Abstract
Morphine has been shown to alter gene expression of the major histocompatibility complex, class II (MHC-II) in circulating rat immunocytes. Here, we demonstrate that a single morphine injection (10 mg/kg) reduces basal MHC-II protein expression on circulating B lymphocytes by 33%, while also impairing the ability of B lymphocytes to increase MHC-II upon interleukin-4 induction. As these data implicate opioids in the regulation of antigen presentation, studies were undertaken to examine the potential mechanisms through which morphine exerts this suppressive effect. Central injection studies utilized Tyr-D-Ala-Gly-(me) Phe-Gly-ol (DAMGO), an opioid receptor agonist, which mimicked morphine’s effect on MHC-II, while D-Phe-Cys_Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) pretreatment, prior to morphine, blocked the suppression of MHC-II. As central opioid receptor activation results in the activation of the hypothalamic–pituitary– adrenal axis, thereby, signaling increased circulating corticosterone levels, we examined whether MHC-II expression was suppressed after incubation with corticosterone at concentrations similar to those observed after morphine. Interestingly, corticosterone dramatically decreased basal MHC-II (88%) expression while completely preventing the induction of MHC-II. Additionally, MHC-II suppression was absent in morphine-treated adrenalectomized animals. Since prolonged morphine exposure has previously been shown to result in tolerance to both the steroidogenic and immunosuppressive effects of morphine, the effect of prolonged morphine exposure on MHC-II was also examined. Interestingly, MHC-II expression is no longer suppressed after chronic morphine, while morphine withdrawal results in both a renewed increase in circulating corticosterone levels and a renewed suppression of MHC-II in previously tolerant animals. Taken together, these data strongly implicate corticosterone in mediating the suppressive effects of morphine on circulating B-lymphocyte MHC-II expression.
Keywords: B lymphocytes, morphine, MHC class II, IL-4, HPA, corticosterone
Morphine suppresses immunity in both humans and animal models, with studies demonstrating that a single morphine injection induces lymphopenia and alters normal immune function (e.g., lymphocyte proliferation, antibody production, natural killer cell activity) (Bayer et al. 1990; Lockwood et al. 1994). To better understand the effect of morphine on immunity, previous studies conducted by our group examined morphine-induced gene expression changes in circulating rat leukocytes. Results indicated that morphine altered the expression of several genes related to antigen presentation, specifically genes coding for proteins associated with the major histocompatibility complex, class II (MHC-II) (Beagles et al. 2004). MHC-II molecules are important regulators of both immune cell development and function. Studies of MHC-II knockout mice demonstrate that the loss of MHC-II produces a marked reduction in CD4+ T-lymphocyte number (Madsen et al. 1999). In addition, MHC-II is a key molecule involved in immune cell development, activation, and survival; therefore, the suppression of MHC-II by morphine may represent a significant deficit in the animal’s ability to mount an immune response (Forsgren et al. 1984; Lokshin et al. 2002; Friedl et al. 2005). Given the important role that MHC-II plays in immune cell development, activation, and proliferation, the suppression of MHC-II expression by morphine may provide insight into the immune dysfunction that has been observed in both clinical and animals studies after morphine exposure.
Several measures of morphine-induced immunosuppression have been shown to be modulated through activation of central opioid receptors. It remains to be determined how morphine treatment decreases MHC-II and if the mechanism involves activation of central opioid receptors or direct activation of immune cell opioid receptors. In addition, while direct activation of lymphocyte opioid receptors could easily account for decreased MHC-II expression after morphine, it remains unclear how central opioid receptor activation might effect changes in MHC-II expression on peripheral immune cells. A potential mechanism for this change might be due to central opioid receptor activation of the hypothalamic-pituitary-adrenal (HPA) axis and/or the sympathetic nervous system (SNS) leading to increased release of inflammatory mediators, including numerous hormones, neurotransmitters, and cytokines, several of which are known to, by themselves, have effects on MHC-II expression (Deitch et al. 1988; Zwilling et al. 1992; Elftman et al. 2007). Therefore, as previous studies of the effects of stress on MHC-II expression have shown that stress hormones directly signal decreased expression of MHC-II on APCs, including circulating B lymphocytes (Zhang et al. 1998), we sought to determine if morphine signaled a decrease in B-lymphocyte MHC-II expression through increases in HPA-dependent stress hormones. Prolonged drug exposure has been shown to result in tolerance to both the steroidogenic and immunosuppressive effects of morphine; however, lymphocytes from these animals display an enhanced sensitivity to the effects of a novel stressor on measures of lymphocyte proliferation (Bayer et al. 1994). This enhanced sensitivity is also apparent in tolerant animals that are withdrawn from morphine, as these animals display both significantly increased plasma corticosterone and suppressed lymphocyte proliferative responses within 24 h of drug cessation (Avila et al. 2004). Circulating steroid concentrations tolerize to chronic morphine administration while withdrawal from morphine results in renewed increases in circulating corticosterone, we also examined the effect of both chronic morphine treatment and morphine withdrawal on MHC-II expression.
The studies presented here examine whether morphine-induced suppression of MHC-II expression is mediated through central opioid receptors. Additional studies examine whether activation of central opioid receptor-mediated pathways is sufficient to signal a decrease in peripheral B-lymphocyte MHC-II expression observed after morphine administration.
Materials and methods
Animals
Pathogen-free 2–3-month-old male Sprague–Dawley rats (200–225 g upon receipt) were obtained from Taconic Laboratories (Germantown, NY, USA). Animals were housed two to three per cage with microisolater tops and provided food (Purina rat chow) and water ad libitum. The light cycle was regulated automatically (12 h light/dark cycle) and temperature was maintained at 23±1°C. All animals were allowed to acclimate to this environment for 1 week prior to experimental manipulations. The Georgetown University Animal Care and Use Committee approved all animal studies in accordance with the guidelines adopted by the National Institutes of Health.
Adrenalectomized animals
Adrenalectomized Sprague–Dawley rats were purchased from Taconic Laboratories (Germantown). Animals were adrenalectomized 4 days prior to shipment by Taconic and upon receipt were provided saline (0.9%) drinking solution supplemented with corticosterone 21-acetate (25 µg/ml in 0.2% ethanol) to restore basal corticosterone levels. There was no significant difference in weight between the adrenalectomized animals (271±2.9 g) compared to intact control animals (258±2.8 g).
Reagents
Morphine sulfate was generously provided by the National Institute on Drug Abuse (Research Triangle Park, NC, USA). All vehicle and drug solutions were made using sterile, pyrogen-free 0.9% saline. Corticosterone, naltrexone hydrochloride, Tyr-D-Ala-Gly-(me) Phe-Gly-ol (DAMGO), and D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) were also obtained from Sigma-Aldrich (St. Louis, MO, USA). For central injections, naltrexone hydrochloride, DAMGO, and CTOP were diluted in sterile isotonic saline containing 0.1% bovine serum albumin (BSA) (vehicle). Recombinant rat interleukin (IL)-4 (rrIL-4) was purchased from Peprotech Inc (Rocky Hill, NJ, USA).
Drug administration and withdrawal
Acute morphine treatment consisted of a single subcutaneous (s.c.) injection of morphine sulfate (10 mg/kg) dissolved in sterile isotonic saline 2 h prior to sacrifice. Chronic morphine treatment began after an acclimatization period of 1 week and consisted of twice daily morphine administration (s.c.), with doses spaced 10–12 h apart. Morphine doses were as follows (AM/PM dose, in mg/kg): day 1, 10/10; day 2, 10/ 20; day 3, 20/20; day 4, 20/30; day 5, 30/30; day 6, 30/40; day 7, 40/40; day 8, 40/40; and day 9, 10 mg/kg challenge dose. This protocol has been previously demonstrated to produce tolerance to the analgesic and steroid increasing effects of morphine (Bayer et al. 1994). The injection volume for all systemic studies was 1 ml/kg. Control injections consisted of 0.3 ml sterile saline administered subcutaneously.
Central cannulation surgeries and microinjection procedures
Animals were anesthetized with Equithesin anesthesia (3 mg/kg, i.p.) and injected intramuscularly with 0.1 ml gentamicin sulfate (40 mg/ml) to prevent infection. Animals were surgically implanted with a single 5.0-mm cannula into the lateral ventricle. Cannulae were placed in the following coordinates relative to bregma: AP −0.9, ML −1.6, DV −3.1; (Paxinos and Watson 1997). All animals were allowed to recover for approximately 1 week prior to experimentation. Microinjection of drugs was accomplished in freely moving, awake animals using a 28-gauge injection cannula, which extended 1 mm below the guide cannula and was connected to a Hamilton syringe by polyethylene tubing filled with vehicle or test substance. The injection volume for all centrally administered drugs was 5 µl, administered over 2 min using a microinfusion pump (kd Scientific, Model 101, New Hope, PA, USA) set to dispense 2.5 µl/min. Internal cannula remained in place for an additional minute to ensure complete drug dispersal into the ventricle. Animals were then returned to their home cages for the duration of the treatment period. Upon completion of experiments involving injection into the lateral ventricle, the brains were injected with 1% fast green dye (5 µl) through the injection cannula. Blunt dissection of the brain was used to verify cannula placement within the lateral ventricle.
Analgesic testing
Antinociception was measured by the radiant heat tail-flick method, as previously described (Hernandez et al. 1993). All animals were acclimated to handling and the tail-flick device for 2–3 days prior to experimental manipulations. Light intensity was controlled to provide a pre-drug latency between 2 and 3 s. A cut-off of 15 s was employed to prevent damage to tail tissue. These conditions have previously been described as a high intensity stimulus readily altered by µ-receptor agonists (Millan 1989). For studies employing opioids, nociception was measured 30 min after either morphine or agonist infusion.
Leukocyte isolation
Animals were sacrificed via rapid decapitation and whole trunk blood was collected in 50 ml conical tubes containing 100 µl of heparin (1,000 U/ml). On average, approximately 7–10 ml of trunk blood was collected per animal. Blood was diluted 1:2 with sterile isotonic saline and placed on a NycoPrep™ 1.077A gradient (Oxoshield, Norway) (5 ml NycoPrep™ for every 6 ml of diluted blood). The gradient was then centrifuged for 20 min at 500 × g, and the buffy coat containing the circulating peripheral blood mononuclear cells (PBMC) was obtained from the interface between the NycoPrep™ and the media. After a series of washes with 1 × phosphate buffered saline (PBS), the cell pellet was re-suspended in RPMI-1640 media supplemented with 1% fetal bovine serum and 0.2% gentamicin. Isolated cells were counted using a Coulter Z1 Counter and re-suspended with supplemented media to a concentration of 2 × 106 cells/ml.
In vitro IL-4
Following leukocyte isolation, 1 × 106 cells were plated out into each cylindrical flat-bottomed well of a 24-well cell culture plate. Cells were then incubated with recombinant rat (rrIL-4; 0–1,000 ng/ml) overnight. Non-adherent cells were removed from the cell culture plate and placed into a 5-ml polystyrene round bottom tube (Becton Dickson, NJ, USA). Adherent cells were then collected from the cell culture plate using 200 µl of ice cold 1% trypsin–EDTA solution. Cells were exposed to trypsin–EDTA for less than 30 s prior to trypsin inactivation with RPMI-1640 media containing 1% fetal calf serum. Adherent cells were added to the cell suspension in the 5-ml tube. Cultured cells were then centrifuged for 5 min at 200 × g. The resulting cell pellet was washed twice in 1 × PBS and re-suspended in 200 µl of supplemented RMPI-1640 media.
Determination of MHC-II expression on leukocyte populations by FACS analyses
Expression of MHC-II RT.1B β on monocytes and B lymphocytes was evaluated by 2-color immunofluorescence. Surface and cytoplasmic staining were performed with the following monoclonal antibodies: fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against rat MHC-II RT.1B β [Becton-Dickinson-Pharmingen (BD) mouse IgG OX-6 clone], FITC-labeled anti-rat CD68 (AbD Serotec mouse IgG ED1 clone), FITC-labeled anti-rat CD68 (Biosource mouse IgG ED1 clone), phycoerythrin (PE)-conjugated monoclonal antibodies against rat CD45RA (Caltag/Invitrogen mouse IgG1 OX-33 clone), and PE-labeled anti-MHC-II RT.1B β (BD mouse IgG1 OX-6 clone). FcR blocking was not used as the culture medium contained fetal calf serum, which serves to reduce nonspecific binding. After two washes with 1 × PBS, 1 × 106/ml cells were re-suspended in 100 µl of RPMI-1640 media containing 1% fetal bovine serum and 0.2% gentamicin. Suspended cells were incubated with MHC-II monoclonal antibodies in addition to counterstaining with cell surface markers for lymphocytes (CD45RA) or monocytes (CD68). Cells were incubated with antibodies for 1 h at room temperature. After incubation with antibodies, cells were washed twice in 1 × PBS and fixed with 1% paraformaldehyde prior to analysis. As positive controls, cell suspensions were incubated separately with each monoclonal-labeled antibody. Samples were protected from light and stored at 4°C until analysis.
Flow cytometry analysis
Flow cytometry analysis was performed using a FACSort flow cytometer (Becton Dickinson FACS system, San Jose, CA, USA) with a single excitation source (200 mW argon laser). Accurate physical and immunological gating was performed using the characteristic side and forward scatter of lymphocytes and monocytes as shown in Supplemental Figure 1. A back gating strategy was employed whereby isolated leukocytes with no fluorescent antibody were used to exclude cell autofluorescence. Single antibody-stained leukocytes were used to set each individual fluorescence gate to allow for back gating. Instrument settings were constant, and alignment for forward scatter and green (FL1) and red (FL2) fluorescence was monitored before each experiment. Flow cytometry allows for individual cell analysis measurement of fluorophore-conjugated antibodies to cell surface or intracellular markers. This measurement is determined by measuring the amount or intensity of the fluorescence excited by a laser on each cell. These measurements are collected to form a population histogram of fluorescent intensity (Supplemental Figure 2). The mean fluorescent intensity (MFI) is a measure of central tendency and as such is subject to all of the caveats typically associated with measures of central tendency (e.g., homogeneous population, normal distribution, etc.). One hundred thousand cells of the population of interest were analyzed per sample, and only double-positive cells identified by dot-plot histograms were used to determine the population histograms and determine the mean fluorescent intensity.
Serum collection and corticosterone measurement
Blood was collected at time of sacrifice in 15 ml tubes, allowed to clot at room temperature, and centrifuged at 500 × g for 20 min at 4°C for serum collection. Serum samples were stored at −20°C until assayed via solid phase 125I corticosterone radioimmunoassay purchased from ICN Pharmaceuticals (Costa Mesa, CA, USA).
Statistical and data analyses
For the central studies, only animals whose cannulae were determined to be accurately placed in the lateral ventricle were used for data analyses. Of the 44 animals used for central cannulation experiments, 9% were omitted from analysis due to incorrect cannula placement. Treatment groups were compared using Student’s t test or one-way ANOVA and Newman–Keuls test for post hoc analysis. Control MFI values may vary for FACS analyses, as each experiment is determined based on a background reading for that particular experiment. Depending on the conditions of each experiment, the background MFI value may alter; however, the relative values in relation to this background value are consistent over all experiments. For this reason when relative comparisons were made, the results are expressed as a percent of the mean control MFI. For all data expressed as a percentage of control, the mean control MFI value for each experiment is given. For all parameters, any value that was greater than two standard deviations from the mean of the entire group was omitted as an outlier. Using these criteria, the incidence of statistical outliers was low with only 9 out of 625 MFI measures (1.44%) for the reported experiments excluded as a statistical outlier. For all studies, a level of p<0.05 was considered statistically significant.
Results
The effects of acute morphine treatment on basal and IL-4-induced B-lymphocyte MHC-II expression
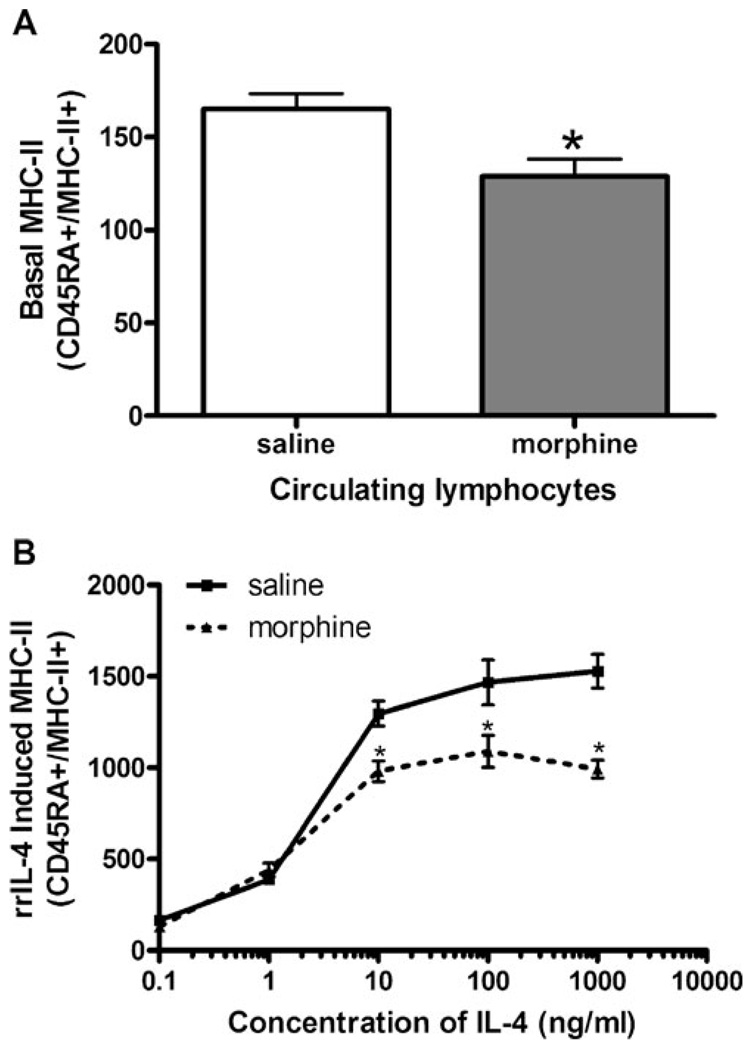
Previously published data from our laboratory have demonstrated that morphine decreases MHC-II gene expression and messenger RNA as determined by gene chip microarray and by reverse transcriptase PCR (Beagles et al. 2004), suggesting that the effects of morphine on antigen-dependent immune functions may be related to decreases in MHC-II expression. Therefore, we examined MHC-II protein expression on circulating APCs after a single subcutaneous injection of morphine (10 mg/kg). Two hours following morphine or saline administration, circulating mononuclear cells from control and morphine-treated animals were isolated and stained using antibodies against MHC-II RT.1B β (FITC- or PE-conjugated) and cell surface markers for either B lymphocytes (CD45RA) or monocytes (CD68). Acute morphine treatment significantly reduced basal MHC-II expression levels by 33% on B lymphocytes compared to saline-treated control animals (Fig. 1a). The effect of morphine was specific to B lymphocytes, as acute morphine treatment had no significant effect on basal MHC-II expression on peripheral monocytes (data not shown). The decrease observed in MHC-II expression was not due to lymphopenia as both saline and morphine treated animals had a similar percentage of B cells and monocytes (data not shown). In addition, the results are not thought to be due to changes in trafficking of B lymphocytes as our previous studies have examined the percentages of different immune cell types in whole blood and spleen following a 2-h morphine treatment using FACS analysis and found no significant differences in the percentages of leukocytes in either blood or spleen (data not shown), suggesting that the decrease in MHC-II is due to a decrease in cell surface protein expression and not changes in trafficking of specific immune cells into different physiological compartments.
Fig. 1.
Basal MHC-II expression after treatment with a single dose of morphine in circulating B lymphocytes. a Groups of Sprague–Dawley rats (n=6) were administered either saline or morphine (10 mg/kg, s. c.). Two hours after morphine injection, the rats were rapidly decapitated and peripheral blood mononuclear cells were isolated from whole blood. Leukocytes were isolated and incubated with fluorochrome-conjugated antibodies as follows: anti-rat CD45RA-PE (lymphocytes) and anti-rat MHC-II RT.1B β FITC (MHC-II). Stained cells were fixed using 1% paraformaldehyde prior to analysis using a FACSort flow cytometer. Data are representative of three separate experiments and are expressed as MFI ± SEM. *p<0.05 compared to all other treatment groups (Student’s t test). b rrIL-4 to induce MHC-II expression in B lymphocytes after a single injection of morphine. Sprague–Dawley rats (n=6 per group) were administered either saline or morphine (10 mg/kg, s.c.). Two hours after the subcutaneous injection, peripheral blood mononuclear cells were isolated and cultured at 37°C and 8% CO2 with rrIL-4 (0.1–1,000 ng/ml). Cells were collected and washed prior to being incubated with fluorochrome-conjugated antibodies, as described above. Fixed cells were analyzed using a FACSort flow cytometer. Data are expressed as MFI ± SEM. Data repeated in two subsequent experiments. *p<0.05 compared to control (ANOVA, Newman–Keuls)
Since morphine treatment reduced basal MHC-II expression, it was of interest to determine if morphine would also inhibit the ability of APCs to increase MHC-II expression upon receiving a standard stimulation signal. While interferon (IFN)-γ is a potent inducer of MHC-II expression on nonlymphoid tissue such as epithelium, endothelium, and macrophages, Roos et al. (1998) previously demonstrated that IL-4 produced a much more robust activation of MHC-II expression on both rat B lymphocytes and monocytes than did IFN-γ. In addition, Grainger et al. (1995) have previously shown that rrIL-4 applied in culture quickly up-regulates the expression of MHC-II on B lymphocytes. Therefore, utilizing this standard stimulation signal, we examined the ability of B lymphocytes from either morphine-(10 mg/kg) or saline-treated animals to up-regulate MHC-II expression upon exposure to increasing concentrations of rrIL-4. As shown in Fig. 1b, B lymphocytes from saline-treated control animals increased MHC-II expression by up to ninefold upon exposure to increasing concentrations of rrIL-4 (10–1,000 ng/ml). However, morphine treatment significantly decreased B-cell up-regulation of MHC-II expression in response to IL-4 when compared with saline-treated controls by as much as 36% (Fig. 1b). Therefore, as acute morphine administration decreased both basal and IL-4-induced MHC-II expression on peripheral B lymphocytes, it was of interest to determine if morphine was acting directly on B lymphocytes or indirectly through central opioid pathways to affect the change in MHC-II expression (Grainger et al. 1995).
Effect of pretreatment with an opioid receptor antagonist, naltrexone, on MHC-II expression in morphine-treated animals
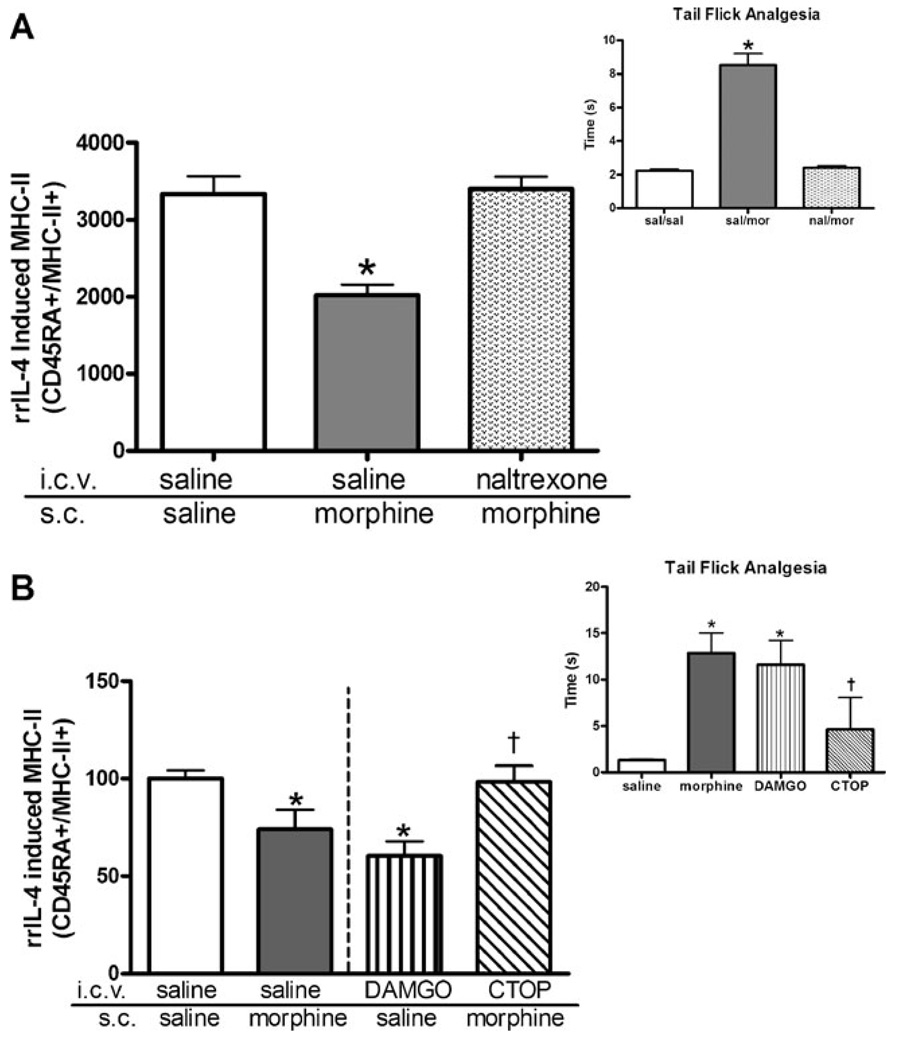
Previous studies examining the immunosuppressive effects of morphine have demonstrated that this suppression is mediated through central µ-opioid receptors (Mellon and Bayer 1998). As direct exposure of cultured naive PBMCs to morphine did not affect either basal or IL-4-induced MHC-II expression (data not shown), studies utilizing a central injection of the opioid receptor antagonist, naltrexone, were undertaken. Animals were administered a single i.c.v. injection of naltrexone (10 µg/5 µl) or vehicle (sterile saline containing 0.1% BSA) 30 min prior to receiving a peripheral challenge injection of either morphine (20 mg/kg) or saline. As shown in Fig. 2a, insert, analgesic responses to morphine were completely antagonized by pretreatment with naltrexone. As previously shown in Fig. 1, acute peripheral injection of morphine significantly suppressed IL-4-induced expression of MHC-II by greater than 30% (Fig. 2a). However, this effect was completely blocked in animals by pretreatment with centrally administered naltrexone as IL-4 stimulation of MHC-II expression was of equal magnitude in B lymphocytes from both saline-treated animals and naltrexonepretreated, morphine-injected animals (Fig. 2a).
Fig. 2.
Central µ-opioid receptors mediate the effect of acute morphine on MHC-II expression. a Central naltrexone blocks the suppressive effect of morphine on IL-4-induced MHC-II expression. Indwelling cannulae were placed in the lateral ventricle of each rat (n=7 per group) 1 week prior to experimentation. A central injection of either saline or naltrexone (10 µg/5 µl/2 min) was administered 30 min prior to the animals receiving a single peripheral injection of either saline or morphine (20 mg/kg, s.c.). Animals were then tested for an analgesic response using tail-flick analgesia, as described in “Materials and methods” (inset). Two hours after the s.c. injection, peripheral blood mononuclear cells were isolated and suspended to a concentration of 2 × 106 cells/ml in supplemented media. Isolated cells (1 × 106 cells/ml) were cultured with rrIL-4 (1,000 ng/ml) overnight. Cultured cells were collected, washed twice in phosphate-buffered saline, and incubated for 1 h at room temperature with fluorochrome-conjugated antibodies against CD45RA and MHC-II RT.1B β. Isolated cells were fixed prior to FACS analysis. Data are expressed as MFI ± SEM. *p<0.05 compared to all other treatment groups (ANOVA, Newman–Keuls). b DAMGO mimics the effects of peripheral morphine injection while a µ-opioid antagonist, CTOP, blocks the effect of peripheral morphine on IL-4-induced MHC-II suppression. Cannulated animals (n=6) were centrally injected with either saline, DAMGO (20 nmol), or CTOP (5 µg/µl). After 30 min, animals were challenged with a peripheral injection of either saline or morphine (20 mg/kg, s.c.) as indicated. Animals were tested for analgesic response using tail-flick analgesia, as described in “Materials and methods” (inset). Two hours after the subcutaneous injection, peripheral blood mononuclear cells were isolated from whole blood prior to being cultured with rrIL-4 (1,000 ng/ml) for 24 h. Cultured cells were collected and washed prior to being incubated for 1 h with fluorochrome-conjugated antibodies against CD45RA and MHC-II RT.1B β. Data were expressed as percent of control MFI ± SEM with the saline-treated control value of 501±21.9. *p<0.05 compared to saline treatment group (Student’s t test). †p<0.05 compared to morphine treatment group (Student’s t test)
Effect of central µ-opioid receptor activation on induced B-lymphocyte MHC-II expression
To further clarify the role of central opioid receptors on the peripheral expression of MHC-II on B lymphocytes, central injections of a µ-opioid receptor-specific agonist were used. Animals received a single central injection of DAMGO, a µ-specific opioid receptor agonist, into the lateral ventricle. A 20-nmol dose of DAMGO was selected as it had previously been shown to significantly decrease lymphocyte proliferation and induce analgesic responses similar to acute morphine treatment (Mellon and Bayer 1998). Centrally administered DAMGO displayed similar analgesic responses as morphine (Fig. 2b). As shown in Fig. 2b, a single central injection of DAMGO alone was sufficient to suppress MHC-II expression on circulating B lymphocytes. DAMGO-injected animals displayed a 40% decrease in MHC-II expression compared to saline-treated controls. Interestingly, animals that received central DAMGO injections display a greater suppression of peripheral MHC-II expression than morphine-treated animals (DAMGO 40% suppression; morphine 31% suppression).
Effect of pretreatment with a central µ-opioid receptor antagonist prior to morphine treatment on induced MHC-II expression
To further elucidate the role of central µ-opioid receptors in the suppression of MHC-II expression on circulating B lymphocytes, a µ-opioid receptor antagonist, CTOP, was administered i.c.v. into cannulated animals prior to a challenge injection of a high dose of morphine (20 mg/kg, s.c.). CTOP is a cyclic somatostatin octapeptide analog with high affinity and selectivity toward µ-opioid receptors. Analgesia was measured using a tail-flick paradigm to ensure that the central injection was effective in blocking the analgesic actions of morphine. As shown in Fig. 2b, CTOP pretreatment blocked the analgesic effects of morphine since the CTOP-pretreated animals demonstrate decreased latency responses similar to saline-treated controls. Two hours after treatment with either morphine or saline, PBMCs were collected and cultured, as previously described in “Materials and methods”, with 1 µg/ml of rrIL-4. Again, peripheral morphine treatment decreased MHC-II expression by as much as 31% on rrIL-4-induced peripheral B lymphocytes (Fig. 2b). Similar to naltrexone, central CTOP pretreatment blocked the suppression of MHC-II expression by morphine in response to IL-4 to levels comparable to saline-treated control animals (Fig. 2b). Collectively, these findings strongly suggest that the decrease in MHC-II expression after morphine administration is centrally mediated through the µ-opioid receptor. As previous studies have shown that central µ-opioid receptor activation mediates peripheral immune function through activation of both the HPA and the SNS, studies were undertaken to examine a potential centrally activated pathway that could be mediating the alterations in peripheral immune cells.
Direct effect of in vitro corticosterone on the basal and induced expression of MHC-II
Morphine administration has been shown to increase circulating corticosterone levels 2 h after morphine administration (Table 1). In addition, central injections of opioid receptor agonists have also been shown to increase circulating corticosterone levels in rats within 2 h after the injection (Table 1) (Mellon and Bayer 1998). As increases in circulating corticosterone levels have previously been associated with decreases in basal MHC-II expression (Weiss et al. 1998; Schwab et al. 2005), experiments were undertaken to determine if the decrease in MHC-II expression after morphine might be mediated by increases in circulating corticosterone levels during 2 h of morphine treatment. Because the effects of corticosterone on MHC-II expression after stimulation with rrIL-4 have not previously been examined, we studied the in vitro effects of corticosterone on basal and IL-4-induced MHC-II expression. In this experiment, the corticosterone concentrations ranged from 100 to 1,000 ng/ml as this reflected the biological levels of corticosterone observed after treatment with morphine in rats (Table 1). In addition, as our purpose was to reproduce the steroid exposure after morphine treatment, we examined the level of MHC-II expression after 2 h of corticosterone treatment. Isolated PBMCs from naive animals were incubated with increasing concentrations of corticosterone (100–1,000 ng/ml) for 2 h and were washed. The cells were incubated with rrIL-4 for 24 h to determine if direct exposure to corticosterone decreased IL-4-induced B-lymphocyte MHC-II expression. Corticosterone decreased basal MHC-II expression on naive B lymphocytes by as much as 88% at all concentrations (100– 1,000 ng/ml) (data not shown). Further, corticosterone blocked the ability of the lymphocytes to up-regulate MHC-II expression at all concentrations examined after treatment with rrIL-4 (data not shown).
Table 1.
Circulating corticosterone levels in rats after experimental manipulations
| Treatment group (i.c.v./s.c.) | Corticosterone (ng/ml) |
|---|---|
| Saline/saline | 30±5 |
| Saline/morphine | 267±41 |
| Naltrexone/saline | 28±3 |
| Naltrexone/morphine | 53±15 |
| DAMGO/saline | 254±38 |
| CTOP/saline | 70±23 |
| CTOP/morphine | 35±20 |
Whole trunk blood was collected from treated animals (n=5–7 per group) at the time of sacrifice in 15 ml tubes and allowed to clot until it could be spun at 500×g for 20 min at 4°C for serum collection. Serum samples were stored at −20°C until assayed via solid phase 125 I corticosterone radioimmunoassay purchased from ICN Pharmaceuticals (Costa Mesa). Data are presented as the mean corticosterone level (ng/ml) ± SEM
Effect of morphine on MHC-II expression in adrenalectomized rats
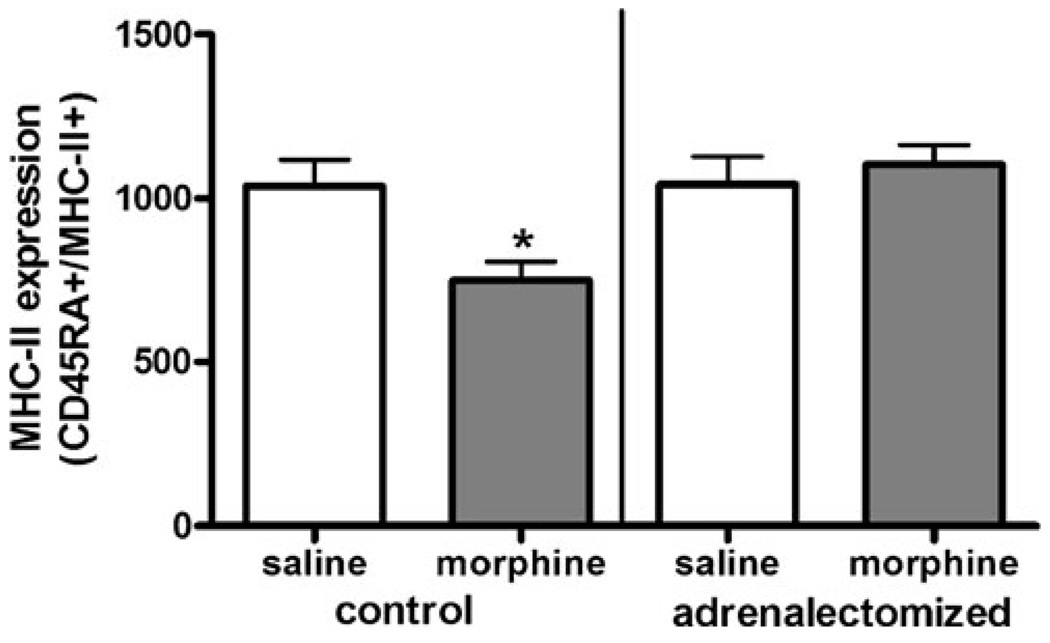
Pursuing this hypothesis, we examined the effect of morphine on B-lymphocyte MHC-II expression in adrenal-ectomized animals. Adrenalectomized animals were provided with water supplemented with corticosterone ad libitum prior to experimentation in order to maintain basal corticosterone levels. On the day of the experiment, animals were injected with either saline or morphine (10 mg/kg) and euthanized 2 h later. Peripheral blood mononuclear cells from these animals were isolated and cultured for 24 h with rrIL-4 (1 µg/ml), as previously described. B-lymphocyte MHC-II expression was significantly suppressed after morphine treatment by 29% in non-adrenalectomized animals, as has been previously shown (Fig. 1). However, in adrenalectomized animals, morphine failed to suppress MHC-II expression as these animals were fully responsive to rrIL-4 induction of MHC-II expression, similar in magnitude to that observed in both saline-treated adrenalectomized and non-adrenalectomized control animals (Fig. 3).
Fig. 3.
IL-4-induced MHC-II expression on B lymphocytes in morphine-treated adrenalectomized animals. Adrenalectomized Sprague–Dawley rats were purchased from Taconic Laboratories and given supplementary corticosterone for 1 week prior to experimentation. On the day of the experiment, animals were injected with either saline or morphine (10 mg/kg, s.c.) and euthanized after 2 h. Non-adrenalectomized intact animals were used as control groups. Two hours after injection, peripheral blood mononuclear cells were isolated then cultured overnight with increasing concentrations of rrIL-4 (0.1– 1,000 ng/ml). Cultured cells were collected and washed prior to being incubated with fluorochrome-conjugated antibodies as described in Fig. 1. Stained cells were fixed using 1% paraformaldehyde prior to analysis using a FACSort flow cytometer. Data are expressed as MFI ± SEM; (n=8). *p<0.05 (one-way ANOVA, Newman–Keuls)
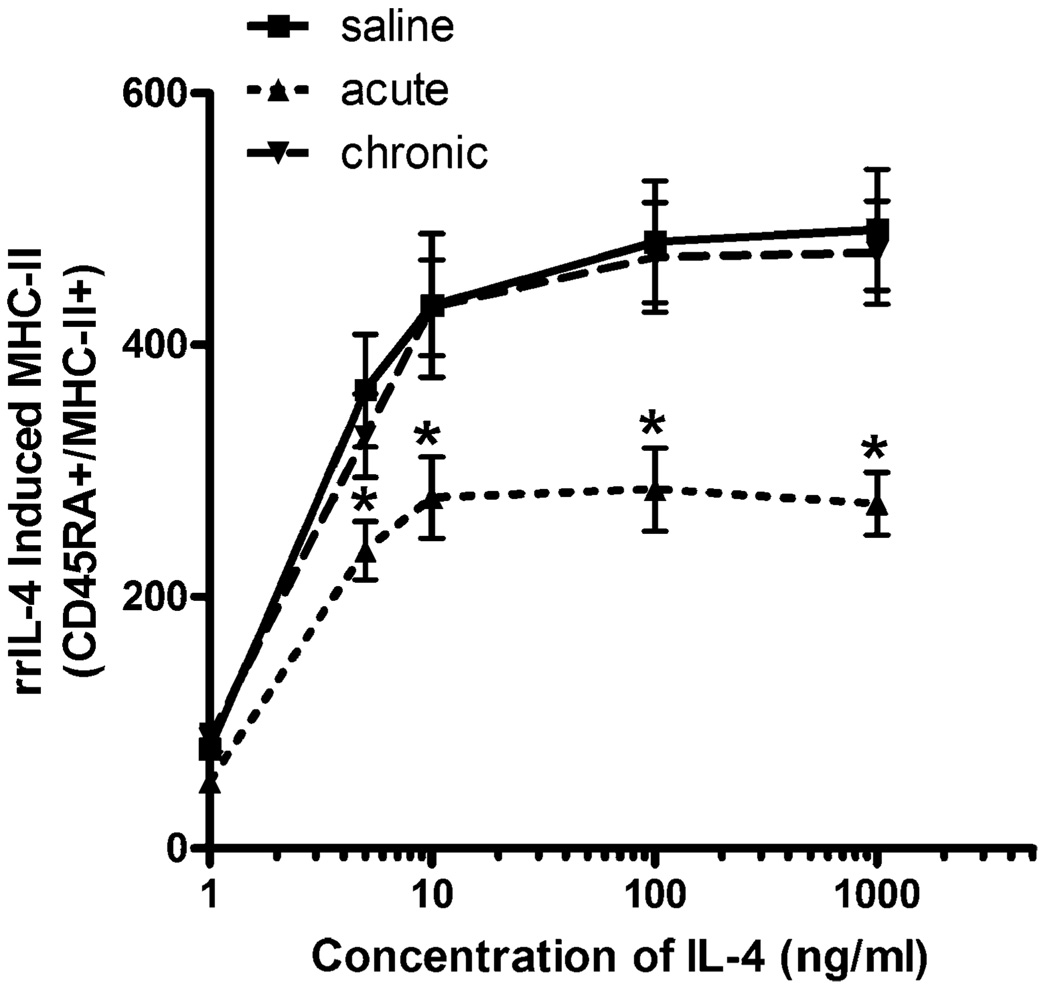
Effect of chronic morphine treatment on B-lymphocyte MHC-II expression
Chronic exposure to morphine has been shown to produce tolerance to a number of effects of acute morphine including analgesia, increased activation of the HPA axis, as well as decreased lymphocyte proliferation in response to mitogen (Bayer et al. 1994). Therefore, we wanted to examine whether the suppressive effects of morphine administration on MHC-II remained after chronic exposure. Animals were injected twice a day with either saline or an escalating dose of morphine (10–40 mg/kg, s.c.) for 8 days. Two hours after a challenge dose of morphine (10 mg/kg, s. c.), PBMCs were isolated and either basal or rrIL-4-stimulated MHC-II expression was measured. Similar to data previously shown in Fig. 1b, the addition of rrIL-4 to isolated PBMCs in culture resulted in a fivefold stimulation of MHC-II expression on B lymphocytes from saline-treated control animals. Following an acute exposure to morphine (2 h), the stimulatory effects of rrIL-4 on MHC-II expression were again significantly suppressed by 34% to 44% at all rrIL-4 concentrations tested (10–1,000 ng/ml). In contrast to this acute suppressive effect, following chronic exposure to morphine, the magnitude of the response to IL-4-induced MHC-II expression was identical to that of the saline-treated control animals (Fig. 4). The suppressive effect on MHC-II expression observed after acute morphine treatment appears to develop tolerance to the effects of morphine as chronically treated animals exhibited no decrease in basal expression compared to similarly treated control animals (Fig. 4). Furthermore, upon induction of MHC-II expression with rrIL-4, chronically treated B lymphocytes were able to increase expression of MHC-II in a similar fashion to saline-treated control animals (greater than fivefold).
Fig. 4.
Effects of chronic morphine exposure on IL-4-induced MHC-II expression in B lymphocytes. Sprague-Dawley rats (n=8) were administered either saline or morphine twice daily using the dosing schedule as described in “Materials and methods”. Acutely treated animals received morphine (10 mg/kg, s.c.) on the day of the experiment. Two hours after the final injection, peripheral blood mononuclear cells were isolated and cultured with rrIL-4 (0.1– 1,000 ng/ml). Stained cells were fixed using 1% paraformaldehyde prior to analysis using a FACSort flow cytometer. Data are expressed as MFI ± SEM. Data repeated in a subsequent experiment. *p<0.05 compared to all other treatment groups (ANOVA, Newman–Keuls)
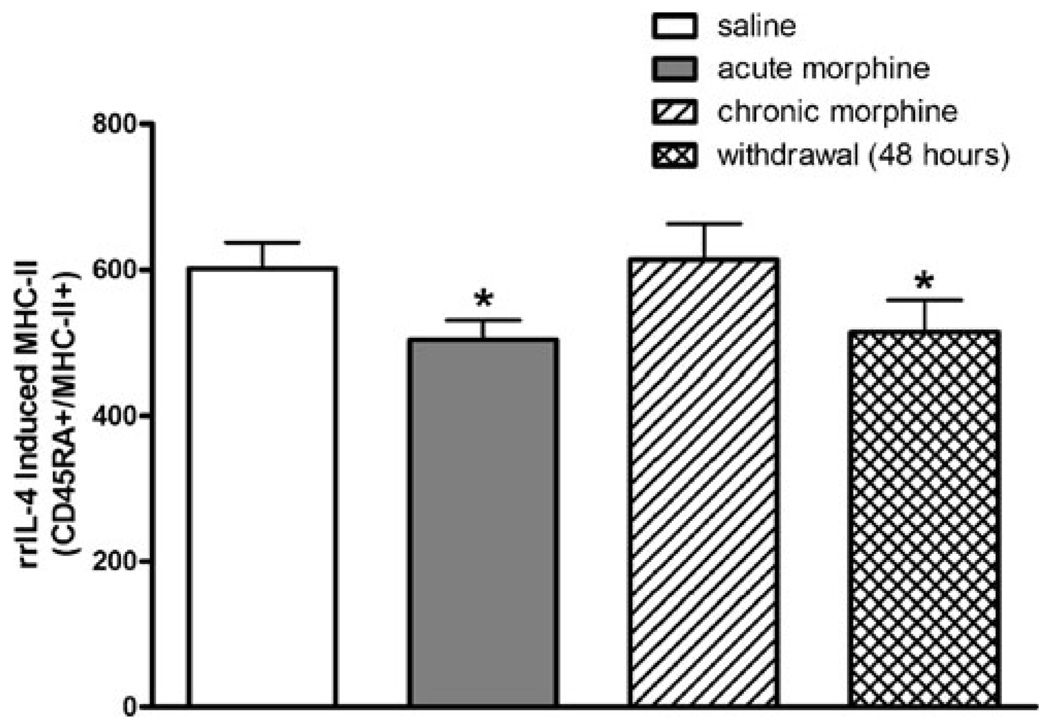
Effect of morphine withdrawal on MHC-II expression
Withdrawal from morphine can be considered a stressor as it has been demonstrated to increase circulating corticosterone levels. Avila et al. (2004) demonstrated that, 24 h after the cessation of morphine treatment, lymphocyte proliferation responses were significantly decreased compared to saline-treated control animals. To examine if the stress of withdrawal after chronic morphine treatment altered the ability of B lymphocytes to present antigens, the present studies examined if MHC-II expression was suppressed after abrupt withdrawal from chronic morphine treatment. Animals were dosed using the chronic morphine-dosing paradigm (see “Materials and methods”). After 8 days of morphine dosing, animals were abruptly switched to twice daily s.c. injections of saline for an additional 2 days after which the MHC-II expression on B lymphocytes was determined. Measurement of plasma corticosterone demonstrated that, similar to acute morphine, 48 h of withdrawal from morphine significantly increased circulating corticosterone levels (Fig. 5). As shown in Fig. 5, the level of rrIL-4-induced MHC-II expression was significantly suppressed by abrupt withdrawal from morphine. This suppression appears to resolve within 1 week after the initiation of morphine withdrawal, since neither basal nor rrIL-4-induced lymphocytes demonstrated a significant decrease in the expression of MHC-II (data not shown). Taken together, these findings suggest that the decrease in MHC-II expression is mediated by morphine-induced increases in corticosterone.
Fig. 5.
Effect of 48-h withdrawal after chronic morphine on rrIL-4-induced B-lymphocyte MHC-II expression. Groups of Sprague–Dawley rats (n=8 per group) were administered either chronic saline or morphine. One subgroup of chronic morphine-treated animals was abruptly withdrawn from chronic morphine treatment by replacing the twice daily injections of morphine with saline for 2 days. Acute morphine-treated animals received morphine (10 mg/kg, s.c.) only on the day of the experiment. Peripheral blood mononuclear cells were isolated and cultured with rrIL-4 (1,000 ng/ml). After rrIL-4 incubation, cells were incubated with fluorochrome-conjugated antibodies as previously described. Stained cells were fixed using 1% paraformaldehyde prior to analysis using a FACSort flow cytometer. Data are represented as MFI ± SEM and are representative of two separate experiments. *p<0.05 compared to saline-treated and chronic morphine-treated groups (ANOVA, Newman–Keuls)
Discussion
Decreases in MHC-II expression on antigen-presenting cells observed after morphine administration may represent a significant impact in the ability to ward off infection. Human studies have shown that diminished MHC-II expression is highly predictive of poor outcomes in septic patients (Venet et al. 2007) with decreased MHC-II expression being predictive of increased mortality (Abe et al. 2008). In addition, decreases in MHC-II expression have been observed in cancer patients. Suppression of MHC-II expression represents a significant impairment in the ability of the host to mount an immune response, as the ability of CD4+ T cells to recognize exogenously derived antigen is dependent upon efficient cell surface presentation of antigen by the MHC-II molecule (Friedl and Brocker 2002). In particular, decreased B-lymphocyte MHC-II expression has been shown to reduce T-cell proliferation in vivo. Investigations into the relative contribution of immune cell-specific MHC-II expression to T-cell proliferation has shown that while dendritic cell MHC-II expression was sufficient to induce CD4+ T-cell activation, B-cell-dependent MHC-II antigen presentation was required to increase T-cell proliferation, clonal expansion, and cytokine production to near normal levels (Kleindienst and Brocker 2005). Studies of chimeric mice, which have MHC-II-deficient B cells but normal dendritic and monocytic MHC-II expression, also found that mice deficient in MHC-II display decreased T-cell proliferation (Crawford et al. 2006). However, a mathematical relationship between the decrease of MHC-II and a functional immunosuppressive effect has yet to be determined. There are currently no studies that have analyzed a relative decrease in MHC-II expression and measures of immune function.
Interestingly, many of the effects of decreased MHC-II are strikingly similar to the immunosuppressive effects of morphine. Despite the fact that MHC-II is a key molecule involved in immune cell communication, few studies have examined whether morphine has effects on MHC-II expression. Immune system function is often determined by the coordinated efforts of different cell types and soluble messengers, which serve to activate cell proliferation in exponential increments. Most studies of the immunosuppressive effects of morphine have examined immune function using ex vivo leukocyte preparations and functional assays, such as stimulated lymphocyte proliferation, analysis of cytokine production, NK cell and cytolytic T-cell cytotoxicity, and directed phagocytosis. Many of these immune system functions are dependent upon MHC-II expression and/or activation. The results presented here demonstrate that a single dose of morphine decreased MHC-II expression by over 30% on circulating B lymphocytes. This effect persisted even after 24 h of ex vivo stimulation with high concentrations of rrIL-4 (1 µg/ml). Whether decreased MHC-II expression contributes to the lack of T-lymphocyte response to mitogen remains to be determined; however, previous studies have shown that mice with decreased levels of MHC-II expression display significantly reduced lymphocyte proliferation and altered levels of TH1 and TH2 cytokines (Kleindienst and Brocker 2005; Crawford et al. 2006). Thus, given the importance of MHC-II to normal immune function, a decrease in the level of MHC-II expression by morphine represents a potential mechanism through which morphine may exert an immunosuppressive effect.
Mitogen-stimulated lymphocyte proliferation has frequently been used as a measure of immune function after experimental manipulation in animals. In our model, the T-cell mitogen concanavalin A (conA) acts as a substitute for MHC-II, thereby activating T-cell proliferation. Using this model, work from our laboratory and others demonstrated that lymphocytes from morphine-treated animals display a decreased ability to proliferate (Bayer et al. 1990; Fecho et al. 1993). Despite studies that have reported that morphine inhibits conA-induced T-cell proliferation through direct interactions with immune cell opioid receptors, extensive studies have demonstrated that these direct effects of morphine were likely due to nonspecific binding due to the high doses of morphine required to produce an effect (Fecho et al. 1996b). In contrast, central injections of N-methyl-morphine, a form of morphine that cannot cross the blood–brain barrier, have been shown to suppress lymphocyte proliferation, strongly suggesting that the suppressive effect of morphine is centrally mediated (Hernandez et al. 1993). Further studies utilizing central injections of µ-opioid-specific agonists and antagonists demonstrated that the immunosuppressive effect of morphine on lymphocyte proliferation was primarily central µ-opioid receptor-dependent (Mellon and Bayer 1998). Hypothesizing that MHC-II expression would be similarly mediated, we utilized central injections of a µ-opioid receptor agonist DAMGO and measured MHC-II expression on circulating B lymphocytes. Similar to the suppression of lymphocyte proliferation, we found that µ-opioid receptor activation was sufficient to signal decreased MHC-II expression similar to that observed after acute morphine. In addition, central pretreatment with opioid antagonists, either CTOP or naltrexone, blocked the suppressive effects of even a high systemic dose of morphine on rrIL-4-induced MHC-II. Interestingly, µ-opioid receptors, the opioid receptor mediating most of morphine’s central effects on the immune system, have been implicated in sepsis where studies have demonstrated that pretreatment with the opioid antagonist naltrexone was protective against LPS-induced septic shock (Hilburger et al. 1997; Greeneltch et al. 2004).
Central opioid receptor activation by morphine has generally been shown to exert effects on the periphery either through activation of the HPA axis or alternatively through sympathetic nervous system activation. Studies examining the effect of increased corticosterone on MHC-II expression have shown that increases in circulating corticosterone levels, whether through direct injections of corticosterone or the application of a stressor, are sufficient to reduce the expression of MHC-II on APCs (Zwilling et al. 1990; Weiss et al. 1996; Schwab et al. 2005). Therefore, it is possible that the increases in circulating corticosterone observed after morphine could mediate the suppressive effect of morphine on MHC-II. The results presented here demonstrate that direct incubation with corticosterone, at concentrations similar to those obtained after morphine, decreased basal MHC-II by 88% in cultured B lymphocytes from naive animals. In addition, corticosterone completely blocked the ability of B cells to increase MHC-II expression upon stimulation with IL-4. This finding is consistent with our central studies: central injections of DAMGO increase circulating corticosterone levels, while the antagonists used in these studies, naltrexone and CTOP, block the increases in corticosterone levels typically observed after morphine (Mellon and Bayer 1998). In addition, morphine-treated animals have been shown to develop tolerance to the steroidogenic and immunosuppressive effects of morphine after periods of prolonged exposure. The development of tolerance is thought to be due to homeostatic changes that counteract the initial effects of the drug, allowing for the appearance of “normal” function on measures of pain tolerance, immune function, circulating steroid levels, and behavioral tests. The chronic dosing paradigm used in our laboratory consists of increasing twice daily doses of morphine escalating from an initial dose of 10 mg/kg to a final dose of 40 mg/kg. Using this dosing paradigm, previous studies have shown that tolerance develops to the effects of a challenge dose of morphine (10 mg/kg) on the activation of the HPA axis, as well as on several measures of immune function, including lymphocyte proliferation (Bayer et al. 1994). In line with these studies, the suppressive effect of morphine on MHC-II expression also was no longer apparent after chronic morphine (Figure 4). This apparent development of tolerance to the effects of chronic morphine treatment coincided with an apparent tolerance to previously observed morphine-induced increases in circulating corticosterone (Table 1). Inversely, morphine withdrawal has previously been demonstrated to result in a renewed immunosuppression, as well as increased activation of the HPA axis leading to a resumption of increased circulating corticosterone levels (Rahim et al. 2003; Avila et al. 2004). Interestingly, upon withdrawal from morphine, MHC-II expression is significantly suppressed within 48 h; this effect implies that it is not a direct effect of morphine on the immune cells, since morphine should be cleared from systemic circulation by this time point. The increases in corticosterone observed after 24 h of withdrawal from morphine are no longer apparent within 72 h after the last dose of morphine (Avila et al. 2004). Similarly, the effect of morphine on MHC-II was no longer apparent after 1 week of withdrawal, as neither basal nor rrIL-4-induced MHC-II appeared suppressed. Although it is likely that the suppression of MHC-II by morphine is mediated in part through elevated corticosterone, there is a discrepancy in the magnitude of the effects obtained with the direct addition of corticosterone to that obtained with morphine. The likelihood that other agents are affecting MHC-II expression would account for the discrepancy in the magnitude of the effects on MHC-II. For example, at plasma levels similar to those induced by morphine, corticosterone completely blocks the up-regulation of MHC-II by IL-4. In contrast, there is only a partial (36%) inhibition of cytokine-induced MHC-II expression after morphine administration. This may be related to several differences when comparing in vivo versus in vitro effects, but may also suggest the possibility of the release of other factors in vivo that may modulate the suppressive effects of corticosterone. Supporting this hypothesis, prior work by Zwilling et al. (1993) demonstrated that restraint stress in adrenalectomized animals continued to exert a suppressive effect on murine peritoneal macrophage MHC-II expression (Zwilling et al. 1993).
Several alternative pathways activated by morphine are also known to exert effects on MHC-II expression. For example, morphine is known to activate the autonomic nervous system, particularly through direct innervation of lymphoid tissues by the SNS. Studies examining the suppression of lymphocyte proliferation after acute morphine have demonstrated that the decrease in lymphocyte proliferative ability was independent of HPA activation, but instead was dependent upon activation of the sympathetic nervous system (Fecho et al. 1996a; Mellon and Bayer 2001). However, the role of the SNS in modulating MHC-II expression levels of peripheral APCs is unclear as previous studies have shown both increases and decreases in MHC-II expression following exposure to increased catecholamines (Bourdoulous et al. 1993; Taniguchi et al. 1993). Furthermore, morphine has been shown to alter the levels of several cytokines including IFN-γ and IL-4, both of which induce MHC-II expression (Wang et al. 2003; Roy et al. 2005). Therefore, it is possible that the moderate suppression we observed after morphine was due to the actions of several factors that have opposing actions on MHC-II expression. Future experiments will be needed to more closely examine the relationship between decreased MHC-II expression and morphine-induced immunosuppression.
Supplementary Material
Acknowledgments
We would like to acknowledge the efforts of Dr. Karen Creswell and Michelle Lombard from the flow cytometry core facility at Georgetown University. Support for this research came from the National Institute of Health, NIDA grant #R01-DA015589-01 and NIDA grant #T32-DA007291. Morphine sulfate was generously provided by the National Institute on Drug Abuse (Research Triangle Park, NC, USA).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11481-010-9218-7) contains supplementary material, which is available to authorized users.
Contributor Information
Alexandria L. Nugent, Email: alexandria.nugent@utsouthwestern.edu, Department of Neuroscience, Georgetown University Medical Center, Research Building, EP-04 3970 Reservoir Road, Washington, DC 20057, USA.
Richard A. Houghtling, Email: rahoughtling@gmail.com, Department of Pharmacology, Georgetown University Medical Center, Research Building, EP-04 3970 Reservoir Road, Washington DC 20057 USA.
Barbara M. Bayer, Email: bayerb@georgetown.edu, Department of Neuroscience, Georgetown University Medical Center, Research Building, EP-04 3970 Reservoir Road, Washington, DC 20057, USA.
References
- Abe R, Hirasawa H, Oda S, Sadahiro T, Nakamura M, Watanabe E, Nakada TA, Hatano M, Tokuhisa T. Up-regulation of interleukin-10 mRNA expression in peripheral leukocytes predicts poor outcome and diminished human leukocyte antigen-DR expression on monocytes in septic patients. J Surg Res. 2008;147:1–8. doi: 10.1016/j.jss.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Avila AH, Alonzo NC, Bayer BM. Immune cell activity during the initial stages of withdrawal from chronic exposure to cocaine or morphine. J Neuroimmunol. 2004;147:109–113. doi: 10.1016/j.jneuroim.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Bayer BM, Daussin S, Hernandez M, Irvin L. Morphine inhibition of lymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology. 1990;29:369–374. doi: 10.1016/0028-3908(90)90096-a. [DOI] [PubMed] [Google Scholar]
- Bayer BM, Brehio RM, Ding XZ, Hernandez MC. Enhanced susceptibility of the immune system to stress in morphine-tolerant rats. Brain Behav Immun. 1994;8:173–184. doi: 10.1006/brbi.1994.1017. [DOI] [PubMed] [Google Scholar]
- Beagles K, Wellstein A, Bayer B. Systemic morphine administration suppresses genes involved in antigen presentation. Mol Pharmacol. 2004;65:437–442. doi: 10.1124/mol.65.2.437. [DOI] [PubMed] [Google Scholar]
- Bourdoulous S, Durieu-Trautmann O, Strosberg AD, Couraud PO. Catecholamines stimulate MHC class I, class II, and invariant chain gene expression in brain endothelium through different mechanisms. J Immunol. 1993;150:1486–1495. [PubMed] [Google Scholar]
- Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Xu D, Bridges RM. Opioids modulate human neutrophil and lymphocyte function: thermal injury alters plasma beta-endorphin levels. Surgery. 1988;104:41–48. [PubMed] [Google Scholar]
- Elftman MD, Norbury CC, Bonneau RH, Truckenmiller ME. Corticosterone impairs dendritic cell maturation and function. Immunology. 2007;122:279–290. doi: 10.1111/j.1365-2567.2007.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecho K, Dykstra LA, Lysle DT. Evidence for beta adrenergic receptor involvement in the immunomodulatory effects of morphine. J Pharmacol Exp Ther. 1993;265:1079–1087. [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Evidence for sympathetic and adrenal involvement in the immunomodulatory effects of acute morphine treatment in rats. J Pharmacol Exp Ther. 1996a;277:633–645. [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. J Pharmacol Exp Ther. 1996b;276:626–636. [PubMed] [Google Scholar]
- Forsgren S, Pobor G, Coutinho A, Pierres M. The role of I-A/E molecules in B lymphocyte activation. I. Inhibition of lipopolysaccharide-induced responses by monoclonal antibodies. J Immunol. 1984;133:2104–2110. [PubMed] [Google Scholar]
- Friedl P, Brocker EB. TCR triggering on the move: diversity of T-cell interactions with antigen-presenting cells. Immunol Rev. 2002;186:83–89. doi: 10.1034/j.1600-065x.2002.18608.x. [DOI] [PubMed] [Google Scholar]
- Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532–545. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- Grainger R, Hart DN, Watson JD, Baird MA. Antigen-pulsed, interleukin-4-treated B cells activate primed T cells in vitro but not naive T cells in vivo. Scand J Immunol. 1995;42:517–523. doi: 10.1111/j.1365-3083.1995.tb03689.x. [DOI] [PubMed] [Google Scholar]
- Greeneltch KM, Haudenschild CC, Keegan AD, Shi Y. The opioid antagonist naltrexone blocks acute endotoxic shock by inhibiting tumor necrosis factor-alpha production. Brain Behav Immun. 2004;18:476–484. doi: 10.1016/j.bbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hernandez MC, Flores LR, Bayer BM. Immunosuppression by morphine is mediated by central pathways. J Pharmacol Exp Ther. 1993;267:1336–1341. [PubMed] [Google Scholar]
- Hilburger ME, Adler MW, Truant AL, Meissler JJ, Jr, Satishchandran V, Rogers TJ, Eisenstein TK. Morphine induces sepsis in mice. J Infect Dis. 1997;176:183–188. doi: 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- Kleindienst P, Brocker T. Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T-cell immunity in vivo. Immunology. 2005;115:556–564. doi: 10.1111/j.1365-2567.2005.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood LL, Silbert LH, Fleshner M, Laudenslager ML, Watkins LR, Maier SF. Morphine-induced decreases in in vivo antibody responses. Brain Behav Immun. 1994;8:24. doi: 10.1006/brbi.1994.1003. [DOI] [PubMed] [Google Scholar]
- Lokshin AE, Kalinski P, Sassi RR, Mailliard RB, Muller-Berghaus J, Storkus WJ, Peng X, Marrangoni AM, Edwards RP, Gorelik E. Differential regulation of maturation and apoptosis of human monocyte-derived dendritic cells mediated by MHC class II. Int Immunol. 2002;14:1027–1037. doi: 10.1093/intimm/dxf073. [DOI] [PubMed] [Google Scholar]
- Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon RD, Bayer BM. Role of central opioid receptor subtypes in morphine-induced alterations in peripheral lymphocyte activity. Brain Res. 1998;789:56–67. doi: 10.1016/s0006-8993(97)01529-1. [DOI] [PubMed] [Google Scholar]
- Mellon RD, Bayer BM. Reversal of acute effects of high dose morphine on lymphocyte activity by chlorisondamine. Drug Alcohol Depend. 2001;62:141–147. doi: 10.1016/s0376-8716(00)00184-8. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Kappa-opioid receptor-mediated antinociception in the rat. I. Comparative actions of mu- and kappa-opioids against noxious thermal, pressure and electrical stimuli. J Pharmacol Exp Ther. 1989;251:334–341. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Rahim RT, Meissler JJ, Zhang L, Adler MW, Rogers TJ, Eisenstein TK. Withdrawal from morphine in mice suppresses splenic macrophage function, cytokine production, and costimulatory molecules. J Neuroimmunol. 2003;144:16–27. doi: 10.1016/s0165-5728(03)00273-x. [DOI] [PubMed] [Google Scholar]
- Roos A, Schilder-Tol EJ, Chand MA, Claessen N, Lakkis FG, Pascual DW, Weening JJ, Aten J. Differential regulation of expression of the MHC class II molecules RT1.B and RT1.D on rat B lymphocytes: effects of interleukin-4, interleukin-13 and interferon-gamma. Immunology. 1998;93:33–40. doi: 10.1046/j.1365-2567.1998.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Wang J, Charboneau R, Loh HH, Barke RA. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J Immunol. 2005;175:6361–6367. doi: 10.4049/jimmunol.175.10.6361. [DOI] [PubMed] [Google Scholar]
- Schwab CL, Fan R, Zheng Q, Myers LP, Hebert P, Pruett SB. Modeling and predicting stress-induced immunosuppression in mice using blood parameters. Toxicol Sci. 2005;83:101–113. doi: 10.1093/toxsci/kfi014. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Nemoto Y, Ota K, Imai T, Tobari J. Participation of poly(ADP-ribose) synthetase in the process of norepinephrine-induced inhibition of major histocompatibility complex class II antigen expression in human astrocytoma-cells. Biochem Biophys Res Commun. 1993;193:886. doi: 10.1006/bbrc.1993.1708. [DOI] [PubMed] [Google Scholar]
- Venet F, Tissot S, Debard AL, Faudot C, Crampe C, Pachot A, Ayala A, Monneret G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: correlation with severity and secondary septic shock. Crit Care Med. 2007;35:1910–1917. doi: 10.1097/01.CCM.0000275271.77350.B6. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Loh HH, Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278:37622–37631. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- Weiss PA, Collier SD, Pruett SB. Role of glucocorticoids in ethanol-induced decreases in expression of MHC class II molecules on B cells and selective decreases in spleen cell number. Toxicol Appl Pharmacol. 1996;139:153–162. doi: 10.1006/taap.1996.0154. [DOI] [PubMed] [Google Scholar]
- Weiss PA, Collier SD, Pruett SB. Effect of ethanol on B cell expression of major histocompatibility class II proteins in immunized mice. Immunopharmacology. 1998;39:61–72. doi: 10.1016/s0162-3109(97)00099-4. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kishihara K, Wang B, Mizobe K, Kubo C, Nomoto K. Restraint stress-induced immunosuppression by inhibiting leukocyte migration and Th1 cytokine expression during the intraperitoneal infection of Listeria monocytogenes. J Neuroimmunol. 1998;92:139–151. doi: 10.1016/s0165-5728(98)00197-0. [DOI] [PubMed] [Google Scholar]
- Zwilling BS, Dinkins M, Christner R, Faris M, Griffin A, Hilburger M, McPeek M, Pearl D. Restraint stress-induced suppression of major histocompatibility complex class II expression by murine peritoneal macrophages. J Neuroimmunol. 1990;29:125–130. doi: 10.1016/0165-5728(90)90154-f. [DOI] [PubMed] [Google Scholar]
- Zwilling BS, Lafuse WP, Brown D, Pearl D. Characterization of ACTH mediated suppression of MHC class II expression by murine peritoneal macrophages. J Neuroimmunol. 1992;39:133–138. doi: 10.1016/0165-5728(92)90182-k. [DOI] [PubMed] [Google Scholar]
- Zwilling BS, Brown D, Feng N, Sheridan J, Pearl D. The effect of adrenalectomy on the restraint stressed induced suppression of MHC class II expression by murine peritoneal macrophages. Brain Behav Immun. 1993;7:29–35. doi: 10.1006/brbi.1993.1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.