Abstract
Study Objectives:
This study investigated the 24-hour variation of macrophage migratory inhibitory factor (MIF), a cytokine which induces insensitivity to the anti-inflammatory effects of glucocorticoids, in patients with untreated obstructive sleep apnea (OSA) as compared to healthy adults with no OSA.
Participants:
Fifty-three men and women with OSA (mean apnea/hypopnea index [AHI] = 39.5) and 24 healthy adults (Non-OSA, AHI = 5.1).
Measurements:
Over a 24-h period, blood was collected every 2 h for MIF and cortisol determination. The following night, sleep was monitored with polysomnography.
Results:
MIF showed a strong 24-h variation, with a peak at 04:00 and a nadir at 22:00. Patients with OSA showed 25% higher MIF levels (area under the curve) over 24 h than healthy controls. Furthermore, MIF levels were significantly associated with AHI and total arousal index (ArI), even after adjusting for BMI. Cortisol showed the expected 24-h variation (peaking at 06:00), but no cortisol differences were observed between OSA and Non-OSA groups.
Conclusion:
MIF is elevated in patients with OSA and is related to OSA severity, while there was no difference in cortisol levels. MIF is a pro-inflammatory cytokine which additionally inhibits the anti-inflammatory effects of glucocorticoids. Thus, elevated MIF levels in OSA may contribute to elevated inflammation.
Citation:
Edwards KM; Tomfohr LM; Mills PJ; Bosch JA; Ancoli-Israel S; Loredo JS; Dimsdale J. Macrophage migratory inhibitory factor (MIF) may be a key factor in inflammation in obstructive sleep apnea. SLEEP 2011;34(2):161-163.
Keywords: Obstructive sleep apnea, MIF, cortisol, inflammation
OBSTRUCTIVE SLEEP APNEA (OSA) IS A PREVALENT SLEEP DISORDER AND IS AN INDEPENDENT RISK FACTOR FOR THE DEVELOPMENT OF CARDIOVASCULAR disease.1 Inflammation has been indicated as a potential mechanistic link and OSA has indeed been associated with elevated levels of various circulating inflammatory markers. However, there is limited consistency between studies regarding elevation of individual specific inflammatory markers and the confounding role of adiposity has led to debate concerning interpretation of these findings. In vitro studies demonstrate that hypoxia, a key characteristic of OSA, increases cellular production of the pro-inflammatory cytokine macrophage migration inhibitory factor (MIF).2 MIF is strongly associated with the hypothalamic pituitary adrenal (HPA) axis and plays a key role in regulation of the inflammatory response. Unusually among pro-inflammatory cytokines, glucocorticoids induce rather than inhibit MIF production3; yet, somewhat counter-intuitively, MIF reduces the sensitivity of cells to the anti-inflammatory actions of glucocorticoids, thereby also indirectly facilitating a pro-inflammatory response in addition to its directly pro-inflammatory actions.4 Thus, glucocorticoids stimulate the production of MIF which then acts to reduce the effects of glucocorticoids. This feedback loop may play a key part in the mechanisms of glucocorticoids' permissive functions at low levels.
Cortisol is the primary human glucocorticoid product of the HPA axis and has powerful anti-inflammatory actions when elevated. Fluctuations in cortisol are intricately related to sleep, and may be a mechanism through which sleep disorders manifest some of their physiologic changes. Despite evidence of increased inflammation in OSA, many studies have failed to find differences in cortisol release and rhythmicity between OSA subjects and normal controls.5 Methodology, (i.e., infrequent sampling) may have limited some reports, especially given that recent studies using more extensive circadian sampling, reported elevated cortisol levels in OSA patients.6
Given the role of MIF in the inflammatory process, its association with HPA axis function, and sensitivity to hypoxia, we examined whether circulating levels of MIF and cortisol were associated with sleep apnea. Both cortisol and MIF display clear circadian rhythms,7 and therefore, valid estimates of circulating levels require repeated sampling throughout the day and night. This is especially relevant in OSA, which is characterized by disrupted sleep and alterations in the circadian rhythms of several important inflammatory factors.8 The current study examined the pattern of 24-h levels of MIF and cortisol in a group of patients with diagnosed OSA and a comparison group of healthy controls (Non-OSA). We hypothesized that MIF and cortisol would be elevated in OSA patients above levels found in Non-OSA patients.
METHODS
Participants
As part of a larger study examining the pathophysiology of the sympathetic nervous system in patients with OSA, participants were recruited via advertisements, word of mouth referral, and referral from local medical practices in the San Diego area. Fifty-three participants with OSA and 23 participants without OSA were included. Exclusion criteria included: history of a major medical illness (with the exception of OSA and hypertension), current psychiatric diagnoses (including alcohol or drug abuse), or receiving psychotropic medications, and blood pressure < 170/105 mmHg for systolic and diastolic. Two patients who were taking hypertensive medication were slowly tapered off their medications for 3 weeks, prior to participation. In both cases, BP remained within the inclusion range for the study, and thus the patients were retained in the sample. The project was approved by the University of California, San Diego (UCSD) Human Subjects Committee.
Procedure
Written informed consent was obtained from all subjects before participation in the study. As described previously, participants were assessed over 2 days.8 The first 24-h period included a blood sample every 2 h, and standard polysomnography (PSG) was recorded on the night of the second day, from 20:00 to 07:00, with lights out at 22:00. Experienced polysomnographic technicians scored PSG sleep records according to the criteria of Rechtschaffen and Kales.9 Apneas, hypopneas, arousals, and transient oxyhemoglobin desaturations followed standard definitions as previously reported. The oxygen desaturation index (ODI) was calculated as the number of transient oxygen desaturations per hour of sleep. Total sleep time (TST) was computed and the numbers of apneas and hypopneas per hour of sleep were calculated to obtain the apnea hypopnea index (AHI). A diagnosis of OSA was given if AHI ≥ 10/h and Non-OSA diagnosis was given if AHI < 9/h.
Statistical Analysis
Analyses were completed using the statistical package SPSS (version 17.0). A P value ≤ 0.05 was considered significant, and all testing was 2-tailed. Group differences were examined using Student t-tests for continuous outcome variables, and the χ2 test for multi-categorical measures. Differences between groups over time were assessed using 2 Group × 12 Time repeated-measures analysis of variance (ANOVA). Hemolyzed samples generate spuriously high MIF values due to presence of MIF in erythrocytes10; therefore hemolyzed samples were excluded, and the missing data point was imputed as the mean of the 2 surrounding time points. Less than 8% of samples were imputed in this manner. If > 2 data points were missing, no imputed value was calculated and the participant was removed from the analysis. Area under curve (AUC) was calculated using the trapezoid rule to create a summary value for 24 h MIF and cortisol levels. Partial correlations were employed to explore the association between sleep indices and MIF and cortisol AUC, with BMI included as a covariate to control for significant group differences.
RESULTS
Sample Characteristics
Overall, the mean age for all subjects was 49 years (SD = 8.6), and the mean BMI was 29.2 kg/m2 (SD = 5.0). The OSA group had a significantly greater BMI (30.0 ± 5.2 vs 27.6 ± 4.0; t74 = −2.18, P = 0.05). There were no significant differences between groups in terms of gender and age (P's > 0.05). Subjects with OSA had higher AHI (39.5 ± 27.8 vs 5.1 ± 2.8), ODI (26.9 ± 3.3 vs 4.4 ± 0.6), and ArI (28.1 ± 6.4 vs 9.6 ± 0.6), and significantly shorter TST (368.8 ± 44.0 vs 403.8 ± 36.7) than those without OSA (P's ≤ 0.001). Percentage of time slept in slow wave sleep (SWS) did not vary significantly between groups (P > 0.05).
Group Differences in MIF
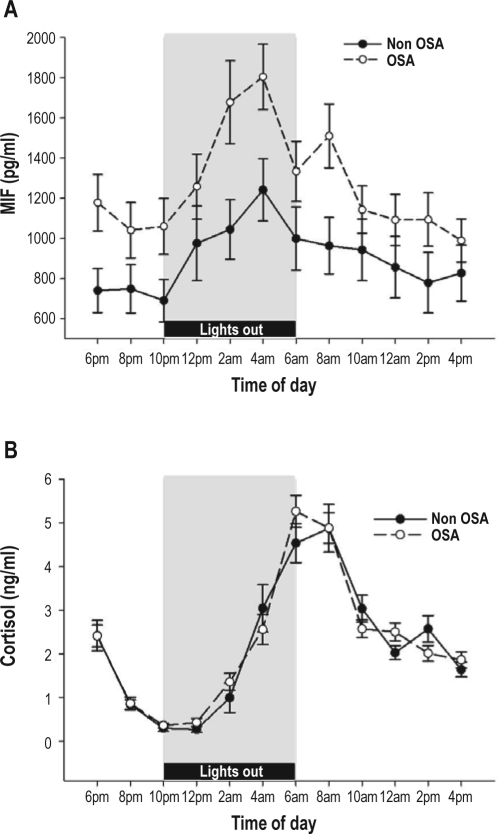
Repeated measures ANOVA revealed a significant effect of time. MIF exhibited a strong day to night variation (Time effect [F11,62 = 3.51, P = 0.001, η2 = 0.384]), with the peak found at 04:00 (see Figure 1a). A significant group difference was also found (F1,72 = 4.32, P = 0.041, η2 = 0.057), with higher concentrations of MIF in the OSA group than in the Non-OSA group (Figure 1a). There was no time by group interaction, indicating that plasma MIF followed the same circadian pattern in OSA and Non-OSA groups. Area under curve (AUC) values were calculated for the full 24-h period and for nighttime (22:00–06:00: period of lights out) and daytime hours (08:00–20:00) to evaluate if differences were confined to nighttime, as has been reported for other inflammatory markers.8 The OSA group had significantly greater MIF 24-h AUC (t71 = −2.32, P = 0.024), at nighttime AUC (t71 = −2.05, P = 0.044), and at daytime AUC (t71 = −2.32, P = 0.025).
Figure 1.
Mean (SE) plasma MIF (A) and cortisol (B) levels in OSA and Non-OSA individuals. Measures were taken every 2 h over a 24-h period starting at 18:00.
Group Differences in Cortisol
The expected circadian effect was seen in plasma cortisol levels (see Fig 1b), with a significant Time effect (F11,60 = 58.0, P = 0.001, η2 = 0.914). However, we did not find any relationship between OSA status and cortisol (P = 0.71), and no interaction was seen between Time and OSA status.
OSA Severity, MIF, and Cortisol
Given the difference in MIF levels in OSA and Non-OSA groups, we examined the relationship between the area under the curve (AUC) for 24-h MIF levels and sleep indices. All correlations were performed covarying for BMI. MIF AUC was significantly positively correlated with AHI (r = 0.351, P < 0.01) and ArI (r = 0.272, P = 0.036), while associations with ODI (r = 0.222, P = 0.088) and % SWS (r = −0.234, P = 0.072) fell short of statistical significance.
We also assessed if cortisol AUC was associated with sleep variables, and found a significant positive association with ODI (r = 0.363, P = 0.004), and a negative association with TST (r = −0.298, P = 0.021) and % SWS (−0.332, P = 0.010).
Relationship between MIF and Cortisol
MIF peaked at 04:00, while cortisol peaked at 06:00. The peak values of MIF and cortisol were significantly associated (r = 0.275, P = 0.023).
DISCUSSION
To our knowledge, this is the first study to examine MIF levels in OSA. The current data showed an approximately 25% higher MIF concentration in patients with untreated OSA than in a control sample of healthy individuals without OSA. This elevation persisted throughout the entire 24-h period, with area under curve for both the nighttime hours (10:00–06:00) and daytime hours (08:00–20:00), showing significantly higher MIF levels in the OSA group. Further, there was a linear association between the severity of OSA (as assessed by AHI) and MIF concentration over the 24-h period, even after adjusting for BMI.
Contrary to our hypothesis, we did not find significant differences in cortisol level or pattern of secretion over 24 h in OSA (versus Non-OSA controls). However, the 24-h aggregate value (area under curve) of plasma cortisol was positively correlated with ODI, and negatively with TST and percent of time in SWS, indicating that increasingly severe sleep disruption is related to higher cortisol levels throughout the 24-h period. This is in line with much of the literature which has examined HPA function in OSA.5,6 A potential limitation of the study was our assessment of plasma cortisol, which reflects total cortisol, whereas only the unbound portion of cortisol, approximately 10%, is biologically active. It cannot be excluded that significant differences in free cortisol may exist between OSA and Non-OSA patients while total cortisol levels appear equivalent and future studies may therefore additionally assess glucocorticoid binding proteins such as albumin and cortisol-binding globulin (CBG).
The relationship between MIF and cortisol is reflected in the current data by the correlation between peak values of MIF and cortisol and their similar associations with markers of OSA. Future work should examine whether successful treatment of OSA has the potential to normalize elevated levels of circulating MIF. Given the pro-inflammatory role of MIF as an inhibitor of glucocorticoid effects, it is possible that the greater up-regulation of MIF seen in OSA is important in the development of inflammatory processes that play an important role in the progression of cardiovascular diseases. However, additional data including broader examination of inflammatory makers and vascular assessments are needed to examine this potential pathway.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ancoli-Israel has consulted for and/or been on the advisory board of Ferring Pharmaceuticals, GlaxoSmithKline, Merck, NeuroVigil, Neurocrine, Pfizer, Respironics, Sanofi-Aventis, Sepracor, and Schering-Plough. She has received research support from Sepracor and Litebook. Dr. Dimsdale has received research support from Sepracor. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants HL073355, HL44915, HL36005, AG08415 and CA23100.
REFERENCES
- 1.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 2.Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signalling role of white adipose tissue. Arch Physiol Biochem. 2008;114:267–276. doi: 10.1080/13813450802306602. [DOI] [PubMed] [Google Scholar]
- 3.Calandra T, Bernhagen J, Metz CN, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 4.Bucala R. MIF re-discovered: pituitary hormone and glucocorticoid-induced regulator of cytokine production. Cytokine Growth Factor Rev. 1996;7:19–24. doi: 10.1016/1359-6101(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 5.Schmoller A, Eberhardt F, Jauch-Chara K, et al. Continuous positive airway pressure therapy decreases evening cortisol concentrations in patients with severe obstructive sleep apnea. Metabolism. 2009;58:848–853. doi: 10.1016/j.metabol.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Pejovic S, Zoumakis E, et al. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2007;92:4199–207. doi: 10.1210/jc.2007-0774. [DOI] [PubMed] [Google Scholar]
- 7.Petrovsky N, Socha L, Silva D, et al. Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol. 2003;81:137–43. doi: 10.1046/j.0818-9641.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 8.Mills PJ, Natarajan L, von Kanel R, Ancoli-Israel S, Dimsdale JE. Diurnal variability of C-reactive protein in obstructive sleep apnea. Sleep Breath. 2009;13:415–20. doi: 10.1007/s11325-009-0268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Los Angeles: Brain Information Service/Brain Research Institute, University of California Los Angeles; 1968. [Google Scholar]
- 10.Mizue Y, Nishihira J, Miyazaki T, et al. Quantitation of macrophage migration inhibitory factor (MIF) using the one-step sandwich enzyme immunosorbent assay: elevated serum MIF concentrations in patients with autoimmune diseases and identification of MIF in erythrocytes. Int J Mol Med. 2000;5:397–403. doi: 10.3892/ijmm.5.4.397. [DOI] [PubMed] [Google Scholar]