Abstract
S-Nitrosylation is a ubiquitous protein modification in redox-based signaling and forms S-nitrosothiol (SNO) from nitric oxide (NO) on cysteine residues. Dysregulation of (S)NO signaling (nitrosative stress) leads to impairment of cellular function. Protein kinase C (PKC) is an important signaling protein that plays a role in the regulation of vascular function and it is not known whether (S)NO affects PKC’s role in vascular reactivity. We hypothesized that S-nitrosylation of PKC in vascular smooth muscle would inhibit its contractile activity. Aortic rings from male C57BL6 mice were treated with auranofin or 1-chloro-2,4-dinitrobenzene (DNCB) as pharmacological tools, which lead to stabilize S-nitrosylation, and propylamine propylamine NONOate (PANOate) or S-nitrosocysteine (CysNO) as NO donors. Contractile responses of aorta to phorbol-12,13-dibutyrate (PDBu), a PKC activator, were attenuated by auranofin, DNCB, PANOate, and CysNO. S-Nitrosylation of PKCα was increased by auranofin or DNCB and CysNO as compared to control protein. Augmented S-nitrosylation inhibited PKCα activity and subsequently downstream signal transduction. These data suggest that PKC is inactivated by S-nitrosylation and this modification inhibits PKC-dependent contractile responses. Since S-nitrosylation of PKC inhibits phosphorylation and activation of target proteins related to contraction, this post-translational modification may be a key player in conditions of decreased vascular reactivity.
Keywords: S-Nitrosylation, Protein kinase C, Vascular contraction
INTRODUCTION
S-Nitrosylation is the post-translational modification of a cysteine residue on target proteins by nitric oxide (NO).1 In the S-nitrosylation reaction, NO is converted to dinitrogen trioxide (N2O3) with an electron acceptor such as oxygen, followed by formation of S-nitrosothiol (SNO) in the protein’s cysteine thiol group. Additionally, as a transnitrosylation reaction, a NO equivalent is transferred from one molecule to another (from a thiol and a SNO).2 There are over 100 S-nitrosylated proteins including channels, transporters, kinases, signaling proteins, and transcription factors.3 These proteins are activated or inactivated by SNO. For example, the activity of p21ras is increased by S-nitrosylation,4 but endothelial NO synthase (eNOS) is inhibited.5
The levels of S-nitrosylation are regulated indirectly and directly by several enzymatic systems. NOS, for example, functions as an indirect enzymatic regulator via NO production, which is added to the thiol group, leading to the formation of SNO. Direct enzymatic systems to regulate S-nitrosylation include enzymes for denitrosylation to remove SNO. Two enzymatic systems, thioredoxin/thioredoxin reductase6, 7 and S-nitrosoglutathione reductase (GSNOR),8, 9 are involved in the denitrosylation process. Thioredoxin reductase is a redox-based enzyme and catalyses the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reduction of the oxidized redox protein thioredoxin. The reduced thioredoxin by thioredoxin reductase denitrosylates the S-nitrosylated proteins.7 Stamler and colleagues8, 10, 11 have investigated the effects of GSNOR, which degrades S-nitrosoglutathione (GSNO) to oxidized glutathione and ammonia, and lowers the levels of S-nitrosylation.
Vascular smooth muscle contraction is associated with the regulation of vascular resistance and blood pressure, and its dysregulation could lead to hypertension. Protein kinase C (PKC) is a well characterized enzyme in agonist-induced vascular smooth muscle contraction.12–15 In vascular smooth muscle, several signal cascades activated by physiological agonists are regulated by PKC. Activated PKC phosphorylates CPI-17 (PKC-potentiated phosphatase inhibitor protein-17 kDa),16 which inhibits myosin light chain (MLC) phosphatase resulting in vascular contraction. In addition, PKC phosphorylates other proteins such as calponin, caldesmon, or mitogen-activated protein kinase (MAPK) to cause vascular contraction.17, 18 PKC also affects membrane-bound regulatory proteins such as MARCKS (myristoylated, alanine-rich C-kinase substrate) and inhibitory GTP-binding protein Gi in vascular smooth muscle.19, 20
PKC is a target of NO, which leads to PKC’s functional inactivation.21, 22 It is possible that in this reaction, PKC is being modified to S-nitrosylated PKC. In the vasculature, the action of S-nitrosylation is unknown. We hypothesized that PKC-mediated vascular contractile response is inhibited by S-nitrosylation. To test this hypothesis, we determined whether vascular reactivity and PKC signal transduction is modified by increased levels of S-nitrosylation.
MATERIALS AND METHODS
Drugs and Reagents
Phorbol-12,13-dibutyrate (PDBu) was purchased from Calbiochem (San Diego, CA). Propylamine propylamine NONOate (PANOate) was obtained from Cayman Chemical (Ann Arbor, MI). S-nitrosocysteine (CysNO) was synthesized from L-cysteine using acidified nitrite. Auranofin was purchased from BioMol (Plymouth Meeting, PA). 1-Chloro-2,4-dinitrobenzene (DNCB) and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
Male, 12–14 weeks-old, C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were used in this study. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education. The animals were housed on a 12-hour light/dark cycle and fed a standard chow diet with water or saline ad libitum.
Isolation of aortic rings and functional studies
After mice were euthanized, thoracic aorta were excised, cleaned from fat tissue and cut into 2 mm length-rings in an ice-cold physiological salt solution consisting of the following: 130 mM NaCl, 4.7 mM KCl, 1.18 mM KH2PO4, 1.18 mM MgSO4·7H2O, 1.56 mM CaCl2·2H2O, 14.9 mM NaHCO3, 5.6 mM glucose, and 0.03 mM EDTA. Aortic rings were mounted in a myograph (Danish Myo Technology A/S, Aarhus, Denmark) containing warmed (37 °C), oxygenated (95% O2/5% CO2) physiological salt solution. The preparations were equilibrated for at least 1 hour under a passive force of 5 mN. After the equilibration period, arterial integrity was assessed by stimulation of vessels with 120 mM KCl and, after contraction reached a plateau, the rings were washed. Subsequently, the rings were stimulated with phenylephrine (0.1 μM) followed by relaxation with acetylcholine (1 μM), which was used as an evidence of an intact endothelium. Cumulative concentration-response curves to PDBu using 10−9 to 10−6 M were performed.
Detection of S-nitrosylation
The biotin-switch method to detect S-nitrosylation was performed with the S-nitrosylated protein detection assay kit (Cayman Chemical, Ann Arbor, MI) modified from the method of Jaffrey et al.23 Biotin-labeled proteins were used for western blot analysis or pulled down by streptavidin agarose beads (Thermo Scientific, Rockford, IL) to specifically detect PKCα S-nitrosylation and then western blot analysis was performed. Ten percent of biotin-labeled proteins were used to measure total PKCα protein expression.
Western blot analysis
Aortas were treated with auranofin (1 μM) or DNCB (4 μM) for 1 h, and CysNO (1.5 mM) for 30 min in Dulbecco’s modified Eagle’s medium (DMEM). Proteins (40 μg) extracted from aorta in lysis buffer (20 mM Tris-HCl, pH 7.4, 5 mM Na2P2O7, 100 mM NaF, 2 mM Na3VO4, 1% NP-40, protease inhibitor cocktail, and 1 mM PMSF) were separated by electrophoresis on a 12% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk in Tris-buffered saline solution with Tween-20 for 1 h at room-temperature. Membranes were then incubated with primary antibodies overnight at 4 °C. Antibodies were as follows: Phospho-CPI-17, CPI-17 (Millipore, Temecula, CA), Phospho-caldesmon, caldesmon (Santa Cruz Biotechnology, Santa Cruz, CA), PKCα (BD Biosciences, San Jose, CA), and β-actin (Sigma-Aldrich). After incubation with secondary antibodies, signals were exposed with chemiluminescence, visualized by autoradiography, and quantified densitometrically.
PKC activity assay
To obtain specific PKCα isoform, immunoprecipitation (IP) was performed before the activity assay.24 Protein extracts (500 μg) were incubated with 2 μg of anti-PKCα antibody (BD Biosciences) and 40 μl of protein A/G plus-agarose (Santa Cruz Biotechnology) at 4 °C, overnight. The beads were collected and extensively washed six times with phosphate buffered saline (PBS). PKC activity assay was performed with the IP samples via PKC assay kit (Calbiochem).
Statistical analysis
Values are mean ± standard error of the mean (SEM), and n represents the number of animals used in the experiments. Contractions were recorded as changes in the displacement (mN) from baseline and were expressed as percentage change normalized to 120 mM KCl-contraction values. Concentration-response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 4.0; GraphPad Software, San Diego, CA), and 2 pharmacological parameters were obtained: the maximal effect generated by the agonist (or Emax) and EC50 (molar concentration of agonist producing 50% of the maximum response). Statistical differences were calculated by Student’s t-test and a P value less than 0.05 was considered to be statistically significant.
RESULTS
Role of S-nitrosylation in PKC-induced contraction
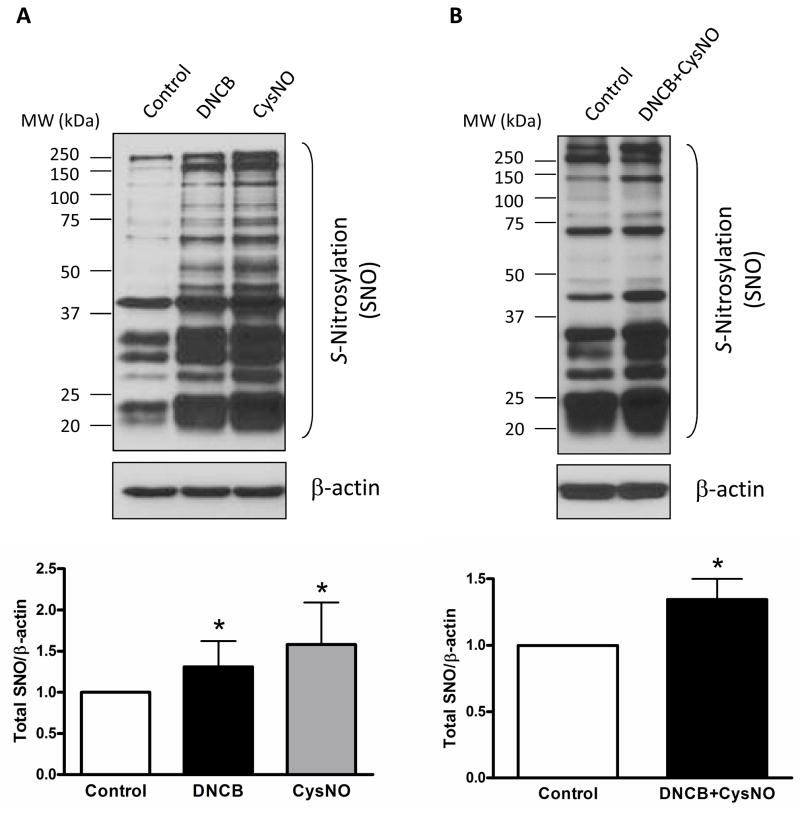
We first determined the content of total S-nitrosylation proteins in mouse aorta. The vascular contents of total S-nitrosylation proteins were increased after incubation with CysNO, an exogenous SNO source, or DNCB as a pharmacological tool leading to stabilize S-nitrosylation, and when the two drugs were combined (Figure 1).
Figure 1.
S-Nitrosylated proteins (SNO) are increased in the presence of DNCB, CysNO, or DNCB plus CysNO in mouse aorta. A and B: Representative western blot images of S-nitrosylated proteins (top) and corresponding bar graphs showing the relative content of S-nitrosylated proteins after normalization to β-actin expression (bottom). Results are presented as mean ± SEM for n=3 in each experimental group. *, p<0.05 compared with control.
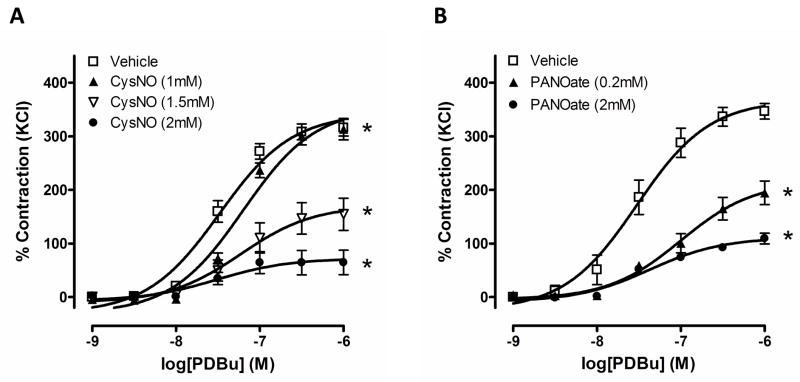
To test the effect of S-nitrosylation generated by NO on vascular contraction, two exogenous SNO sources were used to induce S-nitrosylation. PANOate is a NO donor and CysNO mediates transnitrosylation (the switch between SNO and a thiol).25 To measure PKC-dependent contraction, PDBu, a PKC activator, was used in endothelium-intact aortic rings. Incubation with PANOate significantly attenuated PDBu-induced contractile responses in aortic rings, compared with vehicle, control (Figure 2A). In addition, contraction to PDBu in CysNO-treated rings was decreased in a concentration-dependent manner (Figure 2B).
Figure 2.
Vascular contraction to PDBu is attenuated by S-nitrosylation. Concentration-dependent contractile responses to PDBu (10−9 to 10−6 M) were measured after incubation with PANOate (A, n=6 to 8) or CysNO (B, n=6 to 8) at the indicated concentrations for 15 minutes in mouse aorta. Experimental values of contraction were calculated relative to the contractile response produced by 120 mM KCl, which was taken as 100%. Results are presented as mean ± SEM in each experimental group. *, p<0.05 compared with vehicle.
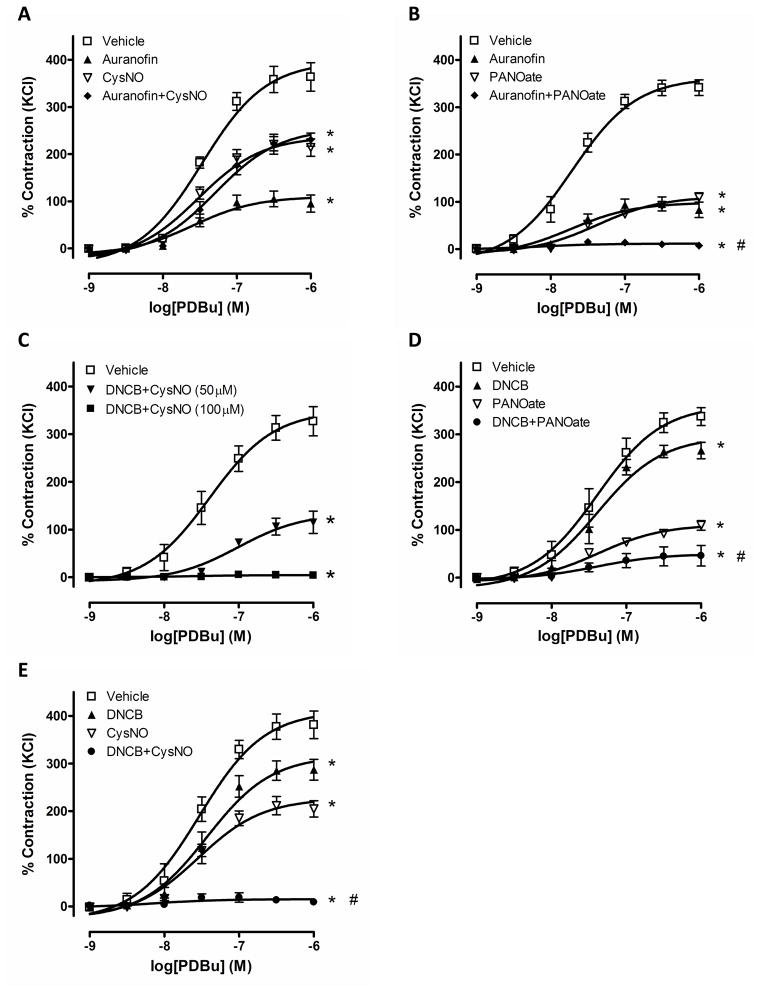
To increase S-nitrosylation, we used two experimental approaches: increased exposure to SNO sources and inhibition of denitrosylation enzymes. Thioredoxin reductase is one of the denitrosylation enzymes and two inhibitors, auranofin and DNCB,6, 7 were used to demonstrate whether S-nitrosylation changes PKC-mediated contractile responses. Auranofin and DNCB significantly reduced PDBu-induced contractions in endothelium-intact aortic rings. To further increase S-nitrosylation, aortic rings were incubated with each inhibitor plus either PANOate or CysNO and the simultaneous addition of these compounds also diminished PDBu-induced contractions compared to control group (Figure 3).
Figure 3.
PDBu-induced contraction in mouse aorta is abolished by increased S-nitrosylation. Concentration-dependent contractions to PDBu (10−9 to 10−6 M) were measured after incubation of the aortic rings with vehicle, auranofin (1 μM, 40 min), DNCB (4 μM, 40 min), PANOate (2 mM, 15 min), CysNO (1.5 mM, 15 min) and auranofin or DNCB plus PANOate or CysNO (1.5 mM or indicated concentration). Experimental values of contraction were calculated relative to the contractile response produced by 120 mM KCl, which was taken as 100%. Results are presented as mean ± SEM in each experimental group. *, p<0.05 vs. vehicle. A: #, p<0.05 vs. PANOate (n=6 to 10). B: (n=6). C: #, p<0.05 vs. DNCB or PANOate (n=6 to 8). D: #, p<0.05 vs. DNCB or CysNO (n=6 to 7). E: (n=6).
Effect of S-nitrosylation on PKC activity
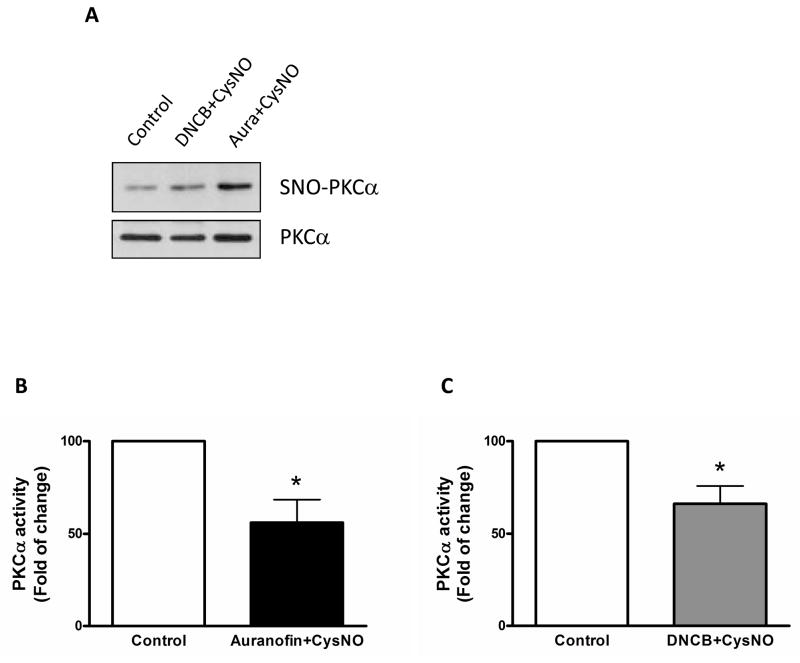
Considering that PKC-induced vascular contraction is modified with S-nitrosylation, we determined protein expression of total and S-nitrosylation of PKC. The levels of S-nitrosylation were determined by the biotin-switch method and western blot analysis as described in methods section. To confirm whether PKCα S-nitrosylation is associated with its activity, PKCα activity assay was determined with IP samples using PKCα antibody. CysNO plus auranofin and CysNO plus DNCB increased PKCα S-nitrosylation and attenuated PKCα activity (Figure 4).
Figure 4.
S-Nitrosylation inhibits PKCα activity. Isolated mouse aortas were treated either with auranofin (Aura; 1 μM, 1 h) or DNCB (4 μM, 1 h) plus CysNO (1.5 mM, 30 min). A: PKCα S-nitrosylation (SNO-PKCα) was detected using the biotin-switch method, streptavidin-precipitation, and western blot analysis with anti-PKCα antibody. Total PKCα expression (PKCα) was detected with biotin-labeled proteins using western blot analysis. B and C: PKCα activity assay. Treated aorta tissues were immunoprecipitated with anti-PKCα antibody (2 μg) and then subjected to the protocol for activity assay. The vehicle-treated group, control, was set to one hundred and bar represents means ± SEM in each experimental group. *, p<0.05 compared with control (B, n=5 and C, n=4).
Effect of PKC S-nitrosylation in vascular signaling proteins
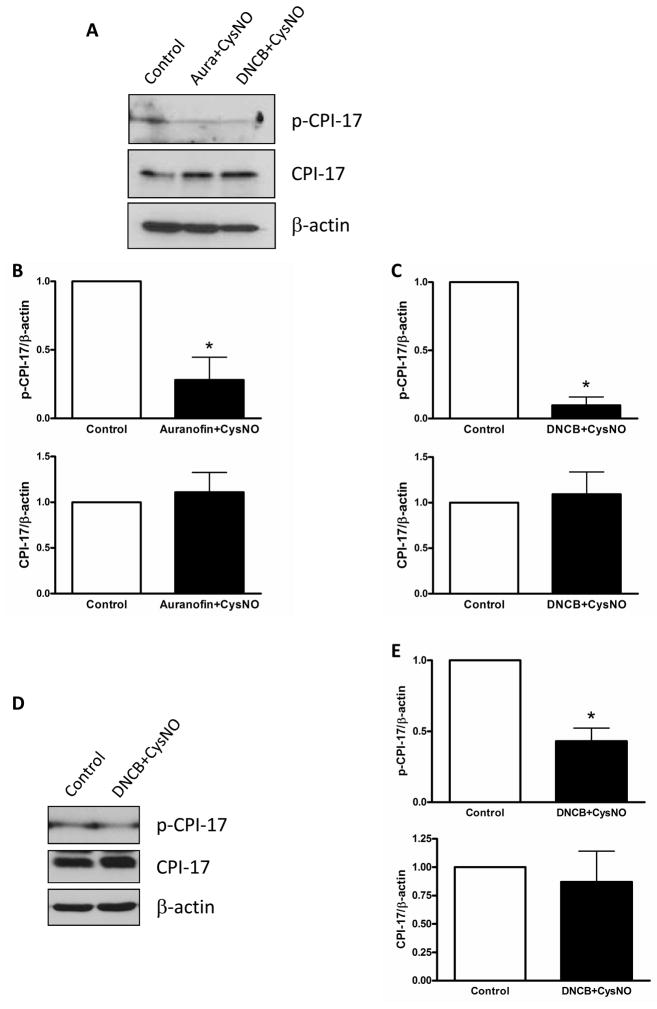
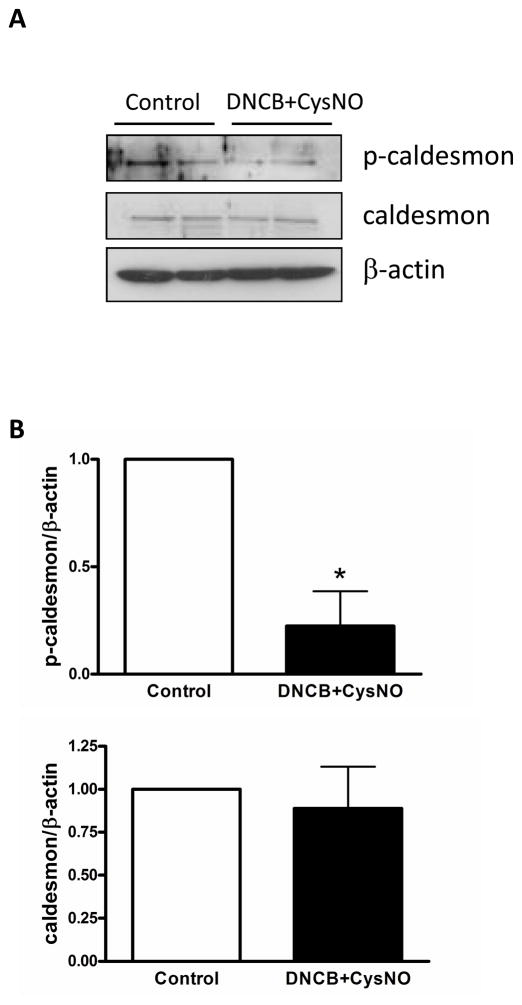
PKC phosphorylates many downstream signaling proteins, such as CPI-17 and caldesmon in smooth muscle. Phosphorylation of CPI-17 was attenuated by treatment with auranofin plus CysNO and DNCB plus CysNO in endothelium-intact aortas (Figure 5). Additionally, another regulatory protein, smooth muscle-specific h-caldesmon, was investigated and phosphorylation of caldesmon was also reduced by incubation with DNCB plus CysNO (Figure 6).
Figure 5.
Phosphorylation of CPI-17 was decreased by S-nitrosylation. Isolated mouse aortas were treated with auranofin (Aura; 1 μM, 1 h) or DNCB (4 μM, 1 h) plus CysNO (A: 1.5 mM, D: 100 μM, 30 min) and then analyzed by western blot with p-CPI-17, CPI-17, and β-actin antibodies. A and D: Western blot analysis. B and C: Bar graphs for A, showing the relative expression of phosphorylation and total proteins after normalization to β-actin expression. E: Bar graphs for D. Results are presented as mean ± SEM in each experimental group. *, p<0.05 vs. control (n=4).
Figure 6.
Phosphorylation of caldesmon was reduced by S-nitrosylation. Isolated mouse aortas were treated with DNCB (4 μM, 1 h) plus CysNO (1.5 mM, 30 min) and then subjected to immunoblot with p-caldesmon, caldesmon, and β-actin antibodies. A: Western blot analysis. B: Bar graphs showing the relative expression of phosphorylation and total proteins after normalization to β-actin expression. Results are presented as mean ± SEM in each experimental group. *, p<0.05 vs. control (n=4).
DISCUSSION
In this study, we investigated whether S-nitrosylation interferes with PKC-mediated vascular contraction. We used endothelium-intact aorta to define this cellular response under normal physiological condition. NO bioactivity is mediated by soluble guanylyl cyclase (sGC), with the subsequent activation of cyclic 3′,5′-guanosine monophosphate (cGMP)-dependent signaling26 or cGMP-independent actions. The cGMP-independent actions of NO include S-nitrosylation of biomolecules to form SNO on target protein.27 Although cGMP-dependent NO bioactivity has been well studied in vascular function, the cGMP-independent mechanisms involving S-nitrosylation have not been demonstrated. Thus, as a new approach, we investigated S-nitrosylation in terms of cGMP-independent NO action in PKC-dependent vasoconstriction.
Our results showed that increased S-nitrosylation abolished PDBu-induced contraction in mouse aorta (Figure 2 and 3). One of the phorbol esters, PDBu was used to measure PKC-mediated vascular contraction. Phorbol esters are potent PKC activators and increase calcium-sensitivity, allowing inhibition of MLC phosphatase in smooth muscle, causing contraction.28, 29 We used several types of drugs to increase S-nitrosylation in proteins (Figure 1) and to evaluate an effect of increased S-nitrosylation on vascular reactivity. Specifically, PANOate and CysNO were used as exogenous SNO sources, but CysNO increases S-nitrosylation in a more specific manner via transnitrosylation than PANOate, a NO donor. Furthermore, CysNO is transported into the cells with higher efficiency, suggesting that the source of SNO may not be just NO.25, 30
PKC is activated when the second messengers or effectors bind to its regulatory domain, such as the conserved C1 and C2 domains, typically at the plasma membrane.31, 32 Phorbol esters bind to C1 domain, which has a cysteine-rich sequence, and this sequence is essential for PKC to bind phorbol esters.33–35 Since S-nitrosylation modifies cysteine residues of target proteins, phorbol esters may not bind or affect the C1 domain of S-nitrosylated PKC, resulting in less PKC activation.
PKC is known as a target of NO and NO decreases PKC activity,21 suggesting that PKC may be modified by S-nitrosylation. As shown in Figure 4, we detected PKC S-nitrosylation and decreased PKC activity in mouse aortas incubated with auranofin or DNCB plus CysNO, indicating that S-nitrosylation is associated with PKC inactivation. In mammals, the PKC family has 12 different isoforms36 and 6 isoforms are present in vascular smooth muscle cells.37 Previous studies reported that different PKC isoforms have specific substrates and functions in different blood vessels and species.20, 38, 39 The present study specifically determined S-nitrosylation of PKCα activity, because PKCα belongs to the conventional PKC subfamily. This enzyme has calcium and diacylglycerol binding regions, therefore representing a key regulator of vascular signal transduction. Several studies have shown that PKCα enhances vascular contraction via calcium sensitization mechanisms and is translocated by calcium from the cytosol to the membrane.38, 40, 41
To determine regulation of target proteins by functional inactivation of PKC, the expression of CPI-17 and caldesmon was assessed. One of the major target proteins for PKC that is important in vascular contraction is CPI-17.16 PKC-induced phosphorylation of CPI-17 inhibits MLC phosphatase, which induces dephosphorylation of MLC, and results in vascular contraction. Caldesmon is another downstream signaling protein of PKC and is an actin-binding protein. Two isoforms of caldesmon exist: a high molecular weight, smooth muscle-specific h-caldesmon, and a low molecular weight, non-muscle l-caldesmon. Phosphorylation of caldesmon induces its detachment from actin, leading to the binding of actin and myosin, and results in vascular contraction. We found that these two proteins, CPI-17 and caldesmon, were dephosphorylated by increased S-nitrosylation, further supporting inactivation of PKC signaling pathway. Noteworthy, the incubation with a low concentration of CysNO (100 μM) and DNCB reduced phosphorylation of CPI-17 only (Figure 5D and 5E), not caldesmon (data was not shown), suggesting that CPI-17 is an important downstream signaling protein for PKC function related to vascular contraction.
In summary, in the present study we have confirmed that the S-nitrosylation leads to decreased PKC function and signaling and impairs vascular contraction. This post-translational modification, and specifically S-nitrosylation of PKC, may represent a key mechanism in conditions associated with abnormal vascular reactivity.
Acknowledgments
This study was supported by grants from the National Institutes of Health (P01HL074167).
Reference List
- 1.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 January 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signaling in cell biology. Mol Interv. 2003 August;3(5):253–263. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- 3.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001 September 21;106(6):675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 4.Lander HM, Hajjar DP, Hempstead BL, Mirza UA, Chait BT, Campbell S, Quilliam LA. A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. J Biol Chem. 1997 February 14;272(7):4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 5.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005 May 20;280(20):19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 6.Holmgren A. Biochemistry. SNO removal. Science. 2008 May 23;320(5879):1019–1020. doi: 10.1126/science.1159246. [DOI] [PubMed] [Google Scholar]
- 7.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008 May 23;320(5879):1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004 February 20;116(4):617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 9.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007 May 4;129(3):511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009 April 14;106(15):6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS, Steverding D. Nitrosative stress: protection by glutathione-dependent formaldehyde dehydrogenase. Redox Rep. 2001;6(4):209–210. doi: 10.1179/135100001101536337. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura J, Khalil RA, Drenth JP, van BC. Evidence for increased myofilament Ca2+ sensitivity in norepinephrine-activated vascular smooth muscle. Am J Physiol. 1990 July;259(1 Pt 2):H2–H8. doi: 10.1152/ajpheart.1990.259.1.H2. [DOI] [PubMed] [Google Scholar]
- 13.Martinez MC, Randriamboavonjy V, Ohlmann P, Komas N, Duarte J, Schneider F, Stoclet JC, Andriantsitohaina R. Involvement of protein kinase C, tyrosine kinases, and Rho kinase in Ca(2+) handling of human small arteries. Am J Physiol Heart Circ Physiol. 2000 September;279(3):H1228–H1238. doi: 10.1152/ajpheart.2000.279.3.H1228. [DOI] [PubMed] [Google Scholar]
- 14.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000 April 7;275(14):9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Dohi Y, Suzuki S, Miyagawa K, Takase H, Kojima M, van BC. Role of Ca2+-sensitive protein kinase C in phenylephrine enhancement of Ca2+ sensitivity in rat tail artery. J Cardiovasc Pharmacol. 2001 September;38(3):347–355. doi: 10.1097/00005344-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001 September 1;535(Pt 2):553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrea JE, Walsh MP. Protein kinase C of smooth muscle. Hypertension. 1992 November;20(5):585–595. doi: 10.1161/01.hyp.20.5.585. [DOI] [PubMed] [Google Scholar]
- 18.Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990 June 15;265(17):10148–10155. [PubMed] [Google Scholar]
- 19.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 April 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 20.Kanashiro CA, Khalil RA. Signal transduction by protein kinase C in mammalian cells. Clin Exp Pharmacol Physiol. 1998 December;25(12):974–985. doi: 10.1111/j.1440-1681.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 21.Kahlos K, Zhang J, Block ER, Patel JM. Thioredoxin restores nitric oxide-induced inhibition of protein kinase C activity in lung endothelial cells. Mol Cell Biochem. 2003 December;254(1–2):47–54. doi: 10.1023/a:1027380828645. [DOI] [PubMed] [Google Scholar]
- 22.Gopalakrishna R, Chen ZH, Gundimeda U. Nitric oxide and nitric oxide-generating agents induce a reversible inactivation of protein kinase C activity and phorbol ester binding. J Biol Chem. 1993 December 25;268(36):27180–27185. [PubMed] [Google Scholar]
- 23.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001 June 12;2001(86):l1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 24.Lin D, Zhou J, Zelenka PS, Takemoto DJ. Protein kinase Cgamma regulation of gap junction activity through caveolin-1-containing lipid rafts. Invest Ophthalmol Vis Sci. 2003 December;44(12):5259–5268. doi: 10.1167/iovs.03-0296. [DOI] [PubMed] [Google Scholar]
- 25.Greco TM, Hodara R, Parastatidis I, Heijnen HF, Dennehy MK, Liebler DC, Ischiropoulos H. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci U S A. 2006 May 9;103(19):7420–7425. doi: 10.1073/pnas.0600729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denninger JW, Marletta MA. Guanylate cyclase and the. NO/cGMP signaling pathway. Biochim Biophys Acta. 1999 May 5;1411(2–3):334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 27.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004 August;287(2):L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 28.Laher I, Vorkapic P, Dowd AL, Bevan JA. Protein kinase C potentiates stretch-induced cerebral artery tone by increasing intracellular sensitivity to Ca2+ Biochem Biophys Res Commun. 1989 November 30;165(1):312–318. doi: 10.1016/0006-291x(89)91071-1. [DOI] [PubMed] [Google Scholar]
- 29.Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca(2+)-sensitizing effect of protein kinase C on vascular smooth muscle: inhibition of myosin light chain phosphatase. J Gen Physiol. 1994 August;104(2):265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci U S A. 2004 May 25;101(21):7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalefski EA, Newton AC. Membrane binding kinetics of protein kinase C betaII mediated by the C2 domain. Biochemistry. 2001 November 6;40(44):13216–13229. doi: 10.1021/bi010761u. [DOI] [PubMed] [Google Scholar]
- 32.Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998 October 30;95(3):307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 33.Hommel U, Zurini M, Luyten M. Solution structure of a cysteine rich domain of rat protein kinase C. Nat Struct Biol. 1994 June;1(6):383–387. doi: 10.1038/nsb0694-383. [DOI] [PubMed] [Google Scholar]
- 34.Kazanietz MG, Wang S, Milne GW, Lewin NE, Liu HL, Blumberg PM. Residues in the second cysteine-rich region of protein kinase C delta relevant to phorbol ester binding as revealed by site-directed mutagenesis. J Biol Chem. 1995 September 15;270(37):21852–21859. doi: 10.1074/jbc.270.37.21852. [DOI] [PubMed] [Google Scholar]
- 35.Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, Nishizuka Y. Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci U S A. 1989 July;86(13):4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998 June 1;332( Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005 November 25;70(11):1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liou YM, Morgan KG. Redistribution of protein kinase C isoforms in association with vascular hypertrophy of rat aorta. Am J Physiol. 1994 October;267(4 Pt 1):C980–C989. doi: 10.1152/ajpcell.1994.267.4.C980. [DOI] [PubMed] [Google Scholar]
- 39.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 October 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 40.Dessy C, Matsuda N, Hulvershorn J, Sougnez CL, Sellke FW, Morgan KG. Evidence for involvement of the PKC-alpha isoform in myogenic contractions of the coronary microcirculation. Am J Physiol Heart Circ Physiol. 2000 September;279(3):H916–H923. doi: 10.1152/ajpheart.2000.279.3.H916. [DOI] [PubMed] [Google Scholar]
- 41.Khalil RA, Lajoie C, Morgan KG. In situ determination of [Ca2+]i threshold for translocation of the alpha-protein kinase C isoform. Am J Physiol. 1994 June;266(6 Pt 1):C1544–C1551. doi: 10.1152/ajpcell.1994.266.6.C1544. [DOI] [PubMed] [Google Scholar]