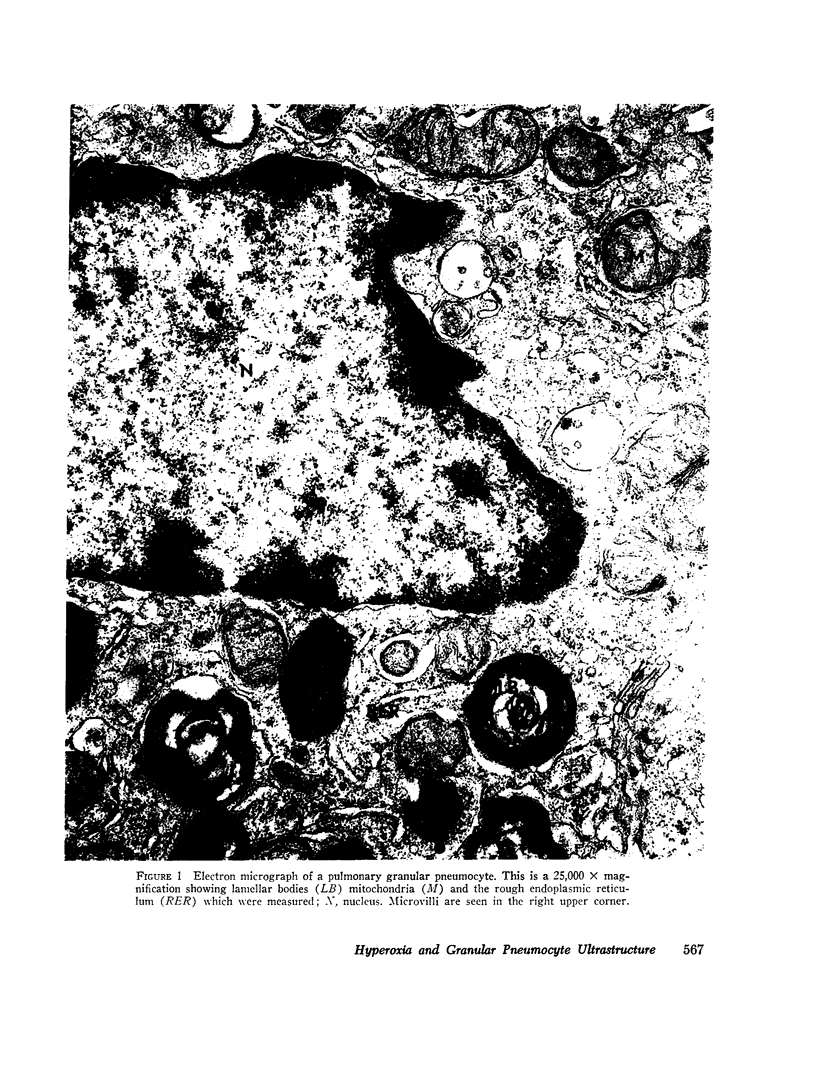
Abstract
We used the technique of lineal analysis to study the influence of 48 h of hyperoxia on cytoplasmic organelles of pulmonary granular pneumocytes with particular reference to their lamellar bodies. We undertook this study because lamellar bodies are considered to be storage granules for pulmonary surfactant and because we had found that hyperoxia decreased [14C]leucine incorporation into protein of a surface-active lung fraction.
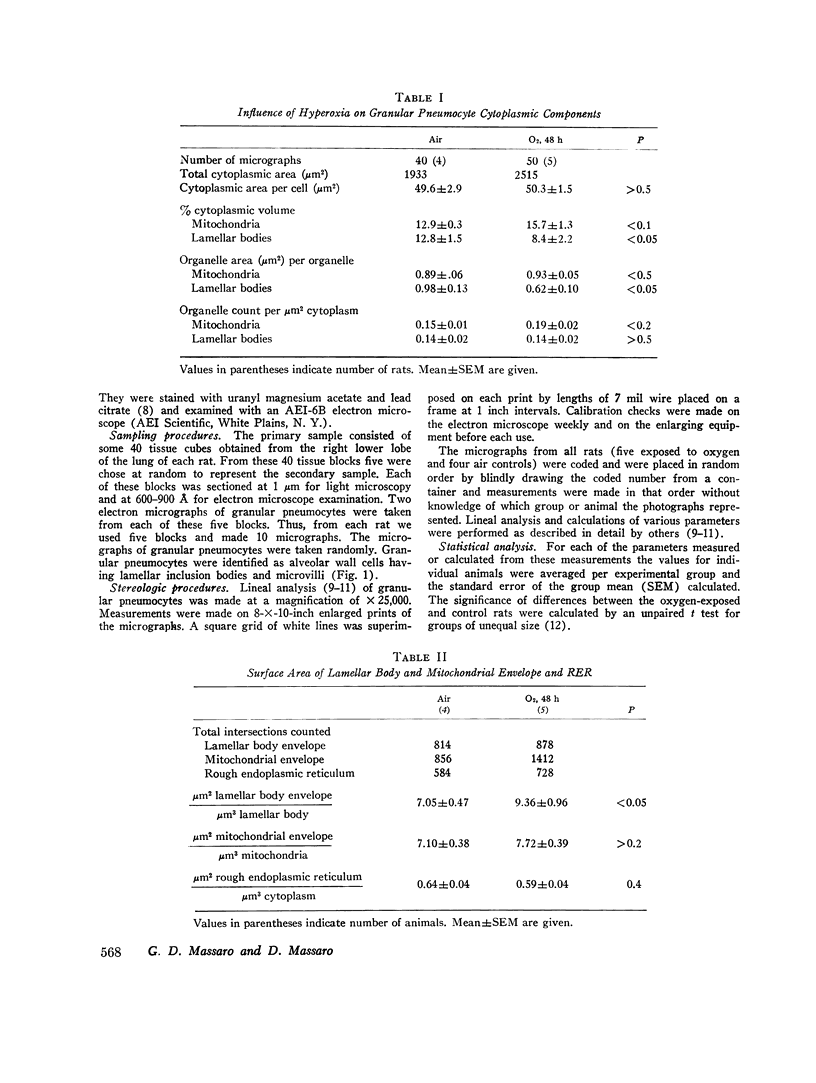
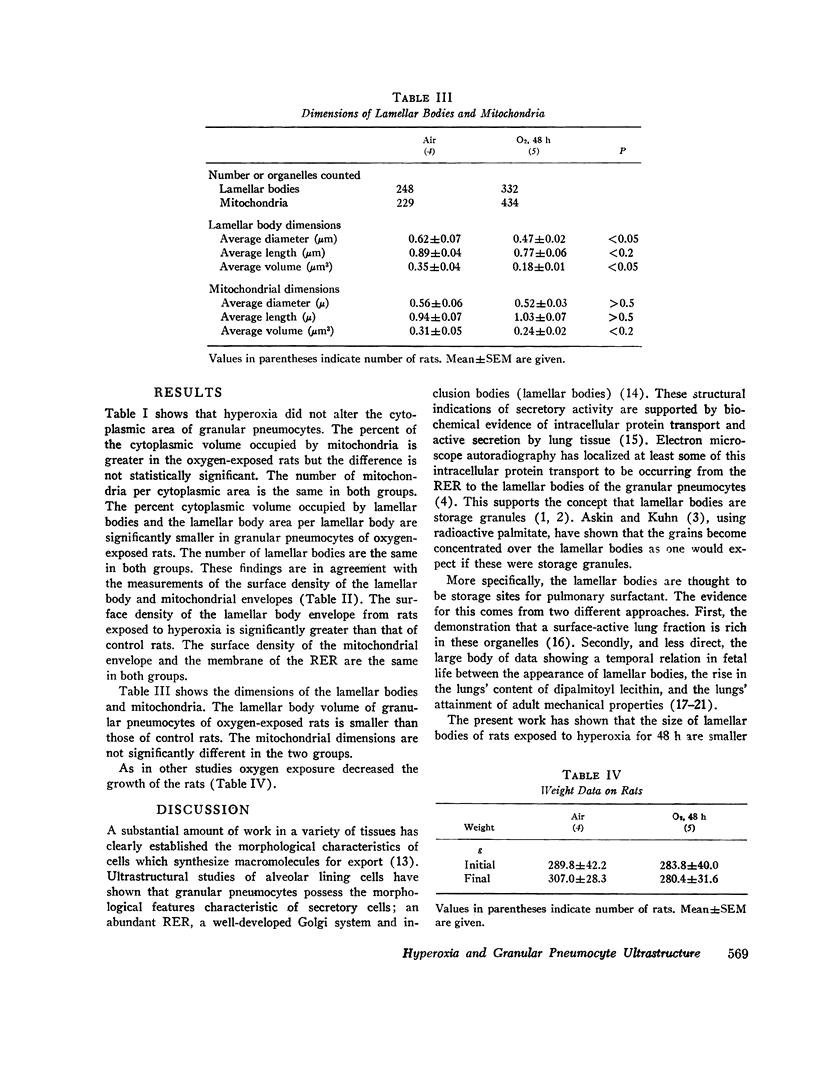
We found that for lamellar bodies the percent cytoplasmic volume was 12.8±1.5 (mean±SEM) and 8.4±2.2, the organelle area (μm2) per organelle was 0.98±0.13 and 0.62±0.10 and the organelle volume (μm2) was 0.35±0.04 and 0.18±0.01, for air- and oxygen-exposed rats, respectively, (P=<0.05). The surface density of the lamellar body membrane was 7.05±0.47 and 9.36±0.96 (P=<0.05) for air- and oxygen-exposed rats. There were no differences in lamellar body number per cytoplasmic area or per pneumocyte between air- and oxygen-exposed rats. There were no statistical differences in these parameters between mitochondria of air- or oxygen-exposed rats. The surface density of the rough endoplasmic reticulum was the same in both groups.
This study indicates that granular pneumocytes of rats exposed to hyperoxia have the same number of lamellar bodies as control rats but the lamellar bodies are smaller. This findings in consistent with the hypothesis that the hyperoxia-induced decrease in protein synthesis by lung represents at least in part a decreased synthesis of the secretory lipoprotein-pulmonary surfactant.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askin F. B., Kuhn C. The cellular origin of pulmonary surfactant. Lab Invest. 1971 Sep;25(3):260–268. [PubMed] [Google Scholar]
- BENSCH K., SCHAEFER K., AVERY M. E. GRANULAR PNEUMOCYTES: ELECTRON MICROSCOPIC EVIDENCE OF THEIR EXOCRINIC FUNCTION. Science. 1964 Sep 18;145(3638):1318–1319. doi: 10.1126/science.145.3638.1318-a. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM S., AVERY M. E. Time of appearance of lung surfactant in the foetal mouse. Nature. 1962 Feb 17;193:688–689. doi: 10.1038/193688a0. [DOI] [PubMed] [Google Scholar]
- Brumley G. W., Chernick V., Hodson W. A., Normand C., Fenner A., Avery M. E. Correlations of mechanical stability, morphology, pulmonary surfactant, and phospholipid content in the developing lamb lung. J Clin Invest. 1967 May;46(5):863–873. doi: 10.1172/JCI105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias H., Hennig A., Schwartz D. E. Stereology: applications to biomedicalresearch. Physiol Rev. 1971 Jan;51(1):158–200. doi: 10.1152/physrev.1971.51.1.158. [DOI] [PubMed] [Google Scholar]
- Frosolono M. F., Charms B. L., Pawlowski R., Slivka S. Isolation, characterization, and surface chemistry of a surface-active fraction from dog lung. J Lipid Res. 1970 Sep;11(5):439–457. [PubMed] [Google Scholar]
- Gacad G., Massaro D. Hyperoxia: influence on lung mechanics and protein synthesis. J Clin Invest. 1973 Mar;52(3):559–565. doi: 10.1172/JCI107216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., Motoyama E. K., Cook C. D. The ultrastructure of the lungs of lambs. The relation of osmiophilic inclusions and alveolar lining layer to fetal maturation and experimentally produced respiratory distress. Am J Pathol. 1965 Nov;47(5):877–903. [PMC free article] [PubMed] [Google Scholar]
- Kotas R. V., Avery M. E. Accelerated appearance of pulmonary surfactant in the fetal rabbit. J Appl Physiol. 1971 Mar;30(3):358–361. doi: 10.1152/jappl.1971.30.3.358. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd Cytochemistry of pulmonary alveolar epithelial cells. Am J Pathol. 1968 Nov;53(5):809–833. [PMC free article] [PubMed] [Google Scholar]
- LOUD A. V., BARANY W. C., PACK B. A. QUANTITATIVE EVALUATION OF CYTOPLASMIC STRUCTURES IN ELECTRON MICROGRAPHS. Lab Invest. 1965 Jun;14:996–1008. [PubMed] [Google Scholar]
- Massaro D., Kelleher K., Massaro G., Yeager H., Jr Alveolar macrophages: depression of protein synthesis during phagocytosis. Am J Physiol. 1970 Jun;218(6):1533–1539. doi: 10.1152/ajplegacy.1970.218.6.1533. [DOI] [PubMed] [Google Scholar]
- Massaro D., Weiss H., Simon M. R. Protein synthesis and secretion by lung. Am Rev Respir Dis. 1970 Feb;101(2):198–206. doi: 10.1164/arrd.1970.101.2.198. [DOI] [PubMed] [Google Scholar]
- Massaro G. D., Massaro D. Granular pneumocytes. Electron microscopic radioautographic evidence of intracellular protein transport. Am Rev Respir Dis. 1972 Jun;105(6):927–931. doi: 10.1164/arrd.1972.105.6.927. [DOI] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Thomas T., Jr, Rhoades R. A. Incorporation of palmitate-1-14C into lung tissue and "alveolar" lecithin. Am J Physiol. 1970 Dec;219(6):1535–1538. doi: 10.1152/ajplegacy.1970.219.6.1535. [DOI] [PubMed] [Google Scholar]
- Wang N. S., Kotas R. V., Avery M. E., Thurlbeck W. M. Accelerated appearance of osmiophilic bodies in fetal lungs following steroid injection. J Appl Physiol. 1971 Mar;30(3):362–365. doi: 10.1152/jappl.1971.30.3.362. [DOI] [PubMed] [Google Scholar]
- Young S. L., Tierney D. F. Dipalmitoyl lecithin secretion and metabolism by the rat lung. Am J Physiol. 1972 Jun;222(6):1539–1544. doi: 10.1152/ajplegacy.1972.222.6.1539. [DOI] [PubMed] [Google Scholar]