Abstract
Background
The fungal pathogen Fusarium graminearum causes Fusarium Head Blight (FHB) disease on wheat which can lead to trichothecene mycotoxin (e.g. deoxynivalenol, DON) contamination of grain, harmful to mammalian health. DON is produced at low levels under standard culture conditions when compared to plant infection but specific polyamines (e.g. putrescine and agmatine) and amino acids (e.g. arginine and ornithine) are potent inducers of DON by F. graminearum in axenic culture. Currently, host factors that promote mycotoxin synthesis during FHB are unknown, but plant derived polyamines could contribute to DON induction in infected heads. However, the temporal and spatial accumulation of polyamines and amino acids in relation to that of DON has not been studied.
Results
Following inoculation of susceptible wheat heads by F. graminearum, DON accumulation was detected at two days after inoculation. The accumulation of putrescine was detected as early as one day following inoculation while arginine and cadaverine were also produced at three and four days post-inoculation. Transcripts of ornithine decarboxylase (ODC) and arginine decarboxylase (ADC), two key biosynthetic enzymes for putrescine biosynthesis, were also strongly induced in heads at two days after inoculation. These results indicated that elicitation of the polyamine biosynthetic pathway is an early response to FHB. Transcripts for genes encoding enzymes acting upstream in the polyamine biosynthetic pathway as well as those of ODC and ADC, and putrescine levels were also induced in the rachis, a flower organ supporting DON production and an important route for pathogen colonisation during FHB. A survey of 24 wheat genotypes with varying responses to FHB showed putrescine induction is a general response to inoculation and no correlation was observed between the accumulation of putrescine and infection or DON accumulation.
Conclusions
The activation of the polyamine biosynthetic pathway and putrescine in infected heads prior to detectable DON accumulation is consistent with a model where the pathogen exploits the generic host stress response of polyamine synthesis as a cue for production of trichothecene mycotoxins during FHB disease. However, it is likely that this mechanism is complicated by other factors contributing to resistance and susceptibility in diverse wheat genetic backgrounds.
Background
Fusarium head blight (FHB) or scab is one of the most important diseases of wheat and other small grain cereals in many wheat growing regions [1]. FHB is caused mainly by F. graminearum, but also by other related Fusaria. A characteristic of FHB disease is the production of trichothecene mycotoxins such as deoxynivalenol (DON) by the fungus in infected heads. Trichothecenes are phytotoxic [2] and their biosynthesis during FHB is necessary for full pathogen virulence, and the spread of the fungus through the infected wheat head [3-5]. Importantly, trichothecenes such as DON are toxic to animals and humans when present in feed and food products, respectively [6]. Because of the undesirable effects of DON on human and animal health, its presence in grain is tightly regulated in many wheat grain markets [7]. Consequently, there is also a strong interest in the development of novel technologies for reducing DON levels in infected wheat either through breeding or via chemical and/or biological control methods [8,9].
One constraint on our ability to reduce DON production by Fusarium pathogens has been a limited understanding of environmental and host-associated genetic factors that regulate the production of trichothecene toxins during the infection process [10]. It is well known that the amount of DON produced by F. graminearum during the infection of living wheat plants is much higher than that observed under common culture conditions, including growth on autoclaved wheat grains. This suggests that specific host factors stimulate DON production during the infection process [11,12]. Several factors that may induce the production of DON by F. graminearum during the infection process have been proposed. These factors include hydrogen peroxide [13], sugars [14], acidic pH [15,16], and fungicides [17]. In particular, we have recently shown in a screen of various nitrogen containing compounds that the most potent inducers of DON production by F. graminearum were metabolites (e.g. arginine, ornithine, agmatine, citrulline and putrescine) of the plant polyamine biosynthetic pathway shown in Figure 1[18]. These metabolites of the polyamine pathway appear far more potent than hydrogen peroxide and sugars at inducing DON production in vitro [18]. Importantly, the levels of DON produced in culture filtrates of F. graminearum after growth in the presence of inducing amines such as agmatine, putrescine and ornithine were extremely high (> 500 ppm) [18]. The primary biosynthetic enzyme in trichothecene biosynthesis in F. graminearum is trichodiene synthase [19] encoded by TRI5 [5] and transcripts of TRI5 were also induced in the fungus by polyamines in culture to levels equivalent to those observed in infected heads [18]. Interestingly, both spermine and spermidine, two very common and abundant plant polyamines, did not induce DON production, suggesting that not all polyamines were inducers of DON [18]. Nevertheless, the stimulation of trichothecene biosynthesis by specific polyamines appears to be a general response in Fusarium pathogens because the DON-inducing polyamine agmatine was also able to induce the production of T-2 toxin, another trichothecene, in F. sporotrichioides [18]. Because of the potency of polyamine inducers in stimulating trichothecene mycotoxins in culture, Gardiner et al. [18] hypothesised that the pathogen may perceive polyamines and related amino acids as cues for the production of toxins during the infection process.
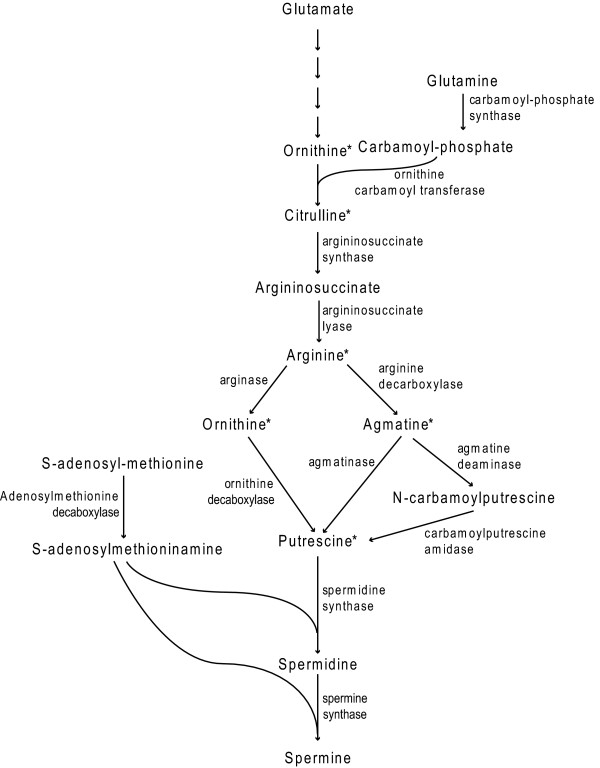
Figure 1.
Principle pathway of polyamine biosynthesis in plants. Expression of all genes encoding enzymes of the pathway were investigated except for agmatine deaminase, carbamoylputrescine amidase and spermine synthase. Metabolites measured in this study included arginine, putrescine, spermine and spermidine. Metabolites indicated by an asterisk were previously shown to have in vitro trichothecene inducing activity [18].
Polyamines are well known as metabolites rapidly induced by diverse abiotic stresses in plants, including salinity, drought, chilling, hypoxia, ozone, heavy metals and UV irradiation [20,21]. The biosynthetic pathway of the principle polyamines of plants is well understood (Figure 1). Two routes of synthesis to the primary amine putrescine have been described with the first steps being decarboxylation of either ornithine or arginine catalysed by ornithine decarboxylase (ODC) and arginine decarboxylase (ADC), respectively. In subsequent reactions aminopropyl groups are generated from S-adenosylmethionine (SAM) by SAM decarboxylase to convert putrescine to spermidine and subsequently spermine. Cadaverine is thought to be produced by the decarboxylation of lysine catalysed by lysine decarboxylase in prokaryotes but in eukaryotes this step is less well defined [22,23]. A role for polyamines in protection against the stress-induced cellular damage has been demonstrated in transgenic plants of rice, Arabidopsis, tobacco, tomato and pears that accumulate high levels of polyamines through the over-expression of key biosynthetic enzymes in the polyamine biosynthetic pathway [reviewed by [24]].
In contrast to abiotic stresses where polyamine accumulation is a general stress response, the response of polyamines to pathogen challenge appears to be more dependent on the host-pathogen system under study. Increases in polyamine content in susceptible barley during rust and powdery mildew disease development [reviewed by [25]] and in rice after infection with the blast pathogen Magnaporthe grisea [26,27] have been reported. In contrast, infection of tobacco with powdery mildew and downy mildew pathogens as well as the necrotroph Alternaria tenuis led to decreased levels of polyamines [28]. Increasing polyamine levels via over-expression of polyamine biosynthetic enzymes has been shown to increase the tolerance of tobacco to F. oxysporum [29]. Conjugates of agmatine, such as hordatine from barley and feruloylagmatine from wheat, are also known to have antifungal activity [30,31]. Furthermore, polyamine biosynthesis appears to be positively regulated by the plant defence hormone methyl jasmonate in wheat and barley but not in rice [32-34]. Polyamine oxidases are also thought to contribute to reactive oxygen species generation during defence against diverse pathogens [26].
Because polyamines are generically induced during plant stress, it is possible that Fusarium pathogens have evolved to recognise these metabolites during the infection of wheat plants to trigger toxin production. However, it is currently unknown what polyamines or related metabolites accumulate during FHB development. Gardiner et al [18] described a preliminary experiment that suggested that putrescine was elevated in wheat heads during FHB disease development, but only one metabolite and one post-inoculation time-point was analysed. In the present study, we have studied the expression of wheat genes that encode polyamine biosynthetic enzymes during FHB development to determine if this host pathway is activated during infection. We have also analysed a spectrum of polyamines and amino acids at multiple time-points after inoculation and across multiple bread wheat genotypes. These experiments have permitted a comparison of the temporal and quantitative patterns of accumulation of polyamines and amino acids during infection with the timing and concentrations of DON in infected heads. The results demonstrate that the core polyamine biosynthetic pathway is activated early on during F. graminearum infection in wheat and that putrescine accumulation occurs prior to toxin production by the pathogen. This latter observation provides additional support to the view that polyamines are not only inducers of toxin production by the pathogen in vitro but may also play a similar role in planta.
Results
Polyamine, amino acid and DON accumulation during FHB disease development
The observation that intermediates of the polyamine pathway are strong inducers of TRI5 expression and DON production by F. graminearum in culture led us to investigate the concentrations of these compounds in wheat heads during infection. Inflorescences of a susceptible wheat cultivar were spray inoculated with conidia of F. graminearum at mid-anthesis and polyamines and free amino acids were analysed in head samples taken daily over a seven-day period.
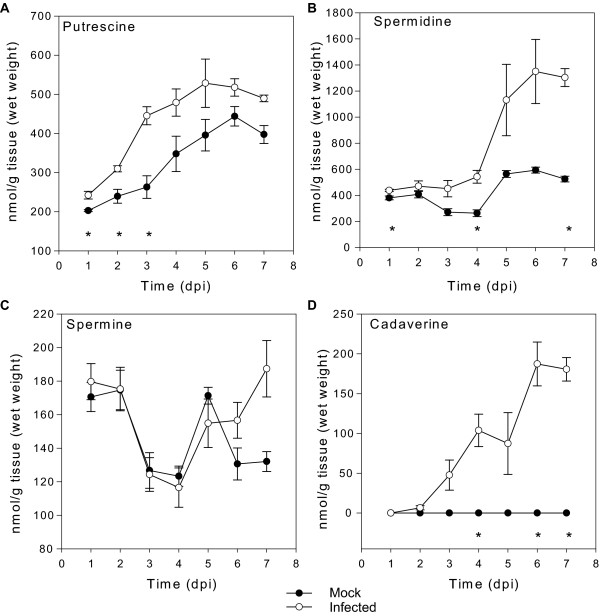
The putrescine concentration in the infected spikes increased rapidly and was almost two fold that of the mock inoculated control at three days post-inoculation and then reached a plateau at approximately 500 nmoles/g fresh weight (Figure 2A). Concentrations of spermidine increased more slowly and appeared to be higher than mock inoculated controls after four to seven days post-inoculation. Spermidine was the most abundant polyamine reaching a level of 1400 nmoles/g fresh weight. Spermine levels were more variable and no significant differences were observed in its concentration in infected relative to mock-inoculated heads (Figure 2C). The levels of putrescine and spermidine increased during the seven-day period even in the mock inoculated heads, indicating that polyamine accumulation may be a normal part of development (Figure 2A-B). Similar increases in these polyamines were observed during the early stages of grain development in field grown wheat [35] and also during development of the rice panicle [36]. Putrescine is an inducer of DON in vitro and it was interesting that inoculation led to a rapid increase in putrescine levels which were significantly higher than those of mock-inoculated controls at one, two and three days post-inoculation (Figure 2A). Agmatine is also a potent DON inducer and a precursor of putrescine synthesis but using our extraction methods we were unable to detect agmatine in wheat heads (data not shown). This is most likely due to the instability of agmatine, as observed by others as well [37]. Interestingly, another polyamine and potent inducer of DON production, cadaverine [15], was not detected in the mock-inoculated heads at all but accumulated in infected heads, with significant increases observed at three days post-inoculation, eventually reaching a concentration of 200 nmoles/g fresh weight. Quantitative RT-PCR measurement of fungal polyamine biosynthetic gene expression indicated a lack of induction of fungal transcripts relative to those of the host during infection (data not shown) and coupled with plant material being the dominant contributor to biomass at all time points, this suggests that the measured polyamines are most likely almost exclusively of plant origin.
Figure 2.
Quantification of polyamines in wheat heads during Fusarium head blight disease development. Asterisks indicate significant differences (t-test P < 0.05). Error bars are the standard error of the mean. n = 4 individual heads.
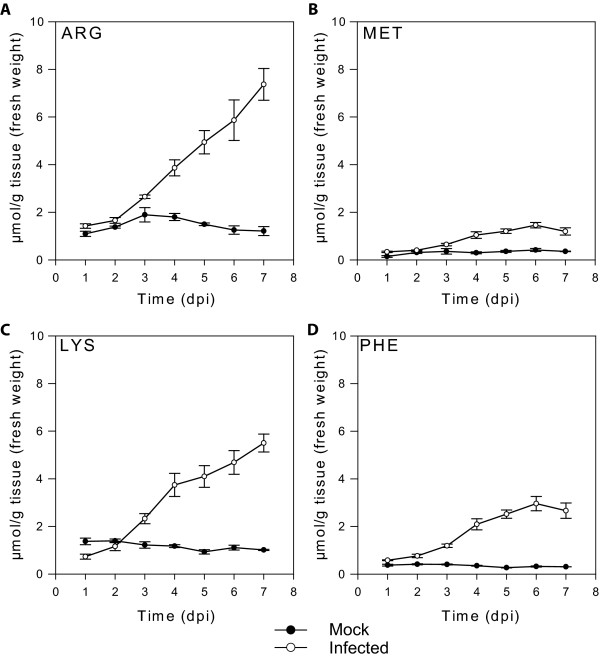
We also analysed the amino acid content of these heads because arginine was a strong DON inducer while some other amino acids such as lysine, methionine and phenylalanine were weak DON inducers [18]. In addition, combinations of amino acids and polyamines (e.g. methionine with putrescine) appeared to act synergistically for DON induction in culture [18]. Unlike polyamines, most amino acids did not show any increase in concentration during the development of the head in mock-inoculated controls. Some amino acids did increase in concentration during FHB disease development and these included glycine, valine, arginine, alanine, phenylalanine, lysine and leucine as well as threonine and/or citrulline which could not be resolved (Figure 3 and Additional File 1). However, a significant increase in the concentration of most of these compounds, and particularly the potent DON inducer arginine, was only observed later in the infection time-course (Figure 3). The concentration of arginine increased from approximately 1 μmol/g fresh weight at one day post-inoculation to up to 7 μmol/g fresh weight at seven day post-inoculation. The only amino acid that rapidly responded to inoculation was isoleucine where an increase was observed at one day post-inoculation but this was not sustained at later stages of infection (Additional File 1). Ornithine is another potent DON inducer [18] but this amino acid was below the detection level in wheat heads using our instrumentation. Using more sensitive equipment (Waters AccQ-Tag Ultra at the Australian Proteome Analysis Facility), we were able to detect trace amounts of ornithine (maximum observed 0.05 μmol/g fresh wt).
Figure 3.
Free amino acids during Fusarium head blight of wheat. Error bars are the standard error of the mean. n = 4 individual heads.
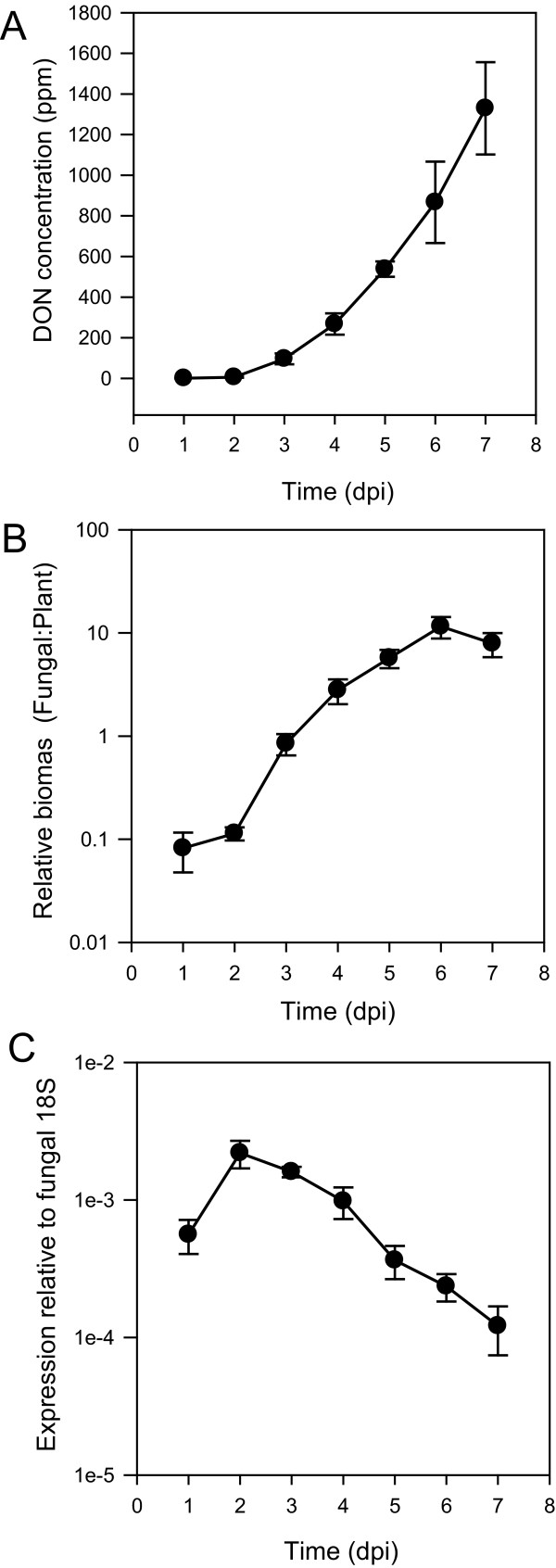
In order to temporally compare the accumulation of potential DON inducing polyamines and amino acids measured in the time-course experiment (Figure 2 and Figure 3) with disease and DON levels, we also measured DON levels and fungal biomass (Figure 4) during disease development. DON was not detectable in the infected heads until three days post-inoculation and then steadily increased, reaching 1200 ppm in fresh head tissue at seven days post-inoculation. Fungal biomass in infected tissue was measured by quantitative PCR of a fungal DNA sequence relative to that of a plant sequence in DNA extracted from infected heads. Similarly, only minor increases in fungal biomass for the first two days post-inoculation was detectable but this increased rapidly thereafter and peaked at five to six days after inoculation. Therefore, these experiments suggest that the induction of putrescine in infected heads preceded the production of DON in the fungus.
Figure 4.
DON concentration (A), fungal biomass (B) and TRI5 expression (C) during Fusarium head blight of wheat. Error bars are the standard error of the mean. n = 4 individual heads.
Induction of genes encoding polyamine biosynthetic enzymes during FHB
The biosynthesis of putrescine, spermine and spermidine from primary amino acid metabolism involves several enzymatic steps, with two potential branches of putrescine synthesis from arginine via either ornithine or agmatine as intermediates (Figure 1). We were interested in knowing which genes of this pathway might be activated by fungal infection and the timing of this response. To determine this, we first identified wheat homologues for 11 of the polyamine biosynthetic enzymes (Figure 1 & Table 1). We used previously annotated rice polyamine sequences as queries in searches of wheat expressed sequence tag clusters available in the WhETS database. Although not a definitive analysis of copy number, based on the clusters produced by WhETS for the query sequences all polyamine pathway genes described in this manuscript appear to be present in single copies in each of the three wheat homoeologous genomes. The wheat sequences identified in these analyses were used to design primers (Table 1) for Quantitative Real-Time Reverse Transcriptase PCR analysis of transcripts in RNA samples from mock- and F. graminearum-inoculated heads of the susceptible wheat cultivar Kennedy during FHB disease development.
Table 1.
Quantitative reverse transcriptase-PCR primers used for detecting expressions from wheat polyamine biosynthesis genes.
| Gene | Forward primer (5'-3') | Reverse primer (5'-3') | Locus | GenBank accession |
|---|---|---|---|---|
| Ornithine decarboxylase (ODC) | AGCGTTACTTCGGGGAGCTT | ATTGTGAAGGCGGTCTCG | Os09g37120 | HM770451 |
| Arginine decarboxylase 1 (ADC1) | CACCAAGATACCAGGCCACT | GTGGAAGTGCAGCAACTTGA | Os04g01690 | HM770446 |
| Arginine decarboxylase 2 (ADC2) | AGGAGGAGGAGCTCGACATT | GCCGAACTTGCCCTTCTC | Os06g04070 | HM770449 |
| Agmatinase/Arginase | GGGAAGAGATTTGGTGTGGA | TCACACCTTCCCCAAGTTTC | Os04g01590 | HM770450 |
| S-adenosylmethionine decarboxylase 2 | GCGTCCTCATCTACCAGAGC | CTTGCCTTCCTTGACCAGAG | Os04g42090 | HM770448 |
| Spermidine synthase 1 | TGATTCAGGACATGCTTTCG | CCCAATTGCACCACTAGGAT | Os02g15550 | HM770442 |
| Spermidine synthase 2 | GCAAAAATCCAATGACACCA | TGTGGCTGCACACACATCTA | Os06g33710 | HM770452 |
| Argininosuccinate synthase | ACGCTGAAGGTTTCATCAGG | TAGATGCCCTTCTCCAGCAT | Os12g13320 | HM770445 |
| Argininosuccinate lyase | GTTGAACAGTTGGAGCGTGA | GAGTCCAGTTCCAGCCAAAG | Os03g19280 | HM770447 |
| Carbamoyl-phosphate synthase | TTGGAAAATTGTTGGTGTTG | CCCATTCATATGGAGCATC | Os02g47850 | HM770444 |
| Ornithine carbamoyl transferase | ATGAAGCCCTGATGGAGATG | ATGGCACCGTCTGTTACCTC | Os02g47590 | HM770443 |
| 18 S rRNA* | CGACCTACTCGACCCTTCGGCCGG | CGATGCCGGAAACACGACCCGG | - | |
| Peroxidase† (TaPERO) | GAGATTCCACAGATGCAAACGAG | GGAGGCCCTTGTTTCTGAATG | - | X56011 |
| 18 S rRNA fungal* | GTCCGGCCGGGCCTTTCC | AAGTCCTGTTTCCCCGCCACGC | - | |
| TRI5* | CACTTTGCTCAGCCTCGCC | CGATTGTTTGGAGGGAAGCC | FGSG_03537 | |
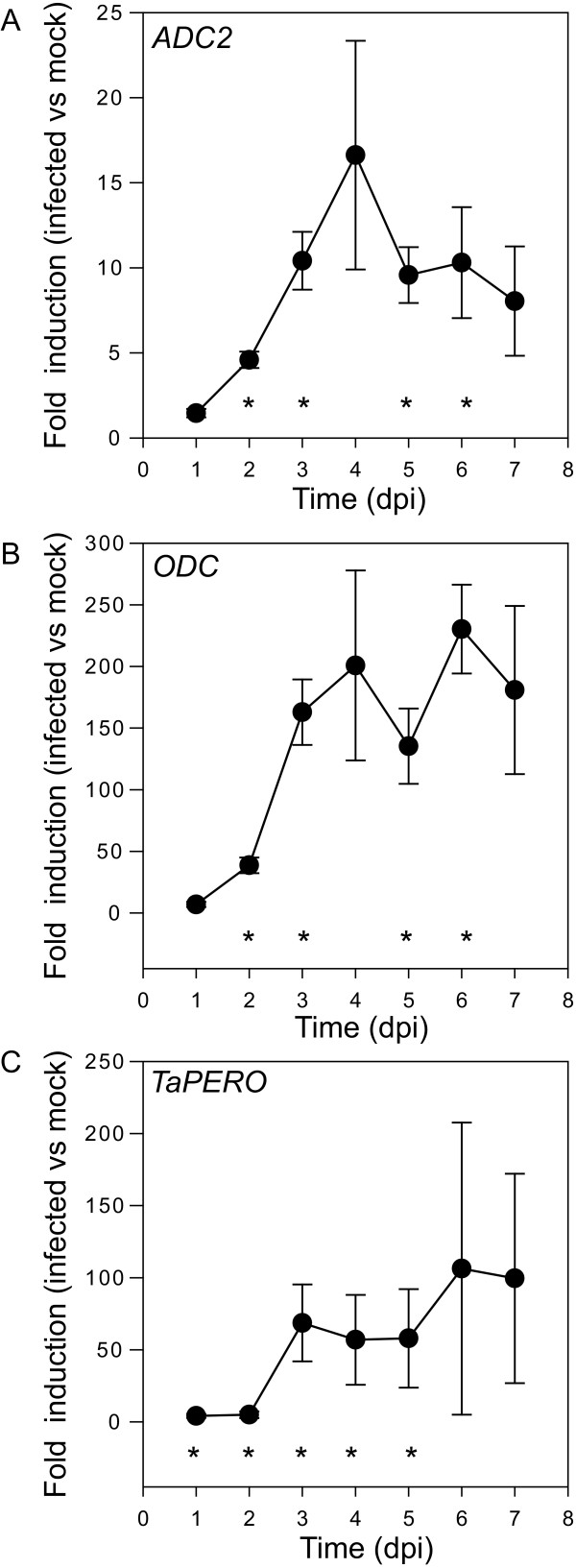
Of the 11 transcripts studied, only two transcripts encoding a wheat orthologue of ornithine decarboxylase (ODC) and an isoform of arginine decarboxylase designated as ADC2 (see below) showed a significant increase following inoculation with F. graminearum (Figure 5). At one day post inoculation, ODC was induced seven fold compared to mock albeit with some variability (P = 0.054). Transcripts for ODC were significantly induced at two days post-inoculation (~40-fold controls, P = 0.003) and continued to increase up to three days post-inoculation where a level >100-fold that of mock controls was reached and maintained for the seven day duration of the experiment.
Figure 5.
Expression of genes of the polyamine biosynthesis pathway during Fusarium head blight infection. Values are the relative expression between infected plants compared to mock inoculated plants. Error bars are the standard error of the mean. n = 4 individual heads. Asterisks indicate statistically significant (t-test P < 0.05) differences between infected and mock treated samples at that time point.
In the model plant Arabidopsis, two genes designated as ADC1 and ADC2 encode separate isoforms of ADC. ADC1 and ADC2 are functionally redundant as mutants for either gene are viable but double mutants are not [38]. Interestingly, ADC2, but not ADC1, has been shown to be responsive to salt and other abiotic stresses in Arabidopsis [39,40]. Similarly, we identified two distinct ADC sequences in wheat. However, based on nucleotide and amino acid comparisons between Arabidopsis and cereal ADC sequences, which one of these sequences is the direct wheat orthologue of the Arabidopsis ADC2 gene could not be established. Of these two sequences, only one was inducible during FHB disease development (Figure 5A and data not shown), suggesting that similarly to Arabidopsis, different ADC isoforms are differentially regulated under stress in wheat. We therefore designated the pathogen-inducible wheat ADC gene as ADC2. This gene was induced three-fold and ~10-fold, relative to mock-inoculated controls, at two and four days post-inoculation (Figure 5A).
To further characterize the relative speed of induction we compared the expression patterns of ODC and ADC2 to those of the peroxidase encoding gene TaPERO, which is known to be transcriptionally induced in wheat following inoculation by F. graminearum, F. culmorum or F. pseudograminearum [41,42]. TaPERO was induced similarly to ODC and ADC2 with three-fold induction observed at one day post-inoculation, followed by a rapid increase between two and three days post-inoculation relative to mock-inoculated controls (Figure 5C). This is consistent with ODC and ADC2 induction being part of the coordinated defence response to FHB.
The polyamine pathway is co-ordinately regulated in the rachis under infection
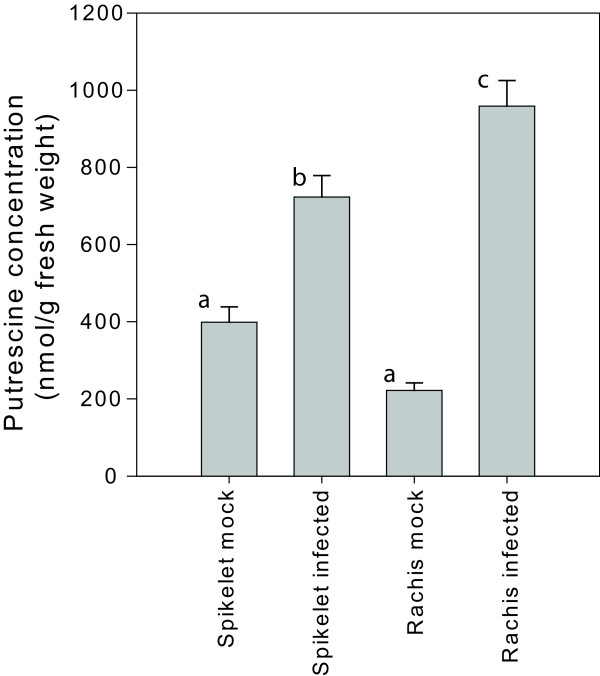
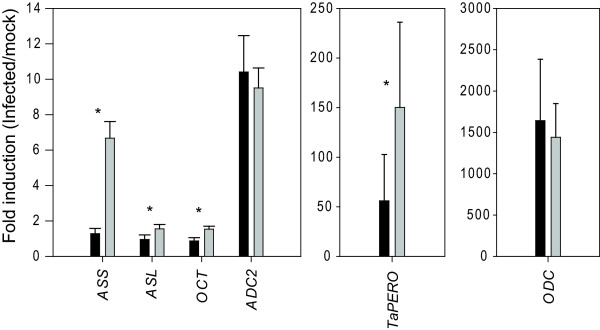
It is now well known that one of the roles that DON plays during FHB development is to allow the fungus to colonise the rachis of infected spikelets and spread into the rachis of the spike [4,43]. Additionally, our previous work has demonstrated TRI5 is strongly expressed in the rachis tissue of wheat [18]. Given the importance of rachis in disease spread within the infected head, we were particularly interested in knowing whether the polyamine pathway is specifically or especially induced in this tissue during FHB development. To test this, we collected the rachis and the spikelets separately from spray- and mock-inoculated heads at six day post-inoculation. Putrescine quantification and quantitative RT-PCR was then carried out on each material. In mock-inoculated heads, putrescine levels were not significantly different in the rachis and spikelets (Figure 6). However, under infection, levels of putrescine in the rachis increased more dramatically than those in the spikelets (Figure 6; difference between infected rachis and spikelets P = 0.011, mock versus infected for both spikelets and rachis P < 0.001). Similar levels of induction of ODC and ADC2 were observed in both rachis and spikelets. However, transcripts encoding for argininosuccinate synthase (P = 2×10-4) and to a lesser extent argininosuccinate lyase (P = 0.04) and orthinine carbamoyl transferase (P = 0.001) all showed significantly higher levels of induction in rachis material (Figure 7). ASS, ASC and OCT showed no significant transcriptional induction in whole head samples. The enzymes encoded by these genes catalyse earlier steps in the polyamine biosynthetic pathway than ODC and ADC (Figure 1) suggesting a more coordinated induction of the pathway may occur in the rachis following infection.
Figure 6.
Putrescine concentration in dissected wheat head material under Fusarium head blight infection. n = 8 individual heads. Letters above bars represent statistically different groupings (Tukey test P < 0.05).
Figure 7.
Comparison of polyamine pathway gene expression in dissected head tissue under Fusarium head blight infection. Values are relative to mock-treated heads. Black bars are spikelet material and grey bars are rachis material. Asterisks indicate statistically significant differences (P < 0.05) as described in the text. Error bars are the standard error of the mean, n = 8 individual heads. ASS, argininosuccinate synthase; ASL, argininosuccinate lyase; OCT, ornithine carbamoyl transferase; ADC2, arginine decarboxylase 2; TaPERO, peroxidase; ODC, ornithine decarboxylase.
Polyamine induction is a general response of resistant and susceptible wheats to FHB
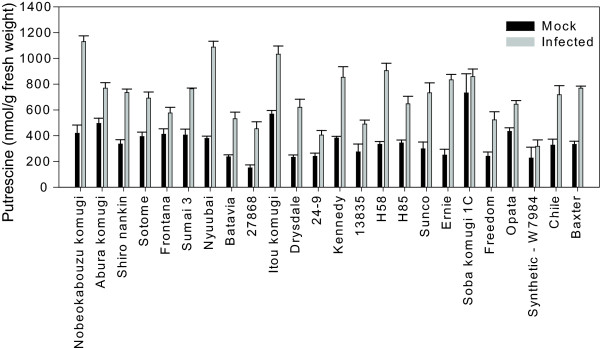
To test whether activation of the polyamine biosynthetic pathway by F. graminearum correlates with resistance or susceptibility to FHB, we investigated the responses of 24 diverse bread wheat lines that have been reported to vary in their response to FHB [44]. As described in the Material and Methods, we first confirmed in independent inoculation experiments that these 24 lines were indeed different in their response in FHB disease symptom development and DON accumulation. Overall, there was a good correlation (r = 0.75) between DON levels and disease susceptibility across these lines, supporting the already known link between DON and disease symptom development in these lines. These lines were also inoculated and whole heads sampled at three days post-inoculation and analysed for polyamine and amino acids and the data for putrescine is shown in Figure 8. Considerable variation in the levels of putrescine, from 150 to 732 nmoles/g fresh weight in mock and from 318 to 1130 nmoles/g fresh weight in inoculated heads, was evident across the genotypes (Figure 8). As described earlier for cv. Kennedy (Figure 2), we found a significant induction of putrescine production in 22 of the wheat lines tested with the exception of Soba komugi 1C and Synthetic-W7984 (Figure 8). Similar inductions were observed for spermidine in all the lines examined (Additional File 2). Cadaverine detection (data not shown) and spermine levels (Additional File 2) were variable across the genotypes. No amino acid, including the potent DON inducer arginine showed any induction upon infection at three days post inoculation in these lines (data not shown). These results, therefore, indicate that the induction of putrescine and spermidine is a general response of wheat to infection and occurs in both resistant and susceptible lines.
Figure 8.
Putrescine levels in mock and inoculated wheat heads of diverse wheat cultivars. Measurements were taken at three days post inoculation. Error bars are the standard error of the mean n≥4. Genotypes are plotted in increasing levels of DON as described in the Materials and Methods.
We tested the correlations between putrescine and spermidine levels found in both mock and inoculated heads and FHB disease ratings and DON levels in these wheat lines (see Material and Methods) but no significant correlation was found (data not shown). These results suggest that polyamine induction is a general response of both FHB resistant and susceptible wheats to infection.
Discussion
Several factors are known to influence the production of trichothecenes by F. graminearum in culture [13-15,18]. However, whether these factors also affect toxin production in planta during pathogenesis is unknown. The plant stress metabolites polyamines are one of the most potent toxin inducing factors in culture and result in toxin and biosynthetic enzyme mRNA levels equivalent to those observed in infected heads [18]. Here we have tested whether it is plausible that polyamines may act as in planta inducers of trichothecene production in wheat. By analysing the temporal changes in polyamine levels and the expression of the genes for their biosynthesis in relation to the biosynthesis of the trichothecene DON during FHB disease development. A key finding was that the induction of expression of key polyamine biosynthetic pathway genes and increase in polyamine (e.g. putrescine) levels following inoculation of wheat heads preceded the detection of DON in infected tissue. Induction of ODC by FHB was also observed in publically available global expression data in both resistant and susceptible near isogenic lines for Fhb1/fhb1 in wheat, and to a lesser extent during barley FHB [45-47]. Furthermore the timing of induction of ADC2 and ODC was similar to that of TaPERO known to be induced in response to Fusarium spp. [41,42]. These observations are consistent with a model where the early stages of fungal challenge of wheat flowers induce polyamine biosynthesis as a generic host stress response and this response is sensed by the pathogen as a cue for boosting trichothecene mycotoxin production such as DON.
It is well established that DON production in the fungus is not required for the initial infection of wheat flowers. However, DON is required as a virulence factor facilitating fungal colonisation and disease spread from the initially infected floret to other florets via the rachis [4,48]. In the absence of fungal DON production, the progress of F. graminearum is halted at the rachis node by plant cell wall thickenings as part of the normal defence response [4]. The model proposed above where the fungus initially stimulates a stress response in the host and then uses this as a trigger for DON production is also consistent with the proposed role for DON later in the infection process rather than it being necessary for initial infection processes.
The importance of the rachis node for induction of DON production in planta has recently been elegantly demonstrated [43]. The tight tissue specificity for DON production observed in the rachis tissue suggests that any DON-inducing factors are likely to be preferentially synthesised in this zone. Interestingly, Peeters et al. [35] detected only putrescine and no other polyamines in the rachis during wheat anthesis, suggesting some tissue specificity in the production of this particular polyamine that is also a potent inducer of DON production. In our analysis of transcripts for polyamine biosynthesis from whole heads we observed significant induction of only ODC and ADC2 but when the rachis was sampled separately there was also a significant induction of transcripts for three other enzymes earlier in the pathway suggesting a coordinated induction of the biosynthetic steps to putrescine. This is also consistent with the model proposed for putrescine as an inducer of DON production during infection.
The concentration of putrescine reached in heads following inoculation was approximately 0.5 mM based on a sample fresh weight basis. In culture, the lower concentration limit observed for the induction of DON by polyamine inducers such as putrescine is approximately 1 mM (data not shown) which is higher than the putrescine concentrations measured here in planta. Although this may argue against a possible in planta DON-inducing role for this metabolite, it should be noted that the actual putrescine concentration at the host-pathogen interface may be higher than the overall tissue level and it is possible that DON is synergistically induced by multiple compounds and conditions as demonstrated previously [15,18]. Also, it is possible that infection hyphae may differ in their sensitivity to inducing compounds when compared to vegetative hyphae growing in batch axenic culture conditions. More definitive information on the role of polyamines may be obtained using transgenic plants silenced for ODC and/or ADC2 that contain significantly reduced levels of polyamines in heads. However, given the central importance of polyamines in many biological processes and the branched biosynthesis pathway in wheat, plants with reduced polyamine levels maybe difficult to generate. Fungal mutants that are non-responsive to the polyamine signals may also be useful to definitively test this hypothesis.
Our analysis of infected wheat heads could not discriminate between polyamines and amino acids of fungal and plant origin. However, the early induction of polyamines such as putrescine, before fungal biomass increases appreciably together with the increases observed in transcripts of genes encoding plant polyamine biosynthetic enzymes all provide evidence that the wheat plant is the major contributor of the metabolic changes observed. Indeed PCR analysis of fungal polyamine biosynthetic genes showed all genes analysed were constantly expressed during infection (data not shown). One polyamine, cadaverine, was not detected in the non-inoculated heads and increased in concentration in parallel with fungal biomass. Cadaverine is produced by decarboxylation of lysine but no clear homologue of the bacterial lysine decarboxylase sequences were identified in the genome sequence of F. graminearum (data not shown). In plants it is highly likely that decarboxylation of lysine is a result of the ODC enzyme acting on lysine as an alternative substrate [49]. This may become significant when ODC transcripts, and presumably enzyme levels, were so strongly induced by infection. This would explain why a delay is observed in the production of cadaverine in infected tissue.
Conclusions
In summary, the in planta induction of DON biosynthesis in F. graminearum is a complex process to which polyamines are likely to contribute. Future work to more specifically define the role of polyamines as inducers of DON during infection will require functional tests with fungal mutants with impaired perception of polyamines as well as tissue specific localisation of metabolites at the host-pathogen interface.
Methods
Plant material and inoculation
The susceptible Australian bread wheat (Triticum aestivum L.) cultivar Kennedy was used for all experiments unless indicated otherwise. Four seeds were sown in 10 cm pots in potting mix amended with osmocote, and grown in a controlled environment room with a photoperiod of 14 h, at 25°C and 50% relative humidity. The light intensity was 500 mmol m-2 s-1. Night-time temperature was set at 15°C, with 90% relative humidity.
Unless specified otherwise, plants were spray inoculated at mid-anthesis with F. graminearum isolate CS3005 [50]. Approximately 2 mL of a 1×106 spores mL-1 or water for mock inoculations was applied to each head using a Preval Sprayer (Precision Valve Corporation, NY, USA). Heads were covered with humidified plastic zip lock bags following treatment. Plastic bags were replaced three days post-inoculation with a glassine bag. All analyses were carried out on individual heads.
A panel of 24 bread wheat accessions that differ for resistance to FHB [44] were selected to screen for differential accumulation of polyamines and DON during FHB following spray inoculation. The response of these genotypes to FHB inoculation and their DON accumulation was assayed following point inoculation of heads as described previously [51], except disease scores and DON assays were conducted 14 days after infection. The genotypes selected and their respective DON accumulation (ppm in fresh head tissue) and disease scores (proportion of spikelets with symptoms) were as follows: Nobeokabouzu komugi (11.3 ppm, 0.127), Abura komugi (13.7, 0.147), Shiro nankin (14.2, 0.158), Sotome (15.7, 0.128), Frontana (16.2, 0.122), Sumai 3 (16.3, 0.173), Nyuubai (17.3, 0.144), Batavia (17.5, 0.171), 27868 (19.7, 0.125), Itou komugi (21.5, 0.165), Drysdale (24.5, 0.349), 24-9 (26.0, 0.145), Kennedy (26.4, 0.344), 13835 (26.5, 0.328), H58 (30.3, 0.123), H85 (31.0, 0.214), Sunco (32.4, 0.182), Ernie (33.1, 0.185), Soba komugi 1C (38.4, 0.370), Freedom (44.4, 0.177), Opata (50.3, 0.331), W7984 (50.9, 0.427), Chile (94.2, 0.537), and Baxter (100.6, 0.381).
Polyamine and amino acid detection and quantification
Polyamines were quantified as previously described [18]. Free amino acids were extracted by grinding the heads under liquid nitrogen and resuspending a known quantity (up to 500 mg) of the powder in 2 mL of methanol, incubated at -20°C overnight and then 8 mL of water added and centrifuged to pellet solid matter. 500 μL of supernatant was dried down under vacuum. This extract was used with the Waters (Milford, MA, USA) AccQ-Tag amino acid detection kit, with quantification by HPLC according to the manufacturer's instructions. In a separate experiment, to allow more sensitive quantification of ornithine, the amino acid analysis was performed using the Waters AccQ-Tag Ultra chemistry at the Australian Proteome Analysis Facility (Sydney, Australia).
Reverse transcriptase quantitative polymerase chain reaction
RT-qPCR was carried out as previously described [11]. To determine the target sequence of wheat polyamine genes for primer design, first the rice locus encoding the target gene was identified by a combination of text querying of putative function using the rice genome and reciprocal blast comparisons with the relevant Arabidopsis loci. The rice loci numbers were then used to query the Wheat Estimated Transcript Server (WhETS) database [52] to determine the orthologous wheat sequences. Where WhETS identified multiple homeologous sequences, ClustalW [53] alignments were used to determine conserved regions of these sequences for design of primers. Where suitable conserved regions could not be identified, multiple primer pairs were utilised. Primers were designed using the Primer3 software [54]. Primer sequences used to amplify wheat polyamine genes and the orthologous rice loci are listed in Table 1. Fungal biomass accumulation was estimated by comparing 18 S rRNA amplification from the fungus and plant respectively using primers listed in Table 1. TRI5 gene expression was measured relative to fungal 18 S using primers listed in Table 1.
Deoxynivalenol quantification
Deoxynivalenol was quantified using the ELISA kit from Beacon Analytical Systems (Saco, Maine, USA) as per the manufacturer's instructions.
Authors' contributions
DMG, KK, SP, FJT, JMM designed the research. DMG, SP and AR performed the experiments and analysed data. DMG, KK and JMM wrote the manuscript. All authors read and approved the manuscript.
Supplementary Material
Free amino acids quantified during Fusarium head blight infection of wheat. Free amino acids quantified during Fusarium head blight infection of wheat. Open circles denote infected samples, closed circles are mock inoculated. Error bars represent the standard error of the mean, n = 4.
Spermidine and spermine concentrations in mock- and Fusarium head blight infected diverse wheat lines. Spermidine (A) and spermine (B) concentrations in mock- and Fusarium head blight infected diverse wheat lines. Error bars are the standard error of the mean n≥4. Genotypes are plotted in increasing order of DON concentration.
Contributor Information
Donald M Gardiner, Email: donald.gardiner@csiro.au.
Kemal Kazan, Email: kemal.kazan@csiro.au.
Sebastien Praud, Email: sebastien.praud@biogemma.com.
Francois J Torney, Email: francois.torney@biogemma.com.
Anca Rusu, Email: anca.rusu@csiro.au.
John M Manners, Email: john.manners@csiro.au.
Acknowledgements
We wish to thank Brendan Kidd and Johanna Bursle for excellent technical assistance. We thank Dr Chunji Liu for providing seed for screening multiple wheat genotypes. This work was jointly funded by CSIRO and Biogemma.
References
- Goswami RS, Kistler HC. Heading for disaster Fusarium graminearum on cereal crops. Mol Plant Pathol. 2004;5(6):515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- Desmond OJ, Manners JM, Stephens AE, Maclean DJ, Schenk PM, Gardiner DM, Munn AL, Kazan K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol Plant Pathol. 2008;9(4):435–445. doi: 10.1111/j.1364-3703.2008.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen P, Maier F, Schäfer W. Trichothecenes and lipases are host-induced and secreted virulence factors of Fusarium graminearum. Cereal Research Communications. 2008;36(0):421–428. doi: 10.1556/CRC.36.2008.Suppl.B.35. [DOI] [Google Scholar]
- Jansen C, von Wettstein D, Schafer W, Kogel K-H, Felk A, Maier FJ. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc Natl Acad Sci USA. 2005;102(46):16892–16897. doi: 10.1073/pnas.0508467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RH, Hohn TM, McCormick SP. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant-Microbe Interact. 1995;8(4):593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- Pestka JJ. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim Feed Sci Technol. 2007;137(3-4):283–298. doi: 10.1016/j.anifeedsci.2007.06.006. [DOI] [Google Scholar]
- van Egmond H, Schothorst R, Jonker M. Regulations relating to mycotoxins in food. Anal Bioanal Chem. 2007;389(1):147–157. doi: 10.1007/s00216-007-1317-9. [DOI] [PubMed] [Google Scholar]
- Boutigny A-L, Richard-Forget F, Barreau C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur J Plant Pathol. 2008;121(4):411–423. doi: 10.1007/s10658-007-9266-x. [DOI] [Google Scholar]
- Yuen GY, Schoneweis SD. Strategies for managing Fusarium head blight and deoxynivalenol accumulation in wheat. Int J Food Microbiol. 2007;119(1-2):126–130. doi: 10.1016/j.ijfoodmicro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Walter S, Nicholson P, Doohan FM. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2010;185(1):54–66. doi: 10.1111/j.1469-8137.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- Mudge AM, Dill-Macky R, Dong Y, Gardiner DM, White RG, Manners JM. A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol Mol Plant Pathol. 2006;69(1-3):73–85. doi: 10.1016/j.pmpp.2007.01.003. [DOI] [Google Scholar]
- Voigt CA, Scheidt B, Gácser A, Kassner H, Lieberei R, Schäfer W, Salomon S. Enhanced mycotoxin production of a lipase-deficient Fusarium graminearum mutant correlates to toxin-related gene expression. Eur J Plant Pathol. 2007;117(1):1–12. doi: 10.1007/s10658-006-9063-y. [DOI] [Google Scholar]
- Ponts N, Couedelo L, Pinson-Gadais L, Verdal-Bonnin M-N, Barreau C, Richard-Forget F. Fusarium response to oxidative stress by H2O2 is trichothecene chemotype-dependent. FEMS Microbiol Lett. 2009;293(2):255–262. doi: 10.1111/j.1574-6968.2009.01521.x. [DOI] [PubMed] [Google Scholar]
- Jiao F, Kawakami A, Nakajima T. Effects of different carbon sources on trichothecene production and Tri gene expression by Fusarium graminearum in liquid culture. FEMS Microbiol Lett. 2008;285(2):212–219. doi: 10.1111/j.1574-6968.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Osborne S, Kazan K, Manners JM. Low pH regulates the production of deoxynivalenol by Fusarium graminearum. Microbiology. 2009;155(9):3149–3156. doi: 10.1099/mic.0.029546-0. [DOI] [PubMed] [Google Scholar]
- Merhej J, Boutigny AL, Pinson-Gadais L, Richard-Forget F, Barreau C. Acidic pH as a determinant of TRI gene expression and trichothecene B biosynthesis in Fusarium graminearum. Food Addit Contam Part A-Chem. 2010;27(5):710–717. doi: 10.1080/19440040903514531. [DOI] [PubMed] [Google Scholar]
- Ochiai N, Tokai T, Takahashi-Ando N, Fujimura M, Kimura M. Genetically engineered Fusarium as a tool to evaluate the effects of environmental factors on initiation of trichothecene biosynthesis. FEMS Microbiol Lett. 2007;275(1):53–61. doi: 10.1111/j.1574-6968.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Kazan K, Manners JM. Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum. Fungal Genet Biol. 2009;46(8):604–613. doi: 10.1016/j.fgb.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Hohn TM, McCormick SP. Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiol Mol Biol Rev. 1993;57(3):595–604. doi: 10.1128/mr.57.3.595-604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 2006;28(23):1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci. 1999;140(2):103–125. doi: 10.1016/S0168-9452(98)00218-0. [DOI] [Google Scholar]
- Paulus TJ, Kiyono P, Davis RH. Polyamine-deficient Neurospora crassa mutants and synthesis of cadaverine. J Bacteriol. 1982;152(1):291–297. doi: 10.1128/jb.152.1.291-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Cho YD. Identification of essential active-site residues in ornithine decarboxylase of Nicotiana glutinosa decarboxylating both L-ornithine and L-lysine. Biochem J. 2001;360(3):657–665. doi: 10.1042/0264-6021:3600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio A. Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231(6):1237–1249. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- Walters DR. Polyamines and plant disease. Phytochemistry. 2003;64(1):97–107. doi: 10.1016/S0031-9422(03)00329-7. [DOI] [PubMed] [Google Scholar]
- Yoda H, Fujimura K, Takahashi H, Munemura I, Uchimiya H, Sano H. Polyamines as a common source of hydrogen peroxide in host- and nonhost hypersensitive response during pathogen infection. Plant Mol Biol. 2009;70(1-2):103–112. doi: 10.1007/s11103-009-9459-0. [DOI] [PubMed] [Google Scholar]
- Parker D, Beckmann M, Zubair H, Enot DP, Caracuel-Rios Z, Overy DP, Snowdon S, Talbot NJ, Draper J. Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J. 2009;59(5):723–737. doi: 10.1111/j.1365-313X.2009.03912.x. [DOI] [PubMed] [Google Scholar]
- Edreva A. Tobacco polyamines as affected by stresses induced by different pathogens. Biol Plant. 1997;40(2):317–320. doi: 10.1023/A:1001093209229. [DOI] [Google Scholar]
- Waie B, Rajam MV. Effect of increased polyamine biosynthesis on stress responses in transgenic tobacco by introduction of human S-adenosylmethionine gene. Plant Sci. 2003;164(5):727–734. doi: 10.1016/S0168-9452(03)00030-X. [DOI] [Google Scholar]
- Jin S, Yoshida M. Antifungal compound, feruloylagmatine, induced in winter wheat exposed to a low temperature. Biosci Biotechnol Biochem. 2000;64(8):1614–1617. doi: 10.1271/bbb.64.1614. [DOI] [PubMed] [Google Scholar]
- Smith TA, Best GR. Distribution of the hordatines in barley. Phytochemistry. 1978;17(7):1093–1098. doi: 10.1016/S0031-9422(00)94295-X. [DOI] [Google Scholar]
- Peremarti A, Bassie L, Yuan D, Pelacho A, Christou P, Capell T. Transcriptional regulation of the rice arginine decarboxylase (Adc1) and S-adenosylmethionine decarboxylase (Samdc) genes by methyl jasmonate. Plant Physiol Biochem. 2010;48(7):553–559. doi: 10.1016/j.plaphy.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Haggag WM, Abd-El-Kareem F. Methyl jasmonate stimulates polyamines biosynthesis and resistance against leaf rust in wheat plants. Arch Phytopathol Plant Protect. 2009;42(1):16–31. doi: 10.1080/03235400600914355. [DOI] [Google Scholar]
- Walters D, Cowley T, Mitchell A. Methyl jasmonate alters polyamine metabolism and induces systemic protection against powdery mildew infection in barley seedlings. J Exp Bot. 2002;53(369):747–756. doi: 10.1093/jexbot/53.369.747. [DOI] [PubMed] [Google Scholar]
- Peeters KMU, Geuns JMC, Van Laere AJ. Free polyamines in senescing wheat (Triticum aestivum L.) J Exp Bot. 1993;44(11):1709–1715. doi: 10.1093/jxb/44.11.1709. [DOI] [Google Scholar]
- Yang J, Yunying C, Zhang H, Liu L, Zhang J. Involvement of polyamines in the post-anthesis development of inferior and superior spikelets in rice. Planta. 2008;228(1):137–149. doi: 10.1007/s00425-008-0725-1. [DOI] [PubMed] [Google Scholar]
- Bencsik K, Kremmer T, Boldizsár M, Tamás J, Mák M, Páldi E. High-performance liquid chromatographic determination and standardization of agmatine. J Chromatogr. 1998;824(2):175–180. doi: 10.1016/S0021-9673(97)00193-3. [DOI] [Google Scholar]
- Urano K, Hobo T, Shinozaki K. Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett. 2005;579(6):1557–1564. doi: 10.1016/j.febslet.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi-Shinozaki K, Shinozaki K. Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun. 2004;313(2):369–375. doi: 10.1016/j.bbrc.2003.11.119. [DOI] [PubMed] [Google Scholar]
- Perez-Amador MA, Leon J, Green PJ, Carbonell J. Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol. 2002;130(3):1454–1463. doi: 10.1104/pp.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond OJ, Edgar CI, Manners JM, Maclean DJ, Schenk PM, Kazan K. Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol. 2005;67(3-5):171–179. doi: 10.1016/j.pmpp.2005.12.007. [DOI] [Google Scholar]
- Pritsch C, Muehlbauer GJ, Bushnell WR, Somers DA, Vance CP. Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol Plant-Microbe Interact. 2000;13(2):159–169. doi: 10.1094/MPMI.2000.13.2.159. [DOI] [PubMed] [Google Scholar]
- Ilgen P, Hadeler B, Maier FJ, SchÃfer W. Developing kernel and rachis node induce the trichothecene pathway of Fusarium graminearum during wheat head infection. Mol Plant-Microbe Interact. 2009;22(8):899–908. doi: 10.1094/MPMI-22-8-0899. [DOI] [PubMed] [Google Scholar]
- Li H, Xie G, Ma J, Liu G, Wen S, Ban T, Chakraborty S, Liu C. Genetic relationships between resistances to Fusarium head blight and crown rot in bread wheat (Triticum aestivum L.) Theor Appl Genet. 2010;121(5):941–950. doi: 10.1007/s00122-010-1363-0. [DOI] [PubMed] [Google Scholar]
- Jia H, Cho S, Muehlbauer GJ. Transcriptome analysis of a wheat near-isogenic line pair carrying Fusarium bead blight resistant and susceptible alleles. Mol Plant-Microbe Interact. 2009;22(11):1366–1378. doi: 10.1094/MPMI-22-11-1366. [DOI] [PubMed] [Google Scholar]
- Boddu J, Cho S, Kruger WM, Muehlbauer GJ. Transcriptome analysis of the barley-Fusarium graminearum interaction. Mol Plant-Microbe Interact. 2006;19(4):407–417. doi: 10.1094/MPMI-19-0407. [DOI] [PubMed] [Google Scholar]
- Wise RP, Caldo RA, Hong L, Shen L, Cannon E, Dickerson JA. In: Plant Bioinformatics. Edwards D, editor. Vol. 406. Totowa, NJ: Humana Press; 2008. BarleyBase/PLEXdb: A Unified Expression Profiling Database for Plants and Plant Pathogens; pp. 347–363. full_text. [Google Scholar]
- Bai GH, Desjardins AE, Plattner RD. Deoxynivalenol non-producing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia. 2002;153(2):91–98. doi: 10.1023/A:1014419323550. [DOI] [PubMed] [Google Scholar]
- Ohe M, Scoccianti V, Bagni N, Tassoni A, Matsuzaki S. Putative occurrence of lysine decarboxylase isoforms in soybean (Glycine max) seedlings. Amino Acids. 2009;36(1):65–70. doi: 10.1007/s00726-008-0029-6. [DOI] [PubMed] [Google Scholar]
- Akinsanmi OA, Mitter V, Simpfendorfer S, Backhouse D, Chakraborty S. Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Aust J Agric Res. 2004;55(1):97–107. doi: 10.1071/AR03090. [DOI] [Google Scholar]
- Gardiner DM, Kazan K, Manners JM. Novel genes of Fusarium graminearum that negatively regulate deoxynivalenol production and virulence. Mol Plant-Microbe Interact. 2009;22(12):1588–1600. doi: 10.1094/MPMI-22-12-1588. [DOI] [PubMed] [Google Scholar]
- Mitchell RAC, Castells-Brooke N, Taubert J, Verrier PJ, Leader DJ, Rawlings CJ. Wheat Estimated Transcript Server (WhETS): a tool to provide best estimate of hexaploid wheat transcript sequence. Nucl Acids Res. 2007;35(suppl_2):W148–151. doi: 10.1093/nar/gkm220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal × version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. In: Bioinformatics Methods and Protocols: Methods in Molecular Biology. Krawetz S, Misener S, editor. Totowa, NJ: Humana Press; 2000. Primer3 on the WWW for general users and for biologist programmers; pp. 365–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Free amino acids quantified during Fusarium head blight infection of wheat. Free amino acids quantified during Fusarium head blight infection of wheat. Open circles denote infected samples, closed circles are mock inoculated. Error bars represent the standard error of the mean, n = 4.
Spermidine and spermine concentrations in mock- and Fusarium head blight infected diverse wheat lines. Spermidine (A) and spermine (B) concentrations in mock- and Fusarium head blight infected diverse wheat lines. Error bars are the standard error of the mean n≥4. Genotypes are plotted in increasing order of DON concentration.