Abstract
Objectives
To assess effects of a localized anastomosis between the aorta and left lower lobe pulmonary artery (LLLPA) on flows through central vessels, and on vascular reactivity of small PAs distal or contralateral to the shunt.
Methods
Flow rates in major vessels and tensions from small PAs from left and right lower lobes were determined 48 hours after creation of an end-to-side anastomosis of the LLLPA to the aorta.
Results
Anastomoses increased flow through the LLLPA from 194 ± 6 to 452 ± 18 ml/min immediately after anastomosis and to 756 ± 19 ml/min by the time of harvest (n=88, p<0.05). Flow rates in main PAs from hosts with anastomoses were lower (557 ± 26 versus1033 ± 244 ml/min) while aortic root flows were not different from controls (1370 ± 53 compared to 1120 ± 111 ml/min; p = 0.07). Wet-to-dry weights of the both lungs and aortic flow rates were proportional to shunt flow rates. PA rings harvested from the right (unshunted) lobe of high flow hosts exhibited increased reactivity to the thromboxane agonist U46619 and phenylephrine relative to those of left PAs from the same animal or those of control hosts.
Conclusions
Our studies are the first to identify enhanced reactivity of PAs in a lung contralateral to a localized high output shunt between an aorta and pulmonary artery. These observations suggest that patients with localized systemic-to-pulmonary shunt could exhibit modified vascular tone in remote pulmonary arteries.
Keywords: pulmonary vascular resistance, arteriovenous shunt, phenylephrine, U46619
Introduction
Sustained high flow increases pulmonary arteriolar vasomotor activity within hours to days of exposure, presumably through a shift in the balance toward constrictor factors over compensatory relaxants, and ultimately yields vascular remodeling (1-6). Very little is known about the subacute (greater than 6 hours but less than 4 weeks) effects of systemic-pulmonary artery shunts on the relative reactivity of arteries distal to the anastomosis. We are not aware of any studies of PA reactivity from the lung contralateral to a localized systemic to pulmonary artery shunt. Systematic studies of the effects of a localized anastomosis on flow rates in the connected vessels immediately before and after the procedure, as well as on the flow rates through the heart and great vessels of the chest are similarly lacking.
We developed a model of high flow in an isolated lung lobe of young pigs characterized by neointimal lesions and medial thickening developing within 8 weeks of anastomosis of the left lower lobe artery to the aorta (7). We applied the same surgical intervention to characterize physiological changes brought about by high-flow after 48 hours. Our first goal was to measure flow in shunted lobar arteries and great vessels in order to establish the size of the shunt and effect on flows through the PAs, aortas, and right lung. Second, we measured reactivity of left and right lower lobe PAs (LLPAs) in hosts with in vivo exposure to high flow shunts.
Methods and Materials
Materials
U46619 (cat# PG-023, Biomol International, L.P., Plymouth Meeting, PA) Transonic Flow Probes (MB-Series Handle Style Cat# 3MB, Transonic systems Inc, Ithaca, NY).
Surgical Anastomosis and Procedures
Weanling infant pigs of either gender 3-6 weeks of age and ~7 kg in weight underwent creation of an aorto-left lower lobe pulmonary artery anastomosis as described in our publication (7). The surgical protocol and postoperative care were in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health and approved by the Animal Studies Committee of the Zablocki Veterans Administration Hospital and the Medical College of Wisconsin. Sedation was achieved with acepromazine (1.5 mg/kg) and ketamine (30 mg/kg) intramuscularly, fentanyl 2 μg/kg intravenously with an isoflurane gas mixture to achieve general anesthesia. Heart rate, end tidal CO2 and depth of sedation were monitored during surgery by a veterinarian to assure hemodynamic stability and adequate pain control.
A left thoracotomy was performed through a mid-thoracic interspace. The left lower lobe pulmonary artery was separated posteriorly from the adjacent lower lobe bronchus. The descending pulmonary artery was ligated just distal to the upper lobe branches and divided. The distal left lower lobe pulmonary artery was sewn end-to-side to the descending thoracic aorta. Blood loss was consistently less than 20 cc, therefore no transfusions were required or administered. Intramuscular cefazolin (25 mg/kg) and furosemide (1 mg/kg) were given postoperatively once to prevent infection and pulmonary edema respectively.
Definition of 3 study groups and flow measurements
Measurements of flow through the shunt were obtained with 3 mm probes immediately before and after creation of the shunt as well as at the time of harvest. For purposes of these analyses, “high flow” was defined as flow rates through the anastomoses at harvest which were either twice that of the right lower lobe pulmonary artery at harvest, or twice that of the left lower lobe PA pre-anastomosis. In some animals, flow data and tension measurements in PA rings were obtained without anastomosis; these subjects are referred to as “control”. Similarly, in some animals identified as “low flow controls” low flow shunts were deliberately created by narrowing the lumen of the anastomoses between the LLPA. Harvest flows were less than ½ that of the unshunted right LLPA or the left LLPA prior to shunt creation in this low flow group. Only measurements of weight and flow through the anastomotic left lower lobe artery at the time of harvest were obtained in every animal.
Flow measurements at harvest
Two days after the creation of the anastomoses, the pigs were sedated with intramuscular acepromazine and ketamine, then pentobarbital (5 mg/kg) to achieve general anesthesia. After intubation, a thoracotomy was performed and flow probes placed around the main (8 mm), left and right pulmonary arteries (3 mm), as well as the proximal aorta (8 mm) above the anastomoses for determination of flow rates. Pressure measurements in the anastomoses were attempted by positioning a needle connected to a transducer in the anastomosis. These results were highly position-dependent, dropping from systemic pressures at the opening of the anastomosis by >30 mmHg upon advancing the needle 1 cm distally and were therefore not pursued or reported.
Ring tension studies
After flow measurements were obtained, animals were euthanized through exsanguination under deep anesthesia. The heart and lungs were removed en bloc and transported on ice to the laboratory where pulmonary arteries 1-2 mm in diameter, 2-3 mm in length and ~5-7 centimeters distal to the aorto-pulmonary shunt were micro-dissected free of adherent lung tissue for tension studies. Tension measurements were obtained according to methods previously published by us (8). Pulmonary arteries were held in ice-cold physiological saline solution (PSS in mM: NaCl 130, CaCl2 2.5; NaHCO3 15; MgSO4 1.2; NaH2PO4 1.2; KCl 4.7; glucose 5.5, HEPES 10 and EDTA 0.026, pH 7.4) after dissection until use, generally within 90 minutes. Some rings were denuded by gently scraping the inner endothelial lining with a blunt forcep followed by rinsing the rings with PSS. Effective denuding was confirmed by absence of relaxation to acetylcholine or carbachol. Rings were mounted on tungsten wires, one connected to a fixed holder and the other to a force displacement transducer (Model FT03E, Grass Instruments) for continuously measuring isometric tension. The apparatus was immersed in pH-adjusted, oxygenated PSS solution (95% O2-5% CO2) at 37°C. Tension data were relayed from transducers to a signal amplifier and acquired and analyzed using CODAS software (DataQ Instruments, Inc.). Rings were preloaded with 0.4g of passive tension based upon preliminary studies with PAs from shunted and unshunted lobes to maximize active tension development, then equilibrated for an additional 30 minutes before the studies began. Standard pulmonary vasoconstrictors U-46619 (a thromboxane agonist) and phenylephrine were used to increase tension and evaluate the underlying vasoactive state of the pulmonary arteries. Binding to plasma membrane thromboxane receptors increases inositol phosphate turnover leading to enhanced activation of smooth muscle. Phenylephrine acts through alpha receptors to increase intracellular calcium which increases myosin light chain phosphorylation. Viability of all rings was confirmed by measuring the contractile response to the addition of 80 mM KCl in the bath at the end of the experiments. Data from rings that did not show at least 2 fold increase in tension to KCl were eliminated from the analysis (<20% of those initially dissected and mounted for PAs from both left and right lower lobes).
Histology and wet-to-dry lung weights
Distal sections of lung from shunted and non-shunted lobes were immersion fixed in neutral formalin for two to four days, after which time samples were embedded in paraffin, sectioned and reacted with a Movat pentachrome stain (9). Digital images were captured at magnifications of 200 or 400X with SPOT Advanced image acquisition software on an inverted Nikon microscope. Randomly selected pieces of left and right lungs were obtained for determination of wet to dry lung weights (7).
Statistical Analyses
Experimental results for PA rings from the same lobe (typically 4-5 from the left and right each) and same test conditions (e.g. a single dose of U46619 and vehicle pretreatment) were averaged for each “n”. Hence the “n”s provided in the results section represent individual animals rather than individual PA rings. Values are expressed as the means ± standard errors from 4 or more animals in each experiment. Least squares regression analysis was performed to examine the relationship between shunt and aortic flow rates as well as wet to dry lung wets and shunt flow rates. Comparisons between controls and treatments (or left versus right) were analyzed by two way repeated measure ANOVA followed by post hoc tests when permitted. Values for paired studies (e.g. left lower lobe PA flows immediately before and after anastomoses or maximum contraction to KCl left versus right lower lobe PAs) were compared by T-test. Values for p<0.05 were considered significant.
Results
Immediate alterations in flow by surgical anastomosis in high flow subjects
Table #1 contains data regarding the weight and flow rates. In control pigs, aortic and main PA flow rates were very similar. Also flow rates through left and right lower lobe pulmonary arteries were not different. In 88 pigs which met the criteria for “high flow” at the time of lung harvest (48 hours after anastomosis), flow rates through the left LLPA prior to anastomoses were 194 ml/min. Immediately after the end-to-side anastomosis to the aorta, flows increased to 452 ml/min, more than double baseline (p<.001) and increased over that of left LLPAs in control pigs (223 ml/min; n=7 controls; p<0.005; Table 1).
Table 1.
Weights, aortic, LLL RLL, and main PA flow rates in control pigs, high flow pigs pre-anastomosis, high flow pigs immediately post anastomosis and at harvest, and low flow pigs at harvest. Heart rates (mean 160-170), oxygen saturations (96-98%), and end tidal CO2 (32-39 mmHg) during surgery for lung harvest were not different between study groups.
| mean +/- SEM | Weight (kg) | Aortic flow (ml/min) | LLL flow: (ml/min) | RLL flow: (ml/min) | Main PA flow (ml/min) |
|---|---|---|---|---|---|
| Control (n=7) | 6.5 ± 0.3 | 1120 ± 111 | 223 ± 57 | 207± 51 | 1033 ± 244 |
| High flow, Pre-anastomosis (n=88) | 7.0 ± 0.1 | 194 ± 6 | |||
| High flow, post-anastomosis (n=88) | 452 ± 18* p<0.001 compared to pre- | ||||
| High flow, at harvest 48 hours post anastomosis | 1371 ± 53 (n=29) | 756 ± 19 (n=88)** p<0.01 compared to pre- and post | 270 ± 8 (n=88) | 557 ± 26 (n=29)* | |
| Low flow, at harvest 48 hours post anastomosis (n=9) | 7.1 ± 0.18 | 82 ± 38 | 380 ± 27* |
p relative to control
p pre- and post-anastomosis
Harvest flow rates high flow subjects
Forty eight hours after creation of high flow anastomoses the flow in the shunted left LLPA had increased to a mean flow of 756 ml/min, compared to a flow of 270 ml/min in the unoperated right pulmonary arteries and 452 ml/min immediately after creation of the anastomosis in LLLPAs (both p<0.001). Therefore, the flow through the surgically created shunts increased 1.7 fold over the two days between formation of the anastomosis and harvesting in high flow animals.
In the high flow hosts, right lower lobe PA flows at harvest (270 ml/min) were not different from those of the right lower lobe in control animals (p=ns). Proximal aortic flow rates in high flow animals were not different than aortic flows in the control hosts (p= 0.07).
Harvest flow rates low flow subjects
In 9 pigs that met criteria for “low flow”, left pulmonary artery flow at the time of harvest was 82 ± 38 ml/min, compared to 380 ± 27 ml/min in the right lower lobe pulmonary artery (p<.001; Table 1). Low flow was attempted in several additional animals, but not achieved because flows at the time of harvest had increased beyond the above defined thresholds; these animals were eliminated from analysis. Flow rates through the right lower lobe PAs were higher in low flow than in high flow animals (see Table 1).
Correlation of flow rates in the left lower lobe
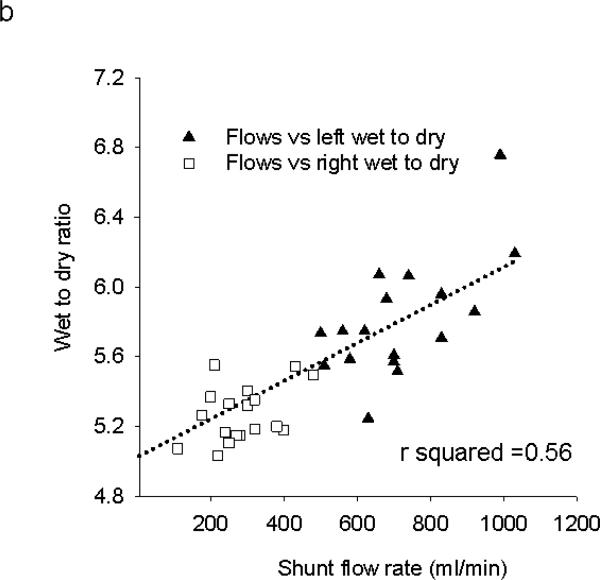
In all shunted subjects (high and low flow), flow through the shunted left lower lobe pulmonary artery was directly correlated to cardiac output as estimated by aortic flow. These data appear in figure 1a. The ratio of wet to dry weight of lung tissue in paired samples from right and left lungs from 19 randomly selected high flow hosts were obtained, and appear in figure 1b. Wet-to-dry ratios were modestly higher in the shunted lobe as compared to the control lung (left =5.83±0.33, right 5.28±0.16, n= 19 animals, p<0.001), indicating there was mild increase in edema and/or hemorrhage in shunted lobes. There was a positive correlation between the flow rate in the left lower lobe and wet:dry weight ratios in all hosts (figure 1b).
Figure 1.
a. Aortic flows rates in ml/min as a function of flow rates through the anastomosed left lower lobe pulmonary artery 48 hours after creation of the shunt in 35 pigs. The regression line shows the least squares fit for these data points, with an r-squared value of 0.60. b. Flow rates through the left (▲) or right (□) lower lobe pulmonary artery at the time of harvest are plotted as a function of wet-to-dry weights of peripheral lungs in 38 samples. The least squares regression line has an r-squared value of 0.56.
Contractility to U-46619 and phenylephrine is higher in unshunted than shunted PAs
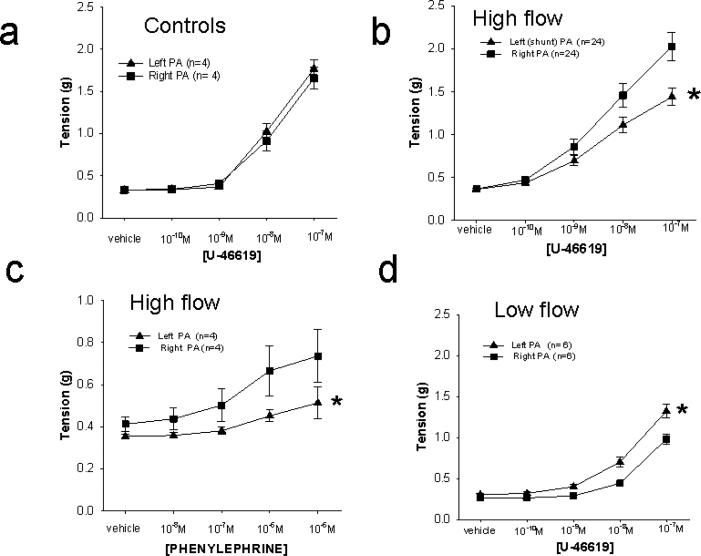
Isolated PA rings from the shunted (left) and unshunted (right) lobes, as well as those from control hosts were tested for reactivity. There was a concentration-dependent increase in tension in all the rings to the thromboxane mimetic U-46619 (10-10 – 10-7 M) as appears in Figure 2. In control hosts, there was no difference in reactivity of left and right PAs to U-46619 (figure 2a). In contrast, PAs from the unshunted right lower lobes of high flow hosts 48 hours after anastomosis exhibited significantly higher tension to the same concentrations of U-46619 as rings from the left lower lobe (n=24; see figure 2b). Similarly, increased tension to phenylephrine (PE 10-8 to 10-5M) in PA rings derived from unshunted right compared to those derived from left lower lobes was observed (figure 2c).
Figure 2.
Values for PAs from the right lower lobe are represented by filled squares (■). Those from the left lower lobe are represented by filled triangles (▲). Asterisks denote concentration curves that are different one from the other based upon two way repeated measure ANOVA (p<.05).
a. PAs from left and right lower lobes from control pigs develop equal tension to U46619 (n=4) in the concentration range of 10-10 through 10-7. This concentration range was chosen to develop a full tension response curve.
b. PAs from the right lower lobe of pigs with high flow shunts developed more tension to U46619 than did PAs from the left lower lobe (n=24; p<.005).
c. Right lower lobe PAs from pigs with high flow shunts exhibited increased tension to phenylephrine over left lower lob PAs from the same pigs (n=4; p<.05).
d. Left lower lobe PAs from low flow shunts exhibited very similar tensions to U46619 as those from high flow pigs, but lower tension in PAs from the right lower lobes (n=6; p<.01).
e. Right LLPAs from high flow hosts developed higher tension to U46619 than those from control (unoperated) hosts (n= 24 high flow, 6 control).
f. Tension to U46619 of left LLPAs from control and high flow hosts are not different (n=24 high flow, 6 control).
In contrast to results from high flow hosts, unshunted right LLPAs from low flow animals developed less tension than PAs from the same hosts harvested from the left lower lobe (figure 2d), as well as less than that of control right LLPAs. The tension to U46619 was similar in left LLPAs regardless of whether they derived from high or low flow pigs, or from control hosts. These data support an effect on tension development in unshunted PAs in this model (see figures 2e and 2f).
Denuding endothelium eliminates difference in U46619 contractility between left and right PAs
PAs from high flow hosts denuded of endothelium were equally responsive to U46619 when comparing the left and right lower lobes (p>0.2; n=4 each left and right) suggesting that an endothelial derived factor contributes to the increase in contractility of PAs from non-shunted lobes.
High flow does not alter depolarization induced contraction of the PAs to high potassium
Rings were constricted with 80 mM KCl to examine non-receptor or endothelial independent contraction. Contraction to KCl was identical in left and right PAs from lungs exposed to high flow (n=34, p=0.326). Similarly, contractility of left and right PAs to KCl in control hosts was identical to that of shunted hosts.
High flow causes limited histological changes to lungs 48 hours post anastomoses
There were no differences in the histological appearance of lungs from control, high flow or low flow animals. Lung sections stained with hematoxylin and eosin showed scattered perivascular edema from both left and right lungs, and no vascular occlusion or neointimal hyperplasia characteristic of established pulmonary hypertension (3,10) and as develops in this model eight weeks after shunt creation (7). Pleuritis along the outer surfaces of the lung, which is commonly associated with surgery, was observed in many samples from high and low flow hosts.
Discussion
To our knowledge, our data are the first to report quantitative flow measurements at the level of the aorta, main PA, and left and right lower lobe PAs 48 hours after creation of an aorto-pulmonary anastomosis which substantially increased flow to the left LLPA. Although it is well recognized that sustained exposure to high pressure/flow shunts in the lung in vivo produces characteristic vasculopathic changes (2,3,11), little attention has been focused on short term vascular effects of this “injury”. Moreover, there is very limited information regarding the effects of such an anastomosis on cardiac output and flows through the unshunted lung, and particularly the effect of flow on reactivity of the unshunted pulmonary vasculature. For example, De Canniere et al (1) reported increased pulmonary vascular resistance and cardiac output 8 weeks after surgical creation of an anastomosis between the aorta and main PA. The goals of our studies, therefore, were to characterize flows in the large intrathoracic vessels and reactivity of small PAs 48 hours after creation of a localized high flow anastomosis. “Low flow” shunts were deliberately created in some pigs in order to examine the effects of surgery with an anastomosis carrying blood with high oxygen tension but low flow rates.
We observed that flow through the surgical anastomosis increased by 1.7 fold between the initial creation of the connection and 48 hours later at harvest (see Table 1). In fact, due to substantial and consistent increments in flow rates through the anastomosed left LLPA between creation of the connection and harvest 48 hours later, experience on the part of the surgical team was required to create an anastomosis that was not so large that it caused death likely due to heart failure in this time frame. This evolution is consistent with the development of a high flow state after the creation of a traumatic AV fistula (12), though the rapid time frame and quantitation of these flows in our animal model is new information. Next, the creation of high flow shunts was associated with a modest and insignificant increase in the proximal aortic flow rates. Although additional numbers of aortic flows in control pigs would likely have resulted in statistically significant increases in aortic flow rates of high flow pigs over that of controls, we doubt the physiological significance of a 20-25% increase in cardiac output as determined by proximal aortic flow rates. As expected, aortic flow rates were reasonably correlated to the size of the shunt and flow through the LLLPA at the time of harvest in our model (fig 1a).
A key finding from our studies is enhanced reactivity of PAs from the unshunted, right lobe over those from the shunted, high flow left lower lobe of the same hosts. Flow to the right lower lobe in high flow hosts was not different from that of right LLPAs in control hosts. Because flows to the unshunted right lung were not significantly elevated over controls, exposure to increased shear should not account for the differences in reactivity of right PAs to standard constricting agents. Identical contractility to KCl in shunted and unshunted arteries argues that the contractile apparatus was not affected by the anastomosis. Removal of the endothelium eliminated the differences in contractility. Finally, reactivity of unshunted right PAs appears to be increased over that of right PAs from control (unshunted) hosts. Together these observations suggest that circulating, endothelial derived- factors from the shunted lobe may trigger changes in vasoactive endothelial factors in remote pulmonary arteries (e.g. decrease in eNOS activation or expression, blunted prostacyclin production) to result in increase reactivity. High partial pressures of oxygen, pressure (as distinct from flow) or even denervation (13) in shunted lobes may contribute to differences in reactivity of shunted and unshunted PAs.
With shunt induced increments in flow to the left lower lobes (and right in low flow animals), wet to dry weights of lung tissue increased as anticipated (figure 1b). Though statistically significant, these increases were very modest. We speculate that this minimal increase in lung water does not account for differential reactivity in shunted compared to unshunted PAs, but cannot completely exclude a contribution of perivascular edema in modifying contractility of shunted PAs.
In summary we have induced high flow localized to a single lobe of a lung in juvenile pigs. Flows through these anastomoses increased substantially in 48 hours after surgical connection. Enhanced reactivity of pulmonary arteries in the lung contralateral to the shunt may have clinical implications. Though rare, either traumatic or naturally occurring systemic-to-pulmonary arterial fistulas (14,15) may be associated with high flow shunting to a pulmonary lobe. Further studies to better define mechanisms underlying and biochemical perturbations associated with changes in vascular reactivity induced by subacute changes in flow are needed. In the accompanying paper (16), we explore the contribution of NOS and eicosanoids to vascular reactivity in this model.
Acknowledgements
The authors acknowledge substantial contributions to this work by Tim Lowry, Daling Zhu and Ying Gao.
Grant support: NHLBI: HL068627 and HL-49294 (ERJ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ultramini abstract. A localized high flow shunt to the left lower lobe pulmonary artery from the aorta results in substantial increases in flow through the anastomosis over 48 hours and enhanced reactivity of PAs contralateral to the shunt relative to ipsilateral or control PAs.
References
- 1.De Canniere D, Stefanidis C, Brimioulle S, Naeije R. Effects of a chronic aortopulmonary shunt on pulmonary hemodynamics in piglets. J Appl Physiol. 1994 Oct;77(4):1591–6. doi: 10.1152/jappl.1994.77.4.1591. [DOI] [PubMed] [Google Scholar]
- 2.Botney MD. Role of hemodynamics in pulmonary vascular remodeling: Implications for primary pulmonary hypertension. Am J Respir Crit Care Med. 1999 Feb;159(2):361–4. doi: 10.1164/ajrccm.159.2.9805075. [DOI] [PubMed] [Google Scholar]
- 3.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958 Oct;18(4 Part 1):533–47. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- 4.Steinhorn RH, Russell JA, Lakshminrusimha S, Gugino SF, Black SM, Fineman JR. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001 Jan;280(1):H311–7. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- 5.Storme L, Rairigh RL, Parker TA, Kinsella JP, Abman SH. Acute intrauterine pulmonary hypertension impairs endothelium-dependent vasodilation in the ovine fetus. Pediatr Res. 1999 Apr;45(4 Pt 1):575–81. doi: 10.1203/00006450-199904010-00018. [DOI] [PubMed] [Google Scholar]
- 6.Vitvitsky EV, Griffin JP, Collins MH, Spray TL, Gaynor JW. Increased pulmonary blood flow produces endothelial cell dysfunction in neonatal swine. Ann Thorac Surg. 1998 Oct;66(4):1372–7. doi: 10.1016/s0003-4975(98)00835-2. [DOI] [PubMed] [Google Scholar]
- 7.Bousamra M, 2nd, Rossi R, Jacobs E, Parviz M, Busch C, Nelin LD, et al. Systemic lobar shunting induces advanced pulmonary vasculopathy. J Thorac Cardiovasc Surg. 2000 Jul;120(1):88–98. doi: 10.1067/mtc.2000.106654. [DOI] [PubMed] [Google Scholar]
- 8.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res. 2003 May 16;92(9):992–1000. doi: 10.1161/01.RES.0000070881.65194.8F. [DOI] [PubMed] [Google Scholar]
- 9.Garvey W, Fathi A, Bigelow F, Carpenter B, Jimenez C. Improved movat pentachrome stain. Stain Technol. 1986 Jan;61(1):60–2. doi: 10.3109/10520298609110708. [DOI] [PubMed] [Google Scholar]
- 10.Tuder RM, Lee SD, Cool CC. Histopathology of pulmonary hypertension. Chest. 1998 Jul;114(1 Suppl):1S–6S. doi: 10.1378/chest.114.1_supplement.1s-a. [DOI] [PubMed] [Google Scholar]
- 11.Fullerton DA, Jones SD, Jaggers J, Piedalue F, Grover FL, McIntyre RC., Jr Effective control of pulmonary vascular resistance with inhaled nitric oxide after cardiac operation. J Thorac Cardiovasc Surg. 1996 Apr;111(4):753, 62. doi: 10.1016/s0022-5223(96)70335-5. discussion 762-3. [DOI] [PubMed] [Google Scholar]
- 12.Bajraktari G, Rexhepaj N, Bakalli A, Shaqiri G, Osmani E, Vokrri L, et al. Remission of high-output heart failure after surgical repair of 30-month arteriovenous femoral fistula: Case report. Heart Surg Forum. 2005;8(2):E118–20. doi: 10.1532/hsf98.20041172. [DOI] [PubMed] [Google Scholar]
- 13.Juratsch CE, Jengo JA, Castagna J, Laks MM. Experimental pulmonary hypertension produced by surgical and chemical denervation of the pulmonary vasculature. Chest. 1980 Apr;77(4):525–30. doi: 10.1378/chest.77.4.525. [DOI] [PubMed] [Google Scholar]
- 14.Loebl EC, Platt MR, Mills LJ, Estrera AS. Pulmonary resection for a traumatic pulmonary arteriovenous fistula. Case report. J Thorac Cardiovasc Surg. 1979 May;77(5):674–6. [PubMed] [Google Scholar]
- 15.Yamanaka A, Hirai T, Fujimoto T, Hase M, Noguchi M, Konishi F. Anomalous systemic arterial supply to normal basal segments of the left lower lobe. Ann Thorac Surg. 1999 Aug;68(2):332–8. doi: 10.1016/s0003-4975(99)00533-0. [DOI] [PubMed] [Google Scholar]
- 16.Pfister S, Somberg L, Lowry T, Gao Y, Medhora M, Jacobs ER. Mechanisms underlying increased reactivity of pulmonary arteries contralateral to a localized high-flow anastomosis. Journal of Thoracic and Cardiovascular Surgery. 2010 doi: 10.1016/j.jtcvs.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]