Abstract
Background
Inflammation and oxidative stress are associated with atrial fibrillation (AF). Statins have antioxidant and anti-inflammatory properties. We tested if atorvastatin reduced AF recurrence after DC cardioversion (CV) by modifying systemic oxidative stress and inflammation. (NCT00252967)
Methods and Results
In a randomized, double-blinded, placebo-controlled trial, patients with atrial fibrillation/flutter (AF) were randomized to receive either atorvastatin 80 mg (n=33) or placebo (n=31) before CV. Treatment was continued for 12 months or until AF recurred. Serum oxidative stress markers (ratios of oxidized to reduced glutathione and cysteine, derivatives of reactive oxygen species, isoprostanes) and inflammatory markers [ high sensitivity C- reactive protein (hs-CRP), interleukin-6 (IL-6), interleukin-1β(IL-1β), tumor necrosis factor-α (TNFα)] were measured at baseline and on follow-up. AF recurred in 22 (66.7%) of atorvastatin and 26 (83.9%) of placebo group (p=0.2). The adjusted hazard ratio of having recurrence on atorvastatin versus on placebo was 0.99 (95% CI: 0.98-1.01, p=0.3). There was no significant difference in the time to recurrence using Kaplan-Meier survival estimates (median (IR): 29 (2-145) days vs. 22 (7-70) days, p=0.9). While no significant effect was seen on oxidative stress, 2 of 4 inflammatory markers, IL-6 (adjusted OR: 0.59, 95% CI: 0.35-0.97, p= 0.04) and hs-CRP (adjusted OR: 0.59, 95% CI: 0.37-0.95, p=0.03) were significantly lowered with atorvastatin. Cholesterol levels significantly decreased with atorvastatin (p=0.03).
Conclusions
High dose atorvastatin did not reduce the recurrence of AF after CV. It reduced selective markers of inflammation without affecting systemic oxidative stress. Failure of atorvastatin to prevent AF recurrence may be due to its failure to affect oxidative stress.
Keywords: atrial fibrillation, statin, cardioversion, inflammation, oxidative stress
Introduction
Atrial fibrillation (AF) is the most commonly encountered sustained arrhythmia in clinical practice, with a prevalence of approximately 0.4 to 1% in general population, increasing to >8% in those over 80 years of age.1, 2 The longer AF persists, the more difficult it becomes to restore and maintain sinus rhythm.3 These observations suggest that age-related changes can initiate AF and there exists a positive feedback loop that maintains the arrhythmia.4
There is evidence to support the role of systemic and cardiac inflammation and related oxidative stress in the initiation and maintenance of AF.5, 6, 7 It has been demonstrated that patients with AF have elevated levels of inflammatory markers such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α). 8,9,10 CRP has been shown to be a marker of AF persistence, AF recurrence after electrical cardioversion (CV), and post-operative occurrence of AF. 8, 11, 12 Gene transcriptional profiles of atrial tissue from AF patients have shown elevated pro-oxidative gene expression. 13
Hydroxy-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors or statin drugs are recognized to have pleiotropic effects.14, 15 Recent studies indicate that statins may play a role in preventing or reversing atrial remodeling in AF, 16 possibly through their anti-inflammatory and antioxidant properties.14 In a randomized double-blind placebo-controlled trial, we tested the hypothesis that high dose statins maintain sinus rhythm following successful CV in persistent AF by modifying systemic oxidative stress and inflammation.
Methods
Study design and patient recruitment
In a randomized, double-blind, placebo-controlled trial (NCT00252967), patients with atrial fibrillation/ flutter (AF) were screened for eligibility. Subjects of both genders, with age ≥18 years and electrocardiographically (ECG) documented AF requiring CV, were enrolled from clinics at the Atlanta Veterans Affairs Medical Center and Emory University affiliated hospitals. Atrial fibrillation was defined as the absence of P waves, presence of continuous atrial electrical activity, and irregular RR intervals on a standard ECG. Atrial flutter was defined as an absence of P waves and the presence of continuous, regular atrial electrical activity. The protocol of the study was approved by the appropriate Institutional Review Boards. A written informed consent was obtained from all subjects. Exclusion criteria included age <18 years, enrollment in another ongoing trial, paroxysmal AF, hemodynamic instability, AF ablation within 6 months of enrollment, a contraindication for anticoagulation, severe valvular heart disease, the presence of a single lead implantable cardioverter defibrillator, unstable angina, NYHA Class IV heart failure, hyperthyroidism, uncontrolled hypertension (blood pressure >180/100 at rest) on medications, an illness that would limit life expectancy to less than 1 year, use of statins within the previous 30 days, significant coronary artery disease or lipid abnormalities necessitating statin therapy, implanted devices for active management of arrhythmias by pacing or defibrillation, lack of access to a telephone, illicit drug use, alcohol abuse, hypersensitivity to atorvastatin by history, pregnancy, sexually active female subjects not on contraception or surgically sterilized, and nursing mothers. Subjects with chronic liver disease or abnormal liver function (elevated transaminases 1.5 times the upper limit of normal (ULN) of laboratory reference range), severe renal disease (creatinine >200 mmol/L), inflammatory muscle disease or creatine kinase (CK) >3 times ULN, and concurrent treatment with cyclosporine, fibrates, or high-dose niacin were also excluded from the study.
Clinical Data
Baseline demographic and clinical data, including use of antiarrhythmic agents, was obtained from the medical records and on interview at the index visit. Blood samples were drawn at the time of entry into the study and on subsequent visits. A baseline transthoracic echocardiogram was obtained at the time of entry. Chamber dimensions, existing valvular disease, estimated left ventricular (LV) ejection fraction, LV wall thickness, and the presence of thrombi were documented.
Interventions
Patients were randomized to enter either the atorvastatin or the placebo limb of study in a 1:1 manner prior to performing CV. Active drug and placebo were administered in identical appearing tablets and all research personnel and subjects were blinded to the treatment drug. Identification codes were not broken during the trial. Atorvastatin at a dose of 80 mg daily was started at the time of randomization. After reconfirming AF, CV was performed 0-7 days after randomization by an independent cardiologist. ECG documentation of the resulting rhythm was obtained immediately after CV and at 1 hour. Successful CV was defined as sinus rhythm (SR) achieved and maintained for at least 1 hour after shock delivery. All subjects were anticoagulated at the time of CV. For subjects who were not therapeutically anticoagulated (INR>2 for 3 weeks) prior to CV, transesophageal echocardiogram was performed immediately before CV to exclude the presence of left atrial appendage (LAA) thrombus. If no LAA thrombus was noted, intravenous (IV) heparin was administered before CV, and a standard anticoagulation regimen was instituted. If no recurrence of AF was noted after 1 month after CV, anticoagulation was discontinued at the discretion of the investigator. Atorvastatin/placebo was continued for 12 months or until the recurrence of AF. Follow-up visits were undertaken at 1 month, at the time of recurrence, and when any subject complained of symptoms suggestive of recurrence. At these visits, a 24-hour Holter recording was performed. Compliance with the study medication was confirmed at each visit by pill count and refills were provided as needed.
Measurement of systemic oxidative stress and inflammation
Plasma levels of markers of systemic oxidative stress and inflammation were obtained at baseline, 1 and 12 months and upon recurrence, whichever occurred sooner. Markers of systemic oxidative stress included the ratio of oxidized to reduced cysteine (CyS/CySS), the ratio of oxidized to reduced glutathione (GSH/GSSG), F2-Isoprostanes (IsoPs), and derivatives of reactive oxygen metabolites (DROMs). 17 Standard methods for measurement of the ratios of oxidized to reduced cysteine and glutathiones were used.18 Redox potentials were calculated using the Nernst equation.17 More negative values corresponded to increased oxidation. Concentration of DROMs was determined using spectrometry (505 nm).19 IsoPs were quantified by gas chromatography/mass spectrometry using computer interference. 20
Systemic inflammatory markers measured included high sensitivity CRP (hs-CRP), interleukin-6 (IL-6), interleukin 1β (IL-1β), and tumor necrosis factor-α (TNF-α). All inflammatory markers were measured using commercially available assay kits.
Primary Endpoint
The primary endpoint of the study was time to first ECG documentation of recurrence of AF. Subjects with a 12-lead ECG demonstration of AF or ≥ 30s duration of the arrhythmia on Holter or telemetry recordings were considered to have reached the primary endpoint.
Statistical Analysis
Sample size was calculated based on the assumption that the 12-month recurrence rate of AF after CV in controlled trials ranged between 50% with the use of antiarrhythmic drugs to 70% in control group. 21 To observe a significant difference with α level of 0.05 and a power of 0.80, 25 patients were needed in each group. Categorical variables were compared using Fischer exact/Chi square test. All continuous data were checked for normal distribution. Continuous variables with normal distribution were analyzed using Student t test while those with skewed distribution were compared using Mann-Whitney test. Cox proportional hazards regression models were used to compute univariate and multivariate hazard ratios for AF recurrence in the two groups. Time to recurrence was analyzed using the Kaplan-Meier survival curves for placebo and treatment groups and compared using the log rank test.
Results
Sixty-four of 524 subjects screened for the eligibility criteria were randomized to receive either atorvastatin 80 mg (n=33) or placebo (n=31). Four hundred twenty eight screened subjects did not meet the eligibility criteria, mostly secondary to current statin use or an indication for statin use, and 32 subjects declined to participate in the study. Baseline demographic and clinical characteristics of the study subjects are presented in Table 1. The two groups were statistically similar in all baseline variables.
Table 1.
Comparison of baseline characteristics in the atorvastatin 80 mg and placebo groups
| Patient characteristics | Atorvastatin 80 mg (n=33) |
Placebo (n=31) |
P value |
|---|---|---|---|
| Age (years) | 58.2±11.6 | 58.9±13.9 | 0.91 |
| Gender (male) | 27 (81.8%) | 26 (83.9%) | 1.0 |
| Race (white) | 28 (84.8%) | 26 (83.9%) | 1.0 |
| Current smokers | 8 (24.2%) | 5 (16.1%) | 0.54 |
| BMI (kg/m2) | 33.1±12.2 | 34.4±12.2 | 0.67 |
| Hypertension | 17 (51.5%) | 15 (48.4%) | 1.0 |
| Diabetes mellitus | 4 (12.1%) | 1 (3.2%) | 0.36 |
| Ischemic heart disease | 4 (12.1%) | 4 (12.9%) | 1.0 |
| Previous cardiac surgery | 1 (3%) | 2 (6.5%) | 0.61 |
| Previous cardioversion | 18 (54.5%) | 12 (38.7%) | 0.22 |
| Heart rate (/minute) | 84.8±20.5 | 86.8±18.9 | 0.67 |
| Systolic BP (mm Hg) | 131.8±26.7 | 126.8±21.3 | 0.41 |
| Diastolic BP (mm Hg) | 82.5±16.0 | 78.9±11.3 | 0.30 |
| Ejection fraction (%) | 49.2±15.0 | 46.9±15.7 | 0.62 |
| LA dimension (mm) | 46.5±6.2 | 45.8±8.1 | 0.79 |
| Total cholesterol (mg/dl) | 183.5±37.3 | 163.3±38.4 | 0.07 |
| Baseline medical therapy | |||
| Antiarrhythmic | 17 (51.5%) | 16 (51.6%) | 1.0 |
| Coumadin | 29 (87.9%) | 28 (90.3%) | 1.0 |
| β blockers | 16 (48.5%) | 21 (67.7%) | 0.14 |
| RAAS inhibitors | 11 (33.3%) | 16 (51.6%) | 0.22 |
| Diuretics | 9 (27.3%) | 13 (41.9%) | 0.29 |
Data are presented as means ± standard deviation for continuous variables and numbers (percentages) for categorical variables. All continuous variables presented in the table have a normal distribution. BMI, Body mass index; BP, Blood pressure; LA, Left atrium; RAAS, Renin angiotensin aldosterone system.
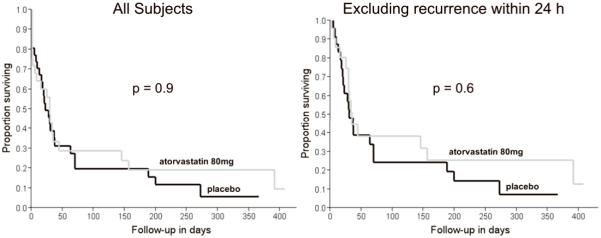
The duration between randomization and CV ranged between 0-7 days in both groups. There were no cases of spontaneous conversion to sinus rhythm between randomization (i.e., start of the study drug) and CV in either group. Kaplan-Meier survival curves for both groups are shown in Figure 1. Survival estimates showed no significant difference in the time to AF recurrence with atorvastatin 80 mg and placebo. (median (IR, interquartile range): 29 (2-145) days versus 22 (7-70) days, p=0.9). Using Cox proportional hazards regression analysis, there was no difference in AF recurrence in atorvastatin 80 mg and placebo groups on both univariate analysis (unadjusted hazard ratio: 1.0, 95% CI: 0.99-1.0, p=0.17) and after adjusting for the available potentially confounding demographic, clinical, and pharmacological variables (adjusted hazard ratio: 0.99, 95% CI: 0.98 -1.01, p=0.14). Eight (24.2%) patients on atorvastatin 80 mg and 7 (22.6%) on placebo had a recurrence in AF within the first 24 hours following successful CV (p=1.0). Even on excluding these patients, there was no significant difference in the time to recurrence of AF in the two groups (median (IR): 34 (25-391) days versus 30 (18-70) days, p=0.6) (Figure 1).
Figure 1.
Kaplan-Meier survival curves showing the time to AF recurrence in the atorvastatin 80 mg and placebo groups after successful cardioversion. (Left) for all trial subjects (p=0.9). (Right) excluding subjects with a recurrence within 24 hours (p=0.6).
Measures of systemic oxidative stress and inflammation at baseline and 1 month after CV are compared between the two study groups in Table 2. While there was no significant difference in the systemic oxidative stress at 1 month between the atorvastatin and placebo groups, 2 of 4 inflammatory markers, IL-6 (unadjusted OR: 0.69, 95% CI: 0.48-0.99) and hs-CRP (unadjusted OR: 0.79, 95% CI: 0.65-0.95), were significantly lower in the atorvastatin group. The difference in effect sizes for IL-6 and hs-CRP was maintained in a multivariate analysis even after adjustment for age, gender, body mass index (BMI), smoking status, chronic medical conditions, and renin-angiotensin system inhibitors (IL-6, adjusted OR: 0.59, 95%CI: 0.35-0.97; hs-CRP, adjusted OR: 0.58, 95% CI: 0.37-0.94). The efficacy of the study drug was assessed by comparing total cholesterol levels at baseline and on 1-month follow-up visit. While there was no change in the placebo group (163.3 ± 38.4 versus 166.3 ± 47.2 mg/dl, p=0.17), patients on atorvastatin 80 mg showed a significant 23% reduction in the mean cholesterol levels (183.5 ± 37.3 versus 142.0 ± 45.4 mg/dl, p=0.03). Safety was monitored by an independent board, and no major side-effects requiring discontinuation of treatment or reduction in dosage were reported during the study period.
Table 2.
Comparison of systemic oxidative stress and inflammation markers in atorvastatin 80 mg and placebo groups
| Serum markers | Baseline |
1 month follow-up |
||||
|---|---|---|---|---|---|---|
| atorvastatin 80mg | placebo | P value | atorvastatin 80mg | placebo | P value | |
| Oxidative stress markers | ||||||
| Eh Cys (mV) | −65.3 (−71.3 to −58.8) | −61.2 (−71.2 to −54.8) | 0.28 | −62.6 (−68.0 to −58.0) | −64.5 (−69.8 to −58.5) | 0.72 |
| Eh GSH (mV) | −125.3 (−138.0 to −117.3) | −116.7 (−134.9 to −110.2) | 0.43 | −129.0 (−138.2 to −113.4) | −124.3 (−142.1 to −109.0) | 0.55 |
| DROM (Carr units) | 382 (322 to 467) | 384 (276 to 422) | 0.75 | 345 (304 to 406) | 400 (338 to 450) | 0.15 |
| IsoP (pg/ml) | 1062 (817 to 1421) | 1259 (1040 to 1738) | 0.62 | 1240 (953 to 1397) | 1146 (964 to 1408) | 0.29 |
| Inflammatory markers | ||||||
| IL-6 (ng/ml) | 4.0 (1.7 to 6.6) | 5.3 (2.1 to 9.2) | 0.26 | 2.2 (1.7 to 3.6) | 5.6 (3.2 to 8.6) | 0.03 |
| IL-1 (ng/ml) | 0.30 (0.17 to 0.47) | 0.29 (0.20 to 0.44) | 0.82 | 0.28 (0.21 to 0.32) | 0.25 (0.17 to 0.32) | 0.41 |
| hs-CRP (mg/l) | 6.3 (2.2 to 11.8) | 9.6 (1.7 to 15.0) | 0.42 | 2.3 (1.3 to 4.7) | 12.3 (2.8 to 14.4) | 0.001 |
| TNFα (ng/ml) | 1.8 (1.4 to 2.5) | 2.0 (1.2 to 2.9) | 0.41 | 1.5 (1.2 to 2.9) | 2.6 (1.7 to 3.8) | 0. 47 |
All continuous variables in the table had a skewed distribution, and data are presented as median (IR, 25 to 75 interquartile range). P values were derived from non-parametric tests. Eh Cys, redox potential for cysteine; Eh GSH, redox potential for glutathione; DROM, Derivatives of reactive oxygen metabolites; IsoP, Isoprostanes; IL-6, Interleukin-6; IL-1, Interleukin-1; hs-CRP, High sensitivity C-reactive protein; TNFα, Tumor necrosis factor alpha.
Discussion
Inflammation, oxidative stress, and atrial fibrosis have all been considered as novel potential therapeutic targets for upstream therapy in the treatment and prevention of AF. Statins have been shown to have pleiotropic effects.14, 15 Nevertheless, clinical trials conducted so far have shown conflicting results with regards to the benefits of statins in the prevention of recurrence of AF after CV.22, 23, 24, 25 In this study, we showed that, despite reducing some inflammatory markers and total cholesterol in the study group, high dose atorvastatin did not affect the recurrence of AF after CV.
Our findings are consistent with those of recent trials. In an open, controlled multicenter study, 24 there was no reduction in the recurrence rate of AF after CV with pravastatin 40 mg daily. In another recent randomized, placebo controlled, multicenter study, atorvastatin 80 mg showed no benefit over placebo in maintaining SR in patients after successful CV.25 A subgroup analysis of the Antihypetensive and Lipid Lowering Treatment to prevent Heart Attack Trial (ALLHAT), found that assignment to pravastatin did not alter the incidence of AF in the study population.26
Systemic and cardiac oxidative stress and increased cardiac NADPH oxidase activity have been associated with AF.5,17,27,28,29 Statins have activity against NADPH oxidases.30, 31 Nevertheless, statin use did not alter systemic oxidative stress in our trial. It is possible that the NADPH oxidase is not the major source of oxidative stress in the myocardium, unlike in blood vessels. Alternatively, it may be that the major source of oxidative stress in persistent AF shifts away from the NADPH oxidase over time. The GISSI-HF group compared the effect of rosuvastatin 10 mg with placebo on the incidence of new AF in heart failure patients. Though they demonstrated a statistically significant improvement in the new AF group, chronic AF patients showed no benefit with statin therapy.32 However, consistent with our data, statin therapy did lower hs-CRP levels in this study. Increased NADPH oxidase activity may contribute to the initiation of AF, but, once established, the oxidative stress driving AF may be derived from sources that are not affected by statins. Thus, NADPH oxidase may have a role in acute onset AF as seen after surgery, 31 but not in established AF. This could explain why agents altering NADPH oxidase signaling, such as renin-angiotensin inhibitors and statins, would be more effective in acute setting like post-operative AF and less effective in chronic longstanding AF. Consistent with this idea, a recent large trial looking at angiotensin receptor blockade in prevention of recurrence of AF showed no benefit in established AF.33
While 2 of 4 markers of systemic inflammation were lowered with high dose statin in our study, there was no significant effect on the time to recurrence after CV. This may imply that though high dose statins have a systemic anti-inflammatory action, it may not necessarily translate into reduction in AF recurrence. The findings, however, do not rule out the role of localized or specific forms of cardiac inflammation in the initiation, maintenance, and recurrence of AF. Moreover, the cause and effect relationship of inflammation and AF is not clear. The current evidence is inconclusive whether inflammation causes AF or the AF itself generates inflammation. 34, 35
Observational studies that showed a benefit of statin therapy on recurrence rates of AF after CV involved a patient population that was older with a significant prevalence of coronary artery disease. 36 It may be possible that statins have greater efficacy against vascular rather than cardiac inflammation or that statins do not affect the relevant inflammatory cascades responsible for perpetuation of chronic AF. Subjects with coronary artery disease were largely excluded in our study, since these patients had a preexisting indication for statin use.
Oxidative stress measures were not altered in our trial. Thus, it remains to be determined if oxidative stress contributes to maintenance of AF. Systemic oxidative stress has been linked to persistent AF. 17 Several antioxidants including ascorbate, Probucol and N-acetylcysteine have been studied in AF. 37,38,39,40 While these studies support the role of oxidative stress in pathophysiology of AF, they do not clarify its source.
Limitations
Although a large number of patients were screened, the ubiquitous use and indications for statin therapy limited enrollment. Even if statins were to prove efficacious in larger trials; based on our findings, it is unlikely that the effect would be through alterations in systemic inflammation or oxidative stress. On the other hand, we excluded subjects with significant coronary artery disease and dyslipidemia. The effect of statin use on recurrence of AF in this population remains unknown. Finally, this study is relevant only to patients with persistent AF, and as discussed above, post-operative or new onset AF may respond differently.
Recurrence rate was slightly higher in our study when compared to that seen in other trials, with a significant portion of recurrence early after CV.24, 25, 26, 40 It is possible that the study sample was derived from a high risk population. Long-standing AF leads to anatomical and electrical remodeling, and fibrosis of atrial tissue causing chronicity and high recurrence rate.
Conclusions
High dose atorvastatin did not reduce the recurrence of AF after CV. It reduced selective markers of inflammation without affecting systemic oxidative stress. Failure of atorvastatin to prevent AF recurrence may be due to its failure to affect oxidative stress.
Acknowledgments
This work was supported by a grant from Pfizer, Inc, and NIH R01 HL085558, R01 HL073753, P01 HL058000 (SCD).
Footnotes
Other authors: No disclosures.
References
- 1.ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevelance, age, distribution, and gender of patients with atrial fibrillation: analysis and implication. Arch Intern Med. 1995;155:469–73. [PubMed] [Google Scholar]
- 3.Wijffels MC, Kircchof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 4.Anyukhovsky EP, Sosunov EA, Chandra P, Rosen TS, Boyden PA, Danilo P, Jr, Rosen MR. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66:353–63. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 6.Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res. 2004;62:105–11. doi: 10.1016/j.cardiores.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Ozaydin M, Dogan A, Varol E, Kucuktepe Z, Dogan A, Ozturk M, Altinbas A. Statin use before by-pass surgery decreases the incidence and shortens the duration of postoperative atrial fibrillation. Cardiology. 2007;107:117–21. doi: 10.1159/000094589. [DOI] [PubMed] [Google Scholar]
- 8.Chung MK, Martin DO, Sprecher D. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 9.Sata N, Hamada N, Horinouchi T, Amitani S, Yamashita T, Moriyama Y, Miyahara K. C-reactive protein and atrial fibrillation. Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J. 2004;45:441–5. doi: 10.1536/jhj.45.441. [DOI] [PubMed] [Google Scholar]
- 10.Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, Burzotta F, Iacoviello L, Donati MB, Schiavello R, Maseri A, Possati G. The 2174G/C interleukin- 6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108:195–9. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 11.Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375–80. doi: 10.2143/AC.56.6.2005701. [DOI] [PubMed] [Google Scholar]
- 12.Conway DS, Buggins P, Hughes E, Lip GY. Predictive value of indices of inflammation and hypercoagulability on success of cardioversion of persistent atrial fibrillation. Am J Cardiol. 2004;94:508–10. doi: 10.1016/j.amjcard.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Lim DS, Lee JH, Shim WJ, Ro YM, Park GH, Becker KG, Cho-Chung YS, Kim MK. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp Mol Med. 2003;35:336–49. doi: 10.1038/emm.2003.45. [DOI] [PubMed] [Google Scholar]
- 14.Lefer DJ. Statins as potent anti-inflammatory drugs. Circulation. 2002;106:2041–2. doi: 10.1161/01.cir.0000033635.42612.88. [DOI] [PubMed] [Google Scholar]
- 15.Riesen WF, Engler H, Risch M, Korte W, Noseda G. Short-term effects of atorvastatin on C-reactive protein. Eur Heart J. 2002;23:753–830. doi: 10.1053/euhj.2001.2967. [DOI] [PubMed] [Google Scholar]
- 16.Shiroshita-Takeshita A, Brundel BJ, Burstein B, Leung TK, Mitamura H, Ogawa S, Nattel S. Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc Res. 2007;74:75–84. doi: 10.1016/j.cardiores.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–7. doi: 10.1373/clinchem.2006.083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trachtenberg BH, Hare JM. Biomarkers of oxidative stress in heart failure. Heart Fail Clin. 2009;5:561–77. doi: 10.1016/j.hfc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Cornelli U, Terranova R, Luca S, et al. Bioavailability and antioxidant activity of some food supplements in men and women using the DROM tests as a marker of oxidative stress. J. Nutr. 131:3208–11. doi: 10.1093/jn/131.12.3208. [DOI] [PubMed] [Google Scholar]
- 20.Roberts LJ, Milne GL. Isoprostanes. J Lipid Res. 2009;50:S219–23. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ, Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 22.Siu CW, Lau CP, Tse HF. Prevention of atrial fibrillation recurrence by statin therapy in patients with lone atrial fibrillation after successful cardioversion. Am J Cardiol. 2003;92:1343–5. doi: 10.1016/j.amjcard.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Ozaydin M, Varol E, Suleyman MA, Kucuktepe Z, Dogan A, Ozturk M, Altinbas A. Effect of Atorvastatin on recurrence rates of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2005:1491–3. doi: 10.1016/j.amjcard.2005.11.082. [DOI] [PubMed] [Google Scholar]
- 24.Tveit A, Grundtvig M, Gundersen T, Vanberg P, Semb AG, Holt E, Gullestad L. Analysis of pravastatin to prevent recurrence of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2004;93:780–2. doi: 10.1016/j.amjcard.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Almroth H, Hoglund N, Boman K, Englund A, Jensen S, Kjellman B, Tornvall P, Rosenqvist M. Atorvastatin and persistent atrial fibrillation after cardioversion:a randomized placebo controlled multicentre study. Eur Heart J. 2009;30:827–33. doi: 10.1093/eurheartj/ehp006. [DOI] [PubMed] [Google Scholar]
- 26.Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, Williard A, ALLHAT Collaborative Research Group Atrial fibrillation at baseline and during follow up in ALLHAT( Antihypertensive and Lipid Lowering treatment to prevent Heart Attack Trial. J Am Coll Cardiol. 2009;54:2023–31. doi: 10.1016/j.jacc.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Cai H, Li Z, Goette A, Mera F, Honeycutt L, Feterik K, Wilcox JN, Dudley SC, Jr, Harrison DG, Langberg JJ. Downregulation of Endocardial Nitric Oxide Synthase Expression and Nitric Oxide Production in Atrial Fibrillation. Potential Mechanisms for Atrial Thrombosis and Stroke. Circulation. 2002;106:2854–8. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 28.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamondopoulos L, Fukai T, Harrison DG, Dikalov LI, Langberg JJ. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–73. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 29.Goette A, Bukowska A, Lendeckel U, Erxleban M, Hammwöhner M, Strugala D, Pfeiffenberger J, Röhl F, Rocken C. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation. 2008;117:732–42. doi: 10.1161/CIRCULATIONAHA.107.730101. [DOI] [PubMed] [Google Scholar]
- 30.Briones AM, Rodríguez-Criado N, Hernanz R, García-Redondo AB, Rodrigues-Díez RR, Alonso MJ, Egido J, Ruiz-Ortega M, Salaices M. Atorvastatin prevents angiotensin II–induced vascular remodeling and oxidative stress. Hypertension. 2009;54:142–9. doi: 10.1161/HYPERTENSIONAHA.109.133710. [DOI] [PubMed] [Google Scholar]
- 31.Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol J. Statins are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol. 2002;40:611–7. doi: 10.1097/00005344-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, Nicolosi GL, Porcu M, Cosmi F, Stefanelli S, Tognoni G, Tavazzi L, The GISSI-HF Investigators Effect of rosuvastatin on atrial fibrillation: ancillary results of the GISSI-HF trial. Eur H J. 2009;30:2327–36. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 33.The GISSI-AF Investigators Valsartan for Prevention of Recurrent Atrial Fibrillation. N Engl J Med. 2009;360:1606–17. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- 34.Pepys MB, Hirschfiled GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD, Polara A, Cobb AJ, Ley SV, Aquilina JA, Robinson CV, Sharif I, Gray GA, Sabin CA, Jenvey MC, Kolstoe SE, Thompson D, Wood SP. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 35.Tveit A, Seljeflot I, Grundvold I, Abdelnoor M, Smith P, Arnesen H. Effect of candesartan and various inflammatory markers on maintenance of sinus rhythm after electrical cardioversion for atrial fibrillation. Am J Cardiol. 2007;99:1544–8. doi: 10.1016/j.amjcard.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Humphries KH, Lee M, Sheldon R, Ramanathan K, Dorian P, Green M, Kerr CR, CARAF Investigators Statin use and recurrence of atrial fibrillation after successful cardioversion. Am Heart J. 2007;154:908–13. doi: 10.1016/j.ahj.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Carnes CA, Chung MK, Nakayama T, Nakayama, Baliga R, Piao S, Kanderian A, Pavia S, Hamlin RA, McCarthy PM, Bauer JA, Van Wagoner DA. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:e32–e38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 38.Korantzopoulos P, Kolettis TM, Kountouris E, Dimitroula V, Karanikis P, Pappa E, Siogas K, Goudevenos JA. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol. 2005;102:321–6. doi: 10.1016/j.ijcard.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Sheng L, Li W, Liu W, Gong Y, Xue H, Shan H. Probucol attenuates atrial structural remodeling in prolonged pacing-induced atrial fibrillation in dogs. Biochem Biophysical Res Comm. 2009;381:198–203. doi: 10.1016/j.bbrc.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Ozaydin M, Peker O, Erdogan D, Kapan S, Turker Y, Varol E, Ozguner F, Dogan A, Ibrisim E. N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective,randomized, placebo-controlled pilot study. Eur Heart J. 2008;29:625–31. doi: 10.1093/eurheartj/ehn011. [DOI] [PubMed] [Google Scholar]