Abstract
Morbidity and mortality estimates due to methicillin-resistant Staphylococcus aureus (MRSA) infections continue to rise. Therapeutic options are limited by antibiotic resistance. Anti-pathogenic compounds, which inhibit quorum sensing (QS) pathways, may be a useful alternative to antibiotics. Staphylococcal QS is encoded by the agr locus and is responsible for the production of δ-hemolysin. Quantification of δ-hemolysin found in culture supernatants permits the analysis of agr activity at the translational, rather than transcriptional, level. We employed RP-HPLC techniques to investigate the anti-QS activity of 168 extracts from 104 Italian plants through quantification of δ-hemolysin. Extracts from three medicinal plants (Ballota nigra, Castanea sativa, and Sambucus ebulus) exhibited a dose-dependent response in the production of δ-hemolysin, indicating strong anti-QS activity in a pathogenic MRSA isolate.
Keywords: quorum sensing, MRSA, medicinal plants, δ-toxin, δ-hemolysin, agr
Introduction
Emerging infections due to methicillin resistant Staphylococcus aureus (MRSA) pose a significant threat to hospital patients as the rates of nosocomial infection steadily rise [1]. Moreover, healthcare-associated MRSA (HA-MRSA) are often multidrug-resistant (MDR) and therapeutic options are rapidly becoming more limited as new resistant phenotypes surface. One approach to drug discovery for the treatment of MRSA is through natural products research. Most research on natural botanic products activity for MRSA is focused on growth inhibition, while some have focused on inhibition of the MDR mechanisms, such as efflux pumps [2–5]. No studies on the agr-inhibiting or quorum sensing inhibiting (QSI) activity of natural botanic products on MRSA have been conducted thus far. Inhibition of staphylococcal QS pathways could potentially limit the degree of pathogenicity posed by some MRSA strains by blocking the production of certain virulence factors. Moreover, the inhibition of staphylococcal pathogenesis could be accomplished without growth inhibition, thus potentially avoiding selective pressures for drug-resistance.
The staphylococcal QS system is a cell-density-dependent mechanism for controlling protein expression, including the production of staphylococcal virulence factors such as the α-, β, and δ-hemolysins. It is encoded by the agr locus, which is a quorum-sensing gene cluster of five genes (hld, agrA, agrB, agrC and agrD) [6].
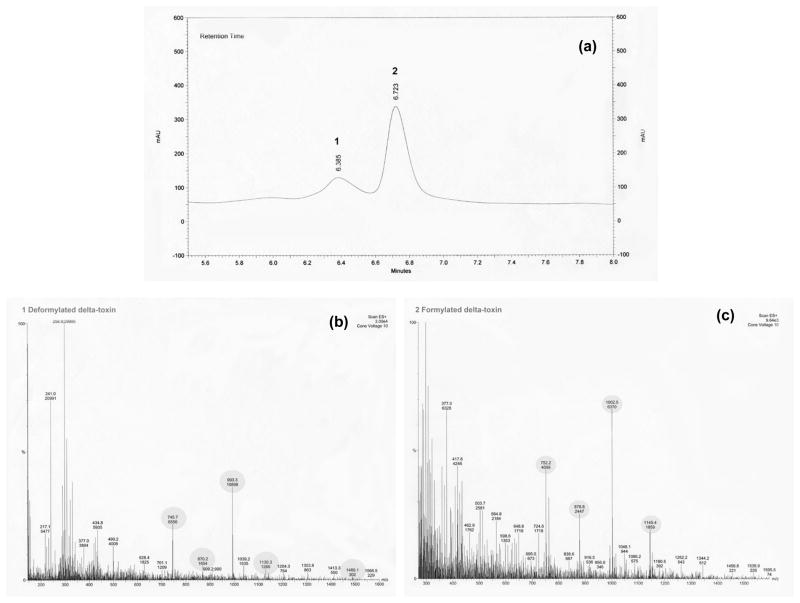
Staphylococcal δ-hemolysin, or δ-toxin, is a translational protein product of RNAIII. It is a 26-aminoacid polypeptide with surfactant-like properties [7]. Translation of hld, the gene for δ-hemolysin, occurs about one hour after transcription of RNAIII. There are two forms of δ-toxin that can be found in the culture supernatant: formylated (with an N-terminal methionine) and deformylated. These forms are represented by two distinct peaks in the RP-HPLC chromatogram (Figure 1). δ-Toxin accumulates in the culture medium in both forms, and is approximately 90% formylated and 10% deformylated. This ratio is due to the arrest of deformylated δ-toxin production during the post-exponential growth phase, whereas formylated δ-toxin continues to accumulate. Sommerville et al. [8] suggest that this change may be linked to iron availability in the culture medium.
Fig. 1S.
Mass spectroscopic analysis of HPLC fractions containing derformylated and formylated δ-toxin. Peaks matching the spectrogram presented in the study by Somerville et al. [8] are highlighted. (a) Absorbance at 280nm of NRS385 (PFT USA500) supernatant fractionated by HPLC. (b) Mass spectrogram of peak 1, deformylated δ-toxin (molecular mass 2979.2 Da). (c) Mass spectrogram of peak 2, formylated δ-toxin (molecular mass of 3007.4 Da).
Quantification of δ-toxin produced by S. aureus and found in the culture supernatants allows for the analysis of agr activity at the translational, rather than transcriptional, level. The identification of agr-inhibiting drugs, or staphylococcal QS-inhibitors, has been proposed by several research groups as a potential anti-staphylococcal therapy [9–13]. In 2000, Otto and Götz [7] provided a fast method for δ-toxin quantification using RP-HPLC techniques for the analysis of staphylococcal culture filtrates. We apply this method for the first time as a screening tool for identifying plant extracts with QSI activity for a strain of HA-MRSA known as pulsed-field type (PFT) USA500.
USA500 isolates are SCCmecIV and MLST ST8. They are highly multidrug-resistant and tend to be associated with nosocomial transmission [14, 15]. USA500 is associated with the production of many virulence factors, including enterotoxins A and B, as well as δ-hemolysin, among others. There is a critical need for novel therapeutic options in the treatment of highly virulent, MDR staphylococcal infection, such as those caused by USA500.
We quantify the amount of δ-hemolysin found in the supernatant of MRSA cultures treated with plant extracts as a means of measuring the impact of plant products on the staphylococcal quorum sensing (QS) system. We examine 168 crude extracts made from 104 Italian plants, representing 44 plant families.
Materials and Methods
Plant material and extraction
Ethnobotanical surveys of plants used in the traditional pharmacopoeia of the Vulture-Alto Bradano region of Basilicata, southern Italy were conducted and results are described in previous works [16–19]. Bulk and voucher specimens were collected and identified in 2006 by C. Quave. Voucher specimens of plants were deposited at the Herbarium Lucanum (HLUC) in Potenza, Italy and Fairchild Tropical Botanic Gardens (FTG) in Miami, FL, USA.
Dry plant materials were ground into a fine powder using a homogenizer. Ethanolic extracts of all plant samples were made by soaking in 95% denatured EtOH using a ratio of 1g (plant material):10 mL (EtOH) for 72 h. Flasks were agitated daily. Water extracts were made by boiling 1g (plant material): 50 mL (dH2O) for 30 minutes. Extracts were vacuum filtered and rotary-evaporated, then frozen and lyophilized. Stock concentrations of 10 mg/mL of dry extract in the excipient (DMSO or dH2O) were prepared, sterile filtered (0.2 μm) and stored in the dark at 4°C. The excipient (DMSO or dH2O) made up less than 5.1% of the final test solution for MIC assays and less than 2.5% for δ-toxin assays.
Bacteria and culture conditions
HA-MRSA PFT USA500 (NRS385) was obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) repository [14]. Bacteria were grown on Tryptic Soy agar plates for 18 h at 37°C. A 1:20 dilution of a standardized inoculum (0.5 McFarland Standard) was used to create final inoculum densities of 5–8 × 105 CFU/mL from overnight cultures using the direct suspension method [20] for MIC and δ-toxin assays. Inoculum densities were confirmed by taking colony counts using the spread plate method at the time of inoculation.
Determination of minimum inhibitory concentrations (MICs)
MICs were determined by the microtiter broth method [21] in sterile flat-bottom 96-well polystyrene plates. We used serial dilution techniques to determine the MIC50 and MIC90 of extracts at concentrations of 8–512 μg/mL after 18 h growth. We included negative controls (cells + TSB), positive controls (cells + TSB + antibiotics − vancomycin, ampicillin, and trimethroprim-sulfamethoxazole), vehicle controls (cells + TSB + DMSO), and media controls (TSB). All tests were performed in triplicate. Optical density readings were taken using a KC4 microplate reader at 600 nm at 0 and 18 hours post-inoculation. Results are reported as the MIC for growth at 18 hours post-inoculation. To account for the effect of extract color on the OD600nm reading, a formula for calculating percent inhibition was used. The mean % inhibition of replicate tests was used to determine the final MIC values.
ODt18 = optical density (600 nm) of the test well at 18 hours post-inoculation
ODt0 = optical density (600 nm) of the test well at 0 hours post-inoculation
ODgc18 = optical density (600 nm) of the growth control well at 18 hours post-inoculation
ODgc0 = optical density (600 nm) of the growth control well at 0 hours post-inoculation
Quantification of δ-toxin production
Polystyrene 24-well culture plates were prepared with a total volume of 1 mL per well of TSB, an initial sub-MIC test concentration of 64 μg of extract suspended in DMSO (<1% DMSO in total well volume) and bacteria. Extracts demonstrating significant activity, as exhibited by lower δ-toxin levels, were also investigated at a range of test concentrations from 8–256 μg/mL. Controls for media, growth, and growth in the carrier solvent (DMSO) were also performed. Liquid test cultures were grown for 15 hours at 37°C and aerated by shaking at 150 rpm. All tests were performed in triplicate.
Aliquots of bacteria (2 mL) were centrifuged for 5 min at 14,000 × g with a microcentrifuge. Supernatants were removed and stored at −20 °C until HPLC analysis. The concentration of δ-toxin was measured by RP-HPLC with a 1-mL Resource PHE column (GE Healthcare, Uppsala, Sweden) as previously described [7], except that 200 μL of supernatant (as opposed to 500 μL) was injected onto the column using a Thermo Spectra-System HPLC apparatus, equipped with a Diode Array Detector and autosampler (Thermo Electron Corporation, San Jose, CA) and ChromQuest 4.1 software.
δ-Hemolysin elutes at a retention time of about 6.4 minutes (deformylated) and 6.8 minutes (formylated) after sample injection as two distinct peaks. Integration of the δ-toxin peak area was performed at 280 nm. We confirmed the identity of δ-toxin peaks by peak fractionation and LC-mass spectrometry (Fig. 1S) using a Thermo Finnigan Deca XP max ion trap mass spectrometer and surveyor LC with autosampler and diode array detector (Thermo Electron Corporation, San Jose, CA) using conditions previously described [8]. The peak areas were calculated using ChromQuest software and the mean percent inhibition of δ-toxin production for the replicate tests was calculated in relation to the mean peak area of the excipient (DMSO) growth controls.
Statistical analysis
All experiments were carried out in triplicate. Data were analyzed using Microsoft Excel and SPLUS software. Differences between the means of the experimental and control groups were evaluated with two-sample t-tests using SPLUS software.
Results
There was a broad low level response of δ-toxin inhibition to the screening test concentration of 64 μg/mL. QSI activity was apparent in 90% of the extracts tested (Table 1). No QSI activity was apparent in aqueous extracts. This suggests that the active QSI components are predominantly nonpolar in nature. Extracts were not effective at inhibiting growth of this multidrug-resistant strain of HA-MRSA (USA500/NRS385). Only 6% of extracts demonstrated a MIC50 at concentrations of 256–512 μg/mL. None demonstrated a MIC90 at concentrations ≤ 512 μg/mL.
Table 1.
Inhibition of δ-toxin and minimal inhibitory concentrations of plant extracts against MRSA (strain I.D. NRS385/PFT USA500).
| Family | Botanic Name | Voucher ID | Plant Part | Ethno-botanical Use* | Extract Solvent | Percent Inhibition of δ-toxin Production** | MIC50*** |
|---|---|---|---|---|---|---|---|
| Adoxaceae | Sambucus ebulus L. | CQ-168 | inflorescence | N | EtOH | 45 | - |
| leaves | S | EtOH | 48 | - | |||
| stems | N | EtOH | 28 | - | |||
| Sambucus nigra L. | CQ-151 | woody parts | R | EtOH | 29 | - | |
| leaves | S | EtOH | 36 | - | |||
| dH2O | - | - | |||||
| inflorescence | S; R | EtOH | 38 | - | |||
| dH2O | - | - | |||||
| infructescence | F | EtOH | 34 | - | |||
| Alliaceae | Allium cepa L. | CQ-206 | leaves; bulbs; roots | S; M; F | EtOH | 22 | - |
| Apiaceae | Daucus carota L. | CQ-215 | leaves; stems | N | EtOH | 2 | - |
| inflorescence; infructescence | N | EtOH | 39 | - | |||
| Foeniculum vulgare ssp. piperitum (Ucria) Coutinho | CQ-192 | leaves; stems | M; F | EtOH | - | - | |
| Foeniculum vulgare ssp. vulgare Mill. | CQ-196 | leaves; stems | M | EtOH | 8 | - | |
| Tordylium apulum L. | CQ-101 | flowers; leaves; roots; stems | N | EtOH | 25 | - | |
| Apocynaceae | Vinca major L. | CQ-117 | flowers; leaves; roots; stems | M | EtOH | 26 | - |
| Aracaeae | Arum italicum Mill. | CQ-175 | stems | N | EtOH | 28 | - |
| fruits | N | EtOH | 15 | - | |||
| stalks | N | EtOH | 2 | - | |||
| leaves | S | EtOH | 22 | - | |||
| Asphodelaceae | Asphodelus microcarpus_Salzm. & Viv. | CQ-109 | inflorescence | N | EtOH | 19 | - |
| leaves | N | EtOH | 17 | - | |||
| Asteraceae | Achillea ageratum L. | CQ-219 | leaves; stems; flowers | M | EtOH | 66 | 512 |
| Achillea millefolium L. | CQ-176 | inflorescence | M | EtOH | 41 | - | |
| leaves; stems | M | EtOH | 23 | - | |||
| leaves; stems; flowers | M | EtOH | 38 | - | |||
| Anacyclus tomentosus DC. | CQ-167 | leaves; stems; flowers | N | EtOH | 36 | - | |
| Cichorium intybus L. | CQ-106 | basal leaves; roots | F | EtOH | 23 | - | |
| dH2O | - | - | |||||
| leaves; stems; flowers | F | EtOH | 8 | - | |||
| Matricaria recutita L. | CQ-118 | flowers; leaves; roots; stems | S; M | EtOH | 29 | 512 | |
| dH2O | - | - | |||||
| Scolymus hispanicus L. | CQ-199 | leaves; stems; flowers | N | EtOH | 23 | - | |
| Tussilago farfara L. | CQ-202 | leaves; stems; roots | S | EtOH | 16 | - | |
| Urospermum dalechampii (L.) Scop. | CQ-134 | flowers; leaves; roots; stems | N | EtOH | 14 | - | |
| Boraginaceae | Anchusa officinalis L. | CQ-128 | leaves; stems; flowers | N | EtOH | 34 | - |
| Borago officinalis L. | CQ-100 | flowers; leaves; roots; stems | M | EtOH | 54 | - | |
| dH2O | - | - | |||||
| Cerinthe major L. | CQ-110 | flowers; leaves; roots; stems | N | EtOH | 48 | - | |
| Echium italicum L. | CQ-162 | leaves; stems; flowers | N | EtOH | 32 | - | |
| Brassicaceae | Brassica rapa subsp. rapa | CQ-104 | flowers; leaves; roots; stems | F | EtOH | 27 | - |
| Cardaria draba (L.) Desv. | CQ-140 | flowers; leaves; roots; stems | N | EtOH | 12 | - | |
| Eruca sativa Mill. | CQ-102 | flowers; leaves; roots; stems | N | EtOH | 13 | - | |
| Sisymbrium officinale (L.) Scop. | CQ-131 | flowers; leaves; roots; stems | N | EtOH | 20 | - | |
| Caprifoliaceae | Lonicera alpigena L. | CQ-213 | woody parts | N | EtOH | 28 | - |
| leaves | N | EtOH | 25 | - | |||
| Caryophyllaceae | Saponaria officinalis L. | CQ-210 | leaves; stems; flowers | N | EtOH | 4 | - |
| Silene alba (Mill.) E.H.L. Krause | CQ-123 | leaves; stems; flowers | N | EtOH | 43 | - | |
| Silene nutans L. | CQ-125 | leaves; stems; flowers | N | EtOH | 43 | - | |
| Cucurbitaceae | Ecballium elaterium (L.) A. Richard | CQ-169 | leaves; stems; flowers | S | EtOH | 21 | - |
| Dennstaedtiaceae | Pteridium aquilinium (L.) Kuhn | CQ-211 | leaves | N | EtOH | - | - |
| stems | N | EtOH | 24 | - | |||
| Dipsacaceae | Dipsacus fullonum L. | CQ-201 | leaves; stems | N | EtOH | 28 | - |
| flowers | N | EtOH | 28 | - | |||
| Knautia arvensis Coult. | CQ-190 | leaves; stems; flowers | N | EtOH | 48 | - | |
| Knautia lucana Lacaita & Szabo | CQ-166 | leaves; stems; flowers | N | EtOH | 6 | - | |
| Equisetaceae | Equisetum arvense L. | CQ-226 | stems; leaves | N | EtOH | 22 | - |
| Fabaceae | Acacia dealbata Link | CQ-115 | inflorescence | O | EtOH | 56 | - |
| stems | O | EtOH | 38 | - | |||
| leaves; stems | O | EtOH | 21 | - | |||
| Anthyllis vulneraria L. | CQ-147 | leaves; stems; flowers | N | EtOH | 28 | - | |
| Astragalus monspessulanus L. | CQ-112 | leaves; stems; flowers; roots | N | EtOH | 36 | - | |
| Coronilla emerus L. | CQ-137 | leaves; flowers | N | EtOH | 33 | - | |
| woody stems | N | EtOH | 14 | - | |||
| Melilotus alba_Medik. | CQ-193 | leaves; stems; flowers | N | EtOH | 43 | - | |
| Robinia pseudoacacia L. | CQ-155 | woody parts | N | EtOH | 32 | - | |
| leaves | N | EtOH | - | - | |||
| inflorescence | N | EtOH | 21 | - | |||
| Spartium junceum L. | CQ-144 | leaves; stems; flowers | A | EtOH | 22 | - | |
| Trifolium repens L. | CQ-138 | leaves; stems; flowers; roots | N | EtOH | 4 | - | |
| Vicia craca L. | CQ-149 | leaves; stems; flowers; roots | N | EtOH | 19 | - | |
| Vicia faba L. | CQ-103 | leaves; stems; flowers; roots | F | EtOH | 14 | - | |
| Vicia sativa subsp. angustifolio | CQ-124 | leaves; stems; flowers | N | EtOH | 22 | 512 | |
| Vicia sativa subsp. sativa | CQ-119 | leaves; stems; flowers | N | EtOH | 29 | - | |
| Wisteria sinensis (Sims) Sweet | CQ-126 | inflorescence | O | EtOH | 36 | - | |
| stems | O | EtOH | 39 | - | |||
| leaves | O | EtOH | 41 | - | |||
| Fagaceae | Castanea sativa Mill. | CQ-191 | inflorescence | N | EtOH | 20 | - |
| leaves | N | EtOH | 70 | 512 | |||
| woody parts | A | EtOH | 32 | 512 | |||
| Quercus cerris L. | CQ-228 | leaves | N | EtOH | 27 | - | |
| stems; fruits | N | EtOH | 37 | - | |||
| Gentianaceae | Centaurium pulchellum (Sw.) Druce | CQ-217 | leaves; stems; flowers; roots | N | EtOH | 21 | - |
| Geraniaceae | Erodium ciconium (L.) L’Hér. | CQ-142 | leaves; stems; flowers; roots | N | EtOH | 34 | - |
| Erodium malacoides (L.) L’Hér. ex Aiton | CQ-121 | leaves; stems; flowers | N | EtOH | 7 | 512 | |
| Geranium columbinum L. | CQ-129 | leaves; stems; flowers | N | EtOH | - | - | |
| Hyacinthaceae | Leopoldia comosa (L.) Parl. | CQ-105 | bulbs | M; F | EtOH | 21 | - |
| dH2O | - | - | |||||
| leaves; inflorescence | N | EtOH | 31 | - | |||
| Hypericaceae | Hypericum perforatum L. | CQ-183 | leaves; stems; flowers | S | EtOH | 36 | - |
| Juglandaceae | Juglans regia L. | CQ-181 | immature fruits | S; C | EtOH | - | - |
| leaves | R | EtOH | 39 | - | |||
| woody parts | N | EtOH | 17 | - | |||
| Juncaceae | Juncus articulatus L. | CQ-216 | leaves; fruits | N | EtOH | 32 | - |
| Lamiaceae | Ballota nigra L. | CQ-160 | stems | S; M | EtOH | 76 | - |
| roots | N | EtOH | 37 | - | |||
| leaves | S; M | EtOH | 47 | - | |||
| leaves; stems; flowers | S; M | EtOH | 47 | - | |||
| dH2O | - | - | |||||
| Clinopodium vulgare L. | CQ-182 | leaves; stems; flowers | N | EtOH | 40 | - | |
| Marrubium vulgare L. | CQ-170 | leaves; stems; flowers | S; M | EtOH | 40 | - | |
| dH2O | 6 | - | |||||
| roots | N | EtOH | 40 | - | |||
| Mentha pulegium L. | CQ-200 | leaves; stems; flowers; roots | F | EtOH | 36 | - | |
| Mentha spicata L. | CQ-224 | leaves; stems; flowers | F | EtOH | 28 | - | |
| Origanum heracleoticum L. | CQ-207 | leaves; stems; flowers | F | EtOH | 30 | - | |
| Phlomis herba-venti L. | CQ-168 | leaves; stems; flowers | N | EtOH | 16 | - | |
| Rosmarinus officinalis L. | CQ-113 | leaves; stems; flowers | F; S | EtOH | 58 | 256 | |
| Salvia pratensis L. | CQ-165 | leaves; stems | N | EtOH | 23 | - | |
| inflorescence | N | EtOH | 58 | - | |||
| Salvia virgata Jacq. | CQ-127 | leaves; stems; flowers | N | EtOH | 42 | 256 | |
| Stachys tymphaea Hausskn. | CQ-189 | leaves; stems; flowers | N | EtOH | 41 | - | |
| Liliaceae | Lilium candidum L. | CQ-174 | leaves; stems | N | EtOH | 37 | - |
| inflorescence | N | EtOH | 30 | - | |||
| Malvaceae | Alcea rosea L. | CQ-205 | leaves; stems; flowers; roots | O | EtOH | 18 | - |
| Malva sylvestris L. | CQ-156 | stems | S; M | EtOH | 22 | - | |
| dH2O | - | - | |||||
| flowers | S; M | EtOH | 53 | - | |||
| leaves | S; M | EtOH | 34 | - | |||
| dH2O | - | - | |||||
| Moraceae | Ficus carica L. | CQ-173 | leaves | N | EtOH | 24 | - |
| woody parts | N | EtOH | 21 | - | |||
| immature fruits | S; F | EtOH | 41 | - | |||
| Myrsinaceae | Cyclamen hederifolium Aiton | CQ-186 | tubers | M | EtOH | 10 | - |
| Nyctaginaceae | Mirabilis jalapa L. | CQ-222 | leaves; flowers; fruits | N | EtOH | 16 | - |
| Oleaceae | Olea europaea L. | CQ-197 | leaves | N | EtOH | 40 | - |
| woody parts | A | EtOH | 24 | - | |||
| Orchidaceae | Aceras anthropophora R. Br. | CQ-153 | leaves; stems; flowers; roots | N | EtOH | 51 | - |
| Orchis italica Poir. | CQ-133 | inflorescence; leaves; stems | N | EtOH | 49 | - | |
| Orchis purpurea Huds. | CQ-132 | inflorescence; leaves; stems | N | EtOH | 57 | - | |
| Papaveraceae | Fumaria officinalis L. | CQ-107 | leaves; stems; flowers; roots | N | EtOH | 44 | - |
| Papaver rhoeas subsp. rhoeas | CQ-145 | leaves; stems; flowers; roots | F | EtOH | 25 | - | |
| Papaver somniferum L. | CQ-178 | leaves; stems; flowers; roots | M; R | EtOH | 36 | - | |
| Plantaginaceae | Digitalis ferruginea L. | CQ-227 | leaves; stems; flowers | N | EtOH | 32 | - |
| Linaria vulgaris Hill | CQ-223 | leaves; stems; flowers; roots | N | EtOH | 25 | - | |
| Plantago major L. | CQ-225 | leaves; stems; flowers; roots | S; M | EtOH | 12 | - | |
| Poaceae | Agropyron repens (L.) P. Beauv. | CQ-208 | leaves; stems; roots | M | EtOH | 19 | - |
| Arundo donax L. | CQ-146 | stem internodes | A; R | EtOH | 22 | - | |
| stem nodes | S | EtOH | 35 | - | |||
| dH2O | - | - | |||||
| leaves; stems | A; R | EtOH | 10 | - | |||
| Polygonaceae | Rumex crispus L. | CQ-171 | leaves; stems; fruits | S | EtOH | 25 | - |
| Pottiaceae | Syntrichia ruralis (Hedw.) Web. & Mohr | CQ-229 | whole plant | N | EtOH | 45 | - |
| Ranunculaceae | Delphinium fissum Waldst. & Kit. | CQ-187 | leaves; stems; flowers; fruits | N | EtOH | 29 | - |
| Ranunculus acris L. | CQ-135 | leaves; stems; flowers | N | EtOH | 34 | - | |
| Rosaceae | Crataegus monogyna Jacq. | CQ-116 | leaves; stems; flowers | M | EtOH | 57 | - |
| Prunus spinosa L. | CQ-163 | woody parts; leaves | M | EtOH | 33 | - | |
| fruits | N | EtOH | 29 | 512 | |||
| Rosa canina var. canina | CQ-152 | fruits | N | EtOH | 16 | - | |
| woody parts | N | EtOH | 44 | - | |||
| leaves; stems | N | EtOH | 14 | 512 | |||
| Rubus ulmifolius Schott | CQ-164 | leaves; stems; flowers | S | EtOH | 10 | - | |
| leaves | S | EtOH | 17 | - | |||
| roots | M | EtOH | 21 | - | |||
| woody stems | N | EtOH | 16 | 512 | |||
| Rubiaceae | Galium verum L. | CQ-177 | leaves; stems; flowers | N | EtOH | 27 | - |
| Scrophulariaceae | Verbascum sinuatum L. | CQ-218 | leaves; stems; flowers | N | EtOH | 34 | - |
| Verbascum thapsus L. | CQ-172 | stems | M | EtOH | 31 | - | |
| leaves | M | EtOH | 40 | - | |||
| inflorescence | M | EtOH | 38 | - | |||
| Ulmaceae | Ulmus minor L. | CQ-195 | leaves | N | EtOH | 23 | - |
| woody parts | M | EtOH | - | - | |||
| Urticaceae | Parietaria diffusa Mert. & Koch | CQ-212 | leaves; stems; fruits; roots | M | EtOH | 19 | - |
| Urtica dioica L. | CQ-179 | leaves; stems; flowers | S; M; F | EtOH | 36 | - | |
| Valerianaceae | Centranthus ruber (L.) DC. | CQ-143 | leaves; stems; inflorescence | M | EtOH | 31 | - |
| Vitaceae | Vitis vinifera var. aglianico | CQ-209 | wine | S; F | - | - | |
| stems | N | EtOH | - | - | |||
| fruits | F | EtOH | 21 | - | |||
| leaves | N | EtOH | 22 | - |
Ethnobotanical use of specific plant part(s) in the study region: S = medicinal application to skin; M = medicinal application not involving the skin; C = cosmetic applications; A = agricultural tool; O = ornamental; R = ritual or spiritual use; F = food; N = no reported use.
percent inhibition for δ-toxin based on an initial screening concentration of 64 μg/mL for MRSA PFT USA500.
MIC values are reported as μg/mL for MRSA PFT USA500.
“-”signifies no inhibitory activity
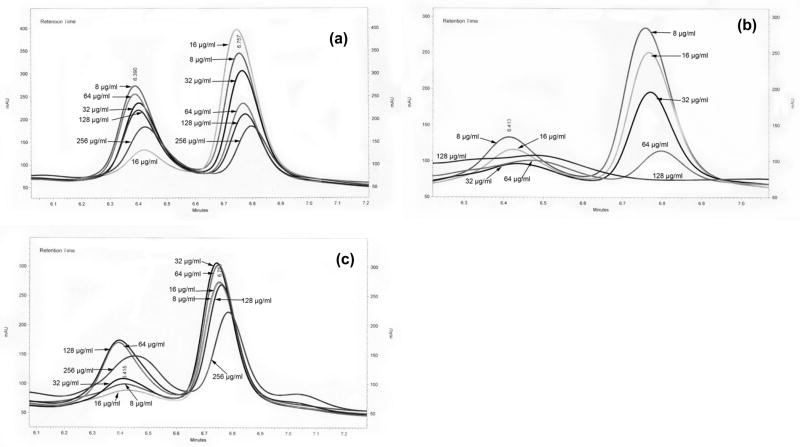
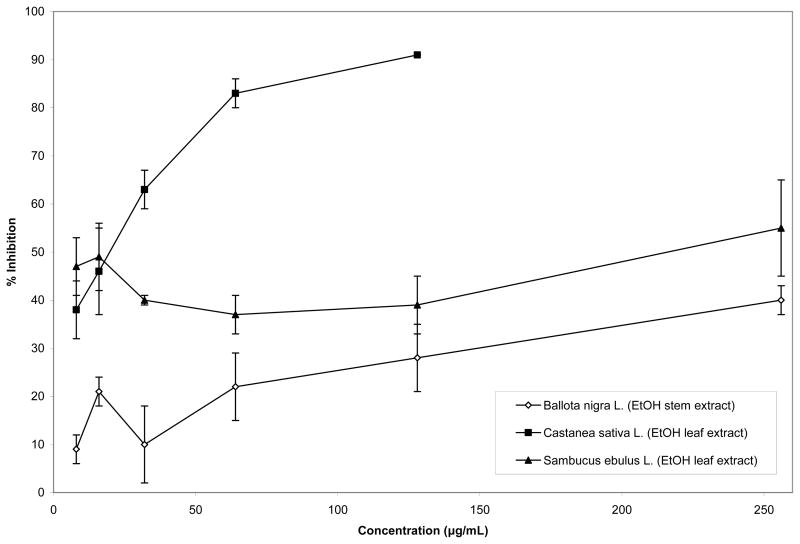
Three ethanolic extracts demonstrated significant δ-toxin inhibition, and come from the following plant species: Ballota nigra, Castanea sativa, and Sambucus ebulus. Interestingly, each of these species is applied in south Italian folk remedies for skin and soft tissue infection [19]. The HPLC chromatograms and graphs of the percent inhibition of δ-toxin (by measure of peak area) for these species demonstrate a strong dose-dependent response (Fig. 2S and 3).
Fig. 2S.
HPLC chromatogram of δ-toxin after treatment with different concentrations of plant extract. (a) EtOH extract of Ballota nigra stems. (b) EtOH extract of Castanea sativa leaves. (c) EtOH extract of Sambucus ebulus leaves.
Fig. 3.
Percent inhibition of δ-toxin peak area after treatment with extracts of Ballota nigra, Castanea sativa and Sambucus ebulus.
Discussion
Quantification of δ-hemolysin in the supernatant of staphylococcal cultures can be used as a measure of agr system, or QS, activity [7–9]. The agr system controls approximately 150 genes and is critical to S. aureus virulence [22]. While the staphylococcal QS system is a useful target for the discovery and development of new anti-pathogenic drugs, the dynamic nature of the agr system must not be overlooked. A better understanding of the effect that agr manipulation can have on the development of infection in vivo is necessary. For example, inhibiting agr activity during certain times in the infection process can lead to deleterious effects, such as increased biofilm formation [23].
Based on analyses of δ-hemolysin production, we have offered the first reports of plant extracts interfering with QS pathways in MRSA. These results indicate that some degree of QSI activity is evident in 90% of the 168 Italian plant extracts screened, including those extracts with no growth inhibitory activity.
The validity of plant-based therapies for infection that do not exhibit activity in the standard in vitro bacteriostatic or bactericidal assays is oftentimes questioned. These data, however, support the idea that other mechanisms of action may be in play, which do not necessarily impact bacterial growth, but virulence mechanisms, instead. These data give validity to the use of south Italian folk remedies incorporating Ballota nigra, Castanea sativa, and Sambucus ebulus for the treatment of skin and soft tissue infection. Further investigation, including the fractionation and isolation of active components from these three species is recommended.
Acknowledgments
This work was funded by NIH, NCCAM (Grant # F31AT004288, PI: C.L. Quave). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Center for Complementary and Alternative Medicine or the National Institutes of Health. Additional support for the project was provided by Botany in Action (C. L. Quave), and Anne Chatham Fellowship in Medicinal Botany (C.L. Quave). We thank Dr. Carmine Colacino (Universitá della Basilicata, Potenza, Italy) and Dr. Andrea Pieroni (University of Gastronomic Sciences, Pollenzo/Bra, Italy) for assisting in the taxonomic identification of plants collected. Special thanks to Dr. Michael Otto (National Institutes of Health/National Institute of Allergy and Infectious Diseases, Bethesda, USA) for assisting in the setup of the experiment. We also thank Dr. Horacio Preistap and Myron Georgiadis (Florida International University, Miami, USA) for technical support in the use of HPLC and MS equipment, respectively.
References
- 1.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;43:387–388. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons S, Oluwatuyi M, Kaatz GW. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J Antimicrob Chemother. 2003;51:13–17. doi: 10.1093/jac/dkg044. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons S, Oluwatuyi M, Veitch NC, Gray AI. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry. 2003;62:83–87. doi: 10.1016/s0031-9422(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons S, Udo EE. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother Res. 2000;14:139–140. doi: 10.1002/(sici)1099-1573(200003)14:2<139::aid-ptr608>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Pereda-Miranda R, Kaatz GW, Gibbons S. Polyacylated oligosaccharides from medicinal Mexican morning glory species as antibacterials and inhibitors of multidrug resistamce in Staphylococcus aureus. J Nat Prod. 2006;69:406–409. doi: 10.1021/np050227d. [DOI] [PubMed] [Google Scholar]
- 6.Sakoulas G, Eliopoulos GM, Moellering RC, Wennersten C, Venkataraman L, Novick RP, Gold HS. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to Vancomycin. Antimicrob Agents Chemother. 2002;46:1492–1502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto M, Götz F. Analysis of quorum sensing activity in staphylococci by RP-HPLC of staphylococcal delta-toxin. BioTechniques. 2000;28:1088–1096. doi: 10.2144/00286bm07. [DOI] [PubMed] [Google Scholar]
- 8.Somerville GA, Cockayne A, Durr M, Peschel A, Otto M, Musser JM. Synthesis and deformylation of Staphylococcus aureus δ-Toxin are linked to Tricarboxylic Acid Cycle Activity. J Bacteriol. 2003;185:6686–6694. doi: 10.1128/JB.185.22.6686-6694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto M. Quorum-sensing control in Staphylococci - a target for antimicrobial drug therapy? FEMS Microbiol Lett. 2004;241:135–141. doi: 10.1016/j.femsle.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Dell’Acqua G, Giacometti A, Cirioni O, Ghiselli R, Saba V, Scalise G, Gov Y, Balaban N. Suppression of drug-resistant staphylococcal infections by the quorum-sensing inhibitor RNAIII-inhibiting peptide. J Infect Dis. 2004;190:318–320. doi: 10.1086/386546. [DOI] [PubMed] [Google Scholar]
- 11.Balaban N, Goldkorn T, Nhan RT, Dang LB, Scott S, Ridgley RM, Rasooly A, Wright SC, Carlson JR. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science. 1998;280:438–440. doi: 10.1126/science.280.5362.438. [DOI] [PubMed] [Google Scholar]
- 12.Otto M, Submuth R, Vuong C, Jung G, Gotz F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 1999;450:257–262. doi: 10.1016/s0014-5793(99)00514-1. [DOI] [PubMed] [Google Scholar]
- 13.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, Muir TW. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci USA. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NARSA. The Network on Antimicrobial Resistance in Staphylococcus aureus. [Accessed March 20, 2010]; Available at http://www.narsa.net.
- 15.McDougal LK, Steward CD, Killgore GE, Chaitram J, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: Establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieroni A, Nebel S, Quave CL, Munz H, Heinrich M. Ethnopharmacology of liakra: traditional weedy vegetables of the Arberëshë of the Vulture area in southern Italy. J Ethnopharmacol. 2002;81:165–185. doi: 10.1016/s0378-8741(02)00052-1. [DOI] [PubMed] [Google Scholar]
- 17.Pieroni A, Quave CL, Nebel S, Heinrich M. Ethnopharmacy of the ethnic Albanians (Arbëreshë) of northern Basilicata, Italy. Fitoterapia. 2002;73:217–241. doi: 10.1016/s0367-326x(02)00063-1. [DOI] [PubMed] [Google Scholar]
- 18.Pieroni A, Quave CL. Traditional pharmacopoeias and medicines among Albanians and Italians in southern Italy: A comparison. J Ethnopharmacol. 2005;101:258–270. doi: 10.1016/j.jep.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Quave CL, Pieroni A, Bennett BC. Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy. J Ethnobio Ethnomed. 2008;4:5. doi: 10.1186/1746-4269-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isenberg HD. Clinical Microbiology Procedures Handbook. 2. Washington, D.C: ASM Press; 2004. [Google Scholar]
- 21.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Loman V, editor. Antibiotics in laboratory medicine. Baltimore, MD: Williams and Wilkins; 1996. pp. 52–111. [Google Scholar]
- 22.Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Brown EL, Zagursky RJ, Shlaes D, Projan SJ. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harraghy N, Kerdudou S, Herrmann M. Quorum-sensing systems in staphylococci as therapeutic targets. Anal Bioanal Chem. 2007;387:437–444. doi: 10.1007/s00216-006-0860-0. [DOI] [PubMed] [Google Scholar]