Abstract
Chronic aldosterone/salt treatment (ALDOST) is accompanied by an adverse structural remodeling of myocardium that includes multiple foci of microscopic scarring representing morphologic footprints of cardiomyocyte necrosis. Our previous studies suggested that signal-transducer-effector pathway leading to necrotic cell death during ALDOST includes intramitochondrial Ca2+ overloading, together with an induction of oxidative stress and opening of the mitochondrial permeability transition pore (mPTP). To further validate this concept, we hypothesized mitochondria-targeted interventions will prove cardioprotective. Accordingly, 8-wk-old male Sprague-Dawley rats receiving 4 wks ALDOST were cotreated with either quercetin (Q), a flavonoid with mitochondrial antioxidant properties, or cyclosporine A (CsA), an mPTP inhibitor, and compared to ALDOST alone or untreated, age-/sex-matched controls. We monitored: mitochondrial free Ca2+ and biomarkers of oxidative stress, including 8-isoprostane and H2O2 production; mPTP opening; total Ca2+ in cardiac tissue; collagen volume fraction (CVF) to quantify replacement fibrosis, a biomarker of cardiomyocyte necrosis; and employed TUNEL assay to address apoptosis in coronal sections of ventricular myocardium. Compared to controls, at 4 wks ALDOST we found: a marked increase in mitochondrial H2O2 production and 8-isoprostane levels, an increased propensity for mPTP opening, and greater concentrations of mitochondrial free [Ca2+]m and total tissue Ca2+, coupled with a 5-fold rise in CVF without any TUNEL-based evidence of cardiomyocyte apoptosis. Each of these pathophysiologic responses to ALDOST were prevented by Q or CsA cotreatment. Thus, mitochondria play a central role in initiating the cellular-molecular pathway that leads to necrotic cell death and myocardial scarring. This destructive cycle can be interrupted and myocardium salvaged with its structure preserved by mitochondria-targeted cardioprotective strategies.
Keywords: Aldosteronism, Mitochondria, Intracellular calcium overloading, Oxidative stress, Mitochondrial permeability transition pore
INTRODUCTION
Chronic inappropriate (relative to dietary Na+) elevations in plasma aldosterone occurs with congestive heart failure (CHF), low-renin hypertension or primary aldosteronism, and contribute to an adverse structural remodeling of the myocardium including the appearance of multiple foci of microscopic scarring, footprints of prior cardiomyocyte necrosis (1–4). Pathophysiologic responses involved in the appearance of this replacement fibrosis have been examined in our well-established rat model of chronic aldosteronism, where plasma aldosterone levels are raised to those found in human CHF, together with a 1% NaCl diet. Cardiac pathology first appears at wk 4 of aldosterone/salt treatment (ALDOST) encompassing both the right and left atria and ventricles (5, 6). Through a series of experimental interventions in rats with ALDOST, we identified intracellular Ca2+ overloading leading to the induction of oxidative stress as contributory to cardiac myocyte necrosis and hence foci of scarring (7–10).
The cellular-molecular mechanisms involved in the signal-transducer-effector pathway leading to myocardial necrosis during ALDOST were next examined. The excessive intracellular Ca2+ accumulation was found to involve both cytosolic and mitochondrial compartments (11, 12). These studies further identified that Ca2+ overloaded mitochondria are the predominant source of reactive oxygen species (ROS) and consequent dysequilibrium between prooxidants and endogenous antioxidants. These sequelae predispose vulnerable mitochondria to pathologic opening of the mitochondrial permeability transition pore (mPTP) with ensuing osmotic swelling and their degeneration that is detrimental to cardiomyocyte survival (13, 14). Cotreatment with antioxidants, N-acetylcysteine, a Zn2+ ionophore, or ZnSO4 supplement have proven cardioprotective in counteracting the proinflammatory phenotype, oxidative stress and cardiomyocyte necrosis with replacement fibrosis in chronic ALDOST (5, 11, 14). Furthermore, Cai and coworkers have found supplemental Zn2+ to be cardioprotective against the oxidative injury associated with either diabetic or alcohol-induced cardiomyopathy (15, 16).
Hence, mitochondria appear to play a central role in initiating a destructive cycle leading to cell death. This common pathogenic mechanism also underlies several neurodegenerative disorders (17, 18). However, the importance of mitochondria-generated ROS in altering cardiomyocyte redox state and its role in leading to necrotic cell death during ALDOST, albeit CHF, remains uncertain. Accordingly, the present study was undertaken to explore the validity and universality of that central concept. Herein, we hypothesized mitochondria-targeted interventions would prove cardioprotective in rats receiving 4 wks ALDOST. Javadov, et al. (19) have suggested the attenuation of ROS accumulation and inhibition of mPTP opening as two different yet promising pathways. Consequently, we examined responses to cotreatment with either quercetin, a flavonoid with mitochondrial antioxidant properties (20), or cyclosporine A (CsA) an mPTP inhibitor (21). Compared to age-/gender-matched untreated controls, we monitored: total Ca2+ in cardiac tissue; mitochondrial free Ca2+ and biomarkers of oxidative stress that included their production of H2O2 and 8-isoprostane level, an index of lipid peroxidation; mitochondrial mPTP opening; and collagen volume fraction, a marker of necrosis, and TUNEL assay as an estimate of apoptosis in coronal sections of the heart.
METHODS
Animal Model
Eight-week-old male Sprague-Dawley rats were used throughout this series of experiments approved by our institution’s Animal Care and Use Committee. As reported previously and following uninephrectomy, an osmotic minipump containing ALDO was implanted subcutaneously. It releases ALDO (0.75 μg/h) to raise circulating ALDO levels to those commonly found in human CHF that suppresses plasma renin activity and circulating levels of angiotensin II. Drinking water was fortified with 1% NaCl and with 0.4% KCl to prevent hypokalemia. A detailed accounting of this model, including various experimental controls (e.g., uninephrectomy, ALDO, or 1% NaCl treatment alone) have been reported elsewhere (12, 22).
Separate groups of rats received ALDOST plus 4 wks cotreatment with either: a) quercetin (25 mg/kg/day by once-daily gavage); or b) cyclosporine A (10 mg/kg/day by once-daily subcutaneous injection).
Cardiac pathology first appears at wk 4 ALDOST. We therefore restricted the present study to this time point. Rats were killed at 4 wks ALDOST alone or at wk 4 ALDOST plus either cotreatment. Unoperated, untreated gender-/age-matched rats served as controls.
Isolation of Mitochondria
Mitochondria were isolated by differential centrifugation from whole heart homogenates and the purity of the mitochondrial preparation and integrity of their membranes were routinely assessed as we previously reported (11, 13).
Mitochondrial H2O2 Production
We monitored H2O2 released by these organelles in response to succinate stimulation as we previously reported (14).
Mitochondrial 8-Isoprostane
Mitochondrial 8-isoprostane levels were measured using a competitive enzyme immunoassay as we previously reported (11).
Mitochondrial mPTP Opening
mPTP opening was evaluated by 200 μM Ca2+-induced swelling of isolated mitochondria and it was completely prevented by preincubation of the organelles with 30 nM cyclosporine A as we previously reported (13).
Myocardial Total Ca2+
Heart tissue was harvested at wk 4 ALDOST and total tissue Ca2+ concentration determined by flame atomic spectroscopy as we previously reported (23).
Mitochondrial Free [Ca2+]m
Mitochondrial free [Ca2+]m was determined by the ratiometric method using FURA-2 and expressed as nanomolar as we previously reported (14, 24).
Cardiac Morphology
The extent of cardiac fibrosis was assessed by picrosirius red staining in coronal sections (6 μm) of the right and left ventricles and examined by light microscopy as we previously reported (5). Collagen volume fraction found in each of the sections was determined using a computer image analysis as we previously reported (25).
TUNEL Assay for Cardiomyocyte Apoptosis
Apoptosis was detected using an Apop Tag Fluorescein kit (Intergen, Norcross, GA, USA). In brief, cryostat sections (6μm) were fixed in 1% paraformaldehyde, followed by incubation with terminal deoxynucleotidyl transferase (TdT) enzyme. Sections were then incubated with anti-digoxigenin conjugated with fluorescein. Sections were mounted and viewed by fluorescence microscopy (26).
Statistical Analysis
Group data are presented as mean±SEM, and analyzed by one-way ANOVA in SPSS software (ver. 18.0; SPSS, Inc., Chicago, Illinois, USA) with p<0.05 as significant. Multiple-group comparisons between two groups were made by Scheffe’s F-test.
RESULTS
Mitochondrial Oxidative Stress
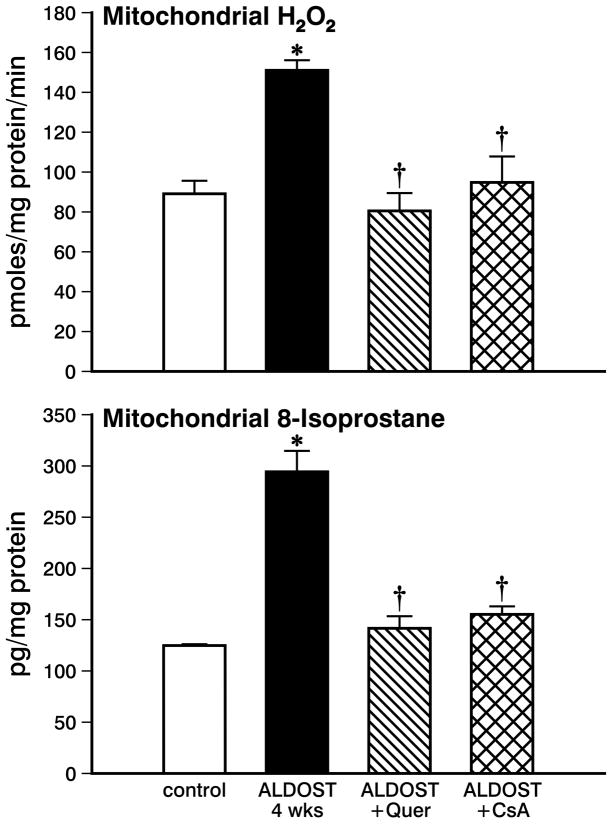
In mitochondria harvested from hearts of rats receiving 4 wks ALDOST alone, succinate-stimulated H2O2 production was significantly increased (p<0.05) compared to untreated controls (see Figure 1, upper panel). A significant increase was also found in mitochondrial 8-isoprostane levels at 4 wks ALDOST (p<0.05) compared to controls (see lower panel, Figure 1). These findings indicate the presence of oxidative stress in cardiac mitochondria during the chronic stressor state that accompanies 4 wks of aldosteronism.
Figure 1.
Mitochondrial H2O2 production and 8-isoprostane levels in organelles harvested from control hearts, with 4 wks aldosterone/salt treatment (ALDOST) alone, with 4 wks ALDOST plus quercetin cotreatment and with 4 wks ALDOST plus cyclosporine A cotreatment. *p<0.05 vs. controls; †p<0.05 vs. 4 wks ALDOST alone.
The increase in mitochondrial H2O2 production and 8-isoprostane levels seen during 4 wks ALDOST were each prevented (p<0.05) in response to cotreatment with the flavonoid, quercetin (see Figure 1, upper and lower panels, respectively) in keeping with its antioxidant properties. Cyclosporine A attenuated (p<0.05) both the increment in mitochondrial H2O2 production and increased level of mitochondrial 8-isoprostane seen during 4 wks ALDOST (see Figure 1, upper and lower panels, respectively).
Mitochondrial Permeability Transition Pore Opening
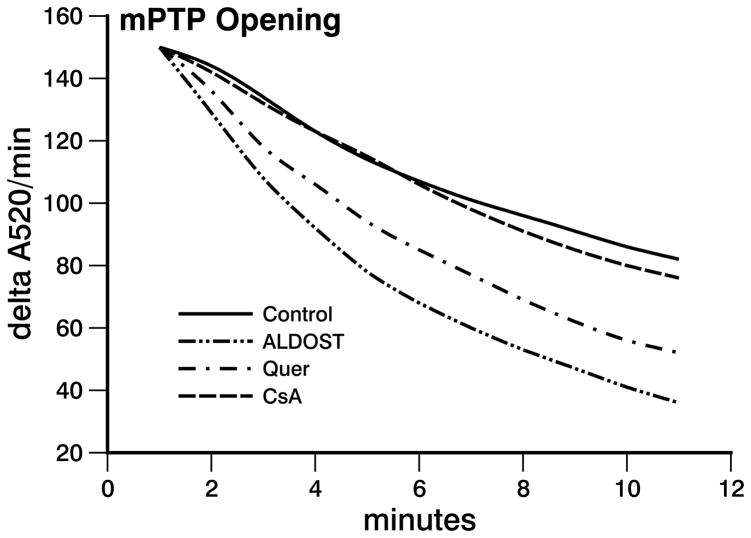
The decrease in spectrophotometric absorbance due to mPTP opening induced by 200 μM CaCl2 in mitochondria harvested at 4 wks ALDOST was greater than that seen in the controls (see Figure 2). This implicates an increased propensity of cardiomyocytes to mPTP opening and a greater susceptibility to necrosis during chronic aldosteronism. In this context, the observed frequency in mPTP opening seen in response to 4 wks ALDOST was attenuated (p<0.05) by quercetin cotreatment (see Figure 2). The increased propensity to mPTP opening in cardiomyocytes that occurs during 4 wks ALDOST was prevented (p<0.05) by CsA cotreatment (see Figure 2).
Figure 2.
Mitochondrial swelling was induced by addition of 200 μmol/l CaCl2 into the reaction chamber. The decrease in rate of changes in adsorption at 520 nm wavelength per minute (A520/min) in absorbance was plotted against time (minutes). Four weeks of ALDOST alone markedly increased mitochondrial transition pore opening (mPTP) compared with control which was significantly attenuated by quercetin cotreatment and completely prevented by cyclosporine A cotreatment.
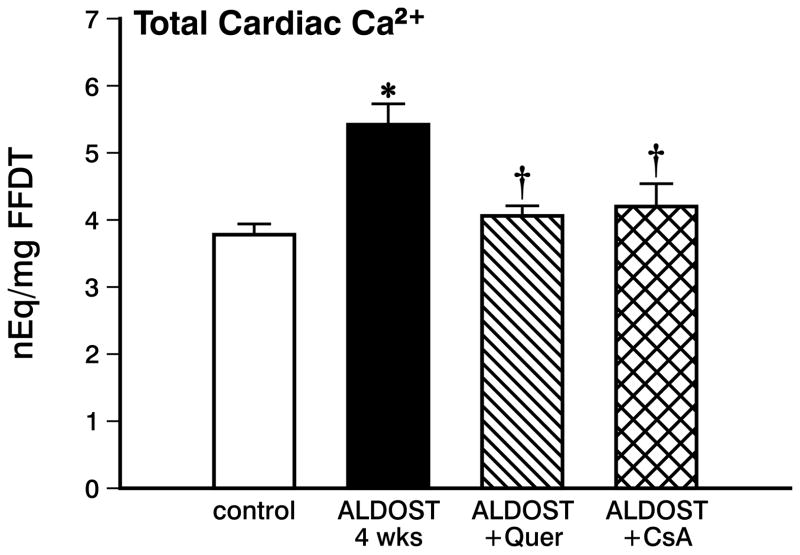
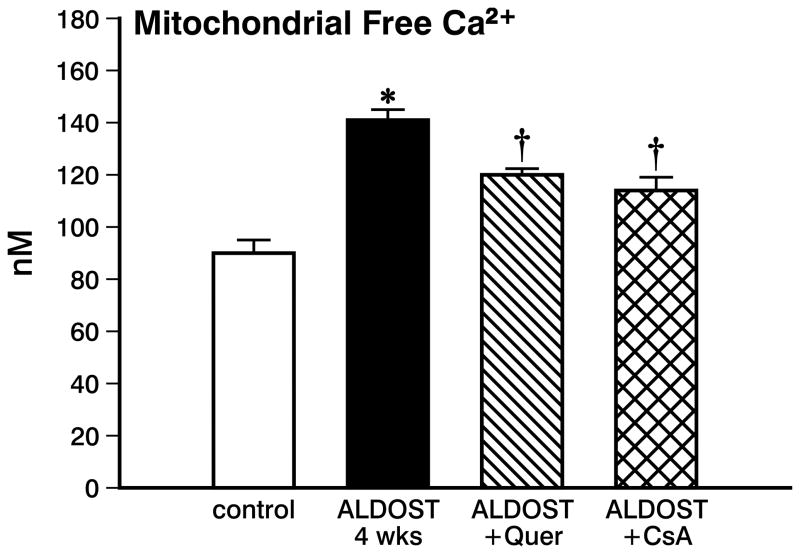
Mitochondrial Total and Free Ca2+
In view of the anticipated intracellular Ca2+ overloading of cardiac myocytes and mitochondria seen during aldosteronism, total Ca2+ in cardiac tissue was significantly increased (p<0.05) at 4 wks ALDOST (see Figure 3). Along with the rise in total cardiac tissue Ca2+, mitochondrial free Ca2+ was also significantly increased (p<0.05) at 4 wks ALDOST (see Figure 4).
Figure 3.
Myocardial total Ca2+ concentration from control hearts, with 4 wks ALDOST alone, with 4 wks ALDOST plus quercetin cotreatment and with 4 wks ALDOST plus cyclosporine A cotreatment. *p<0.05 vs. controls; †p<0.05 vs. 4 wks ALDOST alone.
Figure 4.
Mitochondrial free Ca2+ concentration harvested from control hearts, with 4 wks ALDOST alone, with 4 wks ALDOST plus quercetin cotreatment and with 4 wks ALDOST plus cyclosporine A cotreatment. *p<0.05 vs. controls; †p<0.05 vs. 4 wks ALDOST alone.
Unlike the myocardial Ca2+ overloading seen at 4 wks of ALDOST alone, quercetin cotreatment attenuated the rise of total Ca2+ in cardiac tissue and the levels were virtually indistinguishable from controls (see Figure 3). This would further implicate that reactive oxygen species indeed contribute to the excessive Ca2+ entry into the cardiomyocytes during chronic ALDOST and which could be attenuated by quercetin cotreatment. Likewise, mitochondrial free [Ca2+]m was significantly attenuated (p<0.05) but not totally abrogated with quercetin cotreatment (see Figure 4).
Similar to quercetin, cotreatment with CsA also prevented (p<0.05) intracellular Ca2+ overloading of cardiac tissue. This finding again supports our contention that reactive oxygen species contribute to Ca2+ overloading of myocardium during 4 wks ALDOST (see Figure 3). Furthermore, mitochondrial Ca2+ overloading was significantly attenuated (p<0.05) but not abolished by CsA cotreatment (see Figure 4).
Cardiac Pathology
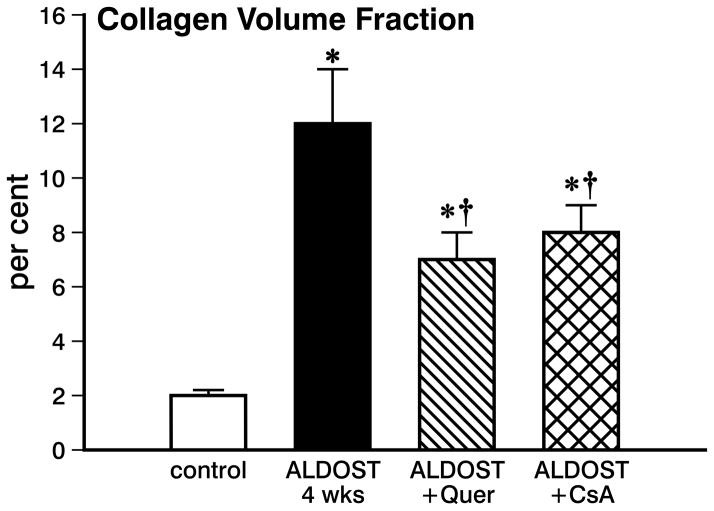
In rats receiving ALDOST for 4 wks, we did not find any evidence of apoptotic cardiomyocytes in either the left or right ventricles. At the same time, however, 4 wks ALDOST was associated with the appearance of replacement fibrosis involving both the right and left heart that structurally represent morphologic footprints of cardiomyocyte necrosis. Myocardial fibrosis was quantified to be a 5-fold increase in collagen volume fraction (p<0.01) found in coronal sections of the right and left ventricles. In response to cotreatment with either quercetin or CsA, myocardial scarring found at 4 wks ALDOST was significantly attenuated (p<0.05) by each of the targeted interventions (see Figure 5). Taken together, our cumulative findings presented herein unequivocally upholds our notion that in targeting mitochondria with either quercetin or cyclosporine A to prevent oxidative stress during aldosteronism, it is feasible to pharmacologically salvage myocardium and its cardiomyocytes.
Figure 5.
Collagen volume fraction found in the coronal section of the right and left ventricles from control hearts, with 4 wks ALDOST alone, with 4 wks ALDOST plus quercetin cotreatment and with 4 wks ALDOST with cyclosporine A cotreatment. *p<0.05 vs. controls; †p<0.05 vs. 4 wks ALDOST alone.
DISCUSSION
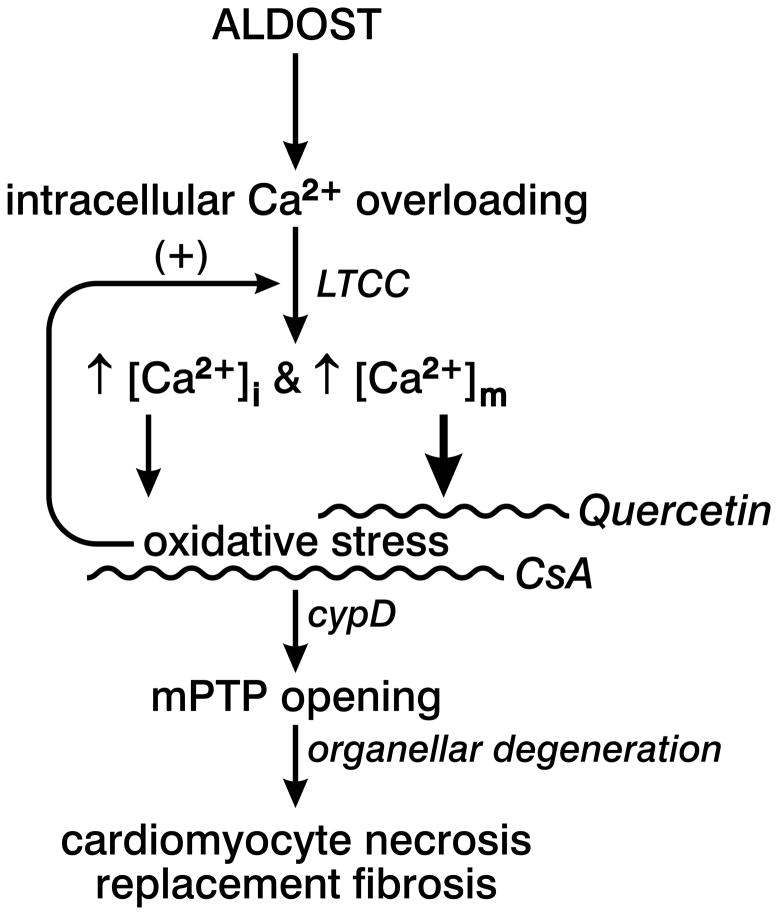
In rats, 4 wks aldosterone/salt treatment (ALDOST) is accompanied by multiple foci of microscopic scarring, a footprint of previous cardiomyocyte necrosis. As depicted in Figure 6, intracellular Ca2+ overloading, including significantly elevated cytosolic [Ca2+]i and mitochondrial [Ca2+]m, together with the accompanying induction of oxidative stress and pathologic opening of the mitochondrial permeability transition pore (mPTP), have been implicated as integral components of a signal-transducer-effector pathway contributing to cardiomyocyte necrosis and subsequent fibrosis during ALDOST. A similar pathophysiologic scenario evolves in ischemia/reperfusion injury (27). The rate of reactive oxygen and nitrogen species (ROS/RNS) generation at wk 1 ALDOST is offset by endogenous antioxidant defenses (13). However, persistent intracellular Ca2+ overloading and marked rise in oxidative stress at wk 4 overwhelms their rate of elimination by these defenses and accounts for the delayed appearance of cardiomyocyte necrosis and fibrosis (7, 8, 13). In fact, persistent mitochondrial Ca2+ overloading, coupled with their ongoing generation of ROS, orchestrate a sustained activation of L-type Ca2+ channels that eventuates in cellular necrosis (28). In turn, activated L-type Ca2+ channels dictate the functional dysregulation of mitochondria via sustained intracellular Ca2+ entry and its organellar influx, thus creating a positive feedback loop (see Figure 6) (29, 30). ROS generation in cardiomyocytes may arise from several sources: a sarcolemmal-bound NADPH oxidase; xanthine oxidase in the cytosol; and most predominantly within the mitochondrial inner membrane. The crucial pathophysiologic roles of mitochondria as a primary source in contributing to cardiac pathology during ALDOST deserves to be further explored. In this context, we targeted mitochondria with pharmacologic interventions to ascertain the role of these organelles in leading to necrosis with scarring and the cardioprotective potential of these agents when given as cotreatment to rats receiving ALDOST. These included: quercetin, a naturally occurring flavonoid found in fruits, having uncoupling activity in rat heart mitochondria (20); and cyclosporine A, an immunosuppressant which is also a mPTP inhibitor (21).
Figure 6.
The schematic governing our central hypothesis representing the pathophysiologic sequence of events in chronic aldosterone/salt treatment (ALDOST) that perpetuate cardiomyocyte necrosis with replacement fibrosis (or scarring) is presented. Intracellular Ca2+ overloading of cardiomyocytes, mediated via L-type Ca2+ channels (LTCC), including cytosolic [Ca2+]i and mitochondrial [Ca2+]m components, leads to the induction of oxidative stress and generation of reactive oxygen species predominantly by mitochondria (heavy arrow) that feeds back to perpetuate LTCC opening. This Ca2+ overloading-mediated redox injury pathway involves the pathologic opening of the mitochondrial permeability transition pore (mPTP)-cyclophilin D (cypD) complex. The ensuing osmotic swelling of these organelles and their degeneration, coupled with impaired mitochondrial generation of high energy phosphates, leads to cardiomyocyte necrosis and subsequent repair with fibrous tissue. Quercetin serves as a potent mitochondria-targeted antioxidant, whereas cyclosporine A (CsA), inhibits CypD-mediated Ca2+-dependent mPTP opening.
Chronic inappropriate (relative to dietary Na+) aldosteronism in rats is associated with a persistent excessive accumulation of Ca2+ in the heart and systemic tissues, including skeletal muscle and peripheral blood mononuclear cells (7, 8, 13, 24). As we demonstrated here, Ca2+ overloading in cardiac tissue and its cardiac myocytes at 4 wks ALDOST includes an increase in mitochondrial free [Ca2+]m coupled with the contemporaneous appearance of oxidative stress in these organelles and expressed by their increased production of H2O2 and levels of 8-isoprostane, coupled with an increased propensity for the opening of their mPTP. In permeabilized cardiomyocytes, an increase in cytosolic [Ca2+]i involves calmodulin and calmodulin-dependent protein kinase II with resultant depolarized mitochondrial membrane potential eventuating in the osmotic destruction of these organelles and leakage of their contents, including calcein (31). These events could be prevented by either chelating cytosolic Ca2+ or reducing mitochondrial Ca2+ entry through the inhibition of the Ca2+ uniporter (31). The mPTP apparatus of the inner membrane importantly includes cyclophilin D, a peptidyl prolyl-cis, trans-isomerase, which is integral to its functional opening and essential to the Ca2+ overloading and oxidative stress-induced pathway regulating cardiomyocyte necrosis (vis-a-vis apoptosis) (32, 33). Collectively, these findings epitomize the notion that mitochondrial Ca2+ homeostasis and redox state are integral to cell survival. As has been implicated, intracellular Ca2+ overloading, in association with the induction of oxidative stress and increased intramitochondrial Ca2+, triggers pathophysiologic responses leading to cardiomyocyte necrosis (34–36). The necrotic death of cardiomyocytes is followed by tissue repair and a replacement fibrosis expressed as an increment in the proportion of myocardium occupied by fibrillar collagen and quantitated histochemically in tissue prepared with a collagen-specific picrosirius red stain as a rise in its a collagen volume fraction. We did not find any evidence of apoptotic cardiomyocytes at 4 wks ALDOST.
Herein, we used a 4-wk course of quercetin cotreatment as a mitochondria-targeted antioxidant. We demonstrated that at wk 4 ALDOST, H2O2 production and 8-isoprostane levels in cardiac mitochondria harvested from rats receiving quercetin cotreatment were indistinguishable from values found in untreated, age-/sex-matched controls. This suggests quercetin had indeed prevented the appearance of oxidative stress in mitochondria. Moreover, the antioxidant properties of quercetin and reduction in ROS generation also attenuated the propensity to pathologic mPTP opening and the appearance of cardiomyocytes necrosis, as inferred from the significant prevention to the rise in myocardial collagen volume fraction. The rise in cardiac tissue total Ca2+ content was prevented by quercetin while the rise in [Ca2+]m was attenuated by this flavonoid. In keeping with the prevention of oxidative stress by quercetin, the attenuation in myocardial Ca2+ overloading supports the premise of redox regulation of L-type Ca2+ channels (28, 30). However, we cannot discount whether ancillary and hitherto unknown properties of quercetin, other than a direct interaction with mitochondria, may be contributory to our cardioprotective findings of this flavonoid.
Flavonoids, in doses corresponding to several glasses of red wine, have been found to be cardioprotective in other experimental models. These include coronary artery ligation-induced myocardial infarction in either normal rats and those with streptozocin-induced diabetes and in rats hearts following ischemia/reperfusion injury (37, 38). In cultured neonatal cardiomyocytes, timed incubation with quercetin resulted in the induction of cellular antioxidant/detoxifying genes, especially an upregulation of phase 2 enzymes (39). In addition to their antioxidant properties, flavonoids are thought to derive additional cardioprotective properties from their ability to chelate iron, which is relevant to doxorubicin-induced cardiomyopathy and iron-dependent downregulation of inducible nitric oxide synthase that accompanies endotoxemia (40–42).
Another mitochondria-directed intervention we examined in our rat model of aldosteronism is cotreatment with CsA, an mPTP opening inhibitor and cyclophilin D target located in the mitochondria matrix and absolute requirement for Ca2+-mediated oxidative injury-induced cardiomyocyte necrosis (33, 36, 43). Previous studies had shown CsA could prevent calcein leakage in permeabilized cardiomyocytes in response to increased cytosolic Ca2+ and isoproterenol-induced Ca2+ overloading (31, 36). We found a 4-wk course of CsA cotreatment prevented the rise in total tissue Ca2+, attenuated the rise in [Ca2+]m and, in turn, the appearance of oxidative stress in mitochondria, as expressed by H2O2 production and 8-isoprostane level, together with the abrogation of cypD-mediated Ca2+-dependent mPTP opening. These responses were accompanied by less microscopic scarring and evidenced by the attenuated rise in CVF, suggestive of less necrosis and fibrosis. However, we could not conclusively establish a direct cause and effect relationship between CsA and mPTP opening since CsA has other important properties, including its inhibition of calcineurin. We could not conclusively establish a direct cause and effect relationship between CsA and mPTP opening since CsA has other pharmacologic properties, besides the inhibition of calcineurin. Although calcineurin-nuclear factor of activated T cell (NFAT) transcription factor-mediated pathways have been implicated in regulating cardiomyocyte growth (44, 45), its putative role in pathologic cardiac remodeling, such as seen in response to chronic aldosteronism, remains largely uncertain at this time. We have considered cotreating rats receiving ALDOST with FK506, a more specific calcineurin inhibitor. Unlike CsA, however, FK506 inhibits aldosterone-stimulated epithelial cell Na+ transport (46). In our model, urinary and fecal Na+ transport is not only fundamental, but also a prerequisite for the ultimate appearance of ionized hypocalcemia with secondary hyperparathyroidism. Parathyroid hormone-mediated intracellular Ca2+ overloading plays the crucial role in inducing cardiac and systemic oxidative stress and subsequent sequelae of chronic aldosteronism (7, 8).
Notwithstanding to the major salutary findings presented here, our study has several limitations. We readily acknowledge that quercetin and CsA each might have other pharmacologic properties which extend beyond that focus solely on mitochondria. Recognizing these wide-ranging nonspecificities, we would not venture out to speculate that our observations in mitochondria are specifically related to their anti-inflammatory or immunosuppressive properties, respectively. It is, therefore, advocated that more specific mitochondria-targeted agents with well-defined modes of action be incorporated in future studies, when they become widely available, to substantiate crucial and salutary cardioprotective potentials of these agents.
In summary, mitochondrial Ca2+ homeostasis and redox state are crucial to cardiomyocyte survival. In chronic aldosteronism, the mitochondrial Ca2+ overloading and oxidative stress-induced mPTP opening pathway inevitably link to cardiomyocyte necrosis and consequent replacement fibrosis (vis-a-vis apoptosis). Although may not be due exclusively to mitochondria-targeted properties, quercetin or CsA have been effective in attenuating oxidative stress and intracellular Ca2+ overloading, and accordingly each proved to be significantly cardioprotective in rats with chronic aldosteronism.
Acknowledgments
This work was supported, in part, by NIH grants R01-HL73043 and R01-HL90867 (KTW). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Authors have no conflicts of interest to disclose.
References
- 1.Karsner HT. Human Pathology. Philadelphia: JB Lippincott; 1955. [Google Scholar]
- 2.Pearlman ES, Weber KT, Janicki JS, Pietra G, Fishman AP. Muscle fiber orientation and connective tissue content in the hypertrophied human heart. Lab Invest. 1982;46:158–164. [PubMed] [Google Scholar]
- 3.Huysman JAN, Vliegen HW, Van der Laarse A, Eulderink F. Changes in nonmyocyte tissue composition associated with pressure overload of hypertrophic human hearts. Pathol Res Pract. 1989;184:577–581. doi: 10.1016/S0344-0338(89)80162-1. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SE, Diaz-Arias AA, Weber KT. Fibrosis of the human heart and systemic organs in adrenal adenoma. Blood Press. 1992;1:149–156. doi: 10.3109/08037059209077510. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart. Role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Ramires FJA, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 7.Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, Smith RA, Gerling IC, Weber KT. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005;111:871–878. doi: 10.1161/01.CIR.0000155621.10213.06. [DOI] [PubMed] [Google Scholar]
- 8.Vidal A, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC, Weber KT. Calcium paradox of aldosteronism and the role of the parathyroid glands. Am J Physiol Heart Circ Physiol. 2006;290:H286–H294. doi: 10.1152/ajpheart.00535.2005. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin KD, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Preventing oxidative stress in rats with aldosteronism by calcitriol and dietary calcium and magnesium supplements. Am J Med Sci. 2006;332:73–78. doi: 10.1097/00000441-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Selektor Y, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Cinacalcet and the prevention of secondary hyperparathyroidism in rats with aldosteronism. Am J Med Sci. 2008;335:105–110. doi: 10.1097/MAJ.0b013e318134f013. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi MS, Deshmukh PA, Kamalov G, Zhao T, Zhao W, Whaley JT, Tichy JR, Bhattacharya SK, Ahokas RA, Sun Y, Gerling IC, Weber KT. Causes and consequences of zinc dyshomeostasis in rats with chronic aldosteronism. J Cardiovasc Pharmacol. 2008;52:245–252. doi: 10.1097/FJC.0b013e3181833eb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamalov G, Deshmukh PA, Baburyan NY, Gandhi MS, Johnson PL, Ahokas RA, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Coupled calcium and zinc dyshomeostasis and oxidative stress in cardiac myocytes and mitochondria of rats with chronic aldosteronism. J Cardiovasc Pharmacol. 2009;53:414–423. doi: 10.1097/FJC.0b013e3181a15e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamalov G, Ahokas RA, Zhao W, Johnson PL, Shahbaz AU, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Temporal responses to intrinsically coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria during aldosteronism. Am J Physiol Heart Circ Physiol. 2010;298:H385–H394. doi: 10.1152/ajpheart.00593.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamalov G, Ahokas RA, Zhao W, Zhao T, Shahbaz AU, Johnson PL, Bhattacharya SK, Sun Y, Gerling IC, Weber KT. Uncoupling the coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria seen in aldosteronism. J Cardiovasc Pharmacol. 2010;55:248–254. doi: 10.1097/FJC.0b013e3181cf0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Wang J, Li XK, Cai L. Zinc and the diabetic heart. Biometals. 2005;18:325–332. doi: 10.1007/s10534-005-3689-7. [DOI] [PubMed] [Google Scholar]
- 16.Cai L. Alcoholic cardiomyopathy: acetaldehyde, insulin insensitization and ER stress. J Mol Cell Cardiol. 2008;44:979–982. doi: 10.1016/j.yjmcc.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes requirement for stored Ca2+ and sustained opening of the permeability transition pore. J Cell Sci. 2002;115:1175–1788. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- 18.Brini M. Ca2+ signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium. 2003;34:399–405. doi: 10.1016/s0143-4160(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 19.Javadov S, Karmazyn M, Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther. 2009;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- 20.Trumbeckaite S, Bernatoniene J, Majiene D, Jakstas V, Savickas A, Toleikis A. The effect of flavonoids on rat heart mitochondrial function. Biomed Pharmacother. 2006;60:245–248. doi: 10.1016/j.biopha.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Waldmeier PC, Zimmermann K, Qian T, Tintelnot-Blomley M, Lemasters JJ. Cyclophilin D as a drug target. Curr Med Chem. 2003;10:1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- 22.Weber KT. From inflammation to fibrosis: a stiff stretch of highway. Hypertension. 2004;43:716–719. doi: 10.1161/01.HYP.0000118586.38323.5b. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya SK, Johnson PL, Thakar JH. Reversal of impaired oxidative phosphorylation and calcium overloading in the in vitro cardiac mitochondria of CHF-146 dystrophic hamsters with hereditary muscular dystrophy. J Neurol Sci. 1993;120:180–186. doi: 10.1016/0022-510x(93)90271-y. [DOI] [PubMed] [Google Scholar]
- 24.Ahokas RA, Sun Y, Bhattacharya SK, Gerling IC, Weber KT. Aldosteronism and a proinflammatory vascular phenotype. Role of Mg2+, Ca2+ and H2O2 in peripheral blood mononuclear cells. Circulation. 2005;111:51–57. doi: 10.1161/01.CIR.0000151516.84238.37. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Ratajska A, Weber KT. Inhibition of angiotensin-converting enzyme and attenuation of myocardial fibrosis by lisinopril in rats receiving angiotensin II. J Lab Clin Med. 1995;126:95–101. [PubMed] [Google Scholar]
- 26.Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of αB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- 27.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 28.Hool LC. The L-type Ca2+ channel as a potential mediator of pathology during alterations in cellular redox state. Heart Lung Circ. 2009;18:3–10. doi: 10.1016/j.hlc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Viola HM, Arthur PG, Hool LC. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res. 2007;100:1036–1044. doi: 10.1161/01.RES.0000263010.19273.48. [DOI] [PubMed] [Google Scholar]
- 30.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Odagiri K, Katoh H, Kawashima H, Tanaka T, Ohtani H, Saotome M, Urushida T, Satoh H, Hayashi H. Local control of mitochondrial membrane potential, permeability transition pore and reactive oxygen species by calcium and calmodulin in rat ventricular myocytes. J Mol Cell Cardiol. 2009;46:989–997. doi: 10.1016/j.yjmcc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 33.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 34.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 35.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529 (Pt 1):57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annapurna A, Reddy CS, Akondi RB, Rao SR. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2009;61:1365–1374. doi: 10.1211/jpp/61.10.0014. [DOI] [PubMed] [Google Scholar]
- 38.Brookes PS, Digerness SB, Parks DA, Darley-Usmar V. Mitochondrial function in response to cardiac ischemia-reperfusion after oral treatment with quercetin. Free Radic Biol Med. 2002;32:1220–1228. doi: 10.1016/s0891-5849(02)00839-0. [DOI] [PubMed] [Google Scholar]
- 39.Angeloni C, Leoncini E, Malaguti M, Angelini S, Hrelia P, Hrelia S. Role of quercetin in modulating rat cardiomyocyte gene expression profile. Am J Physiol Heart Circ Physiol. 2008;294:H1233–H1243. doi: 10.1152/ajpheart.01091.2007. [DOI] [PubMed] [Google Scholar]
- 40.Komarov AM, Mattson DL, Mak IT, Weglicki WB. Iron attenuates nitric oxide level and iNOS expression in endotoxin-treated mice. FEBS Lett. 1998;424:253–256. doi: 10.1016/s0014-5793(98)00181-1. [DOI] [PubMed] [Google Scholar]
- 41.Bast A, Haenen GR, Bruynzeel AM, Van der Vijgh WJ. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovasc Toxicol. 2007;7:154–159. doi: 10.1007/s12012-007-0018-0. [DOI] [PubMed] [Google Scholar]
- 42.Kaiserova H, Simunek T, van der Vijgh WJ, Bast A, Kvasnickova E. Flavonoids as protectors against doxorubicin cardiotoxicity: role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim Biophys Acta. 2007;1772:1065–1074. doi: 10.1016/j.bbadis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the holy grail of cardioprotection. Basic Res Cardiol. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 44.Bueno OF, van Rooij E, Molkentin JD, Doevendans PA, De Windt LJ. Calcineurin and hypertrophic heart disease: novel insights and remaining questions. Cardiovasc Res. 2002;53:806–821. doi: 10.1016/s0008-6363(01)00493-x. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 46.Rokaw MD, West ME, Palevsky PM, Johnson JP. FK-506 and rapamycin but not cyclosporin inhibit aldosterone-stimulated sodium transport in A6 cells. Am J Physiol. 1996;271:C194–202. doi: 10.1152/ajpcell.1996.271.1.C194. [DOI] [PubMed] [Google Scholar]