Abstract
Introduction:
Short sleep duration is associated with systemic inflammation and diabetes; however the mechanisms by which reduced sleep leads to these complications are unclear. One possibility is sleep may impact secretion of adipocyte derived hormones that regulate inflammation and insulin resistance. In this study we assessed the association between sleep duration and 3 adipokine levels.
Methods:
A total of 561 adults from the Cleveland Family Study underwent standardized laboratory polysomnography followed by a morning fasting blood draw assayed for leptin, visfatin, and retinol binding protein-4 (RBP4) levels.
Results:
The cohort had an age of 44.5 (16.1) years and total sleep time (TST) of 6.2 (1.3) hours (mean [SD]). Each hour reduction in TST was associated with a 10% increase in leptin (P = 0.01) and a 14% increase in visfatin levels (P = 0.03) in analyses adjusted for age, gender, and race. After additional adjustment for obesity, sleep apnea severity, hypertension, and diabetes, each hour reduction in TST was associated with a 6% increase in leptin (P = 0.01) and a 14% increase in visfatin levels (P = 0.02). Leptin increased by 15% (P = 0.01) and visfatin increased by 31% (P = 0.05) for every 1-h decrease in REM sleep. In contrast, no association between sleep duration and RBP4 was found.
Conclusions:
Reduced sleep and reduced REM sleep are associated with elevations in leptin and visfatin, 2 adipokines associated with inflammation and insulin resistance. Further investigation of the effect of sleep on adipose tissue function should be pursued.
Citation:
Hayes AL; Xu F; Babineau D; Patel SR. Sleep duration and circulating adipokine levels. SLEEP 2011;34(2):147–152.
Keywords: Sleep duration, leptin, retinol binding protein-4, visfatin, adipokine, insulin resistance, adipose tissue, obesity
SEVERAL STUDIES HAVE LINKED SHORT SLEEP WITH BOTH DIABETES AND SYSTEMIC INFLAMMATION.1–5 THE MECHANISMS BY WHICH SHORT SLEEP LEADS to insulin resistance and a pro-inflammatory state and the tissues involved, however, are unclear. Adipose tissue, rather than being simply an inert energy storage depot, is increasingly recognized as having pleiotropic endocrine functions.6–8 Many adipokines (cytokines secreted by adipose tissue) have been associated with systemic inflammation, atherogenesis, regulation of energy homeostasis, and diabetes.9–11 It is possible that adipose tissue function is altered by sleep deprivation and that dysregulation of adipokines leads to the insulin resistance and systemic inflammation observed with chronic sleep deprivation.
This study sought to investigate the association between sleep duration and circulating levels of 3 adipokines, leptin, visfatin, and retinol binding protein-4 (RBP4), in a cohort with standardized sleep phenotyping.
LMETHODS
Subjects
The Cleveland Family Study (CFS) is a longitudinal family-based epidemiological cohort designed to study the natural history and genetic basis of sleep phenotypes. Details of the cohort have been previously described.12 Briefly, families of index probands with a laboratory confirmed diagnosis of sleep apnea as well as control families were recruited. A subset of these individuals was selected for detailed phenotyping based on expected genetic informativity. This was accomplished by choosing pedigrees where siblings had extremes (either high or low) of apnea hypopnea index (AHI). A detailed description of the selection strategy has been previously published.13 Subjects currently using continuous positive airway pressure (CPAP) therapy and those < 16 years of age were excluded from this analysis. The CFS protocol was approved by the University Hospitals Case Medical Center institutional review board, and all participants provided written informed consent.
Phenotype Collection
Data from the most recent phenotyping where subjects underwent overnight laboratory polysomnography (PSG) were used for this analysis. Lights off was enforced at 23:00 and subjects were awoken at 06:45. The PSG utilized both oronasal thermocouple and nasal pressure to assess airflow and inductive plethysmography to assess respiratory effort (Compumedics, Abbotsford, AU). Sleep was monitored using electroencephalography (EEG), electro-oculography (EOG), and chin electromyography. Sleep stages were manually scored in 30-sec epochs using standard criteria.14 Total sleep time (TST) was calculated by summing all epochs scored as any stage of sleep. Apneas were defined as complete cessation of airflow, and hypopneas were defined as a 30% decrease in airflow > 10 seconds associated with a 3% decrease in oxygen saturation. The AHI was computed by dividing the total number of apneas and hypopneas by TST.
Waist circumference (WC) was obtained by measuring the smallest horizontal circumference between the ribs and the iliac crest in duplicate by certified research personnel and averaged. Body mass index (BMI) was calculated as the ratio of weight to height squared. Hypertension was defined as having a systolic blood pressure (BP) ≥ 140 mm Hg, a diastolic BP ≥ 90 mm Hg, a physician diagnosis of hypertension, or usage of an antihypertensive medication. Type 2 diabetes was defined as a fasting glucose ≥ 126 mg/dL, a 2-h glucose ≥ 200 mg/dL on oral glucose tolerance testing, physician diagnosis of type 2 diabetes, or usage of an antidiabetic medication.
Venous blood was obtained at 07:00 the morning following PSG and an overnight fast. Blood samples were centrifuged, aliquotted, and stored at −80°C until assayed. Leptin (R&D Systems, Minneapolis, MN), RBP4 (ALPCO Diagnostics, Salem, NH), and visfatin (ALPCO Diagnostics, Salem, NH) levels in serum were measured by enzyme-linked immunosorbent assay (ELISA). Interassay coefficients of variance ranged from 3.0% to 5.4% for leptin, 9.0% to 15.0% for RBP4, and 4.6% to 7.2% for visfatin.
Statistical Analysis
The primary exposure of interest was TST although times spent in each individual sleep stage (NREM stages N1, N2, N3, and REM sleep) were also assessed as secondary exposures. Demographic characteristics were compared among 5 groups of subjects defined by < 5 h, 5-6 h, 6-7 h, 7-8 h, and ≥ 8 h in TST, using a linear mixed model that assumed a compound symmetric covariance structure to account for within family correlation or by Fisher exact test with multiple outputation.
To model the association between each exposure and adipokine level, 2 models were used. Linear mixed models using a compound symmetry covariance structure to account for within family correlation were used to model leptin and RBP4 levels. To satisfy normality assumptions, both adipokine levels were log transformed prior to analysis. Because the distribution of visfatin was right skewed and 17% of visfatin levels were below the lower threshold of detection for the assay used, a linear mixed model was not applicable. Instead, a Weibull model accounting for the left censored nature of the data15 was used to model visfatin levels on the log transformed scale. This model incorporated a shared Gaussian frailty term to account for within family correlation. While all models adjusted for age, gender, and race, additional models also adjusted for adiposity, sleep apnea severity, hypertension, and diabetes. Because central fat appears to be most metabolically relevant, adiposity was measured primarily using WC.
To investigate the linear association between each exposure and adipokine level, separate sets of models were fit to each exposure in 2 ways. The first method categorized each exposure into intervals defined by quartiles and used a linear orthogonal polynomial contrast for unequally spaced exposure levels to test for linear associations between the exposure and adipokine level. Using this method, the geometric mean of each adipokine level in each interval was estimated. Alternatively, the second method treated each exposure as a continuous variable and used splines to test for a nonlinear association between each exposure and adipokine level. Using this method, no evidence of a nonlinear association was found, so results were interpreted as the geometric mean ratio in the adipokine level for each hour increase in sleep.
Linear mixed effects models were fit using SAS version 9.2 (Cary, NC), while Weibull models were fit using R version 2.9.0 (Vienna, Austria).
RESULTS
A total of 735 subjects underwent the in-laboratory PSG protocol. Individuals currently using CPAP (n = 70) and subjects below the age of 16 years (n = 91) were excluded from this analysis. Eight subjects were excluded due to poor EEG signal quality and 5 for missing WC measurements, leaving 561 subjects available for analysis. Demographic characteristics by sleep duration are presented in Table 1. The average age was 44.5 years, with roughly equal numbers of men and women and Caucasians and African Americans. Since the cohort was designed to study the genetic basis of sleep apnea, the prevalence of obstructive sleep apnea (OSA), obesity, hypertension, and diabetes was high. Subjects with short TST tended to be older (P < 0.001), have a larger WC (P = 0.01), and a higher AHI (P = 0.002). Also, as TST decreased there was a higher prevalence of hypertension (P < 0.001) and type 2 diabetes (P < 0.001).
Table 1.
Demographic characteristics stratified by total sleep time
| < 5 h (n = 82) | 5-6 h (n = 139) | 6-7 h (n = 205) | 7-8 h (n = 96) | ≥ 8 h (n = 39) | P-value | |
|---|---|---|---|---|---|---|
| Age (yrs) | 55.4 ± 18.0 | 50.2 ± 17.3 | 41.3 ± 15.7 | 38.1 ± 15.3 | 34.0 ± 12.5 | < 0.001 |
| Male gender (%) | 47.6 | 42.0 | 38.5 | 39.6 | 43.6 | 0.65 |
| White race (%) | 51.2 | 41.0 | 44.4 | 40.6 | 28.2 | 0.40 |
| Body mass index (kg/m2) | 33.5 ± 8.1 | 32.4 ± 7.7 | 33.1 ± 8.7 | 31.5 ± 7.7 | 30.2 ± 7.1 | 0.07 |
| Waist Circumference (cm) | 102.7 ± 16.4 | 97.7 ± 18.0 | 98.0 ± 18.1 | 94.9 ± 18.0 | 92.5 ± 16.2 | 0.01 |
| Hypertension (%) | 52.4 | 41.7 | 30.7 | 22.9 | 20.5 | < 0.001 |
| Systolic blood pressure (mm Hg) | 128.7 ± 14.2 | 126.5 ± 15.6 | 122.7 ± 15.6 | 120.4 ± 14.5 | 121.8 ± 16.7 | < 0.001 |
| Diastolic blood pressure (mm Hg) | 75.0 ± 9.5 | 75.0 ± 9.0 | 74.3 ± 9.1 | 73.2 ± 9.4 | 74.2 ± 10.4 | 0.56 |
| Antihypertensive medication usage (%) | 45.1 | 38.1 | 24.4 | 18.8 | 12.8 | < 0.001 |
| Type 2 diabetes (%) | 34.2 | 15.8 | 14.6 | 10.4 | 5.1 | < 0.001 |
| Fasting glucose (mg/dL) | 101.4 ± 25.1 | 97.5 ± 23.4 | 98.4 ± 28.8 | 97.0 ± 24.5 | 91.8 ± 9.9 | 0.09 |
| Antidiabetic medication usage (%) | 18.3 | 9.4 | 5.9 | 5.2 | 0.0 | 0.19 |
| Apnea hypopnea index | 19.2 ± 22.4 | 15.1 ± 21.7 | 15.1 ± 21.6 | 9.2 ± 16.1 | 12.3 ± 22.6 | 0.002 |
| N1 (minutes) | 18.1 ± 12.5 | 21.6 ± 21.5 | 18.2 ± 10.9 | 17.2 ± 10.9 | 21.8 ± 16.6 | 0.06 |
| N2 (minutes) | 142.7 ± 46.1 | 201.6 ± 41.5 | 227.3 ± 41.3 | 242.2 ± 46.5 | 291.5 ± 47.1 | < 0.001 |
| N3 (minutes) | 43.4 ± 33.1 | 57.2 ± 38.9 | 68.8 ± 39.4 | 87.8 ± 43.6 | 82.5 ± 41.9 | < 0.001 |
| REM (minutes) | 38.6 ± 25.2 | 53.2 ± 23.1 | 74.8 ± 25.7 | 96.0 ± 20.9 | 118.1 ± 38.8 | < 0.001 |
Values displayed as mean ± standard deviation or proportion (%).
Adipokines and Total Sleep Time
Of the 561 subjects available for analysis, 557 subjects had leptin levels assayed. Short sleep was associated with elevations in leptin (Table 2). For every hour decrease in TST, leptin levels increased by 10% (P = 0.01) after adjusting for age, gender, and race. After further adjustment for WC, this association remained significant (P = 0.01) but was slightly attenuated. Leptin increased by 7% with every 1-h decrease in TST. The association was not altered when AHI, hypertension, and diabetes were added to the model. Leptin levels were similarly associated with TST in the quartile analysis (Figure 1). A progressive decline in the leptin levels was found with increasing TST quartile. In adjusted analyses, the mean leptin level for subjects in the first quartile (< 5.5 h) was 50.4 ng/mL (95% CI: [42.2-60.2]), while the mean leptin level for subjects in the fourth quartile (> 7.0 h) was 39.4 ng/mL (95%CI: [34.2-45.4]) (Figure 1). These findings were unchanged in models which adjusted for adiposity by including BMI instead of WC as a covariate.
Table 2.
Adjusted relative change in the geometric mean of adipokine level per one hour decrease in sleep time
| Leptin |
Visfatin |
RBP4 |
||||
|---|---|---|---|---|---|---|
| Slope (95% CI) | P-value | Slope (95% CI) | P-value | Slope (95% CI) | P-value | |
| Total Sleep Time | ||||||
| Model 1 | 1.10 (1.02, 1.18) | 0.01 | 1.14 (1.01, 1.28) | 0.03 | 1.02 (0.99, 1.04) | 0.21 |
| Model 2 | 1.07 (1.02, 1.12) | 0.01 | 1.14 (1.01, 1.28) | 0.03 | 1.01 (0.99, 1.04) | 0.29 |
| Model 3 | 1.06 (1.01, 1.11) | 0.01 | 1.14 (1.02, 1.29) | 0.02 | 1.01 (0.98, 1.03) | 0.52 |
| Sleep Stagesa | ||||||
| N1 time | 1.02 (0.81, 1.27) | 0.89 | 0.83 (0.48, 1.43) | 0.50 | 1.11 (1.02, 1.22) | 0.03 |
| N2 time | 1.04 (0.97, 1.11) | 0.24 | 1.08 (0.93, 1.25) | 0.32 | 1.01 (0.97, 1.04) | 0.61 |
| N3 time | 1.03 (0.91, 1.15) | 0.67 | 1.23 (0.96, 1.54) | 0.09 | 0.98 (0.94, 1.02) | 0.29 |
| REM time | 1.15 (1.03, 1.28) | 0.01 | 1.31 (1.01, 1.72) | 0.05 | 1.02 (0.98 1.06) | 0.40 |
Model 1 adjusted for age, gender, and race. Model 2 adjusted for age, gender, race, and waist circumference. Model 3 adjusted for age, gender, race, waist circumference, apnea hypopnea index, hypertension, and diabetes.
All sleep stages models adjusted for age, gender, race, waist circumference, apnea hypopnea index, hypertension, and diabetes. RBP4 refers to Retinol Binding Protein-4; CI, confidence interval.
Figure 1.
Leptin levels by quartile of total sleep time. Values displayed as geometric mean concentrations and 95% confidence interval. Models adjusted for age, race, gender, waist circumference, apnea hypopnea index, hypertension, and diabetes. P = 0.01 for test of linear trend.
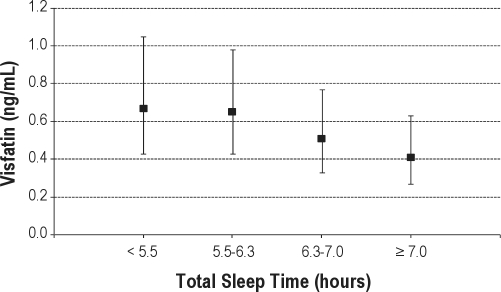
Visfatin was assayed in 493 of the 561 subjects. Similar to leptin, short sleep time was predictive of greater visfatin levels (Table 2). Accounting for age, gender, and race, visfatin levels increased 14% for every hour decrease in TST (P = 0.03). This association was unaffected with further adjustment for obesity, OSA severity, hypertension, and diabetes. As with leptin, mean visfatin levels were highest in the first quartile and lowest in the fourth quartile of TST with a significant linear trend (P < 0.05, Figure 2). Mean visfatin levels were 0.67 ng/mL (95% CI: [0.43-1.05]) in those sleeping < 5.5 h and 0.41 ng/mL (95% CI: [0.27-0.63]) in those sleeping > 7.0 h. Again, adjusting for BMI instead of WC did not materially affect the associations between TST and visfatin.
Figure 2.
Visfatin levels by quartile of total sleep time. Values displayed as geometric mean concentrations and 95% confidence interval. Models adjusted for age, race, gender, waist circumference, apnea hypopnea index, hypertension, and diabetes. P < 0.05 for test of linear trend.
RBP4 levels were available for 517 subjects. No clear association was found between RBP4 and TST in either the linear regression (Table 2) or quartile analyses (data not shown).
Adipokine and Sleep Stages
Secondary analyses were performed testing for associations between adipokine levels and time in specific sleep stages with the hypothesis that adipokine levels would be especially sensitive to the amount of slow wave sleep. In contrast to our expectations, leptin and visfatin levels were not associated with the amount of N3 sleep (Table 2). However the amount of REM sleep was associated with levels of both adipokines. For every 1-h decrease in REM sleep time, leptin levels increased by 15% and visfatin levels increased by 31% after adjusting for age, gender, race, obesity, sleep apnea severity, hypertension, and diabetes (P = 0.01 and P = 0.05, respectively).
The association between REM sleep time and these 2 adipokines persisted after excluding 66 subjects who were taking antidepressant medications. In this subgroup, leptin levels increased 18%, and visfatin levels increased 37% for every 1-h reduction in REM sleep time (P = 0.004 and P = 0.04, respectively). Of note, because of the high correlation between TST and time in REM sleep (Spearman correlation = 0.68), collinearity issues prevented the modeling of both TST and REM sleep time effects simultaneously. As such, it cannot be determined whether the identified associations are due to the effect of REM sleep time or TST.
DISCUSSION
The primary goal of this study was to investigate the relationship between sleep duration and levels of three adipokines associated with inflammation and insulin resistance to assess whether effects of sleep on these adipokines might mediate the adverse health effects of curtailed sleep. Our results suggest short sleep is associated with elevations in both leptin and visfatin levels, but not RBP4.
Leptin is a hormone produced predominantly by subcutaneous fat whose primary physiologic role appears to be involved in signaling energy status to the hypothalamus. It represents the major peripheral satiety signal to the brain reducing appetite and prevention of leptin signaling through mutations in either leptin or the leptin receptor produces a phenotype of unrestrained appetite and severe obesity in both humans and mice.16–18 However, typical obesity in humans is associated with elevated leptin levels and leptin resistance.19 In this setting, elevated leptin levels (independent of overall adiposity level) have an adverse effect. Leptin increases the production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6).20 In prospective studies, elevated leptin levels are an independent risk factor for weight gain, type 2 diabetes, and coronary heart disease.11,21–24 Initial studies on the association of sleep and leptin levels suggested reduced sleep may be associated with lower leptin levels.25,26 The Wisconsin Sleep Cohort study found in a cross-sectional analysis after adjusting for obesity, that reduced sleep duration was associated with lower leptin levels.26 However, sleep duration was assessed with sleep diaries rather than PSG and research suggests differential relationships between self-reported habitual sleep duration and polysomnographically measured acute sleep on cytokine levels.3 Short term partial sleep deprivation has been found to cause reductions in leptin levels in lean men on a controlled diet and receiving a constant glucose infusion.25 However, in subsequent sleep deprivation studies including both men and women who did not receive glucose infusions and were allowed to determine their own caloric intake, leptin levels have been found to increase with sleep curtailment.27–29 In a study of 136 men and women who were given three meals a day and snack foods ad libitum, leptin levels were increased 33% compared to baseline after five nights of 4 hours time in bed.29 Another study of 21 men and women found a 14% increase in 24-hour leptin levels after one night of total sleep deprivation. In this study, subjects were instructed to not change their usual diet and allowed to choose their meals from a hospital menu.28 Our results are in agreement with these more recent experimental studies that allow for a more “real world” setting in terms of access to food, suggesting the impact of sleep curtailment on leptin levels may be modulated by caloric intake. While the increase in leptin levels may suggest a primary increase in leptin secretion, they may also reflect reduced end-organ sensitivity to leptin with a secondary increase in leptin levels in an attempt to overcome this leptin resistance. An effect of reduced sleep on leptin resistance could explain the association between short sleep and increased obesity risk.30–33 However, the fact that the association between sleep duration and leptin levels were unaffected by adjustment for level of adiposity argues against a role for end-organ resistance in mediating this association.
In contrast to leptin, visfatin is an adipokine made primarily by visceral adipose tissue. It has been considered a potential contributor to the development of diabetes because it can bind the insulin receptor and so may serve as a competitive inhibitor of insulin-insulin receptor binding.34 In addition, epidemiologic studies have found that visfatin levels are elevated in both type 1 and type 2 diabetes.35,36 Like leptin, visfatin has pro-inflammatory effects. These include inducing expression of TNF-α, IL-1β, and IL-6 and activation of monocytes.10
Our results identifying an association between short sleep and elevated levels of visfatin confirm findings from a prior small study of 44 subjects by Trakada et al.37 That study, designed to assess the impact of OSA on visfatin, found no relationship with apnea severity measures but did find, similar to our study, a strong inverse correlation between TST and visfatin levels (r = −0.66). Our results further suggest this association is independent of obesity or sleep apnea severity.
RBP4 is an adipokine that is overexpressed in a rodent model of insulin resistance38 and has been associated with both insulin resistance and obesity in human studies.9,39,40 RBP4 has also been associated with inflammation in adipose tissue.41 To our knowledge, no prior study has assessed the potential effect of sleep duration on RBP4 levels. A previous small study found OSA was associated with elevations in RBP4 levels, but the effect of sleep duration was not assessed.42 Our results suggest, unlike leptin and visfatin, sleep duration does not have an important effect on levels of RBP4.
Given the role of slow wave sleep in secretion of growth hormone and other metabolically relevant processes,43–45 we hypothesized adipokine levels may be particularly sensitive to differences in amount of this sleep stage. Surprisingly, our results suggest leptin and visfatin levels were not associated with N3 time. Instead, leptin and visfatin levels were inversely correlated with REM sleep time. However, TST and REM sleep time were highly correlated, so it is unclear whether the observed associations were due to the effect of REM sleep or TST. Several studies suggest REM sleep may have a role in metabolism, obesity, and adipokine regulation.33,46–48 A population-based study found an association between reduced amount of REM sleep and central obesity in women, while a cross-sectional study in children and adolescents found both an increased REM latency and reduced amount of REM sleep were associated with a higher odds of being overweight.33,48 Another study in children with Prader-Willi syndrome, found levels of the insulin-sensitizing adipokine, adiponectin, were associated with an earlier REM latency and greater percentage of REM sleep.47 In rats, selective REM sleep deprivation has been found to lead to elevation in plasma levels of TNF-α, IL-1β, and IL-6, cytokines all made in adipose tissue.49 Thus, more investigation of the relationship between REM sleep and adipokine levels is warranted.
Strengths of our study include the standardized protocol with detailed sleep phenotyping using polysomnography. In addition, unlike prior studies, our cohort comprised a more generalizable population in terms of both gender and race. However, there are several limitations that should be noted. First, because of the cross-sectional nature of the study, the direction of causality cannot be determined. In mice, administration of leptin has been found to reduce the amount of REM sleep, suggesting adipokine levels may influence sleep.50 Second, it should be noted that the associations found were related to acute exposure to sleep immediately preceding blood sampling and thus these findings may not correspond to chronic effects of habitual sleep deprivation. Third, a limitation of this study is that the protocol used a standardized bed and wake time as well as time of blood draw. While this allowed for a standardized comparison of measures relative to clock time, the protocol did not take into account participants' habitual sleep habits, so effects due to altered circadian phase cannot be excluded. Finally, though the analyses were adjusted for multiple potential confounders, the possibility of residual confounding cannot be completely eliminated.
In summary, an inverse association was found between sleep duration and levels of both leptin and visfatin. These associations were independent of both obesity and sleep apnea severity. Furthermore, elevations in both leptin and visfatin levels were associated with reduced amount of REM sleep time, suggesting that the composition of sleep may play an important role in adipokine regulation. These findings suggest reduced sleep may have detrimental effects on adipose tissue function and these effects may help explain the systemic inflammation and insulin resistance associated with curtailed sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Patel has received research support from Health Right Products. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL081385, HL046380, RR000080, and RR024990.
We would like to thank Susan Redline for providing access to data from the Cleveland Family Study.
Footnotes
See commentary: Penev PP. Short sleep and circulating adipokine concentrations: does the fat hit the fire? SLEEP 2011;34:131–132.
REFERENCES
- 1.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 2.Frey DJ, Fleshner M, Wright KP., Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 5.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 6.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. quiz 20. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 9.Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 10.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–58. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 11.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 12.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:682–7. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 13.Palmer LJ, Buxbaum SG, Larkin E, et al. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet. 2003;72:340–50. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 15.Therneau TM, Grambsch PM, Pankratz VS. Penalized survival models and frailty. J Comput Graph Stat. 2003;12:156–75. [Google Scholar]
- 16.Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356:237–47. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–58. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 18.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 19.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 20.Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 21.Chessler SD, Fujimoto WY, Shofer JB, Boyko EJ, Weigle DS. Increased plasma leptin levels are associated with fat accumulation in Japanese Americans. Diabetes. 1998;47:239–43. doi: 10.2337/diab.47.2.239. [DOI] [PubMed] [Google Scholar]
- 22.McNeely MJ, Boyko EJ, Weigle DS, et al. Association between baseline plasma leptin levels and subsequent development of diabetes in Japanese Americans. Diabetes Care. 1999;22:65–70. doi: 10.2337/diacare.22.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Soderberg S, Zimmet P, Tuomilehto J, et al. Leptin predicts the development of diabetes in Mauritian men, but not women: a population-based study. Int J Obes (Lond) 2007;31:1126–33. doi: 10.1038/sj.ijo.0803561. [DOI] [PubMed] [Google Scholar]
- 24.van Rossum CT, Hoebee B, van Baak MA, Mars M, Saris WH, Seidell JC. Genetic variation in the leptin receptor gene, leptin, and weight gain in young Dutch adults. Obes Res. 2003;11:377–86. doi: 10.1038/oby.2003.51. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 26.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;2010:9. doi: 10.1111/j.1365-2869.2010.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;2010:7. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes (Lond) 2008;32:1825–34. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theorell-Haglow J, Berne C, Janson C, Sahlin C, Lindberg E. Associations between short sleep duration and central obesity in women. Sleep. 2010;33:593–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Xie H, Tang SY, Luo XH, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–10. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 35.Dogru T, Sonmez A, Tasci I, et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76:24–9. doi: 10.1016/j.diabres.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, et al. Serum visfatin increases with progressive beta-cell deterioration. Diabetes. 2006;55:2871–5. doi: 10.2337/db06-0259. [DOI] [PubMed] [Google Scholar]
- 37.Trakada G, Steiropoulos P, Nena E, et al. Plasma visfatin levels in severe obstructive sleep apnea-hypopnea syndrome. Sleep Breath. 2009;10:10. doi: 10.1007/s11325-009-0254-6. [DOI] [PubMed] [Google Scholar]
- 38.Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 39.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712–9. doi: 10.1210/jc.2006-1249. [DOI] [PubMed] [Google Scholar]
- 40.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 41.Yao-Borengasser A, Varma V, Bodles AM, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–7. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makino S, Fujiwara M, Suzukawa K, et al. Visceral obesity is associated with the metabolic syndrome and elevated plasma retinol binding protein-4 level in obstructive sleep apnea syndrome. Horm Metab Res. 2009;41:221–6. doi: 10.1055/s-0028-1100411. [DOI] [PubMed] [Google Scholar]
- 43.Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–90. doi: 10.1093/sleep/32.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Cauter E, Latta F, Nedeltcheva A, et al. Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res. 2004;14(Suppl A):S10–7. doi: 10.1016/j.ghir.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Horne J. REM sleep, energy balance and ‘optimal foraging’. Neurosci Biobehav Rev. 2009;33:466–74. doi: 10.1016/j.neubiorev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Joo EY, Hong SB, Sohn YB, et al. Plasma adiponectin level and sleep structures in children with Prader-Willi syndrome. J Sleep Res. 2009;11:11. doi: 10.1111/j.1365-2869.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Forbes EE, Ryan ND, Rofey D, Hannon TS, Dahl RE. Rapid eye movement sleep in relation to overweight in children and adolescents. Arch Gen Psychiatry. 2008;65:924–32. doi: 10.1001/archpsyc.65.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res. 2009;29:393–8. doi: 10.1089/jir.2008.0080. [DOI] [PubMed] [Google Scholar]
- 50.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8:197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]