatographic distribution and immunoglobulin characteristics of human blocking antibody. J Immunol. 1968 Aug;101(2):317–324. [PubMed] [Google Scholar]
- Lichtenstein L. M., King T. P., Osler A. G. In vitro assay of allergenic properties of ragweed pollen antigens. J Allergy. 1966 Sep;38(3):174–182. doi: 10.1016/0021-8707(66)90040-2. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L. A single year of immunotherapy for ragweed hay fever. Immunologic and clinical studies. Ann Intern Med. 1971 Nov;75(5):663–671. doi: 10.7326/0003-4819-75-5-663. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L. Clinical and in vitro studies on the role of immunotherapy in ragweed hay fever. Am J Med. 1968 Apr;44(4):514–524. doi: 10.1016/0002-9343(68)90052-1. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Norman P. S., Winkenwerder W. L., Osler A. G. In vitro studies of human ragweed allergy: changes in cellular and humoral activity associated with specific desensitization. J Clin Invest. 1966 Jul;45(7):1126–1136. doi: 10.1172/JCI105419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., Osler A. G. Studies on the mechanisms of hypersensitivity phenomena. XII. An in vitro study of the reaction between ragweed pollen antigen, allergic human serum and ragweed-sensitive human leukocytes. J Immunol. 1966 Jan;96(1):169–179. [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R., Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy. 1970 Jul;46(1):12–20. doi: 10.1016/0021-8707(70)90056-0. [DOI] [PubMed] [Google Scholar]
- NORMAN P. S., WINKENWERDER W. L. SUPPRESSION OF HAY FEVER SYMPTOMS WITH INTRANASAL DEXAMETHASONE AEROSOL. J Allergy. 1965 May-Jun;36:284–292. doi: 10.1016/0021-8707(65)90087-0. [DOI] [PubMed] [Google Scholar]
- Norman P. S., Winkenwerder W. L., Lichtenstein L. M. Trials of alum-precipitated pollen extracts in the treatment of hay fever. J Allergy Clin Immunol. 1972 Jul;50(1):31–44. doi: 10.1016/0091-6749(72)90077-2. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Kochwa S., Smith C., Ishizaka K., McIntyre O. R. Clinical aspects of IgE myeloma. N Engl J Med. 1969 Nov 27;281(22):1217–1220. doi: 10.1056/NEJM196911272812204. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Pruzansky J. J., Patterson R. Histamine release from leukocytes of hypersensitive individuals. II. Reduced sensitivity of leukocytes after injection therapy. J Allergy. 1967 Jan;39(1):44–50. doi: 10.1016/0021-8707(67)90126-8. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Sadan N., Rhyne M. B., Mellits E. D., Goldstein E. O., Levy D. A., Lichtenstein L. M. Immunotherapy of pollinosis in children: investigation of the immunologic basis of clinical improvement. N Engl J Med. 1969 Mar 20;280(12):623–627. doi: 10.1056/NEJM196903202801201. [DOI] [PubMed] [Google Scholar]
- Tada T., Okumura K. Regulation of homocytotropic antibody formation in the rat. I. Feed-back regulation by passively administered antibody. J Immunol. 1971 Apr;106(4):1002–1011. [PubMed] [Google Scholar]
- Vaz N. M., Prouvost-Danon A. Behaviour of mouse mast cells during anaphylaxis in vitro. Prog Allergy. 1969;13:111–173. doi: 10.1159/000385921. [DOI] [PubMed] [Google Scholar]
- Wide L., Bennich H., Johansson S. G. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967 Nov 25;2(7526):1105–1107. doi: 10.1016/s0140-6736(67)90615-0. [DOI] [PubMed] [Google Scholar]
- Wide L. Radioimmunoassays employing immunosorbents. Acta Endocrinol Suppl (Copenh) 1969;142:207–221. doi: 10.1530/acta.0.062s207. [DOI] [PubMed] [Google Scholar]



