Abstract
Allopregnanolone (ALLO), is a brain endogenous neurosteroid that binds with high affinity to γ-aminobutyric acid type A (GABAA) receptors and positively modulates the action of GABA at these receptors. Unlike ALLO, 5α-dihydroprogesterone (5α-DHP) binds with high affinity to intracellular progesterone receptors that regulate DNA transcription. To investigate the physiological roles of ALLO and 5α-DHP synthesized in brain, we have adopted a mouse model involving protracted social isolation. In the frontal cortex of mice, socially isolated for 6 weeks, both neurosteroids were decreased by approximately 50%. After administration of (17β)-17-(bis-1-methyl amino carbonyl) androstane-3,5-diene-3-carboxylic acid (SKF105,111), an inhibitor of the enzyme (5α-reductase Type I and II) that converts progesterone into 5α-DHP, the ALLO and 5α-DHP content of frontal cortex of both group-housed and socially isolated mice decreased exponentially to 10%–20% of control values in about 30 min. The fractional rate constants (k h−1) of ALLO and 5α-DHP decline multiplied by the ALLO and 5α-DHP concentrations at any given steady-state estimate the rate of synthesis required to maintain that steady state. After 6 weeks of social isolation, ALLO and 5α-DHP biosynthesis rates were decreased to 30% of the values calculated in group-housed mice. Moreover, in socially isolated mice, the expression of 5α-reductase Type I mRNA and protein was approximately 50% lower than in group-housed mice whereas 3α-hydroxysteroid oxidoreductase mRNA expression was equal in the two groups. Protracted social isolation in mice may provide a model to investigate whether 5α-DHP by a genomic action, and ALLO by a nongenomic mechanism down-regulate the action of drugs acting as agonists, partial agonists, or positive allosteric modulators of the benzodiazepine recognition sites expressed by GABAA receptors.
Allopregnanolone (ALLO), (3α5α-tetrahydroprogesterone), and 5α-dihydroprogesterone (5α-DHP) are the most likely candidates among the endogenous neuroactive steroids for a neurophysiological role in maintaining GABAergic transmission efficacy. ALLO is synthesized in brain from progesterone by the sequential action of two enzymes: (i) 5α-reductase, which reduces progesterone to 5α-DHP; and (ii) 3α-hydroxysteroidoxidoreductase (3α-HSOR), which either converts 5α-DHP into ALLO (reductive reaction) or converts ALLO into 5α-DHP (oxidative reaction) (1, 2).
Several lines of evidence have demonstrated that these neurosteroids in nanomolar concentrations regulate GABAergic transmission by two modalities: (i) nongenomic (ALLO), by facilitating γ-aminobutyric acid (GABA) gating of GABA type A (GABAA) receptors after ALLO-specific binding to an allosteric center expressed by these receptors; and (ii) genomic (5α-DHP) by regulating DNA transcription, perhaps including genes encoding for GABAA receptor subunits, after 5α-DHP binding to intracellular progesterone receptors (3, 4).
The influence of these neurosteroids on the physiological efficacy of GABAA receptor function can be documented in vivo and in vitro by administering ALLO and/or 5α-DHP, but the role of endogenously synthesized ALLO and 5α-DHP can be only indirectly inferred from measurements of brain region-specific changes in their biosynthesis rate and of consequent changes in the physiological efficacy of GABAA receptors. Several lines of independent investigation have shown that, in rodents, the efficacy of GABAA receptors is modified during social isolation (5–7). However, research aimed at validating a putative physiological or pathological role for 5α-DHP or ALLO has been hampered by (i) the lack of specific antagonists acting at neurosteroid recognition sites expressed on GABAA receptors as well as by (ii) the insufficient specificity of RIA methods for neurosteroid determination, when these methods are used at the limit of their sensitivity. To overcome these obstacles, we have developed a highly specific gas-chromatographic-negative ion-mass-spectrometric (GC-MS) method that allows for accurate measurement in the attomolar range of specific neurosteroids, and also provides information that defines the neurosteroid stereoisomeric structure (i.e. 3α vs. 3β derivatives). Although specific antagonists of ALLO and of other neurosteroids are not yet available, we have defined the validity of the following pharmacological research strategies: (i) inhibition of the rate-limiting enzyme that converts progesterone into 5α-DHP (5α-reductase) with (17β)-17-(bis-1-methyl amino carbonyl) androstane-3,5-diene-3-carboxylic acid (SKF105,111), which specifically blocks the catalytic activity of both 5α-reductase Type I and Type II (8, 9); and (ii) use of fluoxetine (10) to increase ALLO biosynthesis without changing the brain content of the precursor 5α-DHP (10), by selectively increasing the catalytic activity of 3α-HSOR (11), the enzyme that converts 5α-DHP into ALLO.
In a recent study, we reported that the symptoms of severe unipolar depression (Hamilton score) were ameliorated by a fluoxetine treatment for 8–10 weeks (12). This improvement was correlated with a normalization of the cerebrospinal fluid (CSF) ALLO content, which before fluoxetine treatment was lower than control level (12). These data suggest that, in humans, a decrease of ALLO expression in specific brain areas may be associated with the onset of anxiety and dysphoria because of a down-regulation of GABAA receptor neurophysiological efficacy.
Mice, socially isolated for 6 weeks, but not for 2 weeks, manifest a decrease of brain ALLO levels (5), which can be reversed with fluoxetine treatment, presumably because fluoxetine activates 3α-HSOR. The ability of fluoxetine to normalize the neurophysiological efficacy of GABAA receptors depressed by the protracted social isolation and ALLO down-regulation can also be inferred by the normalization of the efficacy of pentobarbital and other drugs that act by reinforcing the action of GABA at GABAA receptors after fluoxetine administration to socially isolated mice (5, 6). Thus, these pharmacological studies on socially isolated mice suggest that the increased anxiety and aggressiveness toward an intruder reported in these animals (13, 14) might be attributable to a down-regulation of electrophysiological GABAergic efficacy because of a decrease of brain ALLO expression (5–7). In summary, these data support the hypothesis that endogenously produced brain ALLO may have a permissive role in the physiological modulation of GABAergic tone as related to the function of GABA-gated Cl− channels.
The experiments to be reported were conducted to test whether there is a cause-effect relationship between down-regulation of ALLO and 5α-DHP expression and the neurophysiological efficacy of GABA receptor function by using a mouse model involving protracted social isolation in which the down-regulation of ALLO and 5α-DHP is proposed to reflect long term adaptation to the stress of social isolation.
The results demonstrate that social isolation for 6 weeks reduces the brain content of 5α-DHP and ALLO by down-regulating 5α-reductase Type I expression.
Methods
Social Isolation.
Male Swiss–Webster mice (Harlan Breeders, Indianapolis), 22–25 g body weight, maintained under a 12-hr dark/light cycle, and food and water ad libitum, were used for all of the experiments. Animals were housed either in groups of five to six per cage (24 × 17 × 12 cm) or individually (socially isolated) in a cage of the same size for 6 weeks before the start of our measurements (5). The housing temperature was regulated to be around 24°C, the humidity around 65%.
Quantitative Analysis of Neurosteroids.
Extraction, derivatization, and gas chromatography-mass spectrometry (GC-MS) analyses of neurosteroids were performed as previously described (5, 10). (i) Brain areas were homogenized in 10 vol of distilled water containing 2–5 fmol/ml of the [3H]neurosteroid (New England Nuclear) of interest or 20 pmol deuterium-labeled 5α-DHP [(5α-DHP (1,2,4,5,6,7-D6; Cambridge Isotope Laboratories, Andover, MA)] to monitor the recovery. The supernatants were extracted with ethyl acetate and, after lyophilization, were purified with HPLC as described by Cheney et al. (8). (ii) The HPLC-eluted fractions containing ALLO, pregnenolone, or progesterone, after the addition of the internal standard (alfaxalone), were derivatized with heptafluorobutyric acid anhydride (HFBA), as previously described (5, 10).
The HPLC fractions containing 5α-DHP and the deuteriated internal standard, were dissolved in 200 μl of pyridine and derivatized with 20 μl of 2% O-2,3,4,5,6-pentafluorobenzyl hydroxylamine hydrochloride (PFBH HCL; Sigma) in pyridine at 65°C for 1 h. The reaction mixture was evaporated to dryness and redissolved in 100 μl of hexane followed by washing with 20 μl of double distilled water. GC/MS separation was carried out by using a methylsilicone capillary column [length 15 m, i.d. 0.25 mm, film thickness 0.05 μm (Quadrex Corporation, Woodbridge, CT)].
Mass spectrometry for the derivatized steroids was performed in the negative ion chemical ionization mode (NICI) by using methane as the reaction gas for the analyses of ALLO, 5α-DHP, and pregnenolone (10). Progesterone was analyzed by using the electron impact (EI) MS as described by Matsumoto et al. (5). For the quantification of ALLO, progesterone, and pregnenolone, the standard curve for the steroid of interest was prepared by combining different known quantities of authentic steroids (Steraloids, Wilton, NH), from 1 to 103 fmol, with a constant amount of internal standard. The area under the peak of the internal standard divided by the area under the peak of a known quantity of each steroid is plotted against the quantity of each steroid to generate the standard curve. The detection limit for ALLO and for the other steroids studied is approximately 10 fmol; the standard curve is linear between 1 and 103 fmol. For quantification of 5α-DHP the m/z ion monitoring mode was 686 for 5α-DHP and 692 for 5α-DHP(1,2,4,5,6,7-D6).
Estimation of Neurosteroid Biosynthesis Rate.
The rate of 5α-DHP or ALLO biosynthesis in various mouse brain parts was estimated by measuring the initial rate of decline after blockade of synthesis with an i.p. injection of 48 μmol/kg of 5α-reductase Types I and II inhibitor SKF105,111 dissolved in 1% DMSO (0.1 ml/10 g body weight). The fractional rate constant for the decline of 5α-DHP or ALLO after blockade of 5α-reductase was calculated from the regression line described by the decrease in 5α-DHP or ALLO concentration with time. The semilogarithmic plot of these data yields a single exponential decline, and k is calculated from the following equation: k = b/0.434, where b is the slope of the decline calculated from the semilogarithmic plot of the data. The derivation and application of this equation was presented in detail previously (15). The significant difference in the slopes was calculated by using the statistical computer package spss for windows, rel. 8.0.0 (SPSS, Chicago). The turnover rate (TR) was calculated by multiplying k h−1 by the ALLO or 5α-DHP concentrations at steady state (15).
Quantitative Reverse Transcription (RT)-PCR Analyses for Determination of 5α-Reductase Type I and Type II and 3αHSOR mRNAs.
The different mRNAs were quantified according to the method of Grayson and Ikonomovic (16). Templates for 5α-reductases Type I and II and 3α-HSOR internal standards were generated by a site-directed mutagenesis using PCR overlapping extension to introduce a restriction enzyme site midway between the amplification primers. Primers for 5α-reductase Type I were as follows: reverse 298–317 (5′-ACCATGACTCATTGCTCCCTGCTT-3′), forward 1–24 (5′-CATCATCAGTGGTACCTCGAGAAG-3′), which were based on the sequenced RT-PCR amplicon¶ obtained by using rat 5α-reductase primers (forward 685–710, reverse 1075–1109; GenBank accession no. J05035); internal standard contained a restriction endonuclease site XbaI, which on digestion generated fragments of 135 and 182 bp. Primers for 5α-reductase Type II were as follows: forward 417 to 441 (5′-CTCCTTCAAGCCTACTACCTGGTT-3′), reverse 837–861 (5′-AGCTGAGCAGTTCCTCCACAGAAA-3′) (GenBank accession no. M95058); internal standard contained a restriction endonuclease site BamHI, which on digestion generated fragments of 219 and 225 bp. Primers for 3α-HSOR were as follows: forward 522–555 (5′-AGGATTCTGAATAAGCCAGGGCTC-3′), reverse 843–876 (5′-GTCCTCTGAAGCCAACTGGAATTC-3′) (GenBank accession no. S57790); internal standard contained a restriction endonuclease site XbaI, which on digestion generated fragments of 184 and 205 bp. The nucleotide sequence‖ for the mouse 3α-HSOR amplification product was 88% identical to that of rat 3α-HSOR and did not match known mouse, rat or human 17β-hydroxysteroid dehydrogenase sequences. Each primer pair annealed to a single RNA template on multiple Blast comparisons and yielded a single band of the correct molecular size following amplification of RNA isolated from mouse brain.
Comparative Western Blot Analysis.
This analysis was conducted as described (17) by using tissue obtained from the same sample used for quantitative RT-PCR analyses. Briefly, two to three aliquots of brain extracts (10 to 40 μg of protein) were resolved on 15% SDS/PAGE gels. After PAGE separation and transfer to nitrocellulose membrane (Hybond ECL, Amersham Pharmacia), blots were reacted with a 5α-reductase Type I isoenzyme antibody raised against the N-terminal region, a generous gift from D. W. Russell (University of Texas, Southwestern Medical Center, Dallas, TX) and the appropriate secondary antibody and developed for 5–10 min with ECL Plus Chemiluminescence Western Blotting kit (Amersham Pharmacia). Protein levels were estimated as optical density ratios against β-actin by using imagequant software on a Fluorescence Storm System from Molecular Dynamics.
Results
Decrease of 5α-DHP and ALLO Steady State and Biosynthesis Rate in Frontal Cortex of Socially Isolated Mice.
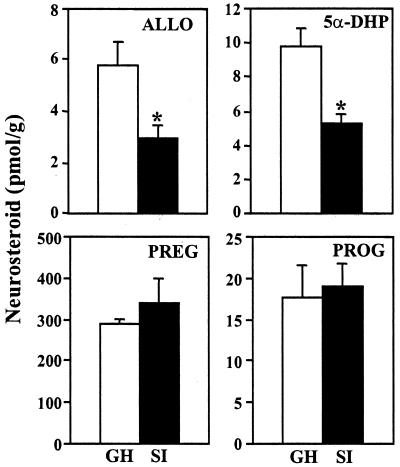
Consistent with our previous report (5), 6 weeks of social isolation decrease ALLO content in frontal cortex by ≈50% (Fig. 1). This ALLO decrease is paralleled by a decrease in 5α-DHP content but is not accompanied by changes in progesterone or pregnenolone content (Fig. 1). To establish whether the decrease of 5α-DHP and ALLO in frontal cortex of social isolated mice reflects a decreased steroid synthesis rate or an increased steroid utilization or degradation rate, we measured the time course of the decline of ALLO and 5α-DHP in frontal cortex group-housed or socially isolated mice treated with SKF105,111, a specific Type I and Type II 5α-reductase inhibitor. In previous experiments (6), we had shown that systemic administration of 48 μmol/kg of SKF105,111 reduced brain 5α-DHP and ALLO content by 80%–90% in 30 min, and that 5α-DHP and ALLO content remained low for at least 6 h.
Figure 1.
Social isolation decreases ALLO and 5α-DHP but not pregnenolone (PREG) or progesterone (PROG) content in the frontal cortex of mice. Each bar represents the mean ± SEM of five to six mice either group-housed (GH, open bars) or socially isolated (SI, filled bars) for 6 weeks. *, P < 0.01 when SI mice were compared with GH mice.
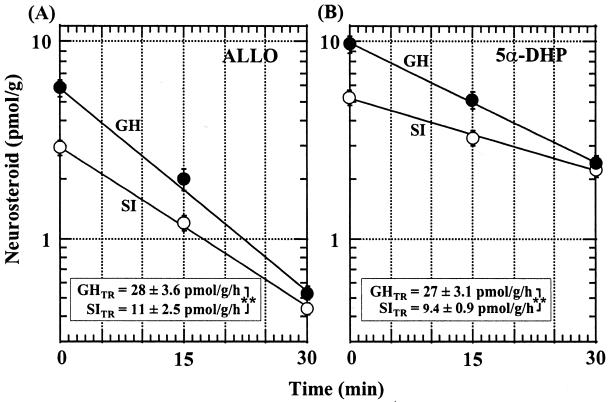
Fig. 2 shows that blockade of 5α-reductase activity caused by injecting 48 μmol/kg SKF105,111 yielded a single exponential decline of ALLO and 5α-DHP during the first 30 min in both group-housed and socially isolated mice. Calculating from the data of Fig. 2, the fractional rate constant (k) for the decline of 5α-DHP was 2.8 h−1 in group-housed mice and 1.7 h−1 in socially isolated mice. The fractional rate constant for ALLO decline was 4.8 h−1 for group-housed mice and 3.9 h−1 for socially isolated mice. The apparent ALLO biosynthesis rate (pmol⋅g−1⋅h−1) was calculated by multiplying the values of the fractional rate constant (k/h) by the respective neurosteroid concentration at steady state. The biosynthesis rate of 5α-DHP was decreased in the frontal cortex of socially isolated mice (9.4 ± 0.90 pmol⋅g−1⋅h−1) when compared with that of the group-housed mice (27 ± 3.1 pmol⋅g−1⋅h−1). A similar decrease was estimated for ALLO biosynthesis in frontal cortex (Fig. 2). Because the brain levels of pregnanolone and progesterone failed to change after SKF105,111 treatment (6), these data suggest that the decrease of 5α-DHP and ALLO steady state observed in socially isolated mice is due to a decrease of 5α-reductase and/or 3α-HSOR and not to a selective increase in the 5α-DHP or ALLO metabolism rate.
Figure 2.
Semilogarithimic plot of the decline of ALLO (A) and 5α-DHP (B) from mouse frontal cortex after i.p. administration of SKF105,111 (48 μmol/kg). (A) The regression equation for ALLO is as follows: log [ALLO]pmol/g = 0.80 − 0.035time in group-housed (GH, ●) mice and log[ALLO]pmol/g = 0.51 − 0.028time in socially isolated (SI, ○) mice. The fractional rate constant (k) of ALLO decline in GH and SI mice is 4.8 h−1 and 3.9 h−1, respectively. (B) The regression equation for 5α-DHP is log[5α-DHP]pmol/g = 0.99 − 0.02time in GH (●) mice and log[5α-DHP]pmol/g = 0.71 − 0.012time in SI (○) mice. The fractional rate constant (k) of 5α-DHP decline in GH and SI mice is 2.8 h−1 and 1.7 h−1, respectively. The neurosteroids were measured at 0, 15, and 30 min after SKF105,111 treatment. Each point is the mean ± SEM of five to six mice. The turnover rate (TR) (pmol⋅g−1⋅h−1) was calculated by multiplying k/h by the ALLO or 5α-DHP concentration at steady state. **, Differences between SITR and GHTR for ALLO or 5α-DHP were statistically significant (P < 0.01, Student's t test).
5α-Reductase and 3α-HSOR Expression in Mouse Brain.
To correlate 5α-DHP and ALLO content with the expression of mRNA encoding for the two key enzymes catalyzing conversion of progesterone into 5α-DHP and 5α-DHP to ALLO, we have developed mutated cRNA internal standards that allow quantitative analyses with competitive RT-PCR (16) of mouse 5α-reductase Type I and Type II and 3α-HSOR mRNA.
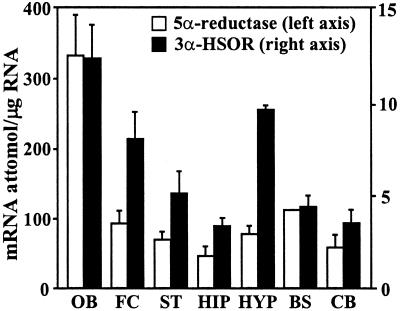
In Fig. 3, we report measurements of 5α-reductase Type I mRNA expression in various brain structures of mice. The highest levels were expressed in the olfactory bulb, significantly lower levels were measured in frontal cortex, and even lower levels were found in cerebellum. In contrast, 5α-reductase Type II mRNA is probably either not expressed in mouse brain or is expressed only in trace amounts that were below the detection capabilities of our method. We also studied the expression of 3α-HSOR mRNA (Fig. 3). The olfactory bulb, frontal cortex, and hypothalamus expressed a higher level of 3α-HSOR than hippocampus or cerebellum. Thus, it appears that the expression level of both 5α-reductase Type I and 3α-HSOR contributes to the brain region-specific differences in the content of 5α-DHP and ALLO. Neurosteroid content is higher in olfactory bulb (ALLO, mean ± SE, 15 ± 1.8; 5α-DHP, 20 ± 2.5 pmol/g; n = 5–6), than in the frontal cortex (ALLO = 5.8 ± 0.80; 5α-DHP = 9.8 ± 1.2), and it is even lower in the cerebellum (ALLO = 3.2 ± 0.025; 5α-DHP = 4.5 ± 1.2).
Figure 3.
Brain distribution of 5α-reductase Type I and 3α-HSOR mRNAs. The mRNAs were determined by competitive RT-PCR with mutant internal standards (16). Each value is the mean ± SE of three to five animals. OB, olfactory bulb; FC, frontal cortex; ST, striatum; HIP, hippocampus; HYP, hypothalamus; BS, brainstem; and CB, cerebellum.
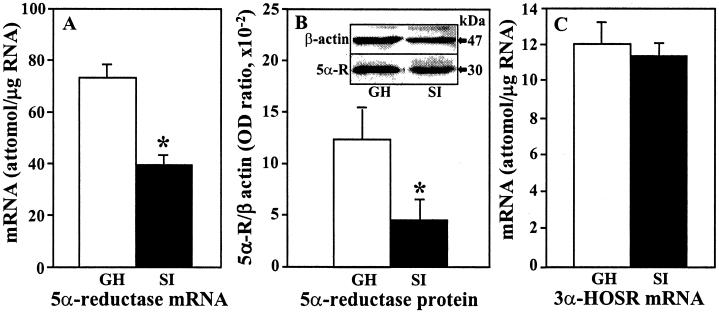
In the frontal cortex of mice socially isolated for 6 weeks, there was about 50% reduction of 5α-reductase Type I mRNA (Fig. 4A) and about 65% decrease of 5α-reductase Type I protein expression (Fig. 4B). The enzyme protein expression was evaluated with Western blot by using a 5α-reductase antibody directed against the N terminus of the Type I enzyme. A similar decrease in 5α-reductase Type I mRNA was also detected in the olfactory bulb (group-housed 315 ± 25; socially isolated 180 ± 18 attomol/μg RNA, n = 5; P < 0.01) whereas no significant difference was observed in cerebella of the same animals (group-housed 35 ± 12, socially isolated 30 ± 9.5 attomol/μg RNA, n = 3). No changes were found in 3α-HSOR mRNA expression (Fig. 4C) after social isolation for 6 weeks.
Figure 4.
5α-reductase Type I mRNA (A), 5α-reductase Type I protein (B), and 3α-HSOR (C) mRNA content in frontal cortex of group-housed (GH, open bars) and socially isolated (SI, filled bars) mice. Each value is the mean ± SE of at least six animals. *, P < 0.01 with Student's t test comparison. mRNAs were quantified by competitive RT-PCR with mutant internal standards (16). (Inset in B) Relative content of 5α-reductase Type I protein (5α-R, molecular mass ≈30 kDa) was estimated by Western blot using OD ratio with β-actin (47 kDa) as a control for overall protein content and blotting efficiency.
Discussion
To investigate a possible physiological role for either 5α-DHP or ALLO expression down-regulation in the genomic and nongenomic control of GABAA receptor function, we have focused on a mouse model of protracted social isolation because, in this model, male mice develop a late adaptation syndrome (anxiety, aggression, and decreased response to GABAmimetic drugs), presumably in response to the initial stress caused by the isolation. We believe that this model is relevant to the study of the molecular substrates of certain psychiatric symptoms associated with human depression (anxiety, dysphoria, and altered response to GABAmimetic drugs) because socially isolated mice, when compared with group-housed mice, exhibit resistance to the sedative and anxiolytic actions of GABAmimetic drugs (5–7), and, similarly to depressed patients (12), they show a marked decrease of ALLO expression.
Similar to depressed patients (12), socially isolated mice respond to the antidepressant drug fluoxetine with a normalization of ALLO brain content (5). In addition, they respond to fluoxetine with a return to normal of the pentobarbital-induced loss of the righting reflex, which reflects a normalization of a depressed GABAA receptor function (5). Because protracted social isolation in mice fails to change brain pregnenolone or progesterone content but results in approximate 50% decrease in brain 5α-DHP and ALLO expression (Figs. 1 and 2), one might infer that pregnenolone or progesterone fail to contribute substantially to the isolation-elicited decrease in ALLO and 5α-DHP brain content. The finding that the expression of 5α-reductase (mRNA and protein) in mouse brain (frontal cortex, olfactory bulb but not cerebellum) is down-regulated during protracted social isolation supports the view that down-regulation of this enzyme is an important contributor to the decrease in ALLO and 5αDHP and to some behavioral and pharmacological aspects of the syndrome induced by protracted social isolation in mice.
In mouse brain, ALLO can be metabolized by two different pathways: it can be converted to either 20-OH ALLO or to 5α-DHP (1, 2, 18). Although the formation of 20-OH-ALLO can be detected in brain in vitro (18), such metabolite is virtually undetectable in vivo. The conversion of ALLO into 5α-DHP by the action of 3α-HSOR acting in the oxidative direction is probably the most important pathway. Thus, an initial aim of our research strategy was to establish whether the decrease in brain ALLO observed in protracted social isolation (5) was associated with an increase or a decrease in 5α-DHP brain content. Because we have now demonstrated that a protracted social isolation of mice results in a marked reduction of 5α-DHP as well as ALLO (Figs. 1 and 2), we can postulate that this reduction is the consequence of a decreased conversion of progesterone into 5α-DHP because of (i) a reduction in Type I 5α-reductase mRNA and protein expression, or (ii) a decrease in synthesis of 5α-DHP as a result of a decrease in the conversion of ALLO into 5α-DHP, when 3α-HSOR operates in the oxidative direction.
In evaluating these two alternatives, a decrease in the steady state of both 5α-DHP and ALLO brain content could be attributed to either an increase or a decrease in their turnover rate. To determine which of these two alternatives was operative, we compared the turnover rate of 5α-DHP and ALLO in socially isolated and group-housed mice after administration of the 5α-reductase inhibitor SKF105,111 (48 μmol/kg) to block 5α-reductase activity (6). When the rate constants (k h-1) of decrease were used to measure turnover rates of 5α-DHP and ALLO, we found that the turnover rates for both neurosteroids decreased in an interdependent manner. A decrease in the turnover rate of both 5α-DHP and ALLO is consistent with the finding that the expression of 5α-reductase, but not that of 3α-HSOR, is decreased in socially isolated mice. This finding prompts the hypothesis that the decrease of ALLO turnover rate may be the consequence of a decrease in the biosynthesis rate of 5α-DHP. When 5α-DHP is produced at a decreased rate, its levels fail to saturate 3α-HSOR, which then produces ALLO at a rate lower than normal.
We have tested other blockers of 5α-reductase such as finasteride, which is a specific Type II 5α-reductase inhibitor. As expected by the virtual absence of 5α-reductase Type II in mouse brain, the potency and efficacy of this drug was significantly inferior to that of SKF 105,111. The efficacy and rapidity with which the levels of 5α-DHP and ALLO decreased in the mouse brain after administration of SKF 105,111 suggest that, by virtue of its high affinity and inhibitory potency on 5α-reductase Type I, SKF 105,111 produces an almost instantaneous and complete blockade of 5α-DHP and ALLO biosynthesis that lasts several hours (5, 6, 8, 9). Thus, GC-MS measurement of ALLO and 5α-DHP at steady state and measurement of their fractional rate constants after blockade of 5α-reductase activity with SKF 105,111 allows an estimate with good approximation for the rate of synthesis of ALLO and 5α-DHP in different brain areas of the same animal (6).
To establish whether in addition to a decrease of 5α-reductase Type I there is also a reduction of 3α-HSOR expression contributing to the down-regulation of ALLO turnover rate in socially isolated mouse brain, we directly measured the expression of 3α-HSOR mRNA in the same animals in which we measured 5α-reductase mRNA and protein content. Unlike human brain, the rodent brain expresses only one form of 3α-HSOR (2, 11). This enzyme, depending on the relative concentration of substrates (5α-DHP or ALLO) and on the type of cofactors (NADPH/NADP or NADH/NAD) present in the milieu of the cell, can either catalyze the reduction of 5α-DHP into ALLO or, vice versa, the oxidation of ALLO into 5α-DHP (1, 2, 11). When we started this work, we had access to rat 5α-reductase Type I, Type II, and 3α-HSOR cDNA bank sequences; however, the mouse mRNA sequences were in part unknown. Thus, we first established the sequence of mouse 5α-reductase Type I and Type II and 3α-HSOR mRNAs by PCR amplification by using the corresponding rat primers, and, after sequencing the amplicons, we developed mutated internal standards for the quantitative determination of the respective mouse mRNAs, by using competitive PCR assay (16). These probes allowed us to determine that 5α-reductase Type I expression was decreased by approximately 50% in the frontal cortex of socially isolated mice when compared with group-housed mice, whereas the expression of 3α-HSOR remained unchanged. Because the cognate protein of the 5α-reductase TypeI mRNA was also decreased in socially isolated mice, our data taken together suggest that protracted social isolation is associated with a selective decrease in the expression of 5α-reductase. We measured 5α-reductase Type I expression in the olfactory bulb, a brain region that contains a very large amount of this enzyme, and found that social isolation produces a decrease of 5α-reductase Type I mRNA expression also in this brain region. However, in cerebellum, where the enzyme is expressed at a significantly lower level, mRNA expression was virtually identical in group-housed and socially isolated mice. Thus, the down-regulation of 5α-reductase during social isolation appears to be region-specific, suggesting that mechanisms related to neuronal activity may control the transcriptional regulation of this enzyme.
Although, after 2 weeks of social isolation, ALLO, progesterone, and pregnenolone brain levels are not changed (5), we cannot exclude that an initial response to social isolation stress may involve the pituitary-adrenal axis and an altered secretion of glucocorticoids that could produce a brain region-specific alteration of 5α-reductase Type I expression. The next step is to evaluate the time course of the down-regulation of 5α-reductase by varying the duration of social isolation.
In conclusion, our data demonstrate that, in socially isolated mice, there is a decrease of brain 5α-DHP biosynthesis rate that may be associated with an alteration of gene expression whereas the concomitant down-regulation of ALLO biosynthesis may be responsible for a nongenomic action at GABAA receptors. In situations in which there is a decrease of brain ALLO content, such as depression in humans (12), progesterone withdrawal in rats (19, 20), and protracted social isolation in rat (7) and mice (5), it is important to measure brain-specific changes of 5α-DHP concentrations to avoid attributing to ALLO what may instead be a genomic action mediated by an alteration of 5α-DHP expression.
Acknowledgments
We thank Dr. Fulton Crews, Pharmacology and Psychiatry, Skipper Bowles Center for Alcohol Studies-University of North Carolina, Chapel Hill, NC, and Dr. Synthia Mellon, Ob/Gyn, University of California, San Francisco, for constructive criticisms and suggestions in the preparation of the manuscript. This manuscript was supported in part by National Institutes of Health Grants MH49486 and MH56890 to A.G.
Abbreviations
- ALLO
allopregnanolone
- 5α-DHP
5α-dihydroprogesterone, 3α-HSOR, 3α-hydroxysteroid oxidoreductase, SKF105,111, (17β)-17-(bis-1-methyl amino carbonyl) androstane-3,5-diene-3 carboxylic acid
- GABA
γ-aminobutyric acid
- RT
reverse transcription
Footnotes
DNA sequence of mouse 5α-reductase, PCR amplification product: 5′-CATCATCAGTGGTACCTCGAGAAGTTTGAAGATTACCCCAAAACAAGAAAAATACTAATTCCATTCCTGCTTTAGTGTGCTGTCCATGCTGTTGTCTTCCATAAGCTGAGTGTCTGTCTTCCCAGTGGCTTTGCTCTGAGCACATACAAGTGAATTGTTTTCCTTATTTCTCCTGCAGTTCCATAGTTCTCAGGAAGGGCGTCCCCTGGTAAAGGACAAAGCCAAACAAAAACTAATCCACCATGTACAGTTAGGGGCTACACAGTGCCTAGTAGGTCAGAAGCAGGGAGCAATGAGTCATGGTG.
DNA sequence of mouse 3α-HSOR, PCR amplification product: 5′-GGCCAAGTCCATCGGAGTGTCGAACTTTAACTTCAGGCAGCTGGAGACGATTCTGAACAAGCCGGGGCTCAAGTACAAGCCTGTGTGCAACCAGGTAGAATGCCATCTTTATTTAAACCAGAGCCAAATGCTGGACTATTGTAAGTCAAAAGACATCATTCTGGTTTCCTACTGCACATTGGGAAGTTCACGAGACAAAATCTGGGTGGACCAGAAAAGTCCAGTTCTCTTAGATGATCCAGTTCTTTGTGCCATGGCAAATAAGTACAAGCAAACACCAGCACTGATTGCCATTCGTTACCAATTACAGCGTGGAATTGTGGTCCTGACCAGGAGTTTCAAGGAGAAGCGGATCAAAGAGTTCATGAAGGGTTTTTGAATTCCAGTTGGCTTCAGAGGACATGAAAGTCCTGGATGGCTTGCACAGAAATTTAAGATACAATACTGCGAGTTATTTTGATGACCATGAAAG-3′.
References
- 1.Guidotti A, Costa E. Biol Psychiatry. 1998;44:856–873. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 2.Compagnone N A, Mellon S H. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 3.McEwen B S. Trends Pharmacol Sci. 1991;12:141–147. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- 4.Rupprecht R, Holsboer F. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto K, Uzunova V, Uzunov D P, Costa E, Guidotti A. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 6.Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville J M, Costa E, Guidotti A. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 7.Serra M, Pisu M G, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy R H, Biggio G. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheney D L, Uzunov D, Costa E, Guidotti A. J Neurosci. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheney D L, Uzunov D, Guidotti A. NeuroReport. 1995;6:1697–1700. doi: 10.1097/00001756-199508000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Uzunov D P, Cooper T B, Costa E, Guidotti A. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin L D, Mellon S H. Proc Natl Acad Sci USA. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uzunova V, Sheline Y, Davis J M, Rasmusson A, Uzunov D P, Costa E, Guidotti A. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawley J N. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 14.Ojima K, Matsumoto K, Tohda M, Watanabe H. Brain Res. 1995;684:87–94. doi: 10.1016/0006-8993(95)00388-7. [DOI] [PubMed] [Google Scholar]
- 15.Goridis C, Neff N H. J Neurochem. 1971;18:1673–1682. doi: 10.1111/j.1471-4159.1971.tb03740.x. [DOI] [PubMed] [Google Scholar]
- 16.Grayson D R, Ikonomovic S. In Vitro Neurochemical Techniques, Neuromethods 34. Clifton, NJ: Humana; 1998. pp. 127–151. [Google Scholar]
- 17.Longone P, Impagnatiello F, Mienville J M, Costa E, Guidotti A. J Mol Neurosci. 1998;11:23–41. doi: 10.1385/JMN:11:1:23. [DOI] [PubMed] [Google Scholar]
- 18.Korneyev A, Guidotti A, Costa E. J Neurochem. 1993;61:2041–2047. doi: 10.1111/j.1471-4159.1993.tb07440.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith S S, Gong Q H, Hsu F C, Markowitz R S, ffrench-Mullen J M, Li X. Nature (London) 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 20.Concas A, Mostallino M C, Porcu P, Follesa P, Barbaccia M L, Trabucchi M, Purdy R H, Grisenti P, Biggio G. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]