Abstract
Objectives:
a. Explore the stability in sleep/wake patterns of middle-aged adults over a 3-year follow-up period. b. Explore the relationship between objectively measured sleep indices, urinary catecholamines, and salivary cortisol.
Design:
Naturalistic follow-up for sleep/wake patterns (n = 114) by 2-week sleep log and cross-sectional design for objective sleep assessments and hormonal measures (n = 96) at follow-up period nearly 3 years after baseline measurements.
Setting:
Community
Participants:
Healthy middle-aged adults
Interventions:
N/A
Measurements and Results:
There were high correlations between baseline and follow-up period (2.6 ± 0.5 years) on sleep/wake patterns (r = 0.6–0.79) as measured by 2-week sleep log. For wave 2 cross-sectional study, objective poor sleepers (3-day actigraphy sleep efficiency < 85%) had a higher 24-h urinary norepinephrine (NE) level (205.7 ± 105 nmol/d vs 162.1 ± 55.6 nmol/d, P = 0.03) and a nearly significantly higher 24-h urinary epinephrine (E) level (P = 0.12) than good sleepers. There were no differences in 3-day mean salivary awakening cortisol and 24-h urinary catecholamines (NE and E) between short and normal/long sleepers. Linear regression results, however, showed that shorter time in bed and actual sleep time, longer sleep onset latency, and lower sleep efficiency were correlated with higher 24-h urinary E and NE (all P < 0.05) but not salivary cortisol. The effect of poor sleep quality on 24-h urinary catecholamines was stronger in males than females.
Conclusions:
Increased sympathetic activity as measured by 24-h urinary catecholamines might play a critical role in the pathogenesis mediating the relationship of insufficient sleep (quantity and quality) with subsequent cardiovascular and metabolic complications. Salivary awakening cortisol was not associated with sleep quantity and quality in healthy middle-aged adults.
Citation:
Zhang J; Ma RCW; Kong APS; So WY; Li AM; Lam SP; Li SX; Yu MWM; Ho CS; Chan MHM; Zhang B; Wing YK. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. SLEEP 2011;34(2):225-233.
Keywords: Sleep duration, sleep quality, actigraphy, catecholamines, cortisol
IN THE PAST DECADE, INCREASING EVIDENCE HAS SUGGESTED THAT SHORT SLEEP DURATION, WHICH IS AN ENDEMIC PROBLEM IN MODERN SOCIETY, IS associated with a constellation of pervasive cardiovascular and metabolic disturbances.1–7 In addition, insomnia has been correlated with hypertension2 and diabetes,6 and there seemed to be a synergistic effect between insomnia and short sleep duration on increased risk of hypertension.2 Nonetheless, the pathophysiologic mechanism in mediating the relationship between poor sleep and various medical consequences remains unclear.
Sleep deprivation is considered an important stressor.8 The hypothalamic-pituitary-adrenal axis (HPA) and locus ceruleus-norepinephrine-autonomic system are two two major components involved in stress response.9 Normal sleep decreases sympathetic nervous system (SNS) activity and increases parasympathetic nervous system activity.10 Experimental studies have shown that sleep deprivation could lead to SNS hyperactivity.11,12 However, response of HPA axis to sleep deprivation was more variable.8 Overall, these studies indicated that sleep deprivation not only had direct stimulating effects on SNS (and probably HPA axis), but also modulated their responses to other stressors and challenges.8 Nonetheless, most studies have been based on sleep deprivation induced by experimental procedure artificially. This procedure per se could be an independent stressor.8 On the other hand, habitual sleep duration, for example in middle-aged adults, was found to be influenced by a complex and interactive web of factors including socioeconomic status,13 waking activities,14 and their children's daytime activities.13 Limited studies have focused on the natural history of sleep duration/quality and the impact of short/poor sleep on SNS and HPA axis in the general population. Thus, we aimed to (1) explore the level of stability in sleep/wake patterns of middle-aged adults over a 3-year follow-up period; (2) explore the relationship of sleep quantity and quality as measured by actigraphy with 24-h urinary catecholamines and 3-day salivary awakening cortisol.
MATERIALS AND METHODS
Subject Selection
This study was part of an ongoing epidemiologic study, which began in 2003, on sleep problems in children and their parents.15 The protocol was approved by the institutional ethics review committee. All participants gave their consent to this study. The current study was a 2-wave prospective study, which assessed the habitual sleep/wake patterns, personality, psychiatric and medical disorders in middle-aged adults (Figure 1). Wave 1 study started at 2003–2005 and wave 2 study was conducted at 2006–2007. Wave 1 study recorded the baseline subjective sleep/wake pattern and further additional objective sleep and hormonal measures were performed at the wave 2 study. In wave 1 study, a total of 252 adult subjects without any self-reported significant sleep disturbance (≥ 3 times/week) or chronic medical problem as assessed by validated questionnaires were invited to attend a detailed clinical assessment.15–17 There were 14 items for sleep problems in this questionnaire,15 dealing with insomnia symptoms (difficulty initiating sleep, difficulty maintaining sleep, and early morning awakening), sleep related breathing symptoms (snoring, nocturnal mouth breathing, nocturnal sweating, dry mouth in the morning), and functional daytime impairment (fatigue, sleepiness).
Figure 1.
Flow diagram documenting recruitment of subjects
Twenty-four of these 252 subjects (9.5%) were found to have concurrent psychiatric disorder(s) by Structured Clinical Interview for DSM-IV Axis I Psychiatric Disorder (SCID). They were excluded from further study, as psychiatric disorders might be a confounding factor in the relationship among sleep, neuroendocrine and cardiovascular diseases.18
At wave 2 study, a total 114 subjects (50.0%) of the cohort consented to participate in the follow-up assessment in 2006–2007 and completed a 2-week sleep log and actigraphic study. None of the subjects recruited into the wave 2 actigraphic assessment had active psychiatric diagnosis as assessed by SCID interview (Figure 1). Three subjects taking antihypertensive medications and 3 subjects with significant insomnia symptoms (≥ 3 times/week) were excluded from objective sleep measures and biological tests. Compared with the recruited subjects, these drop-outs from wave 2 study were more likely to be male (38.1% vs 21.7% for wave 1 and wave 2 respectively, P = 0.007). There were no differences in age, education level, or sleep duration between these 2 groups.
In addition to the detailed questionnaires, a consecutive 2-week sleep log, and physical examination as measured at wave 1 study, the subjects also completed 3-day actigraphic assessment, 3-day morning salivary awakening cortisol, and 24-h urinary catecholamine collection at wave 2 study.
Physical Examination (both waves)
All participants underwent anthropometric measurements and clinical assessments. Weight was measured to the nearest 0.1 kg (Tanita physician digital scale, Tanita Corp., Tokyo, Japan) while height was measured to the nearest millimeter. Waist circumference was measured at the midpoint between the lower ribs and the iliac crest. Hip circumference was measured horizontally at the level of the largest lateral extension of the hips or over the buttocks. Body mass index (BMI) was calculated as weight divided by height square (kg/m2). Blood pressure was measured once after ≥ 15 min rest in a sitting position. Venous blood was sampled for measurement of plasma creatinine concentrations. All subjects had plasma creatinine < 125 μmol/L.
Sleep/Wake Patterns Assessments (both waves)
The sleep/wake patterns were measured by 2 consecutive weeks sleep log at both waves. Subjects were asked to record the time for going to bed and getting up everyday for 2-week in the sleep log. The bedtime and wake up time were averaged according to 2-week record, while time in bed was calculated as time interval between wake up time and bedtime.
Actigraphy (wave 2)
Sleep-wake estimation in wave 2 study was recorded by wrist actigraphy (Mini Mitter, Actiwatch 16, OR, USA,), which was analyzed with the Actiware-Sleep v 3.0 analysis software (Mini Mitter Co., Inc.). A threshold of 20 was used to distinguish sleep from waking, based on previous validation study.19 Bedtime and wake up time were extracted based on the marker button. Sleep parameters in actigraphy were estimated as follows:
Time in bed (TIB): Interval between bedtime and wake up time as derived from event-marker buttons.
Actual sleep duration (ASD): Interval between sleep start and sleep end, minus wakening duration (defined as sleep quantity in this study).
Sleep onset latency (SOL): Interval between bedtime and sleep start time
Wake after sleep onset (WASO): waking duration during sleep start time and wake up time
Sleep efficiency (SE): Actual sleep duration divided by TIB × 100% (defined as sleep quality in this study)
Subjects with sleep efficiency < 85% (n = 38) were considered to have poor sleep quality (poor sleepers) as similarly defined by previous studies.2,20 As there is a lack of consensus on criteria for defining short sleepers, subjects with the lowest quartile of ASD (≤ 340 min) (n = 23, 24%) were considered short sleepers. Ninety-nine of 114 subjects (86.8%) had at least one day of valid actigraphic results, but 3 subjects taking antihypertensive drugs were excluded. The final analysis was restricted to subjects (n = 96) who had at ≥ 1 day of actigraphic recordings (95 subjects with 2-day recordings and 87 subjects with 3-day recordings).
24-Hour Urinary Catecholamine Assessment (wave 2)
All subjects were instructed to collect 24-h urinary samples during the period of actigraphic assessment to allow real-time association analysis between objective sleep parameters and catecholamines levels. All urine samples were collected into 24-h urine bottles with 0.1L of 0.5M hydrochloric acid as preservative and stored at −20° before analysis. Epinephrine (E) and norepinephrine (NE) in the urine samples were measured by high-performance liquid chromatography with electrochemical detection. Ninety-eight subjects completed 24-h urine collection; 84 of them (including 23 short sleepers and 34 poor sleepers) completed both 24-h urine assessment and actigraphic recordings.
Morning Salivary Awakening Cortisol Assessment (wave 2)
All subjects were asked to collect 3 days awakening salivary cortisol immediately after waking up, and the samples were stored at 4°C with Salivette (Sarstedt, Nʊumbrecht, Germany). Salivary samples were collected using an insert containing a sterile polyester swab (Aktiengesellschaft & Co.). Salivary cortisol concentrations were measured by an automated immunoassay analyzer system (Modular Analytics E, Roche Diagnostics, Basel, Switzerland). The 3-day cortisol levels were averaged for analysis. A total of 101 subjects had valid 3-day cortisol results for analysis; 90 of them (including 23 short sleepers and 38 poor sleepers) completed both 3-day salivary awakening cortisol and actigraphic recordings.
Statistical Analysis
Descriptive statistics were presented as percentages for discrete variables and as means (± standard deviation) for continuous variables. Parametric and nonparametric data were compared using paired/independent t-test and Mann-Whitney U-test, respectively. Chi-square or Fisher exact tests were employed to analyze the difference in proportions between groups. Pearson correlation was employed to explore the correlation of individual sleep/wake patterns as determined by 2-week sleep log between baseline and follow-up. Bland-Altman plots were further used to explore the agreements in sleep/wake patterns between baseline and follow-up.21
The correlations of anthropometric measurements with actigraphic parameters and 24-h urinary catecholamine as well as 3-day salivary awakening cortisol level were tested by Pearson correlation. Linear regression with enter method was used to examine the associations of 24-h urinary catecholamines and 3-day salivary awakening cortisol with TIB, ASD, SE, SOL, and WASO, respectively, after adjusting for age, gender, BMI, education level, waist/hip ratio, systolic and diastolic BP. Two-way ANOVA was used to explore the potential interaction between short sleepers (ASD ≤ 340 min) and poor sleep quality (sleep efficiency < 85%), and between gender and short ASD/poor sleep quality on 24-h urinary catecholamines. P-value < 0.05 was considered a statistically significant level. SPSS 16.0 for Windows (SPSS Inc, Chicago, IL) was used for all tests.
RESULTS
Longitudinal Changes of Sleep/Wake Patterns as Measured by 2-Week Sleep Log in Middle-Aged Adults
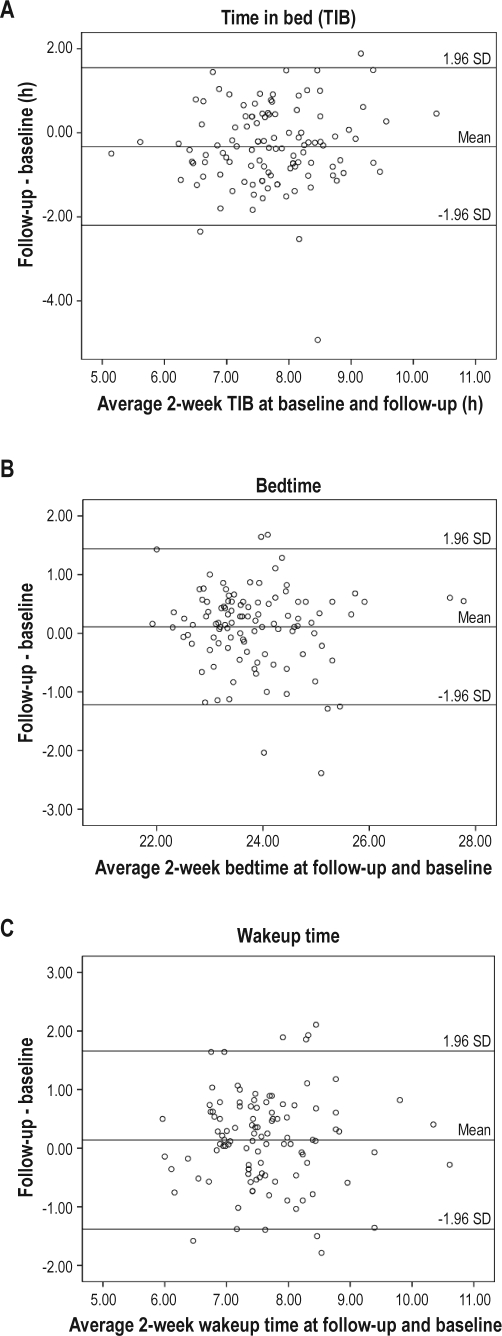
There were high correlations between baseline (wave 1) and follow-up (wave 2) (duration of follow-up = 2.6 ± 0.5 years, range: 1.8–3.5 years) period on various sleep/wake patterns (r = 0.79 for bedtime, r = 0.64 for wakeup time and r = 0.60 for TIB respectively, P < 0.001). There were moderate to high correlations in sleep/wake patterns between 2-week sleep log (wave 2) and 3-day actigraphy at wave 2 study (Spearman r = 0.43, 0.48, 0.81 for wake up time, TIB, and bedtime, respectively; P < 0.001). Figure 2 shows the agreements in sleep/wake patterns between follow-up and baseline period by using Bland and Altman method (comparing the difference between each pair of results with the mean value of each pair).21 No systematic trend was found between the difference and mean of any of the sleep/wake patterns. The numbers of subjects outside the limits of agreements were 4 for TIB, 6 for bedtime, and 8 for wakeup time. Overall, the agreement in sleep/wake patterns as measured by 2-week sleep log between baseline and follow-up period were satisfactory.
Figure 2.
Bland-Altman plots for sleep wake/patterns as measured by 2-week sleep log. Bland-Altman plots of sleep/wake patterns show the differences between baseline and follow-up.21 The mean difference and the limits of agreement (± 1.96 SD) are represented as solid lines.
Cross-Sectional Association of Objective Sleep Indices (3-Day Actigraphy) with Anthropometric and Hormonal Measures at Follow-Up
Among 96 subjects who had actigraphic data, 23 (24.0%) were identified as short sleepers and 38 (39.6%) were identified as poor sleepers. Table 1 shows the sociodemographic characteristics of subjects. The proportion of subjects with tertiary educational level was lower in poor sleepers than good sleepers (13.2% vs 31.0%, P = 0.045); short sleepers were more likely to be males (42.6% vs 16.0%, P = 0.01). Otherwise, there were no differences in sociodemographic characteristics and anthropometric measurements between poor and good sleepers or between short and normal/long sleepers at baseline and follow-up.
Table 1.
Sociodemographic comparisons between short sleepers and normal/long sleepers and between poor sleepers and good sleepers
| ASD ≤ 340 minutes (n = 23) | ASD > 340 minutes (n = 73) | P value | SE < 85% (n = 38) | SE ≥ 85% (n = 58) | P value | |
|---|---|---|---|---|---|---|
| Age, years | 41.1 ± 4.1 | 41.0 ± 4.4 | 0.92 | 41.8 ± 4.9 | 40.4 ± 3.7 | 0.11 |
| Male, N (%) | 9 (42.9) | 12 (16.0) | 0.01* | 9 (23.7) | 11 (19.0) | 0.41 |
| Marital status (married), N (%) | 21 (100) | 70 (93.3) | 0.22 | 37 (97.5) | 55 (94.8) | 0.65 |
| Educational level (tertiary), N (%) | 6 (28.6) | 17 (22.7) | 0.99 | 5 (13.2) | 18 (31.0) | 0.045* |
| Family income> 15,000 HK$/month, N (%) | 8 (38.1) | 18 (24.0) | 0.11 | 10 (26.3) | 16 (27.6) | 0.74 |
| Unemployed or housewife, N (%) | 8 (38.1) | 28 (37.8) | 0.98 | 14 (36.8) | 22 (37.9) | 0.89 |
P < 0.05; ASD, actual sleep duration; SE, sleep efficiency.
Table 2 shows that poor sleepers had higher 24-h urinary NE level (205.7 ± 105.3 nmol/d vs 162.1 ± 55.6 nmol/d, P = 0.03) and a trend of higher E level (37.2 ± 22.9 nmol/d vs 27.4 ± 14.1 nmol/d, P = 0.12). There were no differences in 24-h urinary E and NE levels between short and normal/long sleepers (P > 0.05). No difference was found in 3-day awakening cortisol level between poor and good sleepers (P = 0.52) and between short and normal/long sleepers (P = 0.30). No interaction effect was found between short ASD and poor sleep quality on either 24-h urinary catecholamines or salivary awakening cortisol level by 2-way ANOVA analysis (P > 0.05).
Table 2.
Comparison of anthropometric and biological measures between short sleepers and normal/long sleepers, and between poor sleepers and good sleepers
| ASD ≤ 340 minutes (n = 23) | ASD > 340 minutes (n = 73) | P value | SE < 85% (n = 38) | SE ≥ 85% (n = 58) | P value | |
|---|---|---|---|---|---|---|
| Baseline information | ||||||
| Systolic BP, mm Hg | 118.3 ± 16.9 | 110.4 ± 17.2 | 0.07 | 113.9 ± 19.8 | 112.2 ± 16.6 | 0.65 |
| Diastolic BP, mm Hg | 79.3 ± 10.0 | 74.6 ± 10.9 | 0.07 | 77.8 ± 10.1 | 75.0 ± 11.4 | 0.21 |
| Heart rate | 66.9 ± 10.1 | 69.7 ± 9.9 | 0.26 | 69.3 ± 10.3 | 68.9 ± 9.9 | 0.85 |
| BMI, kg/m2 | 23.7 ± 2.9 | 23.0 ± 2.8 | 0.67 | 23.7 ± 2.7 | 23.1 ± 2.4 | 0.23 |
| Follow-up information | ||||||
| Systolic BP, mm Hg | 118.3 ± 18.7 | 112.0 ± 14.2 | 0.11 | 115.0 ± 16.7 | 113.9 ± 16.0 | 0.75 |
| Diastolic BP, mm Hg | 74.9 ± 14.0 | 73.6 ± 11.5 | 0.69 | 75.2 ± 13.0 | 74.0 ± 11.7 | 0.65 |
| Heart rate | 72.7 ± 9.3 | 71.6 ± 8.4 | 0.61 | 72.7 ± 7.7 | 71.3 ± 9.1 | 0.46 |
| BMI, kg/m2 | 24.7 ± 2.9 | 23.0 ± 2.8 | 0.36 | 23.5 ± 3.0 | 23.0 ± 2.7 | 0.35 |
| Morning salivary cortisolΨ, nmol/L | 14.7 ± 5.3 | 13.4 ± 3.9 | 0.30 | 14.1 ± 5.1 | 14.7 ± 4.8 | 0.52 |
| 24-hour epinephrineΦ#, nmol/d | 34.3 ± 24.0 | 30.0 ± 16.3 | 0.36 | 37.2 ± 22.9 | 27.4 ± 14.1 | 0.12 |
| 24-hour norepinephrineΦ#, nmol/d | 196.8 ± 130.6 | 173.4 ± 58.3 | 0.26 | 205.7 ± 105 | 162.1 ± 55.6 | 0.03* |
P < 0.05; ASD, actual sleep duration; SE, sleep efficiency.
Nonparametric test (Mann-Whitney U test).
N = 90, n = 21 for ASD ≤ 340 minutes, n = 38 for sleep efficiency < 85%.
N = 84, n = 20 for ASD ≤ 340 minutes, n = 34 for sleep efficiency < 85%.
As a previous observational study22 which found significant association between short sleep duration and decreased waking cortisol level was based on the interval between bedtime and wake up time (equivalent to TIB in our study), we also divided the subjects into 2 groups on the basis of their TIB. A lower 3-day awakening cortisol level was found in subjects with short TIB (< 7 h, lowest quartile n = 23) when compared with subjects with normal/long TIB (12.5 ± 3.3 nmol/L vs 15.0 ± 5.3 nmol/L, P = 0.009).
Table 3 shows that short sleepers had delayed bedtime, advanced wake up time, shorter TIB, lower SE, and longer WASO than normal/long sleepers, albeit they had similar SOL with normal/long sleepers. Poor sleepers had shorter ASD, longer SOL, and longer WASO than good sleepers, but bedtime, wake up time, and TIB were similar to those of good sleepers.
Table 3.
Comparison of sleep parameters between short sleepers and normal/long sleepers, between poor sleepers and good sleepers
| ASD ≤ 340 minutes (n = 23) | ASD > 340 minutes (n = 73) | P value | SE < 85% (n = 38) | SE ≥ 85% (n = 58) | P value | |
|---|---|---|---|---|---|---|
| Bedtime | 00:54 ± 01:52 | 23:55 ± 00:47 | < 0.001* | 00:00 ± 01:05 | 00:04 ± 01:00 | 0.85 |
| Wake up time | 06:19 ± 01:21 | 07:20 ± 00:52 | < 0.001* | 07:36 ± 01:01 | 07:35 ± 00:59 | 0.95 |
| Time in bed, min | 401 ± 75 | 478 ± 47 | < 0.001* | 461 ± 77 | 451 ± 60 | 0.48 |
| Actual sleep duration, min | 302 ± 45 | 414 ± 38 | < 0.001* | 342 ± 62 | 405 ± 56 | < 0.001* |
| Sleep efficiency, % | 75.3 ± 11.9 | 86.6 ± 6.2 | < 0.001* | 74.2 ± 8.4 | 89.8 ± 3.2 | < 0.001* |
| Sleep onset latency, min | 15.8 ± 18.8 | 10.4 ± 9.9 | 0.15 | 17.6 ± 18.5 | 8.4 ± 6.4 | < 0.001* |
| Wake after sleep onset, min | 83 ± 16 | 54 ± 21 | 0.02* | 102 ± 43 | 38 ± 15 | 0.005* |
P < 0.05; ASD, actual sleep duration; SE, sleep efficiency.
The unadjusted and adjusted associations of 24-h urinary E and NE levels with objective sleep parameters are shown in Table 4. The models showed that 24-h catecholamines were negatively associated with TIB, ASD, and SE, but positively associated with SOL. For instance, linear model predicted that each 1 minute increase in TIB would result in 0.068 ± 0.027 nmol/d decrease in urinary E and 0.291 ± 0.117 nmol/d decrease in urinary NE. The models also indicated that each 1% increase in sleep efficiency would result in 3.108 ± 0.824 nmol/d decrease in urinary NE. There was a trend for the association between 24-h urinary E and sleep efficiency after adjusting for potential confounders (P = 0.053). However, linear regression analysis showed that sleep parameters, including TIB, were not associated with 3-day awakening cortisol level (data not shown).
Table 4.
Linear regression results for the association between 24-h urinary catecholamines and objective sleep parameters
| 24-hour epinephrine, nmol/L |
24-hour norepinephrine, nmol/L |
|||
|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| Time in bed, min | −0.065 ± 0.027# | −0.068 ± 0.027# | −0.326 ± 0.116# | −0.291 ± 0.117# |
| Actual sleep duration, min | −0.069 ± 0.027# | −0.075 ± 0.029# | −0.333 ± 0.116# | −0.274 ± 0.124# |
| Sleep efficiency (%) | −0.506 ± 0.201# | −0.407 ± 0.207Ψ | −2.912 ± 0.849# | −3.108 ± 0.824# |
| Sleep onset latency, min | 0.550 ± 0.138# | 0.471 ± 0.152# | 3.076 ± 0.564# | 2.990 ± 0.601# |
| WASO, min | 0.044 ± 0.103 | −0.003 ± 0.104 | −0.542 ± 0.445 | −0.579 ± 0.437 |
| Nocturnal time in bed + nap duration, min | −0.064 ± 0.028# | −0.073 ± 0.028# | −0.337 ± 0.124# | −0.359 ± 0.123# |
Adjusted for age, gender, BMI, education level, waist/hip ratio, wave 2 systolic and diastolic BP and plasma creatinine level (enter method).
P < 0.05;
P = 0.053.
Interaction Effects of Sleep Quantity and Quality with Gender on Urinary Catecholamines
Figure 3A-D illustrates the interaction effects between gender and sleep quality/quantity on 24-h urinary catecholamines. The 24-h urinary E and NE were similar between genders when the subjects had good sleep quality (SE ≥ 85%). However, males had a higher NE level than females when they had poor sleep quality (SE < 85%) (P = 0.001) (Figure 3D). There was also a trend for interaction of male gender with 24-h urinary E (P = 0.057) (Figure 3C). However, such kind of interaction effects could not be found between short ASD and gender on 24-h urinary E and NE levels (Figures 3A and 3B) (P = 0.46 and 0.14, respectively). No interaction effect between gender and SE or TIB was found in 3-day morning awakening cortisol level (data not shown).
Figure 3.
Interaction effect between gender and their sleep quantity and quality on 24-h urinary catecholamines. Subjects with the lowest quartile of ASD (≤ 340 min) (n = 23, 24%) were considered short sleepers (A and B). Subjects with sleep efficiency < 85% (n = 34) were considered to have poor sleep quality (poor sleepers) (C and D). ASD, actual sleep duration; SE, sleep efficiency.
Associations of Anthropometric Measurements with Actigraphic Parameters, 24-H Urinary Catecholamines, and Salivary Awakening Cortisol
Among the actigraphic parameters, SOL was significantly correlated with higher BMI, waist/hip ratio, systolic BP, and diastolic BP (correlation coefficients ranged from 0.22 to 0.31; P < 0.05; Table 5). Other sleep parameters were not correlated with anthropometric measurements. Both 24-h urinary E and NE were correlated with waist/hip ratio (r = 0.30 and 0.29, respectively; P for both < 0.01), while 24-h urinary NE was also correlated with diastolic blood pressure (r = 0.29, P < 0.01). No correlation was found between anthropometric measurements and 3-day salivary awakening cortisol.
Table 5.
Correlations of anthropometric measurements with sleep parameters and 24-h urinary catecholamines at follow-up (wave 2)
| BMI | Waist/hip ratio | Systolic blood pressure | Diastolic blood pressure | |
|---|---|---|---|---|
| Nocturnal time in bed | −0.00 | 0.08 | −0.09 | −0.03 |
| Nocturnal sleep duration | −0.02 | 0.05 | −0.10 | −0.05 |
| Sleep efficiency (%) | −0.05 | −0.15 | −0.12 | −0.13 |
| Sleep onset latency | 0.22* | 0.22* | 0.27** | 0.31** |
| Wake after sleep onset | 0.01 | 0.21* | 0.07 | 0.06 |
| Nocturnal sleep duration + nap duration | 0.04 | 0.01 | 0.21 | 0.08 |
| 24-h epinephrine | 0.08 | 0.30** | 0.01 | 0.12 |
| 24-h norepinephrine | 0.20 | 0.29** | 0.16 | 0.29** |
| 3-day morning salivary cortisol | −0.028 | 0.071 | −0.012 | −0.123 |
Pearson correlation coefficients
P < 0.05,
P < 0.01.
DISCUSSION
The merit of current study relies on the exclusion of significant sleep disorders, psychiatric illness, and medical conditions, as well as documentation of objectively measured sleep quantity and quality by actigraphy. In addition, the actigraphy assessment was synchronized with 24-h urinary catecholamines and 3-day awakening cortisol, which allowed us to test the real-time association between habitual sleep/wake patterns and catecholamines and cortisol.
Stability of Sleep/Wake Patterns in Middle-Aged Adults by 2-Week Sleep Log
Little is known about the long-term changes of sleep/wake patterns in middle-aged adults. Middle-aged women were found to sleep 0.4 hour less in a 24-year prospective cohort.23 Using actigraphic measurement, Coronary Artery Risk Development in Young Adults (CARDIA) study found minimal change in inter-individual sleep duration and only mild variability in intra-individual sleep duration in one year interval.24 In general, we found that sleep/wake patterns in this population sample was quite stable across a 3-year period as measured by 2-week sleep log, with correlation coefficients ranging from 0.60–0.79. Bland-Altman plots further showed that there was no systematic trend between the difference and the mean of any of the sleep parameters. Thus, high stability of sleep/wake patterns indicated that the adverse consequences as related to insufficient or poor sleep quality could be persistent and pervasive.
Relationship of Sleep Quantity and Quality with 24-Hour Urinary Catecholamines
Nearly all studies investigating the relationship of sleep deprivation with SNS were based on experimental studies by artificially inducing sleep deprivation in normal subjects.10,11,25 However, sleep deprivation procedure by artificially shortening sleep duration or disrupting sleep was criticized as a potential confounding factor which might be considered an independent stressor.8 The 24-h urinary NE level was considered a reliable indicator of overall sympathetic activity, while 24-h urinary E level was considered an adrenal response to support life-sustaining activity.26 By employing 24-h urinary catecholamines as an indicator of SNS activity, the current study, for the first time, replicated findings of the effects of sleep quantity and sleep quality on SNS among healthy general population. This study with permission of maintaining habitual sleep/wake patterns found consistent results with those experimental studies and suggested that curtailment of sleep duration and poor sleep quality per se were associated with increased SNS activity.8 Nonetheless, the cause-effect relationship between short sleep duration/poor sleep and SNS activity needs further investigation with prospective design.The exact pathophysiology underlying the relationship between insufficient sleep quantity or quality and SNS is unclear. Reduction of REM and SWS in subjects with short sleep duration and poor sleep quality might play a role. Sleep-stage specific effects on plasma catecholamines concentrations were, however, inconsistent in previous studies. While two studies found that plasma NE was lowest in REM sleep, followed by SWS sleep,27,28 another study found no difference in plasma NE and E level among sleep stages, except for a higher NE level during SWS sleep.11 Nonetheless, selective SWS deprivation was found to increase plasma catecholamines25 and decrease in insulin sensitivity,29 which was suggested to be mediated by SNS activity.30
For the relationship between sleep quality and SNS activity, an early study by Vgontzas et al. showed that 24-h urinary norepinephrine output was correlated positively with stage 1 sleep and WASO, but negatively with SWS in 15 chronic insomniacs.31 Subsequently, their group showed that insomniacs had higher nocturnal plasma NE levels than controls and sleep efficiency was negatively correlated with nocturnal NE concentration in insomniacs.32 Sympathetic hyperactivity, as one of the important components of hyperarousal, was suggested to play a critical role in the pathogenesis of insomnia.33 Interestingly, our study found that objectively measured poor sleep quality was correlated with increased SNS activity, even in normal subjects with no complaints of insomnia.
Our study also suggested that male subjects seemed to be more vulnerable than females to the effects of poor sleep quality on their SNS activities (Figure 3). Such gender-dependent effects of catecholamine response were also reported previously.34,35 Compared with women, men were found to have higher plasma catecholamine (NE and E) response to stress (for example, vigorous exercise)34 and alpha2-adrenoceptor blockade.35 Gender-dependent response (male predisposition) of SNS to stress was similar to that found in rodents.36 Another study found that females had higher NE levels than males,37 but such differences could be partly accounted by the difference in the bulk of muscle mass.38 The gender-dependent relationship between catecholamines and sleep quality will need further investigation.
Relationship of Sleep Quantity and Quality with Salivary Cortisol
The effects of sleep deprivation on cortisol level in experimental studies varied from mild elevation, no effect to slight decrease (see more details in review by Meerla et al.8). Overall, sleep deprivation in a less stressful environment showed no effect or even slight decrease,39,40 while more stressful sleep deprivation could lead to increased cortisol levels.12,41 Thus, our study did not show any association between ASD and waking salivary cortisol level (both dichotomized and continuous approaches). In contrast to the results of experimental studies, the Whitehall II study,22 with quite a large sample size of general population, showed that subjects with shorter self-reported sleep duration had lower awakening cortisol level than normal/long sleepers. The lower awakening cortisol apparently contradicted the widely accepted view of conceptualizing short sleep as a potential stressor, which might theoretically lead to higher cortisol levels.41 One proposed explanation was that lower awakening cortisol occurred as a consequence of higher evening cortisol surge in subjects with sleep loss.42 However, the estimation of sleep duration in the Whitehall II study was based on self-reported “sleep duration” derived from the time interval between bedtime and wake up time (equivalent to TIB in this study). In the current study, lower awakening cortisol level was similarly found in those subjects with lowest quartile of TIB, but not in those with shortened ASD. In other words, shortened TIB might be a heterogeneous group of subjects who curtail sleep duration voluntarily or passively and hence with considerable individual differences,43 while ASD might be a combination index of both sleep quantity and quality. In addition, we did not find any association between sleep efficiency and awakening cortisol. Previous studies reported inconsistent relationship between poor sleep/insomnia and awakening cortisol,22,42 while some studies found that insomnia was correlated with increased evening coritsol.44,45 Due to the circadian variability of cortisol secretion, awakening salivary cortisol might not reflect the full profiles of HPA functioning. More sophisticated assessment of the HPA axis, for example, cortisol awakening response, diurnal cortisol slope, or day profile of salivary cortisol46 are needed to investigate the relationship between HPA axis and sleep quality and quantity in future. In this regard, subjects with insomnia symptoms were excluded from our study. Low sleep efficiency (< 85%) indicated poor objective sleep quality rather than insomnia symptoms in this study.
Clinical Implications
The increased SNS activities might mediate the relationship of short sleep duration and poor sleep quality with adverse medical consequences as reflected by their association with the anthropometric measures. As increased SNS activity is suggested to play a role in essential hypertension,47 metabolic syndrome, and obesity,48,49 our findings have significant clinical implications for future intervention and management.
Strengths and Limitations
The strengths of our study stemmed from the exclusion of significant sleep, psychiatric, and medical problems, which would allow us to minimize these confounding influences. Second, objective measures of sleep parameters were more reliable than subjective report, especially for sleep quality and nocturnal awakening time. To the best of our knowledge, this is the largest study testing the association between objective sleep and neuroendocrine variables in general population, but our findings should be replicated in other ethnic groups with a larger sample size. A major shortcoming was the relatively low response rate (50%) with female predominance among participants at the follow-up study, which may limit the conclusion of gender-dependent sensitivity of sleep quality on the activation of SNS. In this regard, a previous study suggested that female subjects slept better than male subjects objectively.50 In our study, both genders had similar sleep/wake patterns as measured by sleep log and 3-day actigraphy, but male subjects tended to have shorter objective sleep duration. Although we excluded those subjects with habitual snoring and other symptoms related to obstructive sleep apnea clinically,15,16 the absence of polysomnography could not allow us to exclude subtle OSA definitely, which might confound the SNS activity.51 Finally, as both cortisol and catecholamines are major components involving in stress response,9 the lack of subjective stress appraisal limited the ability to delineate the detailed mechanism underlying the association among stress, sleep, anthropometric measures, and SNS activity. In addition, the lack of stress assessment could not differentiate those genuine habitual short sleepers from those who curtail their sleep duration under stressful situation passively.
CONCLUSION
Healthy middle-aged adults had a rather stable sleep/wake patterns over a period of nearly 3-year interval as measured by 2-week sleep log. Short sleep duration and poor sleep quality were associated with increased 24-hour urinary catecholamines. The inter-correlations among sleep quantity/quality, 24-hour urinary catecholamines, and anthropometric measurements indicated that increased SNS activity might play a critical role in the pathogenesis underlying the relationship of sleep disturbances with subsequent cardiovascular and metabolic consequences. Further interventional studies will be required to see whether sympathetic hyperactivity could be readily reversed with lengthened sleep duration and improved sleep quality.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. B. Zhang has received research support from AstraZeneca and Pfizer. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank the cooperation and participation of all the participants as well as the logistic support of Ms. Cherry Chiu and Ms. Edith Kwok of endocrine unit.
REFERENCES
- 1.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 2.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–7. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wing YK, Li SX, Li AM, Zhang J, Kong AP. The effect of weekend and holiday sleep compensation on childhood overweight and obesity. Pediatrics. 2009;124:e994–e1000. doi: 10.1542/peds.2008-3602. [DOI] [PubMed] [Google Scholar]
- 8.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 10.Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol. 1997;273:H1761–8. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- 11.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Li AM, Fok TF, Wing YK. The roles of parental sleep/wake patterns, socio-economic status and daytime activities in the sleep/wake patterns of children. J Pediatr. 2010;156:606–12. doi: 10.1016/j.jpeds.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30:1085–95. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Li AM, Kong AP, Lai KY, Tang NL, Wing YK. A community-based study of insomnia in Hong Kong Chinese children: prevalence, risk factors and familial aggregation. Sleep Med. 2009;10:1040–6. doi: 10.1016/j.sleep.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Wing YK, Li RH, Lam CW, Ho CK, Fong SY, Leung T. The prevalence of narcolepsy among Chinese in Hong Kong. Ann Neurol. 2002;51:578–84. doi: 10.1002/ana.10162. [DOI] [PubMed] [Google Scholar]
- 17.Li SX, Zhang B, Li AM, Wing YK. Prevalence and correlates of frequent nightmares: a community-based 2-phase study. Sleep. 2010;33:774–80. doi: 10.1093/sleep/33.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickering TG. Mental stress as a causal factor in the development of hypertension and cardiovascular disease. Curr Hypertens Rep. 2001;3:249–54. doi: 10.1007/s11906-001-0047-1. [DOI] [PubMed] [Google Scholar]
- 19.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 20.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22:85–93. doi: 10.1002/uog.122. [DOI] [PubMed] [Google Scholar]
- 22.Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–9. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjorkelund C, Bengtsson C, Lissner L, Rodstrom K. Women′s sleep: longitudinal changes and secular trends in a 24-year perspective. Results of the population study of women in Gothenburg, Sweden. Sleep. 2002;25:894–6. [PubMed] [Google Scholar]
- 24.Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30:793–6. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiemeier H, Pelzer E, Jonck L, Moller HJ, Rao ML. Plasma catecholamines and selective slow wave sleep deprivation. Neuropsychobiology. 2002;45:81–6. doi: 10.1159/000048681. [DOI] [PubMed] [Google Scholar]
- 26.Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247:E35–40. doi: 10.1152/ajpendo.1984.247.1.E35. [DOI] [PubMed] [Google Scholar]
- 27.Rasch B, Dodt C, Molle M, Born J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology. 2007;32:884–91. doi: 10.1016/j.psyneuen.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30:71–6. doi: 10.1161/01.hyp.30.1.71. [DOI] [PubMed] [Google Scholar]
- 29.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dijk DJ. Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc Natl Acad Sci U S A. 2008;105:1107–8. doi: 10.1073/pnas.0711635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 32.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 33.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Davis SN, Galassetti P, Wasserman DH, Tate D. Effects of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J Clin Endocrinol Metab. 2000;85:224–30. doi: 10.1210/jcem.85.1.6328. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt ME, Matochik JA, Goldstein DS, Schouten JL, Zametkin AJ, Potter WZ. Gender differences in brain metabolic and plasma catecholamine responses to alpha 2-adrenoceptor blockade. Neuropsychopharmacology. 1997;16:298–310. doi: 10.1016/S0893-133X(96)00264-3. [DOI] [PubMed] [Google Scholar]
- 36.Uji M, Yoshida K, Shintani-Ishida K, Morimoto K. Sex difference in norepinephrine surge in response to psychological stress through nitric oxide in rats. Life Sci. 2007;80:860–6. doi: 10.1016/j.lfs.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg U, Frankenhaeuser M. Stress and workload of men and women in high-ranking positions. J Occup Health Psychol. 1999;4:142–51. doi: 10.1037//1076-8998.4.2.142. [DOI] [PubMed] [Google Scholar]
- 38.Masi CM, Rickett EM, Hawkley LC, Cacioppo JT. Gender and ethnic differences in urinary stress hormones: the population-based Chicago Health, Aging, and Social Relations Study. J Appl Physiol. 2004;97:941–7. doi: 10.1152/japplphysiol.00256.2004. [DOI] [PubMed] [Google Scholar]
- 39.Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 1999;51:205–15. doi: 10.1046/j.1365-2265.1999.00763.x. [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Pejovic S, Zoumakis E, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–61. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology. 2004;29:1184–91. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14:239–47. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodenbeck A, Huether G, Ruther E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett. 2002;324:159–63. doi: 10.1016/s0304-3940(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 45.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 46.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–36. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177:275–84. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 48.Snitker S, Macdonald I, Ravussin E, Astrup A. The sympathetic nervous system and obesity: role in aetiology and treatment. Obes Rev. 2000;1:5–15. doi: 10.1046/j.1467-789x.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 49.Mancia G, Bousquet P, Elghozi JL, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–20. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
- 50.Bixler EO, Papaliaga MN, Vgontzas AN, et al. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res. 2009;18:221–8. doi: 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]