Abstract
Study Objectives:
To investigate the within-subject stability in the sleep EEG and the association between the sleep EEG and intellectual abilities in 9- to 12-year-old children.
Design:
Intellectual ability (WISC-IV, full scale, fluid, and verbal IQ, working memory, speed of processing) were examined and all-night polysomnography was performed (2 nights per subject).
Setting:
Sleep laboratory.
Participants:
Fourteen healthy children (mean age 10.5 ± 1.0 years; 6 girls).
Measurements and Results:
Spectral analysis was performed on artifact-free NREM sleep epochs (C3/A2). To determine intra-individual stability and inter-individual variability of the sleep EEG, power spectra were used as feature vectors for the estimation of Euclidean distances, and intraclass correlation coefficients (ICC) were calculated for the 2 nights. Sleep spindle peaks were identified for each individual and individual sigma band power was determined. Trait-like aspects of the sleep EEG were observed for sleep stage variables and spectral power. Within-subject distances were smaller than between-subject distances and ICC values ranged from 0.72 to 0.96. Correlations between spectral power in individual frequency bins and intelligence scores revealed clusters of positive associations in the alpha, sigma, and beta range for full scale IQ, fluid IQ, and working memory. Similar to adults, sigma power correlated with full scale (r = 0.67) and fluid IQ (r = 0.65), but not with verbal IQ. Spindle peak frequency was negatively related to full scale IQ (r = −0.56).
Conclusions:
The sleep EEG during childhood shows high within-subject stability and may be a marker for intellectual ability.
Citation:
Geiger A; Huber R; Kurth S; Ringli M; Jenni OG; Achermann P. The sleep EEG as a marker of intellectual ability in school age children. SLEEP 2011;34(2):181-189.
Keywords: Sleep, trait, intelligence, development, childhood, sleep spindle, power density spectra, intraclass correlation coefficient
A TRAIT REPRESENTS A BEHAVIORAL OR BIOLOGICAL DISPOSITION WHICH IS EMPIRICALLY OR STATISTICALLY ESTABLISHED,1 not specific to certain situations or tasks, and fairly stable over time.2 For instance, intelligence defined as the ability to reason, learn, and solve problems is considered a trait, irrespective of the specific situation and characterized by a long-term intra-individual stability.3 Hertzog and Schaie4 reported stability coefficients for general intellectual ability over 7 years and found that their composite score ranged from 0.89 to 0.96 in populations aged between 25 and 67 years. Furthermore, based on studies in mono- and dizygotic twins, large genetic contributions on intelligence,5 with heritability estimates about 40% to 50% during childhood and about 80% in adulthood6 were reported. Several studies have explored the relationship between intelligence and physiological parameters. For example, Thatcher et al.7 reported correlation coefficients of 0.60 between intelligence quotient (IQ) scores and a combination of parameters derived from the waking EEG, such as amplitude asymmetry, coherence, relative and absolute power, power ratios, and phase delay between a combinations of electrodes within certain regions.7 Overall, the concept of intelligence fits the general definition of a trait from both a psychological and biological perspective.
Human sleep also qualifies for a trait, with characteristic individual sleep durations and chronotypes.8,9 It is likely that certain aspects of sleep architecture and regulation are also under genetic control.10–12 Polysomnographic studies indicated that a significant proportion of the variance in stage 2, stage 4, slow wave sleep, and the density of rapid eye movements in REM sleep are in part genetically determined.13,14 Spectral analyses of the sleep EEG in twins revealed that the sleep EEG is among the most heritable traits in humans.15,16 For example, heritability for the amount of NREM sleep is estimated at about 50%.17 The trait-like stability of sleep stages is surpassed by individual profiles of sleep EEG power spectra.18,19 Furthermore, the intra-individual stability of power maps largely exceeded the effects evoked by experimental manipulations such as sleep deprivation.20 For instance, spindle frequency activity (SFA or sigma power, i.e., spectral power in the 12-15 Hz range) is highly variable across, but highly stable within individuals,21,22 thus representing a trait or phenotype of a given subject. Based on recent data of sleep EEG recordings in mono- versus dizygotic twins, a heritability estimate of 96% for NREM sleep power spectra for frequencies between 8 and 16 Hz was found.16 The spectral composition of NREM sleep EEG was suggested to be suitable for defining endophenotypes.15 Such insights from studies in healthy human subjects and patients with sleep disorders are consistent with investigations in inbred mice, where differences in sleep duration and structure, as well as in the spectral composition of the sleep EEG, showed high estimates of heritability.23,24 In summary, the human sleep EEG has consistently been described as a trait-like “fingerprint” characteristic, probably reflecting traits of the underlying brain anatomy.20 However, trait-like characteristics of the sleep EEG have not been examined in children.
Despite the general acceptance of trait definitions for both sleep and intelligence, surprisingly little research is documented about the potential relationship between the two phenomena. Early studies from the 1930s examined the link between sleep and intelligence and found a negative correlation between sleep duration and children's intellectual ability (reviewed in Geiger et al.25). In fact, our group recently replicated earlier findings and also reported a negative association between sleep duration and intelligence scores in healthy children.25 The few studies in adults that have attempted to link physiological measures of sleep and scores on intelligence tests focused exclusively on sleep spindles or SFA in association with intelligence scores. There is, however, no study that investigates the relationship between spectral power during sleep and intelligence scores in healthy children. In adults, an increase in SFA was reported in highly gifted subjects compared to controls26 as well as positive correlations between intelligence scores and the total number of sleep spindles.27 Moreover, Bodizs and colleagues28 found moderately positive correlations between intelligence scores and the percentage of stage 2 sleep.
From developmental research, it is known that sleep spindle number, density, duration, intra-spindle frequency, and local distribution change with age.21,29,30 However, the precise role and function of sleep spindles in cognitive development is not well understood. Furthermore, it is not known whether the relationship between sleep spindles and intellectual ability shown in adults is present in children. Although there are earlier studies about the relationship between SFA and cognitive ability in developmentally delayed children,31,32 only a single nonclinical study examined the relationship between sleep spindles and intellectual performance during childhood. In this study, Busby and colleagues reported significantly more stage 2 sleep in school age children with higher intelligence scores than those with scores in the normal range.33 The study population, however, only included male subjects grouped into a superior and an average IQ group by median split. This approach ignores the continuous distribution of IQ scores in the population, which limits generalizability of their results. Furthermore, the authors did not report spectral data.
The aim of the present study was to investigate the reliability of inter-individual differences and the intra-individual stability in the sleep EEG (trait-like aspects) and to identify and characterize a potential relationship between the sleep EEG and intellectual ability in healthy children.
METHODS
Participants
Fourteen right-handed healthy children between 9.1 to 12.5 years of age (8 male, 6 female, mean age 10.5 years) participated in this study. Our aim was to recruit a study population with a large variability in intellectual ability. Eight children were recruited from primary schools in the greater Zurich area and 6 from a special school program for gifted children. Inclusion in such a program is not based on IQ testing, but simply based on teacher's recommendation. Exclusion criteria were chronic diseases, neurological or psychiatric diagnoses (e.g., attention deficit hyperactivity disorder), sleep disorders, or taking medication. Socioeconomic status (SES) was determined using the Largo and Pfister34 scale, which combines paternal occupation and maternal education resulting in scores ranging from 2 to 12. In 6 children an upper SES (10-12 points) was observed, while a middle SES was found in 8 individuals (6-9 points). Pubertal development status was assessed with a translated rating scale for pubertal development35 filled in by the parents. According to the questionnaire, which is related to Tanner staging, 12 children were prepubertal, and 2 children were mid-pubertal.
Cognitive and Attentional Variables
The children were assessed with the WISC-IV (German version),36 yielding separate indices for fluid intelligence, verbal intelligence, speed of processing, and working memory, as well as a full scale intelligence quotient (IQ) score. By convention WISC-IV scores are standardized within each age group, such that the mean is always 100 and the standard deviation (SD) is 15 (referred to as age-standardized). A children's test battery for the assessment of attention (KITAP)37 was also administered to examine different aspects of attention, including alertness and go/no-go. The alertness subtest of the KITAP provided median and SD of reaction times based on age-referenced normative data. A combined score reflecting mean reaction time and SD was calculated and referred to as alertness, with an age-standardized population mean of 50 and a SD of 10. The go/no-go subtest of KITAP also provided median reaction time with an age-standardized population mean of 50 and a SD of 10.
To rule out learning effects, only children who had not performed any kind of intelligence testing during at least the last 2 years prior to participation in the study were included.
Procedure
The study was approved by the institutional review board of the University Children's Hospital and the Canton of Zurich and was performed according to the Declaration of Helsinki. All families received a detailed study description and provided written informed consent. After information about purpose and procedure of the study, the WISC-IV (German version) intelligence test36 was administered by a trained psychologist (AG). The participants then performed the 2 subtests alertness and go/no-go of the KITAP.37 One week prior to the recordings, parents were instructed to maintain children's habitual sleep-wake schedule, and to keep a sleep diary with detailed information about bed and wake-up times, caffeine consumption, and medication. Additionally, children were monitored for regular sleep-wake schedules with wrist-worn actigraphs. Daytime sleepiness was assessed by the Pediatric Daytime Sleepiness Score (PDSS), a sum score combining 8 different daytime sleepiness items on a 5-point scale (parent report).38 All children participated in 2 recording sessions, separated by 1 or 2 weeks. Prior to the sleep recordings, children were instructed to refrain from vigorous physical activity.
Sleep Recordings
All sleep recordings were performed at the sleep laboratory of the University Children's Hospital Zurich, starting between 21:00 and 22:30 (habitual bed-time of the subjects) and lasting between 8 and 10 h (524 ± 29 min).
All-night polysomnography was recorded by a portable high density EEG system (128 electrodes net, Electrical Geodesic, Inc.). The net included the electrode positions of the 10-20 system. In addition, the submental electromyogram (EMG) and the electrooculogram (EOG, 2 bipolar derivations) were recorded. EOG electrodes were placed approximately 1 cm below and above the outer canthus. Data were sampled at 500 Hz (0.01 to 200 Hz).
Data Analysis and Processing
Sleep stages were visually scored in 20-s epochs according to standard criteria.39 Analysis was restricted to derivation C3/A2. Data were band-pass filtered (0.1 to 40 Hz; FFT filter) and down-sampled to 250 Hz. EEG power density spectra were calculated for consecutive 20-s epochs (FFTW approach [Matlab, The MathWorks Inc.], Hanning window, averages of five 4-s epochs; frequency resolution 0.25 Hz) and matched with the corresponding sleep stages. Artifacts were excluded on a 4-s basis by visual inspection and semi-automatically. Epochs were excluded whenever power in the 20-40 Hz and the SWA band exceeded a threshold based on a moving average determined over twenty 20-s epochs. Average spectra of NREM sleep (N2 and N3) were calculated for the minimum common length (6.41 h) of sleep. Because of technical artefacts affecting electrode A2, the derivation of C4/A1 was used in one child on both recording nights.
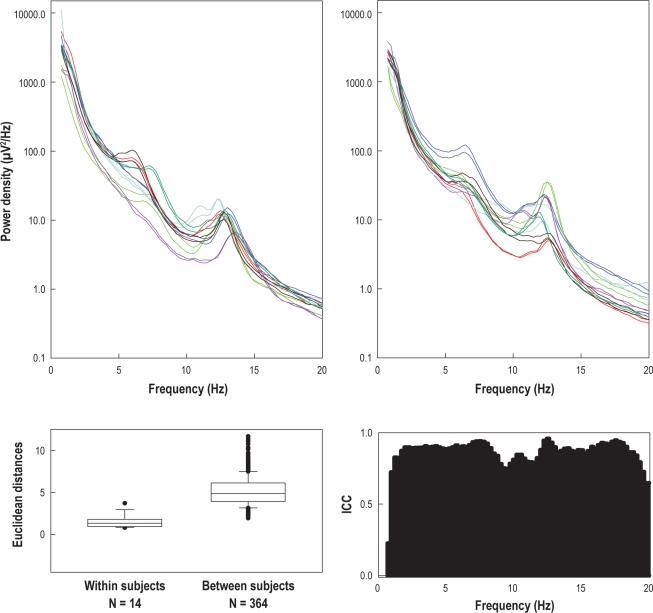
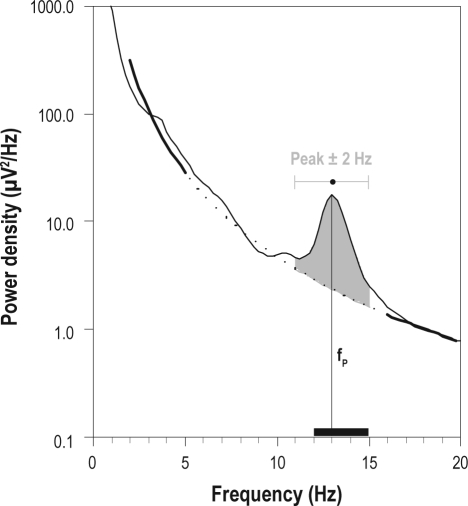
The location of the spindle peaks in the power density spectra varies considerably between individuals (Figure 2). Thus, individual spindle peaks and relative spindle power were determined in each subject based on mean all-night power spectra of stage N2 sleep. Using a manual cursor program, the center frequency of the spindle peak in the power spectrum was marked. To measure the spindle power relative to the background of the power spectrum, a power law function was fit to the spectrum in the range 2-5 Hz and 16-25 Hz, excluding the 5.25-15.75 Hz range (containing theta, alpha, and spindle peaks). If more than one peak was present, the peak with the higher frequency was selected.40 Individual relative spindle power was determined as power in the range of ± 2 Hz around the individual peak minus background power in the same frequency range (see Figure 1 for details). We use the term spindle frequency activity (SFA) or sigma power when referring to the literature where a predefined frequency range, e.g. 12-15 Hz, was used ignoring individual differences.
Figure 2.
Upper panels: Power density spectra of N2 sleep of all subjects (2 nights per subject, presented in the same color); left panel: subjects 1-7, right panel: subjects 8-14. Lower left panel: Distribution (box plot) of Euclidean distances (power density spectra used as feature vector) within (14 values) and between subjects (364 values). Boxes represent the lower quartile, median, and upper quartile. Whiskers above and below the box indicate the 90th and 10th percentiles. Outliers are indicated by •. Lower right panel: Intraclass correlation coefficients (ICC).
Figure 1.
Method used to determine the individual relative sigma power. Example of a mean all-night power spectrum of N2 sleep (single subject). A power law function (bold and dotted line) was fit to the data in the range of 2-5 and 16-25 Hz, excluding the 5.25-15.75 Hz range (containing theta, alpha, and spindle peaks, dotted line). The peak frequency (fP; i.e., average frequency of sleep spindles) was determined using a manual cursor program. Individual relative sigma power: determined as power in the range of ± 2 Hz around the peak minus background power in the same frequency range (grey area). Spindle frequency activity (SFA) or sigma power: power in the “classical” frequency range of 12-15 Hz (indicated by a black bar on the frequency axis), ignoring individual differences in position of the peak.
Statistical Analysis
To compare sleep stage variables of the 2 nights, paired Wilcoxon tests and Spearman rank correlations were calculated. To determine intra-individual stability and inter-individual variability in power density spectra, spectra were used as feature vectors and the Euclidean distance between the vectors was determined. Vectors were log-transformed before calculating distances. Within-subject distances were estimated between the 2 nights of each subject resulting in 14 distance values. Between-subject distances were determined between all possible combinations of recordings resulting in 364 values. Within- and between-subject distances were compared by Mann-Whitney rank sum test. To quantify trait-like aspects in the power density spectra, intraclass correlation coefficients (ICCs) were determined for each frequency bin. ICC values were calculated as the between-subjects variance divided by the sum of the between- and within-subject variances.41
Spearman rank correlations were calculated to investigate associations between different cognitive abilities and spectral power in each frequency bin (average spectra of the 2 nights). Single bins may reach a significant correlation by change, but would not be clustered in a band. Thus, only if ≥ 6 consecutive frequency bins (a range of 1.5 Hz) showed a significant correlation would we consider it relevant for our interpretation.
Spearman rank correlations were also calculated to investigate associations between different cognitive abilities and individual relative sigma power. To control for potential biases from age effects or time being awake before the sleep recording, 2 post hoc multiple linear regression analyses were calculated. One analysis included the predictors age, time awake before sleep recording, and full scale IQ; the second analysis included the predictors age, time awake before sleep recording, and fluid IQ. Individual relative sigma power was used as criterion variable for both multiple linear regression analyses.
RESULTS
Cognitive Variables
Scores of cognitive variables (Table 1) were above population average of the corresponding age group (P < 0.05, 1-sample t-test), but with a similar standard deviation (SD) (P > 0.05). The distributions were unimodal and did not differ from a normal distribution (P > 0.05; Shapiro-Wilks Test). One child scored as moderately gifted (score > 130). Alertness and reaction times in the go/no-go test did not correlate with any of the cognitive and sleep variables (derived from scoring and the sleep EEG).
Table 1.
Cognitive and attentional variables assessed by WISC-IV and KITAP (n = 14)
| M | SD | Range | |
|---|---|---|---|
| IQ | 116.6 | 12.8 | 93–137 |
| Fluid IQ | 112.9 | 12.9 | 91–133 |
| Verbal IQ | 117.6 | 14.7 | 93–144 |
| Speed of processing | 116.4 | 12.7 | 100–141 |
| Working memory | 116.5 | 12.0 | 84–138 |
| Alertness | 57.6 | 8.2 | 40–70 |
| Reaction time Go/No-go | 59.5 | 7.0 | 50–71 |
Population norms (age-standardized) for full scale IQ and indices fluid IQ, verbal IQ, speed of processing, and working memory are mean (M) = 100 and standard deviation (SD) = 15. Population norms (age-standardized) for alertness and go/no-go are M = 50, SD = 10.
Sleep Variables Derived from Visual Scoring
Sleep stage distribution, wake after sleep onset (W), sleep latency (SL; defined as the first occurrence of N2), REM sleep latency (RL; defined as the first occurrence of REM sleep), and sleep efficiency (SE, defined as total sleep time as % of time in bed) did not differ between the 2 nights (paired Wilcoxon test). However, the total sleep time (TST) was longer for the second night (Z = −2.4, P < 0.05, paired Wilcoxon test, Table 2). There was no effect of age or time awake before the recording on any of the sleep variables (F2,10 = 0.3–3.8, P > 0.05, multiple linear regression analysis). Daytime sleepiness assessed as sum score of the PDSS was low (7.5, SD = 4.1 [a PDSS score of 16 corresponds to the 50th percentile]).
Table 2.
Sleep variables derived from visual scoring (n = 14; 2 nights per subject)
| Night 1 |
Night 2 |
Paired Wilcoxon test | Correlations | |||
|---|---|---|---|---|---|---|
| MD | Range | MD | Range | |||
| % stage N1 sleep | 8.3 | 1.7–16.0 | 8.3 | 4.5–16.5 | n.s. | 0.75** |
| % stage N2 sleep | 40.4 | 34.3–61.0 | 46.4 | 36.2–53.6 | n.s. | 0.68** |
| % stage N3 sleep | 26.2 | 13.6–35.0 | 26.6 | 15.8–35.1 | n.s. | 0.64* |
| % stage R sleep | 20.8 | 12.6–28.8 | 19.8 | 12.9–27.8 | n.s. | 0.57* |
| % stage N sleep | 70.4 | 62.7–79.8 | 72.2 | 61.1–79.3 | n.s. | 0.76** |
| Wake after sleep onset (min) | 27.4 | 12.7–128.3 | 18.3 | 4.0–72.3 | n.s. | n.s. |
| Sleep latency (min) | 23.2 | 0–62.0 | 16.0 | 6.7–43.7 | n.s. | n.s. |
| R sleep latency (min) | 140.7 | 53.0–215.7 | 143.2 | 51.3–178.3 | n.s. | n.s. |
| Total sleep time (min) | 442.2 | 388.3–502.3 | 477.3 | 385.0–521.7 | Z = −2.04* | n.s. |
| Sleep efficiency (%) | 90.5 | 72.0–96.8 | 92.0 | 77.6–98.0 | n.s. | n.s. |
Percentages of stage N1, N2, N3, R, and N refer to total sleep time. Sleep latency is defined as the first occurrence of N2. Sleep efficiency is total sleep time as % of time in bed. Stage N sleep refers to stage N2 plus stage N3. MD, median. Paired Wilcoxon tests are based on between-subjects comparisons (night 1 vs. night 2). Correlations are calculated between the 2 nights.
P < 0.05;
P < 0.01.
To compare both nights in terms of sleep stage distribution, Spearman correlations between the 2 nights were calculated. All variables related to sleep stage distributions showed significant correlations, with the highest correlation observed for the percentage of NREM sleep. W, SL, RL, TST, and SE did not correlate between the 2 nights (Table 2).
Power Density Spectra
Total power (0.75-20 Hz) of the NREM sleep EEG did not differ between the 2 nights (Night 1: 2887.89 μV2, Night 2: 2503.22 μV2; P = 0.78, paired t-test). In general, the power density spectra were characterized by a large variability between, but a small variability within subjects. Power density spectra of 2 nights of a given subject largely overlapped (Figure 2, upper panels), while considerable differences between subjects were present.
ICCs were determined for each frequency bin (Figure 2, lower right panel). Values ranged from 0.72 to 0.96, reflecting substantial to almost perfect stability of the spectra of the 2 nights.42 Furthermore, using power density spectra as feature vectors, within-subject and between-subject Euclidean distances were calculated and compared (see Methods). Within-subject distances (MD = 1.3; Figure 2, lower left panel) were smaller than between-subject distances (MD = 4.9; t = 176, P < 0.001, Mann-Whitney rank sum test), indicating trait-like features in power density spectra of the NREM sleep EEG.
Given the relatively high intra-individual stability in terms of sleep stage distribution and spectral power, mean values of 2 nights per subject were calculated and used to analyze associations between cognition and sleep.
Relationship between Cognitive Abilities and the Sleep EEG
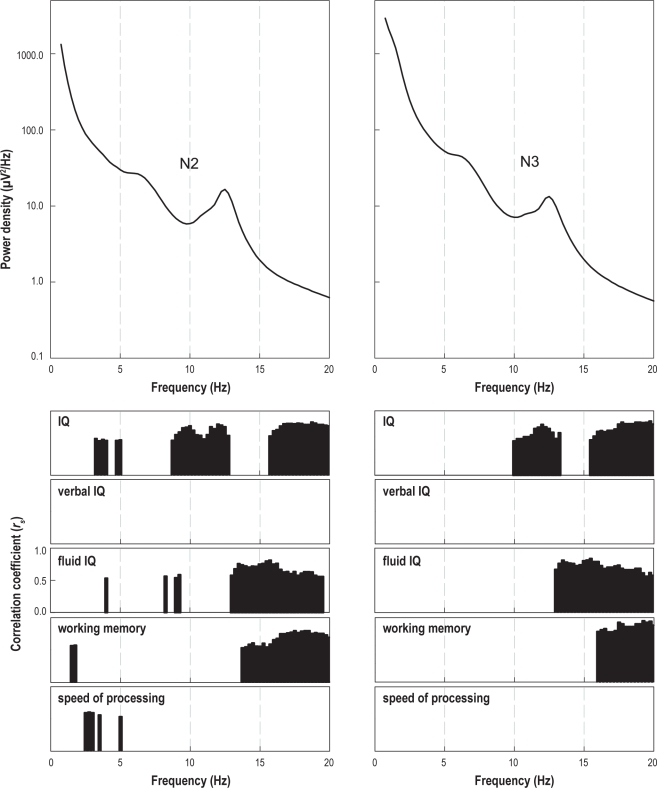
To explore the relationship between cognitive variables and sleep EEG power density spectra, Spearman rank correlations between cognitive variables and spectral power were calculated for each frequency bin (Figure 3; average spectra of the 2 nights; N2 and N3 sleep separately). Several bands of significant correlations between spectral power and cognitive scores were observed, in particular for full scale IQ (10-13.25 Hz; > 15.5 Hz), fluid IQ (> 13 Hz), and working memory (> 16 Hz). These bands were similar for N2 and N3. Verbal IQ and speed of processing did not reveal bands of significant correlations. Positive correlations in the alpha, sigma, and beta range were observed, but not in the delta and theta range. Similar bands of positive correlations were present in REM sleep for full scale IQ, fluid IQ, and working memory (data not shown).
Figure 3.
Spearman rank correlations between spectral power and cognitive variables. Upper left panel: mean power density spectrum (all nights) of N2 sleep. Upper right panel: mean power density spectrum (all nights) of N3 sleep. Lower panels: Significant correlations for different cognitive variables.
We visually identified individual sleep spindle peaks for each subject (in N2 sleep only). Sleep spindle peak frequency (M = 12.5 Hz, SD = 0.4 Hz) correlated negatively with full scale IQ (rs = −0.56, P < 0.05), that is, the higher the IQ, the lower the sleep spindle peak frequency. Peak frequency did not correlate with any other of the cognitive variables. Correlations were also calculated between individual relative sigma power (power ± 2Hz around peak relative to the background EEG) and cognitive variables. Individual relative sigma power correlated positively with full scale IQ (rs = 0.67, P < 0.01) and fluid IQ (rs = 0.65, P < 0.05), accounting for about 45% of the variability in full scale IQ scores and 42% of the variability in fluid IQ scores. Verbal IQ, working memory, and speed of processing were not related to individual relative sigma power, and the observed relationship between individual relative sigma power and intellectual abilities were neither modulated by age nor time awake before the sleep recording (full scale IQ: F3,9 = 1.8, P > 0.05; fluid IQ: F3,9 = 2.4, P > 0.05; multiple linear regression analysis).
DISCUSSION
This study demonstrated for the first time a high within-subject stability of the sleep EEG in 9- to 12-year-old children. Sleep stage distribution and spectral power was highly correlated between two nights of the same child, with a large intra-individual similarity and inter-individual variability. We found a similar characteristic phenotype of the sleep EEG as reported in the adult literature.15–20 Although the analysis was based on a small population and included only two nights per subject, trait-like aspects of the sleep EEG were consistent and corroborated earlier findings in adults. Thus, we are confident that the results reflect a general disposition and not an experimental effect evoked by the sleep laboratory environment. As mentioned earlier,15,20 features of the sleep EEG may represent the functional aspects of the underlying brain anatomy or individual characteristics of brain morphology. Based on these findings we conclude that trait-like aspects reflected by the high within-subject stability in the sleep EEG are present in children.
Apart from the trait-like aspects of children's sleep, we found a relationship between the sleep EEG and intellectual ability in healthy children, which has not been reported previously. Overall, there were positive associations between spectral power in specific frequency ranges and cognitive scores, with significant correlations clustered in the alpha, sigma, and beta range, but not for frequencies in the delta and theta range. Thus, the higher the power in specific frequency bands, the higher the performance of specific cognitive variables. In adults, it has previously been observed that sigma power is related to intelligence scores.26–28 However, the association between alpha and beta power with cognitive variables has never been reported before. Given the lack of data in adults, we cannot state whether our results are unique to children or whether the relationship between alpha or beta power and cognitive variables has simply not been considered in previous studies of adults.
Interestingly, we found a dissociation between different components of intellectual ability in their relationship with physiological correlates—the positive associations between spectral power and cognitive variables were primarily restricted to full scale IQ, fluid IQ, and working memory, but not observed for verbal IQ and speed of processing. The dissociation between full scale IQ and fluid IQ being related and verbal IQ being unrelated to spectral power of the sleep EEG has been observed before, and thus, our results extend and corroborate earlier findings in adults.27
Fluid IQ, the non-verbal dimension of intellectual capacity (e.g., the ability to solve new problems independent of acquired knowledge) was originally defined as “the influence of biological factors on intelligence.”43 Based on this concept, fluid IQ may be understood as a component of intellectual ability that is closely related to neuronal processing; in other words, fluid IQ may reflect the “hardware” of human intelligence. Working memory has been defined as “the ability to hold in mind information in the face of potentially interfering distraction…”,44 which is a subdomain of executive functions. Genetic influences on executive functions have been estimated to reach approximately 50%.45 Moreover, it has been reported that working memory and fluid IQ partially rely on a common process, namely attentional control.46,47 Therefore these processes share a certain amount of variability, with correlation coefficients ranging from 0.36 to 0.7.46,48 Imaging data are pointing towards the same direction: a considerably high overlap of neural circuitries involved in both working memory and fluid IQ, with networks primarily located in lateral prefrontal and the parietal cortex.46,49 In this sense, physiological correlates for those components of intellectual capacity that are more nature as opposed to nurture driven seems plausible.
Despite the large body of evidence on the relationship between waking EEG parameters and cognitive ability,7,50,51 surprisingly little research has been performed to elucidate the association between cognitive ability and the sleeping brain. Until now, the focus has been on the relationship between cognitive ability and SFA with studies reporting positive associations26–28—the higher the SFA, the higher the cognitive ability. This relationship has however, not been shown for children. Our data, which corroborate these earlier findings in adults, allow for extension—the relationship between cognitive ability and SFA is already present in childhood. Studies in the context of sleep and learning also highlighted the importance of sleep spindles or SFA at the state level.52–55
Sleep spindle oscillations have been speculated to play a dominant role in gating plastic changes during sleep and may represent a candidate physiological mechanism for memory consolidation.56 Given this often cited and appealing hypothesis for the functional role of sleep spindles, it is not surprising that the main focus of research for a physiological correlate of intelligence during sleep has been on sleep spindles or SFA. Our data show, however, a more nuanced picture: We found a negative association between spindle peak frequency (i.e., average frequency of spindles) and full scale IQ. Thus, the lower the sleep spindle peak frequency in the power spectra, the higher the full scale IQ scores. Earlier studies have not reported associations between spindle peak frequency and cognitive ability. The spindle peak is subjected to substantial developmental changes, and may therefore be used as a marker of brain maturation. It has been reported that the peak frequency of the sleep spindles increases with age. For example, older children show higher spindle peak frequencies.30,57 If we assume that those children with higher IQ scores are developmentally advanced, our findings seem contradictory at a first glance, because we would expect a positive relationship between spindle peak and IQ scores. However, data on the age-related increase of the spindle peak frequency are based on cross-sectional studies, and must therefore be interpreted with caution. Our data resemble a snap shot in a specific age group. Moreover, it has been reported that adolescent girls may even have faster spindles than adults.58 Finally, it is not clear how chronological age, brain maturation, and cognitive development co-evolve and interact. Our data are, however, in accordance with previous studies in adults.26–28 We found a positive relationship between full scale IQ scores, fluid IQ scores, and individual relative sigma power.
To date, there is only one other study on the relationship between the sleep and intelligence in children, but the reported effects were on IQ scores related to the percentage of stage 2 sleep rather than a direct measure of EEG activity.33 From a clinical point of view, several sleep EEG variables have been proposed to reflect the integrity of the central nervous system. Specifically, it has consistently been reported that alterations in SFA, number of sleep spindles, or percentage stage 2, are affected by pathological changes of the nervous system (e.g., in neurodegenerative disorders such as in Alzheimer and Creutzfeldt Jakob disease59), by ischemic events,40 and in psychiatric diseases such as schizophrenia.60 In addition, children with mental retardation showed either large amounts of SFA or virtually none.61 In light of the previously reported positive association between intelligence and SFA in healthy subjects, an increased SFA in children with mental retardation31,32 appears counterintuitive on the first glance. However, Fogel et al.62 have postulated that the relationship between intelligence and SFA may be U-shaped or curvilinear. Thus, our data would indeed conform with the predictions of this hypothesis—a positive association between IQ scores and SFA in a population with average to superior intellectual ability.
One limitation of the present study was the relatively small sample whose IQ scores were higher than average. Thus, it may be argued that the reported associations between variables of the sleep EEG and intellectual ability that we found may be a property of this specific population. However, because IQ scores covered a reasonably broad range and our standard deviation was similar to that observed in the population (Table 1), we believe that we captured a representative sample (68% of individuals). Furthermore, children were healthy and not sleep deprived (as revealed by screening interviews and validated questionnaires). Thus, we are confident that our results are generalizable up to certain degree. However, the findings are only of a correlational nature and thus have to be interpreted with caution. Finally, the sample size was too small to examine age or gender effects. Moreover, daytime activities such as sports or music were neither controlled nor systematically manipulated. Thus, we cannot rule out the possibility that state-dependent sources of variance may have contributed to inter- and intra-individual differences in the sleep EEG. Nevertheless, stable night-to-night power density spectra were observed within subjects (Figure 2). Future studies are needed to replicate the reported associations and to further differentiate between state- and trait-related sources of variance. For example, longitudinal investigation of a larger group of children with a broad age range in which sleep is systematically manipulated would help elucidate this issue. It also may be worthwhile to follow the approach of Schabus26 and differentiate between trait-related hardwired individual differences in intelligence as opposed to learning-induced short-term changes in their association with SFA.
In sum, we demonstrate that high within-subject stability in the sleep EEG (trait-like aspects) is already present in childhood. Moreover, we found that inter-individual differences of the sleep EEG were related to intellectual ability—full scale IQ, fluid IQ, and working memory. The sleep spindle frequency peak as well as sigma power was particularly associated to full scale and fluid IQ. Many empirical observations have related differences in IQ scores to variations in brain structure and function.63 The neuronal network properties underlying these intellectual differences are supposedly hardwired and thus should be identical regardless of the actual state—be it for the waking or the sleeping brain. Whereas studies performed during wakefulness may be biased by many external influences such as attention, motivation or temporal fluctuations in current mood, studies performed on the sleeping brain may be less affected by these potential sources of interference. Eventually, it may be speculated that the human sleep EEG and intellectual ability are epiphenomena of the same underlying processes, representing two facets of the same individual trait.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Prof. Valentin Rousson and Dr. Luciano Molinari for statistical advice, Thomas Rusterholz for programming the ICC routine, Dr. Roland Dürr for providing the FFT filter, Dr. Leila Tarokh for comments on the manuscript and all children and their families for their participation.
This work was supported by a research grant from the University Research Priority Program (URPP Integrative Human Physiology, to Reto Huber, Oskar G. Jenni and Peter Achermann) of the University of Zurich, the Swiss National Science Foundation (PP00A3-114923, to RH; 320030-130766, to Peter Achermann) and the Schüller Foundation (to OGJ).
Footnotes
See commentary: Tucker AM. The prospects for enhancing sleep across the lifespan. SLEEP 2011;34:135-136.
REFERENCES
- 1.Allport GW. What is a trait of personality. J Abnormal Soc Psychol. 1931;25:368–72. [Google Scholar]
- 2.Chen G, Gully SM, Whiteman JA, Kilcullen RN. Examination of relationships among trait-like individual differences, state-like individual differences, and learning performance. J Appl Psychol. 2000;85:835–47. doi: 10.1037/0021-9010.85.6.835. [DOI] [PubMed] [Google Scholar]
- 3.Conley JJ. The hierarchy of consistency: A review and model of longitudinal findings on adult individual differences in intelligence, personality and self-opinion. Pers Individ Dif. 1984;5:11–25. [Google Scholar]
- 4.Hertzog C, Schaie KW. Stability and change in adult intelligence: 1. Analysis of longitudinal covariance structures. Psychol Aging. 1986;1:159–71. doi: 10.1037//0882-7974.1.2.159. [DOI] [PubMed] [Google Scholar]
- 5.Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Hum Genet. 2009;126:215–32. doi: 10.1007/s00439-009-0655-4. [DOI] [PubMed] [Google Scholar]
- 6.Baltes PB, Staudinger UM, Lindenberger U. Lifespan psychology: theory and application to intellectual functioning. Annu Rev Psychol. 1999;50:471–507. doi: 10.1146/annurev.psych.50.1.471. [DOI] [PubMed] [Google Scholar]
- 7.Thatcher RW, North D, Biver C. EEG and intelligence: relations between EEG coherence, EEG phase delay and power. Clin Neurophysiol. 2005;116:2129–41. doi: 10.1016/j.clinph.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 9.Tucker AM, Dinges DF, Van Dongen HPA. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 10.Andretic R, Franken P, Tafti M. Genetics of sleep. Annu Rev Genet. 2008;42:361–88. doi: 10.1146/annurev.genet.42.110807.091541. [DOI] [PubMed] [Google Scholar]
- 11.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10:549–60. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tafti M, Maret S, Dauvilliers Y. Genes for normal sleep and sleep disorders. Ann Med. 2005;37:580–9. doi: 10.1080/07853890500372047. [DOI] [PubMed] [Google Scholar]
- 13.Linkowski P, Kerkhofs M, Hauspie R, Susanne C, Mendlewicz J. EEG sleep patterns in man: a twin study. Electroencephalogr Clin Neurophysiol. 1989;73:279–84. doi: 10.1016/0013-4694(89)90106-5. [DOI] [PubMed] [Google Scholar]
- 14.Merica H, Gaillard JM. Statistical description and evaluation of the interrelationships of standard sleep variables for normal subjects. Sleep. 1985;8:261–73. doi: 10.1093/sleep/8.3.261. [DOI] [PubMed] [Google Scholar]
- 15.Ambrosius U, Lietzenmaier S, Wehrle R, et al. Heritability of sleep encephalogram. Biol Psychol. 2008;64:344–8. doi: 10.1016/j.biopsych.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.De Gennaro L, Marzano C, Fratello F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–60. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 17.Linkowski P. EEG sleep patterns in twins. J Sleep Res. 1999;8(Suppl 1):11–3. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 18.Buckelmüller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–6. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 19.De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M. An electroencephalographic fingerprint of human sleep. Neuroimage. 2005;26:114–122. doi: 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Finelli LA, Achermann P, Borbély AA. Individual ‘fingerprints’ in human sleep EEG topography. Neuropsychopharmacology. 2001;25:S57–62. doi: 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 21.Scholle S, Zwacka G, Scholle HC. Sleep spindle evolution from infancy to adolescence. Clin Neurophysiol. 2007;118:1525–31. doi: 10.1016/j.clinph.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Werth E, Achermann P, Dijk DJ, Borbély AA. Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalogr Clin Neurophysio. 1997;103:535–42. doi: 10.1016/s0013-4694(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 23.Dauvilliers Y, Maret S, Tafti M. Genetics of normal and pathological sleep in humans. Sleep Med Rev. 2005;9:91–100. doi: 10.1016/j.smrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Franken P, Tafti M. Genetics of sleep and sleep disorders. Front Biosci. 2003;8:e381–97. doi: 10.2741/1084. [DOI] [PubMed] [Google Scholar]
- 25.Geiger A, Achermann P, Jenni OG. Association between sleep duration and intelligence scores in healthy children. Dev Psychol. 2010;46:949–54. doi: 10.1037/a0019679. [DOI] [PubMed] [Google Scholar]
- 26.Schabus M, Hoedlmoser K, Pecherstorfer T, et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–35. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behav Neurosci. 2007;121:1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Bodizs R, Kis T, Lazar AS, et al. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res. 2005;14:285–92. doi: 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 29.Nicolas A, Petit D, Rompre S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol. 2001;112:521–7. doi: 10.1016/s1388-2457(00)00556-3. [DOI] [PubMed] [Google Scholar]
- 30.Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence: a preliminary study. Sleep. 2010;33:801–9. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bixler EO, Rhodes JM. Spindle activity during sleep in cultural-familial mild retardates. Psychophysiology. 1968;5:212. [Google Scholar]
- 32.Gibbs EL, Gibbs FA. Extreme spindles: correlation of electroencephalographic sleep pattern with mental retardation. Science. 1962;138:1106–7. doi: 10.1126/science.138.3545.1106. [DOI] [PubMed] [Google Scholar]
- 33.Busby K, Pivik RT. Sleep patterns in children of superior intelligence. J Child Psychol Psychiatry. 1983;24:587–600. doi: 10.1111/j.1469-7610.1983.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 34.Largo RH, Pfister D, Molinari L, Kundu S, Lipp A, Duc G. Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev Med Child Neurol. 1989;31:440–56. doi: 10.1111/j.1469-8749.1989.tb04022.x. [DOI] [PubMed] [Google Scholar]
- 35.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–5. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 36.Petermann F, Petermann U, editors. Hamburg-Wechsler Intelligenztest für Kinder. Bern: Huber; 2007. [Google Scholar]
- 37.Zimmermann P, Fimm B, editors. Testbatterie zur Aufmerksamkeitsprüfung für Kinder (KITAP) Freiburg: Psytest; 1993. [Google Scholar]
- 38.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26:455–8. [PubMed] [Google Scholar]
- 39.Iber C, Ancoli-Israel S, Chesson AL, Quan S. AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 40.Gottselig JM, Bassetti CL, Achermann P. Power and coherence of sleep spindle frequency activity following hemispheric stroke. Brain. 2002;125:373–83. doi: 10.1093/brain/awf021. [DOI] [PubMed] [Google Scholar]
- 41.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 42.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 43.Horn JL, Cattell RB. Refinement and test of the theory of fluid and crystallized general intelligences. J Educ Psychol. 1966;57:253–70. doi: 10.1037/h0023816. [DOI] [PubMed] [Google Scholar]
- 44.Jarrold C, Towse JN. Individual differences in working memory. Neuroscience. 2006;139:39–50. doi: 10.1016/j.neuroscience.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Rose RJ. Genes and human behavior. Annu Rev Psychol. 1995;46:625–54. doi: 10.1146/annurev.ps.46.020195.003205. [DOI] [PubMed] [Google Scholar]
- 46.Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–22. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 47.Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity. Intelligence. 1990;14:389–433. [Google Scholar]
- 48.Süss HM, Oberauer K, Wittmann WW, Wilhelm O, Schulze R. Working-memory capacity explains reasoning ability - and a little bit more. Intelligence. 2002;30:261–88. [Google Scholar]
- 49.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychol Bull Rev. 2002;9:637–71. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 50.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 51.Neubauer AC, Fink A, Grabner RH. Sensitivity of alpha band ERD to individual differences in cognition. Prog Brain Res. 2006;159:167–78. doi: 10.1016/S0079-6123(06)59011-9. [DOI] [PubMed] [Google Scholar]
- 52.Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13-15 Hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–11. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–35. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt C, Peigneux P, Muto V, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–82. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–23. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 57.Shinomiya S, Nagata K, Takahashi K, Masumura T. Development of sleep spindles in young children and adolescents. Clin Electroencephalogr. 1999;30:39–43. doi: 10.1177/155005949903000203. [DOI] [PubMed] [Google Scholar]
- 58.Nader RS, Smith CT, Muir D, Scharfe E. The existence of ‘super-fast’ spindles in adolescent girls. Sleep. 2003;26:A73. [Google Scholar]
- 59.Petit D, Gagnon JF, Fantini ML, Ferini-Strambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56:487–96. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 61.Shibagaki M, Kiyono S, Watanabe K. Spindle evolution in normal and mentally retarded children: a review. Sleep. 1982;5:47–57. doi: 10.1093/sleep/5.1.47. [DOI] [PubMed] [Google Scholar]
- 62.Fogel SM, Smith CT, Beninger RJ. Too much of a good thing? Elevated baseline sleep spindles predict poor avoidance performance in rats. Brain Res. 2010;1319:112–7. doi: 10.1016/j.brainres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 63.Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–54. doi: 10.1017/S0140525X07001185. discussion 154-87. [DOI] [PubMed] [Google Scholar]