Abstract
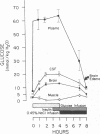
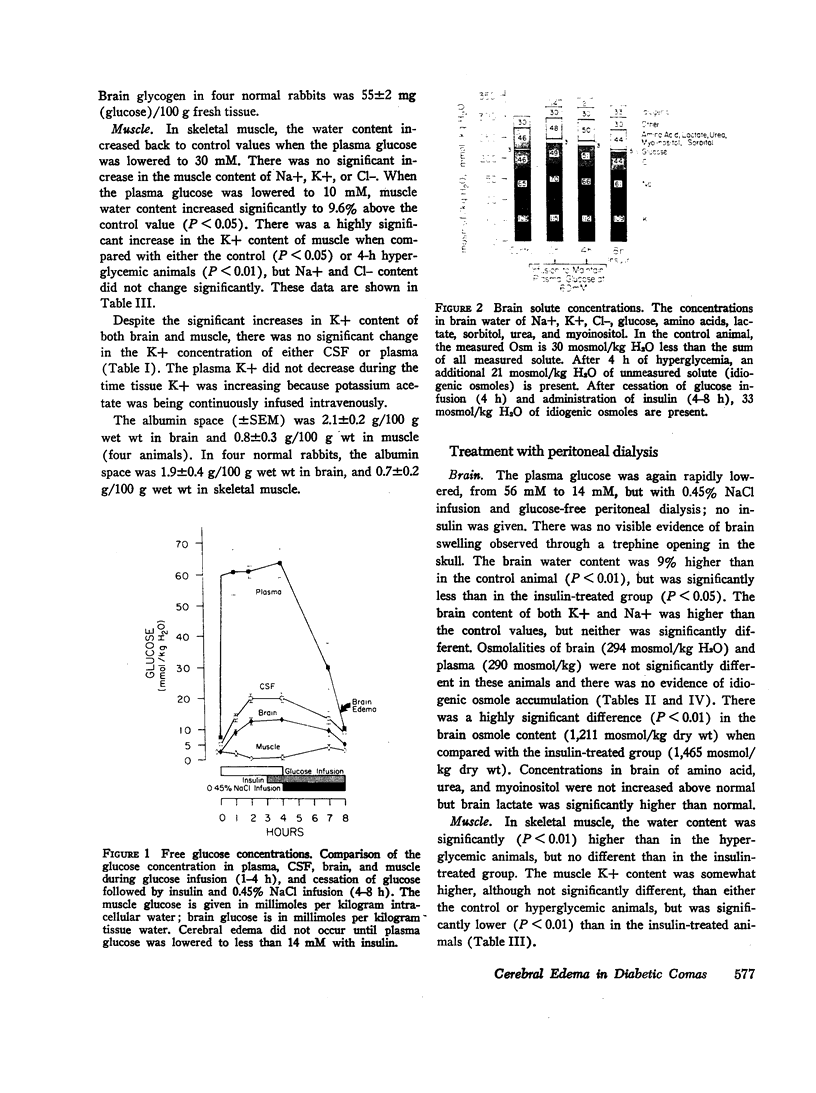
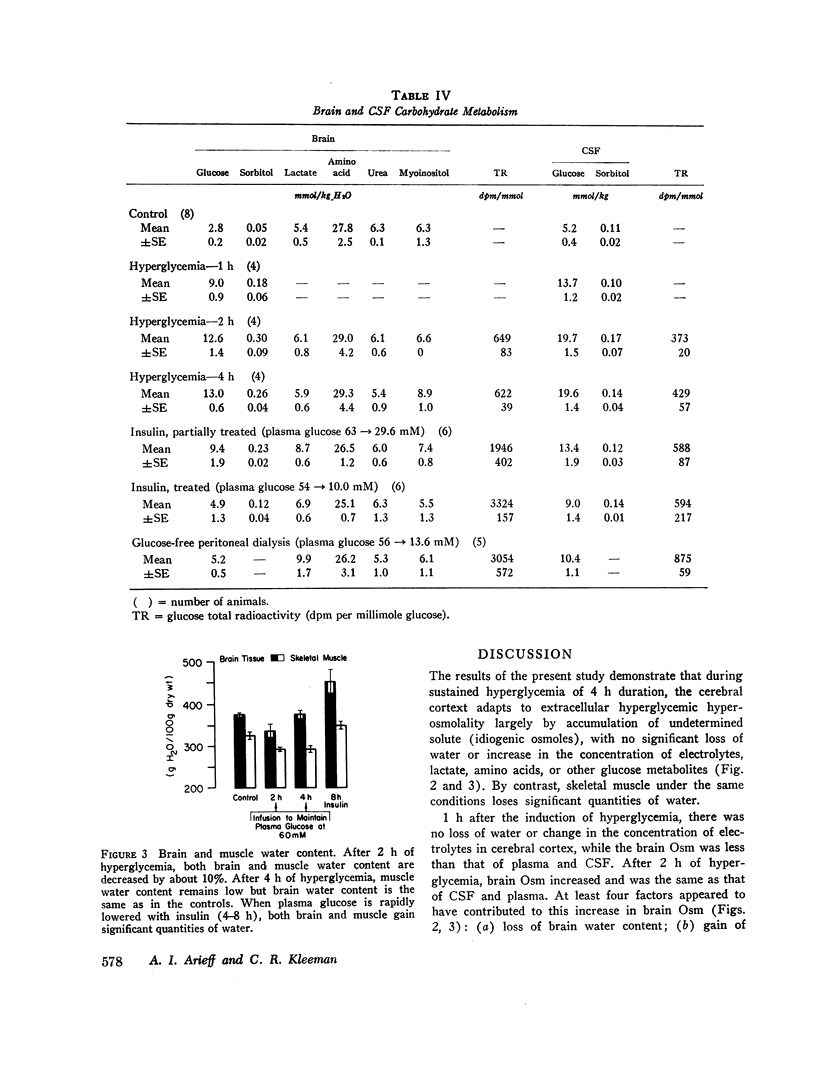
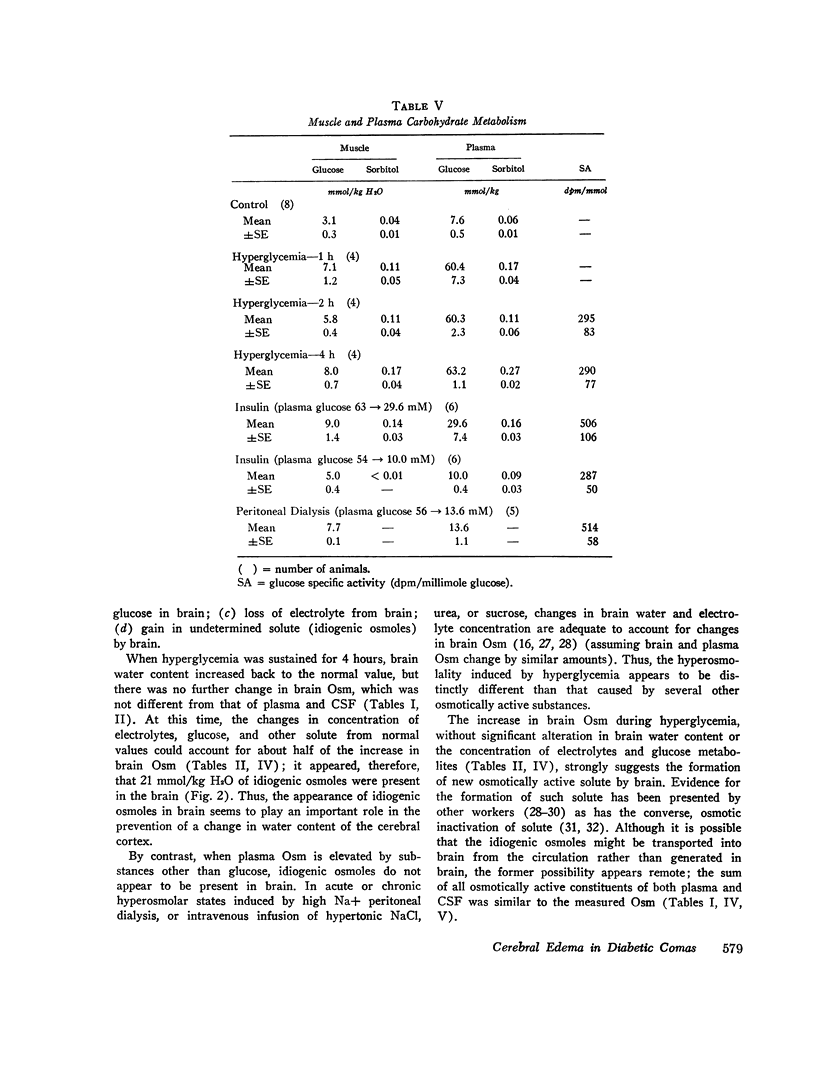
To investigate the pathophysiology of cerebral edema occurring during treatment of diabetic coma, the effects of hyperglycemia and rapid lowering of plasma glucose were evaluated in normal rabbits. During 2 h of hyperglycemia (plasma glucose=61 mM), both brain (cerebral cortex) and muscle initially lost about 10% of water content. After 4 h of hyperglycemia, skeletal muscle water content remained low but that of brain was normal. Brain osmolality (Osm) (343 mosmol/kg H2O) was similar to that of cerebrospinal fluid (CSF) (340 mosmol/kg), but increases in the concentration of Na+, K+, Cl-, glucose, sorbitol, lactate, urea, myoinositol, and amino acids accounted for only about half of this increase. The unidentified solute was designated “idiogenic osmoles”. When plasma glucose was rapidly lowered to normal with insulin, there was gross brain edema, increases in brain content of water, Na+, K+, Cl- and idiogenic osmoles, and a significant osmotic gradient from brain (326 mosmol/kg H2O) to plasma (287 mosmol/kg). By similarly lowering plasma glucose with peritoneal dialysis, increases in brain Na+, K+, Cl-, and water were significantly less, idiogenic osmoles were not present, and brain and plasma Osm were not different. It is concluded that during sustained hyperglycemia, the cerebral cortex adapts to extracellular hyperosmolality primarily by accumulation of idiogenic osmoles rather than loss of water or gain in solute. When plasma glucose is rapidly lowered with insulin, an osmotic gradient develops from brain to plasma. Despite the brain to plasma osmotic gradient, there is no net movement of water into brain until plasma glucose has fallen to at least 14 mM, at which time cerebral edema occurs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Wright R. L., Kowada M., Thurston J. M., Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968 Feb;52(2):437–453. [PMC free article] [PubMed] [Google Scholar]
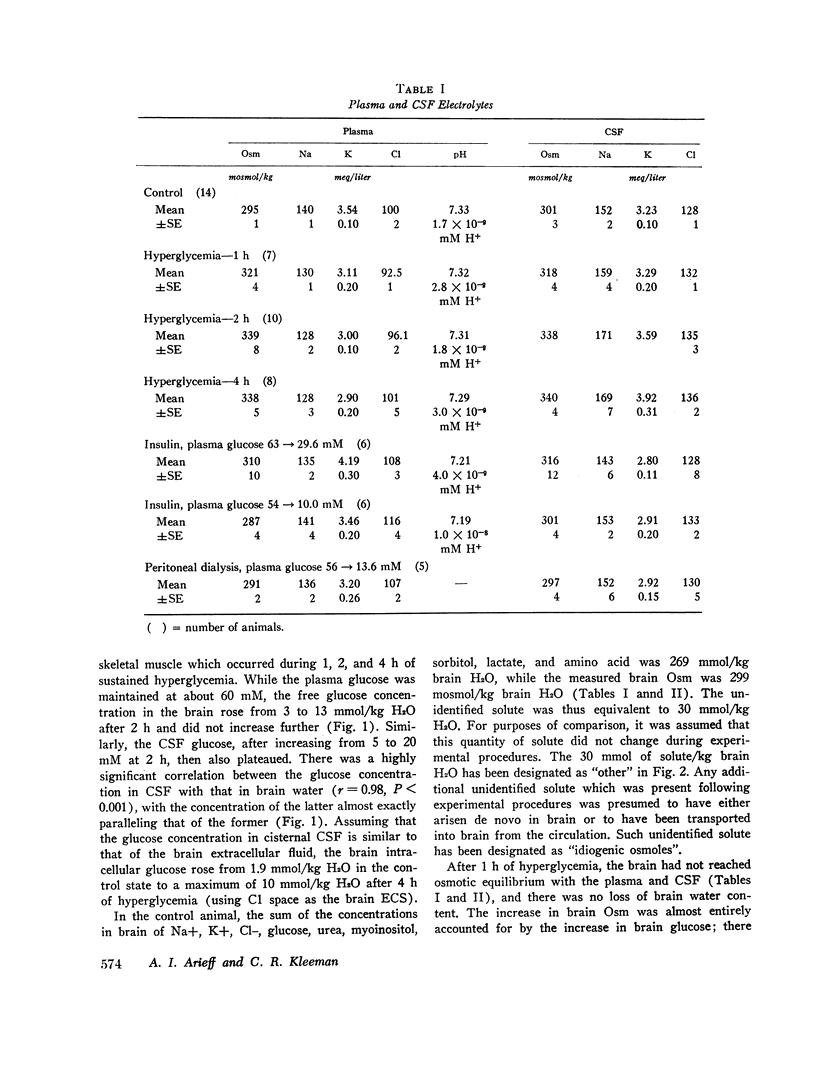
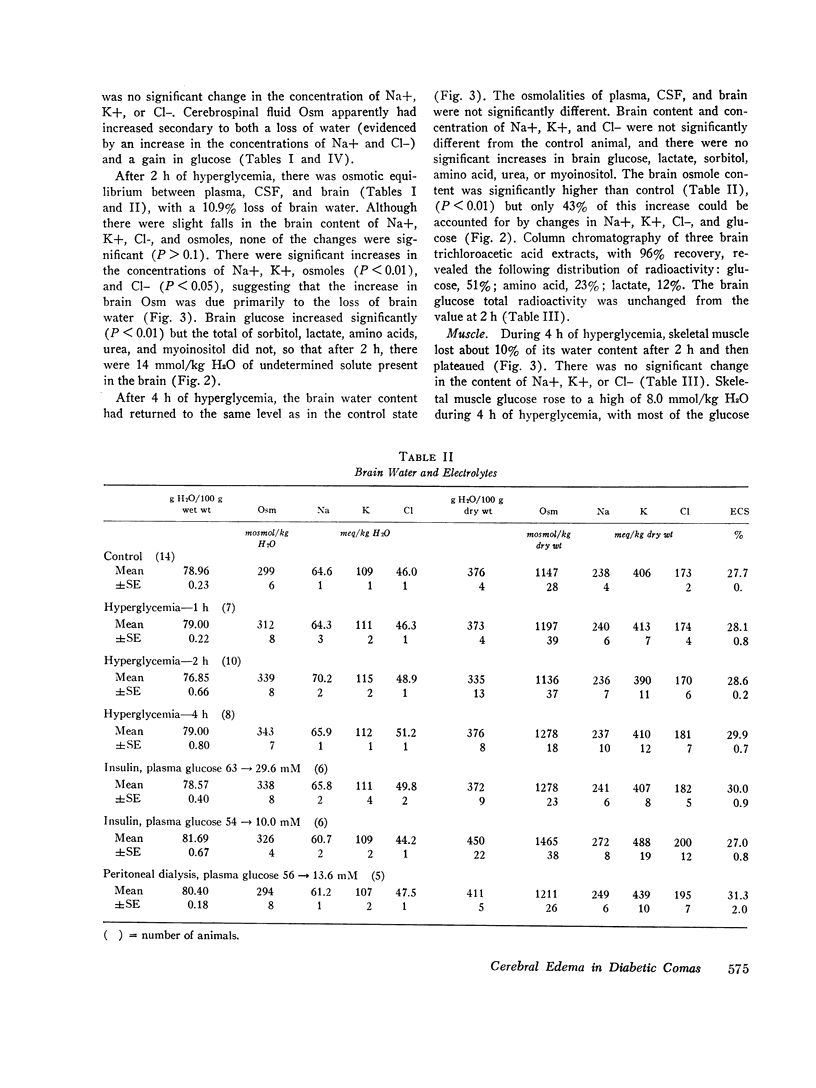
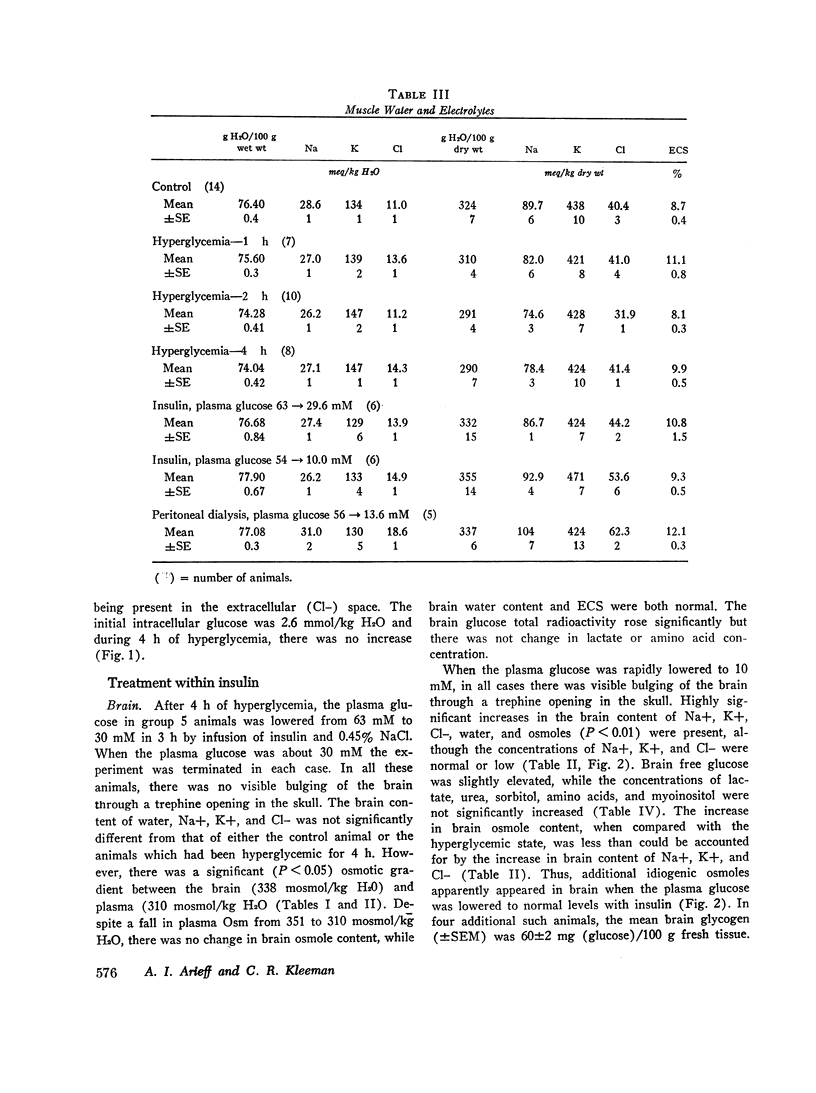
- Arieff A. I., Kleeman C. R., Keushkerian A., Bagdoyan H. Brain tissue osmolality: method of determination and variations in hyper- and hypo-osmolar states. J Lab Clin Med. 1972 Feb;79(2):334–343. [PubMed] [Google Scholar]
- Bradbury M. W., Kleeman C. R. Stability of the potassium content of cerebrospinal fluid and brain. Am J Physiol. 1967 Aug;213(2):519–528. doi: 10.1152/ajplegacy.1967.213.2.519. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Kleeman C. R. The effect of chronic osmotic disturbance on the concentrations of cations in cerebrospinal fluid. J Physiol. 1969 Sep;204(1):181–193. doi: 10.1113/jphysiol.1969.sp008907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL H. J., GOTTERER R., ALTSHULER B. EXCHANGEABLE SODIUM, BODY POTASSIUM, AND BODY WATER IN PREVIOUSLY EDEMATOUS CARDIAC PATIENTS: EVIDENCE FOR OSMOTIC INACTIVATION OF CATION. Circulation. 1965 Aug;32:185–192. doi: 10.1161/01.cir.32.2.185. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Blumenthal S. A., Morrison A. D., Winegrad A. I. Increased cerebrospinal-fluid pressure during treatment of diabetic ketosis. Lancet. 1971 Sep 25;2(7726):671–675. doi: 10.1016/s0140-6736(71)92245-8. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Morrison A. D., Winegrad A. I. Polyol pathway in aorta: regulation by hormones. Science. 1969 Nov 21;166(3908):1007–1008. doi: 10.1126/science.166.3908.1007. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Prockop L. D., Winegrad A. I. Acute cerebral oedema during treatment of hyperglycaemia. An experimentsl model. Lancet. 1968 Aug 17;2(7564):384–386. doi: 10.1016/s0140-6736(68)90597-7. [DOI] [PubMed] [Google Scholar]
- Cotlier E., Beaty C. The transport of 14C-alpha-aminoisobutyric acid in galactose cataracts in rats and rabbit lenses incubated in high galactose media. Invest Ophthalmol. 1968 Feb;7(1):77–87. [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- FINBERG L., LUTTRELL C., REDD H. Pathogenesis of lesions in the nervous system in hypernatremic states. II. Experimental studies of gross anatomic changes and alterations of chemical composition of the tissues. Pediatrics. 1959 Jan;23(1 Pt 1):46–53. [PubMed] [Google Scholar]
- Fernandez J. P., McGinn J. T., Hoffman R. S. Cerebral edema from blood-brain glucose differences complicating peritoneal dialysis. Second membrane syndrome. N Y State J Med. 1968 Mar 1;68(5):677–680. [PubMed] [Google Scholar]
- Fitzgerald M. G., O'Sullivan D. J., Malins J. M. Fatal Diabetic Ketosis. Br Med J. 1961 Jan 28;1(5221):247–250. doi: 10.1136/bmj.1.5221.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock E. V., Tyce G. M., Owen C. A., Jr Glucose metabolism in brains of diabetic rats. Endocrinology. 1969 Sep;85(3):428–437. doi: 10.1210/endo-85-3-428. [DOI] [PubMed] [Google Scholar]
- Flock E. V., Tyce G. M., Owen C. A., Jr Glucose metabolism in brains of eviscerated rats with different blood levels of glucose. Mayo Clin Proc. 1969 Jun;44(6):387–405. [PubMed] [Google Scholar]
- GREENAWAY J. M., READ J. Diabetic coma; a review of 69 cases. Australas Ann Med. 1958 May;7(2):151–158. doi: 10.1111/imj.1958.7.2.151. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Merola L. O., Field R. A. Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science. 1966 Jan 14;151(3707):209–210. doi: 10.1126/science.151.3707.209. [DOI] [PubMed] [Google Scholar]
- Hanig R. C., Tachiki K. H., Aprison M. H. Subcellular distribution of potassium, sodium, magnesium, calcium and chloride in cerebral cortex. J Neurochem. 1972 Jun;19(6):1501–1507. doi: 10.1111/j.1471-4159.1972.tb05093.x. [DOI] [PubMed] [Google Scholar]
- Hayes T. M., Woods C. J. Unexpected death during treatment of uncomplicated diabetic ketoacidosis. Br Med J. 1968 Oct 5;4(5622):32–33. doi: 10.1136/bmj.4.5622.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera F. C. Effect of insulin on short-circuit current and sodium transport across toad urinary bladder. Am J Physiol. 1965 Oct;209(4):819–824. doi: 10.1152/ajplegacy.1965.209.4.819. [DOI] [PubMed] [Google Scholar]
- KINOSHITA J. H., FUTTERMAN S., SATOH K., MEROLA L. O. FACTORS AFFECTING THE FORMATION OF SUGAR ALCOHOLS IN OCULAR LENS. Biochim Biophys Acta. 1963 Aug 13;74:340–350. doi: 10.1016/0006-3002(63)91377-5. [DOI] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Kety S. S., Polis B. D., Nadler C. S., Schmidt C. F. THE BLOOD FLOW AND OXYGEN CONSUMPTION OF THE HUMAN BRAIN IN DIABETIC ACIDOSIS AND COMA. J Clin Invest. 1948 Jul;27(4):500–510. doi: 10.1172/JCI101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTIMORE G. E. Effect of insulin on potassium transfer in isolated rat liver. Am J Physiol. 1961 Jun;200:1315–1319. doi: 10.1152/ajplegacy.1961.200.6.1315. [DOI] [PubMed] [Google Scholar]
- Maccario M., Messis C. P. Cerebral oedema complicating treated non-ketotic hyperglycaemia. Lancet. 1969 Aug 16;2(7616):352–353. doi: 10.1016/s0140-6736(69)92702-0. [DOI] [PubMed] [Google Scholar]
- McDOWELL M. E., WOLF A. V., STEER A. Osmotic volumes of distribution; idiogenic changes in osmotic pressure associated with administration of hypertonic solutions. Am J Physiol. 1955 Mar;180(3):545–558. doi: 10.1152/ajplegacy.1955.180.3.545. [DOI] [PubMed] [Google Scholar]
- Metzger A. L., Rubenstein A. H. Reversible cerebral oedema complicating diabetic ketoacidosis. Br Med J. 1970 Sep 26;3(5725):746–747. doi: 10.1136/bmj.3.5725.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. S., Gotoh F., Ebihara S., Tomita M. Effects of anoxia on cerebral metabolism and electrolytes in man. Neurology. 1965 Oct;15(10):892–901. doi: 10.1212/wnl.15.10.892. [DOI] [PubMed] [Google Scholar]
- Olsson K. E., Saltin B. Variation in total body water with muscle glycogen changes in man. Acta Physiol Scand. 1970 Sep;80(1):11–18. doi: 10.1111/j.1748-1716.1970.tb04764.x. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Otsuki I. Mechanism of muscular paralysis by insulin with special reference to periodic paralysis. Am J Physiol. 1970 Nov;219(5):1178–1182. doi: 10.1152/ajplegacy.1970.219.5.1178. [DOI] [PubMed] [Google Scholar]
- Prockop L. D. Hyperglycemia, polyol accumulation, and increased intracranial pressure. Arch Neurol. 1971 Aug;25(2):126–140. doi: 10.1001/archneur.1971.00490020044005. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- STERN W. E., COXON R. V. OSMOLALITY OF BRAIN TISSUE AND ITS RELATION TO BRAIN BULK. Am J Physiol. 1964 Jan;206:1–7. doi: 10.1152/ajplegacy.1964.206.1.1. [DOI] [PubMed] [Google Scholar]
- Sloviter H. A., Yamada H. Absence of direct action of insulin on metabolism of the isolated perfused rat brain. J Neurochem. 1971 Jul;18(7):1269–1274. doi: 10.1111/j.1471-4159.1971.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Strang R. H., Bachelard H. S. Effect of insulin on levels and turnover of intermediates of brain carbohydrate metabolism in vivo. J Neurochem. 1971 Oct;18(10):1799–1807. doi: 10.1111/j.1471-4159.1971.tb09585.x. [DOI] [PubMed] [Google Scholar]
- Taubin H., Matz R. Cerebral edema, diabetes insipidus, and sudden death during the treatment of diabetic ketoacidosis. Diabetes. 1968 Feb;17(2):108–109. doi: 10.2337/diab.17.2.108. [DOI] [PubMed] [Google Scholar]
- Van den Noort S., Eckel R. E., Brine K. L., Hrdlicka J. Brain metabolism in experimental uremia. Arch Intern Med. 1970 Nov;126(5):831–834. doi: 10.1001/archinte.126.5.831. [DOI] [PubMed] [Google Scholar]
- WELT L. G., ORLOFF J., KYDD D. M., OLTMAN J. E. An example of cellular hyperosmolarity. J Clin Invest. 1950 Jul;29(7):935–939. doi: 10.1172/JCI102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Bradley R. F. Cerebral edema with irreversible coma in severe diabetic ketoacidosis. N Engl J Med. 1967 Mar 23;276(12):665–669. doi: 10.1056/NEJM196703232761204. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Effect of insulin on potassium efflux from rat muscle in the presence and absence of glucose. Am J Physiol. 1960 May;198:1066–1070. doi: 10.1152/ajplegacy.1960.198.5.1066. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]