Abstract
Clinical studies suggest that smoking is a risk factor in the progression of chronic kidney disease, including diabetic nephropathy. The mechanisms involved are not completely understood. We have previously demonstrated that nicotine, one of the compounds present in large amounts in tobacco, promotes mesangial cell proliferation and fibronectin production. In this study, we hypothesized that exposure to environmental tobacco smoke (ETS) promotes the progression of diabetic nephropathy by increasing the expression of pro-fibrotic cytokines such as TGF-β and the extracellular matrix proteins fibronectin and collagen IV. Six-week-old diabetic (db/db) mice were divided into two groups. The experimental group (n=12) was exposed to ETS at a concentration of 30 mg/m3 for 6 hrs/day, 5 days/week for eight weeks. The control group (n=8) was exposed to room air. Urine was collected before euthanasia for albumin (ELISA), and creatinine measurements (mass spectrometry). After euthanasia, the kidneys were harvested for morphometric analysis and Western blot analysis. Serum was saved for cotinine measurements by ELISA. ETS exposure resulted in serum levels of cotinine similar to those found in human smokers. ETS exposure for eight weeks induced significant mesangial expansion (~ 50% increase) that was accompanied by concomitant increases in TGF-β and fibronectin expression (~20 %). ETS however, did not modify results in significant changes in urinary albumin excretion. These studies demonstrate that ETS exposure worsens the progression of diabetic nephropathy by increasing the amount of mesangial expansion and that these effects are likely mediated by increased expression of pro-fibrotic cytokines such as TGF-β.
Introduction
Cigarette smoking is the most important cause of preventable morbidity and mortality in developed countries including the United States 1, 2. In addition to being a major risk factor for cardiovascular disease, lung disease and cancer, cigarette smoking is now recognized as an important independent risk factor in the progression of chronic kidney disease including diabetic nephropathy 3–5, by increasing the rate of transition from microalbuminuria to persistent proteinuria and promoting the progression to ESRD3–5. Furthermore, a recent cross sectional analysis of participants in the National Health and Nutrition Examination Survey (NHANES) demonstrated a strong association between exposure to secondhand smoke and proteinuria, suggesting that passive smokers are also at increased risk of CKD6.
Diabetic nephropathy, the most common cause of end stage renal disease in the United States, is characterized by an initial phase of glomerular hyperfiltration followed by glomerular hypertrophy, mesangial expansion, and increased deposition of extracellular matrix (ECM) proteins including fibronectin and collagen. Fibrosis and progressive mesangial expansion induce irreversible changes in the structure and function of the glomeruli, effectively reducing the glomerular filtration surface,7 and eventually result in glomerulosclerosis and interstitial fibrosis.8 Several clinical and experimental studies have demonstrated the role of transforming growth factor beta (TGF-β) in the pathogenesis of chronic kidney disease, including diabetic nephropathy.9 This cytokine is largely pro-fibrotic, and plays a significant role in diabetic nephropathy by increasing the production of ECM components in the glomerulus.
The db/db mouse is a well validated and extensively-used model of diabetic nephropathy. This mouse has a point mutation of the leptin receptor, which leads to abnormal splicing and defective signaling of the adipocyte-derived hormone leptin in the hypothalamus10. As a result these mice develop persistent hyperphagia, obesity, hyperglycemia, and changes consistent with diabetic nephropathy including glomerular hypertrophy, mesangial expansion and proteinuria10.
In these studies, we tested the hypothesis that exposure to tobacco smoke in db/db mice worsens the progression of diabetic nephropathy by increasing the severity of extracellular matrix deposition and increasing the expression of the pro-fibrotic cytokine TGF-β.
Methods
Environmental Tobacco Smoke (ETS) Exposure
For these studies, we used six-week-old db/db mice (C57BLKS/JLepr, Jackson Labs), which were exposed to either room air (n=8) or to ETS (n=12) for eight weeks. ETS exposures were performed at the Center for Health and the Environment at the University of California-Davis. Mice assigned to ETS were exposed to tobacco smoke from Research Cigarettes (University of Kentucky) at a concentration of 30 μg/m3 for six hours per day, five days per week that resulted in levels of smoke exposure similar to those found in active smokers.11 After eight weeks of exposure to either room air or ETS, and prior to euthanasia, urine collections were performed. After euthanasia, the kidneys were harvested for histology and molecular biology analysis, and serum was saved for cotinine measurements. The animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of California–Davis and the University of Alabama at Birmingham, and were consistent with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Measurement of Glomerular Surface Area
PAS-stained sections (5 μm) were used for morphometric analysis. The surface area and mesangial area (μm2) of a minimum of 20 glomerular sections from each animal were determined from digitally-acquired images using the ImagePro Plus 4.5 software (Media Cybernetics, Inc.). Glomerular and mesangial surface areas were measured from digital images by tracing around the perimeter of the glomerular tuft (glomerular area), and around the perimeter of intraglomerular PAS-positive material (mesangial area). Glomeruli that were incomplete, distorted, tangentially sectioned, or globally sclerosed were excluded from analysis. The analysis software was calibrated to a stage micrometer.
Western blotting
In brief, kidney cortex homogenates were separated by SDS-PAGE (8% acrylamide gel) under reducing conditions and transferred to a nitrocellulose membrane (Hybond ECL). Blots were incubated for 1 hour with rabbit anti-murine polyclonal antibody to fibronectin at a 1:200 dilution (Sigma), TGF-β at a 1:1000 dilution (R&D Systems), collagen IV at a 1:1000 dilution (Abcam), or a polyclonal antibody to β-tubulin at 1:200 dilution (Santa Cruz). After washing, the blots were incubated with goat anti-rabbit antibody (Santa Cruz) for one hour, and the signal detected by horseradish polymerase-catalyzed chemiluminescence.
Serum cotinine measurements
Cotinine was measured in serum by ELISA (E-101-25, Bethyl Labs) according to the manufacturer’s instructions.
Urinary albumin excretion
Urine collections were performed the day prior to sacrifice, and urine saved at − 20° C. Urinary albumin concentration was measured by ELISA (E-101-25, Bethyl Labs) following the manufacturer’s instructions. Urinary albumin excretion was adjusted for urinary creatinine, which was measured by tandem mass spectrometry (Biochemical Genetics Laboratory, University of Alabama at Birmingham).
Statistical analysis
Data are expressed as mean ± SEM (standard error mean). For statistical comparisons, a student’s unpaired t-test was used (Microsoft Excel).
Results
Effects of ETS on mesangial expansion in diabetic mice
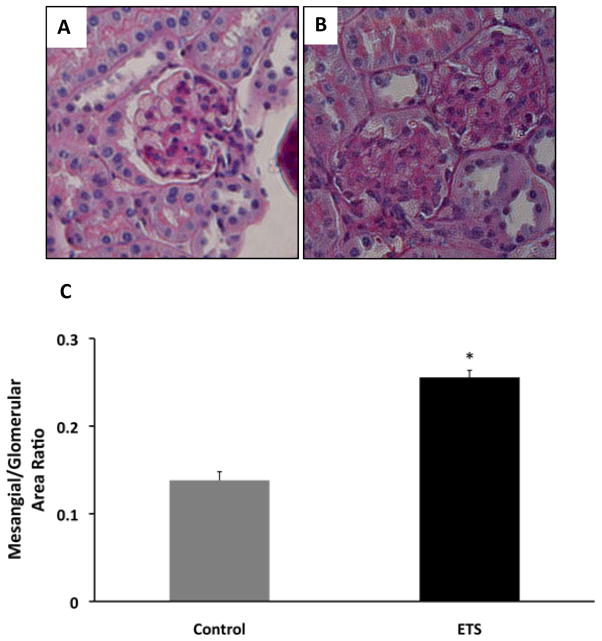
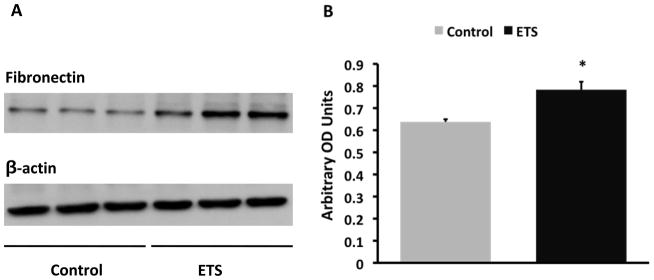
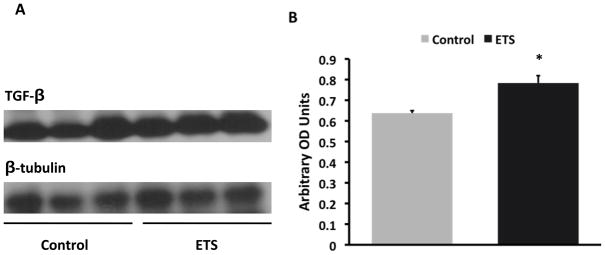
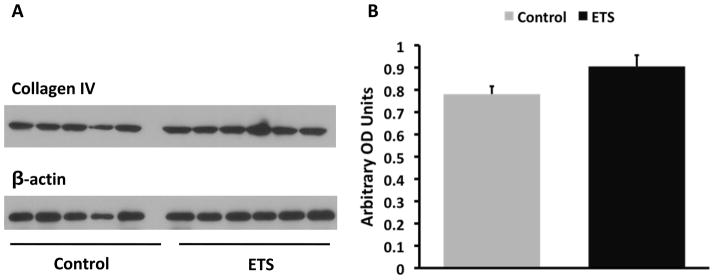
The exposure to ETS did not modify the levels of blood glucose which was > 600 mg/dl in both groups but resulted in lower body weight at the time of sacrifice: Control: 50 ± 0.6 gm vs ETS 45.4 ± 0.9 gm (P < 0.05). Morphometric analysis in PAS-stained sections demonstrated a significant increase in mesangial expansion (mesangial/glomerular area ratio, Figure 1) in mice exposed to ETS, suggesting that ETS exposure worsens extracellular matrix deposition in this mouse model of diabetic nephropathy. To determine whether these changes were accompanied by changes in the expression of the extracellular matrix proteins fibronectin12 and collagen, as well as the pro-fibrotic cytokine TGF-β,13 we performed Western blot analysis in renal cortex homogenates from air-exposed and ETS-exposed diabetic mice. As shown in Figures 2 and 3, ETS exposure resulted in significant increases in the expression of both fibronectin and TGF-β as assessed by Western blot. Additionally, the ETS-exposed group had an increase in collagen IV that however did not reach statistical significance (Figure 4). In the aggregate, these findings demonstrate that chronic ETS exposure increases mesangial expansion in diabetic mice.
Figure 1. Exposure to tobacco smoke worsens mesangial expansion in diabetic mice.
Panel A: Representative photomicrograph of a glomerulus from a db/db mouse exposed to air. Panel B: Representative photomicrograph of a glomerulus from a db/db mouse exposed to tobacco smoke. Panel C: Mesangial/glomerular area ratio from diabetic mice exposed to air (control) and from diabetic mice exposed to tobacco smoke (ETS). N=8 – 12, * P < 0.05 vs control.
Figure 2. Exposure to tobacco smoke increases cortical fibronectin expression in diabetic mice.
Panel A: Representative western blot for fibronectin and β-actin, which was used to control for loading. Panel B: Densitometry data analysis for cortical fibronectin expression (N = 8 –12 per group, * P < 0.05 vs Control).
Figure 3. Exposure to tobacco smoke increases cortical TGF-β expression in diabetic mice.
Panel A: Representative western blot for TGF-β and tubulin, which was used to control for loading. Panel B: Densitometry data analysis for cortical TGF-β expression (N = 8 –12 per group, * P < 0.05 vs Control).
Figure 4. Exposure to tobacco smoke did not significantly increase collagen IV expression in diabetic mice.
Panel A: Representative western blot for collagen IV and β-actin, which was used to control for loading. Panel B: Densitometry data analysis for cortical collagen IV expression (N = 8 –12 per group, P = NS).
Effects of ETS on urinary albumin excretion
To determine whether ETS-induced mesangial cell expansion was associated with changes in urinary protein excretion, we measured albuminuria in air-exposed and ETS-exposed diabetic mice. Air-exposed diabetic mice had urinary albumin excretions in the range reported by others in db/db mice with nephropathy; 10 however, in contrast with our morphometric analysis and Western blot analysis, ETS exposure did not significantly modify the urinary excretion of albumin: Control: 1.03 ± 0.16 μg albumin/μg creatinine vs ETS: 1.04 ± 0.13 μg albumin/μg creatinine (P = NS).
Cotinine levels in ETS-exposed mice
We measured the serum levels of cotinine, a stable metabolite of nicotine, as a marker of ETS exposure. The ETS group had measured plasma cotinine levels of 80.6 ± 7.45 ng/ml, while the control group exhibited undetectable levels. ETS exposure resulted in levels of cotinine similar to those found in the plasma of human smokers.14
Discussion
In these studies, we have demonstrated that exposure to environmental tobacco smoke worsens the severity of renal injury in a mouse model of diabetic nephropathy. These studies were performed in db/db mice, a mouse model of type II diabetes that, after several weeks of hyperglycemia, develops changes that closely resemble diabetic nephropathy in humans, including proteinuria and mesangial expansion.10 These mice however do not consistently develop increases in glomerular basement thickness in relation to albuminuria or progressive increase in albuminuria and do not show the advanced features of diabetic nephropathy in humans such as nodular sclerosis or tubulointerstitial fibrosis. 10 These mice have a leptin receptor mutation, which results in defective signaling of the adipocyte hormone leptin,15, 16 and the development of polyphagia, obesity and insulin resistance followed by diabetes mellitus.15, 16 Although these mice have been used extensively as a model of diabetes in humans, it should be noted that mutations of the leptin receptor are extremely rare in humans. We have recently demonstrated that human mesangial cells are endowed with nicotine receptors,12 and that nicotine worsens renal injury in a rat model of glomerulonephritis7 as well as in diabetic mice17. Our current studies now demonstrate that ETS exposure results in significant mesangial expansion associated with increased expression of the extracellular matrix proteins fibronectin and collagen IV, as well as TGF-β, a pro-fibrotic cytokine that plays a major role in the pathogenesis of chronic kidney disease, including diabetic nephropathy. Importantly, our studies were performed with ETS exposures that resulted in levels of the stable nicotine metabolite cotinine in the range observed in the plasma of active smokers.14 In contrast with our studies utilizing nicotine17, the effects of ETS on diabetic nephropathy were less severe suggesting that differences in the route of administration (smoking machine vs drinking water), hours of exposure (six hours a day, five day a week vs 24 hours a day seven days a week) as well as exposure to other compounds present in ETS may have an impact on the severity of nephropathy.
Several epidemiologic studies have demonstrated that cigarette smoking accelerates the rate of progression of nephropathy in diabetics, 3–5 and is also a risk factor in the deterioration of renal function in patients with hypertension and post-kidney transplant. 18–21 Smoking is also associated with deterioration of renal function in primary renal diseases including lupus nephritis,22 polycystic kidney disease, and glomerulonephritis.23, 24 The National Health and Nutrition Examination Survey (NHANES) also demonstrated a strong association between exposure to secondhand smoke and proteinuria in both diabetics and non-diabetics, suggesting that passive smokers are also at increased risk of CKD6. The mechanisms by which cigarette smoking accelerate the progression of kidney disease are not known.25,24,26,22
There are over 4000 compounds in mainstream cigarette smoke, including reactive oxygen species, carbon monoxide, nitric oxide and stable reactive aldehydes and ketones, among others.27 Among the numerous harmful substances found in tobacco and tobacco smoke, nicotine is one of the highly-active compounds that may be acquired through both active and passive smoking. In addition to being responsible for the addictive properties of tobacco smoking, nicotine has a variety of biological effects that may play an important role in the pathogenesis of vascular and renal disease. In the vasculature, nicotine promotes atherosclerosis and angiogenesis,28 and, as we have recently demonstrated, nicotine worsens glomerular injury and proteinuria in the anti-Thy1 model of proliferative glomerulonephritis.29 Although the epidemiological and clinical evidence strongly suggests that cigarette smoking accelerates the progression of chronic kidney disease the role of tobacco as a primary risk factor in the initiation of chronic kidney disease has not been established and indeed the long term exposure to ETS in normal mice does not result in significant changes in proteinuria or in the development of glomerulosclerosis13. However, in previous studies we have also determined that nicotine promotes mesangial cell proliferation and hypertrophy in vitro12. We hypothesize that the pro-inflammatory conditions that occur when mesangial cells are grown in vitro make these cells susceptible to the effects of nicotine, which are unlikely to take place in vivo in the absence of a predisposiong condition (i.e. glomerulonephritis or diabetes).
In addition we have previously demonstrated that acrolein, a highly-reactive and stable aldehyde present in large amounts in tobacco smoke, promotes endothelial dysfunction and increases the generation of reactive oxygen species in the vasculature30. We hypothesize that several of the multiple biologically active compounds present in tobacco smoke mediate the effects of tobacco on the progression of diabetic nephropathy. Given the role of reactive oxygen species as mediators of diabetic nephropathy and their role in the activation of pathways linked to cell growth and matrix formation31, we hypothesize that increased oxidative stress as a result of ETS exposure plays a major role in the pathogenesis of accelerated progression of nephropathy in smokers. This hypothesis is the subject of ongoing studies in our laboratory.
In the current studies, ETS exposure resulted in increased mesangial expansion and increased expression of the extracellular matrix protein fibronectin, and to a lesser extent collagen IV. These changes were accompanied by significant increases in TGF-β expression, suggesting that this cytokine is an important mediator of the deleterious effects of smoking on the progression of diabetic nephropathy. Although we observed significant extracellular matrix expansion, ETS exposure was not accompanied by significant increases in proteinuria as adjusted by creatinine excretion. Since urine collections were performed only prior to sacrifice, we speculate that at the time of sacrifice, protein excretion was already at its maximum in both groups, or alternatively, that the ETS has more selective effects on matrix expansion pathways and less on the filtration barrier.
Based on our previous studies,12 we hypothesize that nicotine, a compound present in large amounts in tobacco smoke, may be mediating in large part the deleterious effects of ETS on the progression of diabetic nephropathy. However, we also recognize that other biologically active compounds present in tobacco smoke may also be playing an important role on these effects. In summary, our studies demonstrate the effects of ETS on the progression of diabetic nephropathy, unveil some of the mechanisms involved, and may result in the development of novel strategies in the treatment and prevention of diabetic nephropathy in smokers and those affected by chronic exposure to tobacco smoke.
Acknowledgments
Funding Provided By Research Grants from:
Flight Attendants Medical Research Institute
National Institutes of Health NIHES014948
Footnotes
Disclaimer: This work was presented during the nephrology subspecialty scientific session at the SSCI Southern Regional Meetings in New Orleans, LA on February 27, 2010.
References
- 1.Boyle P. Cancer, cigarette smoking and premature death in Europe: a review including the Recommendations of European Cancer Experts Consensus Meeting, Helsinki, October 1996. Lung Cancer. 1997;17:1–60. doi: 10.1016/s0169-5002(97)00648-x. [DOI] [PubMed] [Google Scholar]
- 2.McBride PE. The health consequences of smoking. Cardiovascular diseases. Med Clin North Am. 1992;76:333–53. doi: 10.1016/s0025-7125(16)30356-x. [DOI] [PubMed] [Google Scholar]
- 3.Orth SR. Smoking--a renal risk factor. Nephron. 2000;86:12–26. doi: 10.1159/000045708. [DOI] [PubMed] [Google Scholar]
- 4.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care. 2002;25:859–64. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 5.Stegmayr BG. A study of patients with diabetes mellitus (type 1) and end-stage renal failure: tobacco usage may increase risk of nephropathy and death. J Intern Med. 1990;228:121–4. doi: 10.1111/j.1365-2796.1990.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 6.Whaley-Connell AT, Sowers JR, Stevens LA, et al. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008;51:S13–20. doi: 10.1053/j.ajkd.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Zelmanovitz T, Gerchman F, Balthazar AP, et al. Diabetic nephropathy. Diabetol Metab Syndr. 2009;1:10. doi: 10.1186/1758-5996-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling BN, Matsunaga H, Ma H, et al. Role of growth factors in mesangial cell ion channel regulation. Kidney Int. 1995;48:1158–66. doi: 10.1038/ki.1995.399. [DOI] [PubMed] [Google Scholar]
- 9.Pohlers D, Brenmoehl J, Löffler I, et al. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–56. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–44. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, Chow D, Chiamvimonvat N, et al. Short-term secondhand smoke exposure decreases heart rate variability and increases arrhythmia susceptibility in mice. Am J Physiol Heart Circ Physiol. 2008;295:H632–9. doi: 10.1152/ajpheart.91535.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaimes E, Tian RX, Raij L. Nicotine: The Link Between Cigarette Smoking and the Progression of Renal Injury? Am J Physiol Heart Circ Physiol. 2007;292:H76–82. doi: 10.1152/ajpheart.00693.2006. [DOI] [PubMed] [Google Scholar]
- 13.Elliot SJ, Karl M, Berho M, et al. Smoking induces glomerulosclerosis in aging estrogen-deficient mice through cross-talk between TGF-beta1 and IGF-I signaling pathways. J Am Soc Nephrol. 2006;17:3315–24. doi: 10.1681/ASN.2006070799. [DOI] [PubMed] [Google Scholar]
- 14.Seccareccia F, Zuccaro P, Pacifici R, et al. Serum cotinine as a marker of environmental tobacco smoke exposure in epidemiological studies: the experience of the MATISS project. Eur J Epidemiol. 2003;18:487–92. doi: 10.1023/a:1024672522802. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 17.Hua H, Feng W, Ji S, et al. Nicotine worsens the severity of nephropathy in diabetic mice: impllcations for the progression of kidney disease in smokes. Am J Physiol. 2010 doi: 10.1152/ajprenal.00293.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horner D, Fliser D, Klimm HP, et al. Albuminuria in normotensive and hypertensive individuals attending offices of general practitioners. J Hypertens. 1996;14:655–60. doi: 10.1097/00004872-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Regalado M, Yang S, Wesson DE. Cigarette smoking is associated with augmented progression of renal insufficiency in severe essential hypertension. Am J Kidney Dis. 2000;35:687–94. doi: 10.1016/s0272-6386(00)70017-5. [DOI] [PubMed] [Google Scholar]
- 20.Mimran A, Ribstein J, DuCailar G, et al. Albuminuria in normals and essential hypertension. J Diabetes Complications. 1994;8:150–6. doi: 10.1016/1056-8727(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 21.Bleyer AJ, Shemanski LR, Burke GL, et al. Tobacco, hypertension, and vascular disease: risk factors for renal functional decline in an older population. Kidney Int. 2000;57:2072–9. doi: 10.1046/j.1523-1755.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 22.Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis. The importance of hypertension and smoking. Arch Intern Med. 1992;152:2082–8. [PubMed] [Google Scholar]
- 23.Orth SR, Stockmann A, Conradt C, et al. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int. 1998;54:926–31. doi: 10.1046/j.1523-1755.1998.00067.x. [DOI] [PubMed] [Google Scholar]
- 24.Stengel B, Couchoud C, Cenee S, et al. Age, blood pressure and smoking effects on chronic renal failure in primary glomerular nephropathies. Kidney Int. 2000;57:2519–26. doi: 10.1046/j.1523-1755.2000.00111.x. [DOI] [PubMed] [Google Scholar]
- 25.Ejerblad E, Fored CM, Lindblad P, et al. Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol. 2004;15:2178–85. doi: 10.1097/01.ASN.0000135048.35659.10. [DOI] [PubMed] [Google Scholar]
- 26.Chapman AB, Johnson AM, Gabow PA, et al. Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5:1349–54. doi: 10.1681/ASN.V561349. [DOI] [PubMed] [Google Scholar]
- 27.Huang MF, Lin WL, Ma YC. A study of reactive oxygen species in mainstream of cigarette. Indoor Air. 2005;15:135–40. doi: 10.1111/j.1600-0668.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 28.Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–9. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 29.Jaimes EA, Tian RX, Joshi M, et al. Nicotine worsens renal injury in nephritis. Am J Nephrol. 2008 [Google Scholar]
- 30.Jaimes EA, DeMaster EG, Tian RX, et al. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–6. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 31.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–26. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]