Abstract
Study Objectives:
To test the hypothesis of autonomic nervous system dysfunction in patients with narcolepsy-cataplexy (NC) by assessing the physiologic activations associated with periodic limb movements during sleep (PLMS).
Design:
Sleep and heart rate (HR) were recorded during 1 night of polysomnography.
Setting:
Data were collected at the Sleep Disorders Center, Sacre-Coeur Hospital, Montreal, Canada.
Participants:
Data from 14 patients with NC (6 men, 8 women, mean age: 52.5 ± 11.9 years) were compared with data from 14 healthy control subjects matched for age and sex.
Interventions:
NA.
Measurements and Results:
Analyses included sleep stages, PLMS, microarousals, RR intervals converted into beats per minute on segments lasting 25 heartbeats (10 RR intervals before PLMS and 15 after), and cardiac-activation amplitudes. A Group-by-Heartbeat interaction was noted for PLMS without microarousals; the patients had a tachycardia of lower amplitude and a delayed and lower-amplitude bradycardia, compared with normal control subjects. Similar significant HR modifications were observed for PLMS with microarousals between patients with NC and control subjects. Patients with NC had a reduced magnitude of cardiac activation associated with PLMS with and without microarousals, as compared with control subjects. A negative correlation was noted between cardiac-activation amplitude and age in patients with NC, but no correlation with PLMS index was found in either patients with NC or control subjects.
Conclusion:
A significant reduction in the amplitude of PLMS-related HR responses in both tachycardia and bradycardia was found in patients with NC. These findings favor the physiologic relevance of the action of hypocretin on autonomic function that may be of clinical significance, i.e., increasing the risk of cardiovascular diseases.
Citation:
Dauvilliers Y; Pennestri MH; Whittom S; Lanfranchi PA; Montplaisir JY. Autonomic response to periodic leg movements during sleep in narcolepsy-cataplexy. SLEEP 2011;34(2):219-223.
Keywords: Narcolepsy, periodic leg movements, heart rate, cardioreactivity, hypocretin
NARCOLEPSY WITH CATAPLEXY (NC) IS CHARACTERIZED BY EXCESSIVE DAYTIME SLEEPINESS, CATAPLEXY, AND DISTURBED NOCTURNAL SLEEP, including parasomnias, dissociated rapid eye movement (REM) sleep, and periodic leg movements during sleep (PLMS).1–4 Recent advances in our understanding of the pathophysiology of NC have demonstrated a marked decrease in hypocretin-1 levels in the cerebrospinal fluid, and in the number of hypocretin neurons.1,5,6 Hypocretin neurons are exclusively located in the lateral hypothalamus but project widely throughout the central nervous system, including to the hypothalamic and brainstem structures that are known to play a role in central cardiovascular regulation.7,8
Animal studies have revealed that pharmacologic administration of hypocretin stimulates arousal and elevates arterial blood pressure, heart rate (HR), oxygen consumption, body temperature, and plasma catecholamine levels.8–12 The increased blood pressure (BP) and HR effects have been demonstrated to be mediated by activation of the sympathetic arm of the autonomic nervous system.8–12 Studies have explored the cardiovascular status of hypocretin-deficient mice, with results showing that these mice have lower arterial BP.13
Sleep fragmentation is frequently reported in patients with NC, who show a high frequency of PLMS.2,4 Physiologic activation associated with PLMS represents a model to study the responses of the autonomic nervous system. Hence, studies conducted in patients with restless legs syndrome (RLS) and in healthy control subjects without RLS have reported that HR changes occurring with PLMS are sensitive markers of autonomic responses, characterized by a tachycardia followed by a bradycardia.14,15 To our knowledge, the arousal responses associated with PLMS have never been studied in subjects with NC. Studying interactions between sleep and the cardiovascular system through physiologic activations associated with PLMS is of interest in NC, which is characterized by the absence of hypocretin.
The aim of this study was to measure HR changes associated with PLMS in the presence or absence of a microarousal in patients with NC and in age- and sex- matched healthy control subjects.
METHODS
Subjects
Fourteen patients with sporadic NC (6 men, 8 women) aged between 29 and 67 years (mean age, 52.4 ± 12.0 years) were included in the study. Inclusion criteria for narcolepsy were the presence of excessive daytime sleepiness, cataplexy, HLA DQB1*0602 positivity, at least 2 sleep-onset REM periods, and a mean sleep latency of less than 8 minutes during the Multiple Sleep Latency Test. An additional inclusion criterion was that the subjects have a PLMS index of at least 10 per hour. Exclusion criteria for NC were the presence of any other sleep disorder, especially RLS, REM sleep behavior disorder (based on clinical interview), and sleep apnea syndrome (as defined by an index of respiratory events > 5). Nine patients were drug naïive, and 5 had stopped their medication (psychostimulant, anticataplectic drugs, and any drug known to influence sleep, motor, or autonomic nervous system activities) for at least 1 month prior to the polysomnographic recording in the sleep laboratory.
Data from 14 control subjects (6 men, 8 women) aged between 20 and 65 years (mean age, 51.3 ± 10.8 yrs) matched for age and sex were compared with data from the patients with NC. These subjects were recruited from the general population by announcements placed in newpapers. None of the control subjects had any complaint of daytime sleepiness, insomnia, RLS, or REM sleep behavior disorder. Control subjects were also required to have a PLMS index of at least 10 per hour. Exclusion criteria for healthy normal subjects were the same as for patients with NC. In addition, none of the control subjects reported any symptom of NC.
All participants were free of coronary artery disease, stroke, heart failure, hypertension, or diabetes and none smoked. All subjects signed a consent form approved by the ethics committee of the Sacré-Coeur Hospital.
Procedures
All subjects underwent 1 night of polysomnographic recording in the sleep laboratory. Sleep recording included 4 electroencephalographic leads (C3, C4, O1 and O2), 2 bilateral electrooculograms, 1 electrocardiogram (lead II), and 1 chin electromyogram (EMG). Respiration was assessed by nasal cannula, thoracoabdominal strain gauges, and finger pulse oximetry. Respiratory events were scored according to American Academy of Sleep Medicine recommendations.16 Surface EMG electrodes placed 3 cm apart on the right and left anterior tibialis muscles were used to record PLMS. Sleep was scored by the standard method,16 and PLMS were scored following the criteria set by the International RLS Study Group.17 Only movements lasting 0.5 to 10 seconds, separated by intervals of 5 to 90 seconds and occurring in a series of at least 4 consecutive movements, were counted. The amplitude criterion for detecting leg movements was an increase in EMG to at least 8 μV above the resting baseline for the onset of the movement and a decrease in EMG to less than 2 μV above the resting level for the offset of movement. Microarousals were scored according to standard American Sleep Disorders Association and American Academy of Sleep Medicine criteria.16,18
In addition to the standard criteria for PLMS, only movements separated by at least 20 seconds were selected for cardiovascular analysis in order to avoid overlapping HR response to successive leg movements. Movements were selected in stage 2 sleep to avoid sleep-stage interaction. Only movements free of any physiologic (rhythmic masticatory muscle activity, flow limitation at the nasal cannula, apneas and hypopneas, arrhythmias) or technical factors were selected. Finally, a total of 210 PLMS with microarousals and 490 PLMS without microarousals were selected for cardiovascular analysis in control subjects; 117 PLMS with and 418 without microarousals were selected in patients with NC. HR was measured on segments lasting 25 heartbeats, comprising 10 RR intervals before the movement (−10 to −1) and 15 RR intervals after the movement (+1 to +15). The RR intervals were converted into beats per minute. For each movement, the baseline was defined as the mean value from beat −10 to −6. Changes in HR were then calculating by subtracting baseline from each HR value. In addition, the mean cardiac-activation amplitude was calculated in each subject for PLMS with and without microarousals (amplitude = maximal value during tachycardia – minimal value during bradycardia).
Statistical Analyses
Mann-Whitney tests were performed to compare sleep architecture and PLMS in narcoleptics patients and normal control subjects. Beat-to-beat HR changes associated with PLMS with and without microarousals in patients with NC and control subjects were assessed independently by 2-way analyses of variance with 1 factor (group) and 1 repeated measure (heartbeat) followed by planned comparisons. Greenhouse-Geisser correction for sphericity was applied. Mann-Whitney tests were also performed to assess between-group differences for cardiac-activation amplitude associated with PLMS with and without microarousals. Spearman rank-order correlation coefficients were calculated to assess the relationships among cardiac activation amplitude, age, and PLMS. Differences were considered significant at P < 0.05.
RESULTS
Clinical and Polysomnographic Data
Polysomnographic data from patients and control subjects are shown in Table 1. The mean age of patients and control subjects was similar at the time that polysomnography was performed (52.4 ± 12.0 y vs 51.3 ± 10.8). Patients reported a long duration of disease at the time of the study, mean of 27.8 ± 15.6 years. We found some differences in sleep architecture between patients and control subjects, with a higher percentage of stage 1 sleep, a higher number of awakenings, a lower percentage of stage 2 sleep, a slightly higher percentage of slow wave sleep, and shorter nighttime and REM sleep latencies in patients with NC (Table 1).
Table 1.
Demographic and polysomnographic characteristics of patients with narcolepsy-cataplexy and control subjects
| Characteristic | Patients with NC | Control subjects | P value |
|---|---|---|---|
| n = 14 | n = 14 | ||
| Sex, men/women | 6/8 | 6/8 | |
| Age at polysomnography, y | 52.4 ± 12.0 | 51.3 ± 10.8 | 0.7 |
| Total sleep time, min | 417.3 ± 51.4 | 387.9 ± 50.7 | 0.4 |
| Sleep latency, min | 5.3 ± 3.7 | 13.0 ± 10.3 | 0.01 |
| REM latency, min | 38.8 ± 66.8 | 85.8 ± 49.3 | 0.007 |
| Sleep efficiency, % | 81.6 ± 8.6 | 85.3 ± 10.1 | 0.2 |
| Duration of wake, min | 92.8 ± 43.3 | 67.0 ± 46.4 | 0.1 |
| Awakenings, no. | 57.6 ± 18.2 | 40.9 ± 20.6 | 0.03 |
| Microarousals, no./h | 15.0 ± 8.0 | 13.0 ± 9.8 | 0.3 |
| Sleep stage, % | |||
| 1 | 18.8 ± 6.9 | 12.3 ± 6.9 | 0.009 |
| 2 | 54.0 ± 8.3 | 64.7 ± 6.9 | 0.0004 |
| SWS | 4.8 ± 4.1 | 2.5 ± 4.0 | 0.04 |
| REM | 22.4 ± 9.7 | 20.5 ± 5.7 | 0.9 |
Data are presented as mean ± SD, except sex. NC refers to narcolepsy-cataplexy; REM, rapid eye movement sleep; SWS, slow-wave sleep.
The PLM index during wakefulness was higher in patients with NC, compared with control subjects; however, total PLMS and PLMS in non-rapid eye movement (NREM) sleep indexes were higher in control subjects (Table 2). No difference was seen between NC and control subjects for PLMS duration (2.0 s ± 0.4 vs 2.1 s ± 0.5) and for PLMS intermovement durations (31.9 s ± 5.6 vs 33.1 s ± 3.7). Finally, no between-group difference was reported for microarousals and PLMS with microarousal indexes (Tables 1 and 2).
Table 2.
Characteristics of PLMW and PLMS and cardiac activation related to PLMS in patients with NC and control subjects
| Polysomnographic parameter | Patients with NC | Control subjects | P value |
|---|---|---|---|
| n = 14 | n = 14 | ||
| PLMW index | 89.8 ± 45.8 | 51.1 ± 37.8 | 0.02 |
| PLMS index | |||
| Total | 30.8 ± 13.3 | 43.6 ± 16.6 | 0.05 |
| In NREM sleep | 31.9 ± 12.0 | 49.2 ± 19.1 | 0.02 |
| In REM sleep | 29.2 ± 28.5 | 21.4 ± 33.4 | 0.3 |
| PLMS-MA index | 3.5 ± 2.1 | 4.8 ± 4.9 | 0.9 |
| PLMS associated with MA, % | 14.3 ± 11.9 | 11.8 ± 13.6 | 0.2 |
| Mean cardiac activation amplitude for PLMS, beats/min | |||
| Without MA | 5.7 ± 2.6 | 10.5 ± 3.1 | 0.0007 |
| With MA | 8.6 ± 4.5 | 13.4 ± 4.1 | 0.009 |
Data are presented as mean ± SD. PLMS refers to periodic limb movements during sleep; PLMW, periodic limb movements during wakefulness; NC, narcolepsy-cataplexy; NREM, non-rapid eye movement sleep; REM, rapid eye movement sleep; MA, microarousals.
HR Changes Associated With PLMS
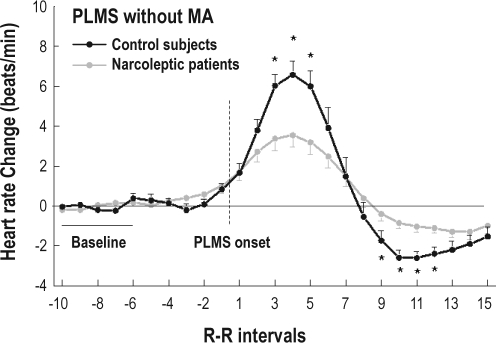
Figure 1 shows the distribution of HR changes associated with PLMS without microarousals in patients with NC, compared with control subjects, for 10 RR intervals before the movement (−10 to −1) and 15 RR intervals after the onset of the movement (+1 to +15). A Group-by-Heartbeat interaction was noted for PLMS without microarousals, with a tachycardia of lower amplitude (significant for beats +3 to +5) and a delayed and lower amplitude of bradycardia (significant for beats +9 to +12) in patients with NC (F19,494 = 6.01, P = 0.001).
Figure 1.
Heart rate changes associated with periodic limb movements during sleep (PLMS) without microarousal (MA) in patients with narcolepsy-cataplexy (NC) (gray) and in control subjects (black). The dashed line represents PLMS onset. Data are presented as mean ± SEM. Asterisks indicate P < 0.05 from baseline.
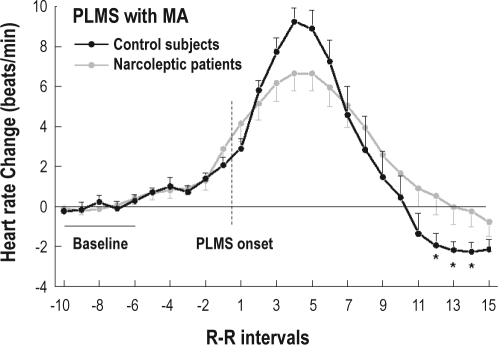
A similar Group-by-Heartbeat interaction was observed for PLMS with microarousals (F19,494 = 2.71, P = 0.05). Again, patients with NC showed a tachycardia of lower amplitude (beats +4) and a delayed and lower amplitude of bradycardia (beats +12 to +14) associated with PLMS with microarousals, compared with control subjects (Figure 2).
Figure 2.
Heart rate changes associated with periodic limb movements during sleep (PLMS) with microarousal (MA) in patients with narcolepsy-cataplexy (NC) (gray) and in control subjects (black). Dashed line represents PLMS onset. Data are presented as means ± SEM. Asterisks indicate P < 0.05 from baseline.
A reduced magnitude of the cardiac activation associated with both PLMS with microarousals (P = 0.009) and PLMS without microarousals (P = 0.0007) was found in patients with NC, compared with control subjects (Table 2). A negative correlation was noted between cardiac-activation amplitude and age in patients with NC (r = −0.54, P = 0.04) but not in control subjects (r = −0.31, P = 0.3). In contrast, no correlation was found between cardiac-activation amplitude and PLMS index for either patients with NC (r = −0.16, P = 0.6) or control subjects (r = −0.25, P = 0.4). Because the PLMS index in NREM sleep was slightly lower in patients with NC than in control subjects, we reanalyzed our cardiac-activation data, removing the data from the 5 control subjects with the highest PLMS indexes. We found no group differences for age and PLMS index within this subset of control subjects (n = 9, mean age = 49.4 ± 12.9 y, and mean PLMS index = 34.8 ± 13.9). Between-group differences in cardiac-activation amplitude remained significant, with patients with NC having a lower amplitude than control subjects for mean cardiac activation for both PLMS without microarousals (5.7 ± 2.6, n = 14 vs 10.7 ± 2.7, n = 9, P = 0.001) and PLMS with microarousals (8.6 ± 4.5, n = 14 vs 13.9 ± 5.0, n = 9, P = 0.02).
DISCUSSION
Our study reports for the first time the autonomic activation associated with PLMS in patients with NC, with a lower magnitude of the changes reported, compared with healthy matched control subjects. The pattern of cardiac acceleration and deceleration was substantially similar for PLMS with and without microarousals, although the changes were greater when microarousals occurred. The increase in HR started before PLMS with or without microarousals in patients with NC and in control subjects.
Reduced PLMS-associated autonomic changes in patients with NC favor a lower sensitivity of the common brainstem generator of cardiac activations.19,20 Similar findings were previously reported in patients with RLS and with the aging process,14,15,21 as also pinpointed in our present study in both patients with NC and control populations. However, we noted in the present study that cardiac-activation amplitude associated with PLMS was independent of the PLMS index in both patients with NC and control subjects.
Reduced autonomic activation associated with PLMS, together with a lower HR variability during sleep and an impairment in both sympathetic and parasympathetic activities in wakefulness, have been reported in patients with idiopathic REM sleep behavior disorder.22,23 These findings were of major interest in terms of pathophysiology, since an increased frequency of REM sleep without atonia, phasic EMG activity, and clinical REM sleep behavior disorder had been previously reported in patients with NC.3,24 We hypothesize that common abnormalities in REM sleep motor regulation and autonomic function exist in both conditions.
Old clinical studies in patients with NC, before the discovery of hypocretins, reported some abnormalities in the autonomic nervous system, including pupillary, erection, and cardiovascular functions.25–27 Hence, 1 study exploring the cardiovascular system in patients with NC revealed a reduced vegetative reactivity in response to muscle contraction and the Valsalva maneuver,27 findings that have not been further confirmed.25 Later on, power spectrum analysis studies of HR and BP variability have suggested only minor changes in NC that failed to find a clear autonomic dysfunction.28,29
Our findings, therefore, have demonstrated, for the first time, a primary change in autonomic function in patients with NC. Autonomic imbalance may be characterized by an impairment in both sympathetic and parasympathetic activities. Recent studies have highlighted the nonsleep actions of hypocretin, including roles in controlling central pathways involved in the regulation of cardiovascular functions.7,30 Pharmacologic studies have revealed that intrathecal hypocretin administration results in increased BP and HR in conscious, as well as anesthetized, animals, an effect that is mediated by enhancement of the sympathetic output.10–12 In addition to acting on brainstem sites related to autonomic function, hypocretin also acts in the hypothalamic paraventricular nucleus and sympathetic preganglionic neurons, increasing the sympathetic tone.31,32 Moreover, cardiovascular studies in orexin-knockout mice have revealed lower arterial BP, as compared with in wild-type mice, differences that are abolished by α-adrenergic or ganglionic blockade.13 Altogether, these results corroborate that the hypocretin system acts as an essential modulator for coordinating autonomic-system circuits, with the predominant sympathetic excitatory effect targeting the autonomic centers of the brain. We hypothesize that the generalized attenuation of PLM-related HR changes observed in human narcolepsy is explained by a lower sympathetic response due to the hypocretin-deficient condition. Although the significance of PLMS and the lower amplitude of the PLMS-related cardiac activations remain unclear, it may be of clinical importance.33 Hence, several studies have shown that a decrease in HR variability, a tool that is frequently used to assess autonomic imbalance, is associated with a higher risk of mortality due to cardiovascular diseases.34,35 These findings require further attention, since NC is often associated with obesity, type 2 diabetes, and the metabolic syndrome.36,37 We also emphasize that patients with NC will be treated with psychostimulants for the rest of their lives, therapy that has a well-known impact on autonomic nervous and cardiovascular systems.38
Several limitations in our study need to be addressed. First, the sample size is small, although sufficient to demonstrate a significant reduction in the amplitude of the PLMS-related HR response. The small sample size is the result of the strict inclusion criteria, which required the presence of enough PLMS to be further analyzed and compared with the PLMS index of a carefully age- and sex-matched population that had no comorbid disease or was taking any medication that may impact the results. Second, we focused on PLMS-related cardiac activations only in stage 2 non-REM sleep. Because most of the PLMS existed in stage 2 sleep, especially in control subjects, we were unable to score enough PLMS to perform between-group HR changes by comparing PLMS in various sleep stages. Finally, no cerebrospinal fluid hypocretin-1 measurement was available in patients. However, we assume that patients were almost all hypocretin deficient because they all had sporadic NC and had severe daytime sleepiness, clear-cut cataplexy, typical results on the Multiple Sleep Latency Test, and HLA DQB1*0602 positivity.
CONCLUSION
Our study pinpointed a significant reduction in the amplitude of PLMS-related HR responses in both tachycardia and bradycardia in patients with NC. This result suggests that the physiologic relevance of the action of hypocretin on autonomic function may be of clinical significance with regard to increasing the risk of cardiovascular diseases.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Dauvilliers has consulted for UCB Pharma, Cephalon, Bioprojet, GlaxoSmithKline, and Boehringer Ingelheim. Dr. Montplaisir has received research support from Sanofi-Aventis, Merck, and GlaxoSmithKline; has participated in speaking engagements for Sanofi-Aventis, Jazz, and Boehringer Ingelheim; and has consulted for Servier and Merck. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Sylvie Rompré and Jean Paquet for helping with data and statistical analysis.
This work was supported by the Canadian Institutes of Health Research (Studentship to Marie-Hélène Pennestri; grants to Paola A. Lanfranchi and Jacques Montplaisir) and the Fonds de la Recherche en Santé du Québec (Scholarship to Paola A. Lanfranchi and studentship to Shirley Whittom).
REFERENCES
- 1.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 2.Dauvilliers Y, Pennestri MH, Petit D, Dang-Vu T, Lavigne G, Montplaisir J. Periodic leg movements during sleep and wakefulness in narcolepsy. J Sleep Res. 2007;16:333–9. doi: 10.1111/j.1365-2869.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 3.Dauvilliers Y, Rompre S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep. 2007;30:844–9. doi: 10.1093/sleep/30.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferri R, Zucconi M, Manconi M, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006;29:1587–94. doi: 10.1093/sleep/29.12.1587. [DOI] [PubMed] [Google Scholar]
- 5.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 6.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 7.Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept. 2002;104:91–5. doi: 10.1016/s0167-0115(01)00352-4. [DOI] [PubMed] [Google Scholar]
- 8.Samson WK, Taylor MM, Ferguson AV. Non-sleep effects of hypocretin/orexin. Sleep Med Rev. 2005;9:243–52. doi: 10.1016/j.smrv.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–7. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira CV, Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Cardiovascular effects of hypocretin-1 in nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2003;284:H1369–77. doi: 10.1152/ajpheart.00877.2002. [DOI] [PubMed] [Google Scholar]
- 11.Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R692–7. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- 12.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–5. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- 13.Kayaba Y, Nakamura A, Kasuya Y, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–93. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- 14.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 15.Gosselin N, Lanfranchi P, Michaud M, et al. Age and gender effects on heart rate activation associated with periodic leg movements in patients with restless legs syndrome. Clin Neurophysiol. 2003;114:2188–95. doi: 10.1016/s1388-2457(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan S. 1st ed. Westchester, IL: Amercian Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events; Rules, Terminology and Technical Specifications. [Google Scholar]
- 17.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 19.Sforza E, Pichot V, Barthelemy JC, Haba-Rubio J, Roche F. Cardiovascular variability during periodic leg movements: a spectral analysis approach. Clin Neurophysiol. 2005;116:1096–104. doi: 10.1016/j.clinph.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Berlucchi G. One or many arousal systems? Reflections on some of Giuseppe Moruzzi's foresights and insights about the intrinsic regulation of brain activity. Arch Ital Biol. 1997;135:5–14. [PubMed] [Google Scholar]
- 21.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–80. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 22.Ferini-Strambi L, Oldani A, Zucconi M, Smirne S. Cardiac autonomic activity during wakefulness and sleep in REM sleep behavior disorder. Sleep. 1996;19:367–9. doi: 10.1093/sleep/19.5.367. [DOI] [PubMed] [Google Scholar]
- 23.Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94. doi: 10.1212/01.wnl.0000038348.94399.f6. [DOI] [PubMed] [Google Scholar]
- 24.Nightingale S, Orgill JC, Ebrahim IO, de Lacy SF, Agrawal S, Williams AJ. The association between narcolepsy and REM behavior disorder (RBD) Sleep Med. 2005;6:253–8. doi: 10.1016/j.sleep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Hublin C, Matikainen E, Partinen M. Autonomic nervous system function in narcolepsy. J Sleep Res. 1994;3:131–7. doi: 10.1111/j.1365-2869.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 26.Karacan I. Erectile dysfunction in narcoleptic patients. Sleep. 1986;9:227–31. doi: 10.1093/sleep/9.1.227. [DOI] [PubMed] [Google Scholar]
- 27.Sachs C, Kaijser L. Autonomic control of cardiovascular reflexes in narcolepsy. J Neurol Neurosurg Psychiatry. 1980;43:535–9. doi: 10.1136/jnnp.43.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferini-Strambi L, Spera A, Oldani A, et al. Autonomic function in narcolepsy: power spectrum analysis of heart rate variability. J Neurol. 1997;244:252–5. doi: 10.1007/s004150050080. [DOI] [PubMed] [Google Scholar]
- 29.Fronczek R, Overeem S, Reijntjes R, Lammers GJ, van Dijk JG, Pijl H. Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J Clin Sleep Med. 2008;4:248–54. [PMC free article] [PubMed] [Google Scholar]
- 30.Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23:3844–54. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1611–20. doi: 10.1152/ajpregu.00719.2002. [DOI] [PubMed] [Google Scholar]
- 33.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 34.Thayer J, Yamamoto S, Brosschot J. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiology. 2010;141:122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 35.Kleiger RE, Miller JP, Bigger JT, Jr., Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 36.Poli F, Plazzi G, Di Dalmazi G, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32:1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 38.Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep. 2005;28:667–72. doi: 10.1093/sleep/28.6.667. [DOI] [PubMed] [Google Scholar]