Abstract
Hypophosphatemia due to inappropriate urinary phosphate wasting is a frequent metabolic complication of the early period following kidney transplantation. Although previously considered to be caused by tertiary hyperparathyroidism, recent evidence suggests a primary role for persistently elevated circulating levels of the phosphorus-regulating hormone, fibroblast growth factors 23 (FGF23). In the setting of a healthy renal allograft, markedly increased FGF23 levels from the dialysis period induce renal phosphate wasting and inhibition of calcitriol production, which contribute to hypophosphatemia. While such tertiary FGF23 excess and resultant hypophosphatemia typically abates within the first few weeks to months post-transplant, some recipients manifest persistent renal phosphate wasting. Furthermore, increased FGF23 levels have been associated with increased risk of kidney disease progression, cardiovascular disease and death outside of the transplant setting. Whether tertiary FGF23 excess is associated with adverse transplant outcomes is unknown. In this article, we review the physiology of FGF23, summarize its relationship with hypophosphatemia after kidney transplantation, and speculate on its potential impact on long term outcomes of renal allograft recipients.
Introduction
The prevalence of end stage renal disease (ESRD) in children has nearly tripled since 1980 (1). With 20-year survival rates greater than 90% (2) compared to only 66% among those who undergo long-term dialysis (3), kidney transplantation is the preferred treatment for ESRD in children. Furthermore, between 1987–1990 and 1999–2002, 5-year allograft survival improved significantly from 75% to 85% in living related donor transplants and from 55% to 80% in deceased donor transplants (4). This remarkable progress is largely the result of improved operative techniques, immune suppression regimens, and prophylaxis against opportunistic infections (5). As a result of improved transplant outcomes, death and disability due to late complications of de novo diabetes mellitus, hypertension, cardiovascular disease, and fracture have emerged as the major threats to patients’ long-term health. These observations have attracted attention to the identification and long-term management of risk factors for cardiovascular, metabolic and skeletal complications of kidney transplantation. A growing body of evidence has linked disordered phosphorus metabolism to increased risk of adverse clinical outcomes in patients with chronic kidney disease (CKD) and kidney transplant recipients often manifest important alterations in phosphorus metabolism. In this review, we explore the potential impact of disordered phosphorus metabolism on pediatric renal transplant recipients.
Post-transplant hypophosphatemia
Hypophosphatemia due to inappropriate urinary phosphate wasting is a frequent metabolic complication of the early period following kidney transplantation. Although hypophosphatemia affects up to 90% of patients (6–8), it is typically self-limited within the first weeks to months post-transplant in most affected individuals. However, 6–27% of patients have persistently low serum phosphate levels for months to years (9–11). In these patients, hypophosphatemia can contribute to complications such as muscle weakness, osteomalacia and metabolic encephalopathy (12–16).
Tertiary hyperparathyroidism – the persistence of severe secondary hyperparathyroidism from the dialysis period into the post-transplant period – has been traditionally thought to drive hypophosphatemia following kidney transplantation but several lines of evidence argue against a primary role for parathyroid hormone (PTH) (17–20). Inappropriate urinary phosphate wasting can occur despite low levels of PTH, such as in patients who have previously undergone parathyroidectomy, and it can persist after elevated PTH levels have normalized (17, 20). Second, the cardinal manifestation of hyperparathyroidism in the setting of normal renal function is hypercalcemia and, in most cases, hypophosphatemia following kidney transplantation is isolated and dissociated from concomitant hypercalcemia. Third, levels of 1,25-dihydroxyvitamin D are often persistently low for several months following transplantation despite excessive PTH, a healthy allograft and hypophosphatemia, each of which should stimulate its production (21).
A study by Green et al. cast further doubt on the role of PTH as the primary mediator of isolated post-transplant hypophosphatemia (17). Compared to sera from healthy volunteers, sera from kidney transplant recipients and patients with CKD and ESRD stimulated significantly less flux of phosphate into opossum renal tubular cells, an in vitro cellular model for tubular sodium-phosphate co-transport. Addition of PTH inhibitors did not modify these effects. This suggested the presence of a circulating factor other than PTH that was responsible for phosphaturia in CKD, ESRD and early post-transplant patients.
Immunosuppressive medications, such as glucocorticoids and calcineurin inhibitors were also hypothesized to contribute to phosphate depletion (22, 23). However, the low incidence of hypophosphatemia following transplantation of other solid organs in which these same agents are used, often at higher doses, argues against their having a primary role (24, 25). Newer agents such as rapamycin were recently reported to induce renal phosphate wasting and hypophosphatemia (26) but a high incidence of post-transplant hypophosphatemia was reported long before this class of drugs was introduced.
The finding of severe hypophosphatemia due to isolated urinary phosphate wasting and inappropriately low levels of 1,25-dihydroxyvitamin D for the degree of hypophosphatemia is reminiscent of the clinical phenotype of X-linked hypophosphatemia and related hereditary rachitic disorders (27). This suggested a common pathogenesis across these syndromes. The discovery of fibroblast growth factor 23 (FGF23), the characterization of its physiological effects, and the determination that FGF23 excess is the central mechanism in the pathogenesis of hereditary hypophosphatemic rickets syndromes suggested novel mechanisms to explain disordered phosphorus metabolism following kidney transplantation.
Discovery of FGF23
Fibroblast growth factor 23 is an endocrine hormone that regulates phosphorus and vitamin D metabolism in conjunction with PTH. FGF23 was discovered in 2000 as the latest member of the FGF family based on the homology of its N-terminus with other FGFs (28). FGF23 is transcribed as a 251 amino acid peptide with the first 24 acting as a signal peptide that is cleaved intracellularly such that secreted FGF23 contains 227 amino acids (29, 30). At the time of its initial discovery, the function of FGF23 and its role in health and human disease was unknown. Shortly after its discovery, genetic studies of families with autosomal dominant hypophosphatemic rickets (ADHR) demonstrated mutations in the coding region of the FGF23 gene on chromosome 12p13 (29). Later it was determined that these mutations alter the amino acid sequence in the cleavage site of FGF23 thereby protecting it from degradation (30). Shortly after its strong linkage to ADHR was discovered, high FGF23 expression was identified in mesenchymal tumors that cause tumor-induced osteomalacia (TIO), a syndrome with a virtually identical phenotype as ADHR: hypophosphatemia, inappropriate urinary phosphate wasting, inappropriately low levels of 1,25-dihydroxyvitamin D and osteomalacia (30). In vitro, animal and human studies confirmed that the physiological actions of FGF23 account for the phenotype in ADHR and TIO (29–31). Subsequently, inappropriately high FGF23 levels were also reported in X-linked hypophosphatemia and its animal analog, the Hyp mouse (31–33).
FGF23 and phosphate homeostasis
FGF23 is expressed primarily by osteocytes (34). Due to lower affinity for extracellular heparan sulfate, FGF23 is free to circulate as an endocrine hormone in contrast to most other FGFs that bind to matrix and thus exert their actions through paracrine and autocrine pathways exclusively (35). Although FGF receptors are found on all cells, the specificity of FGF23 for its target cells is mediated by klotho, which acts as its co-receptor (36, 37). Klotho is primarily expressed in the kidneys and parathyroid glands, which are thus the primary sites of action of FGF23.
In the kidney, FGF23 inhibits renal tubular phosphate reabsorption by suppressing the expression of luminal sodium-phosphate co-transporters (NaPi) 2a and 2c (38). FGF23 also reduces levels of 1,25-dihydroxyvitamin D through its genomic effects on the hydroxylases involved in vitamin D synthesis and degradation: FGF23 inhibits the anabolic CYP27B1 (1-α hydroxylase) and stimulates the catabolic CYP24 (24-hydroxylase) (39, 40). By lowering 1,25-dihydroxyvitamin D levels, FGF23 likely reduces absorption of dietary phosphate in the intestine. Thus, states of high FGF23 levels are characterized by phosphaturia and low serum phosphate and 1,25-dihydroxyvitamin D levels. Under normal conditions, FGF23 binding to klotho-FGF receptor complexes in the parathyroid glands inhibits PTH secretion (41).
A high dietary phosphorus intake and 1,25-dihydroxyvitamin D stimulate FGF23 secretion (39, 42). The genomic effect of calcitriol acts through a vitamin D-responsive element on the FGF23 promoter that is mediated by a vitamin D receptor-dependent pathway; administration of 1,25-dihydroxyvitamin D did not increase FGF23 levels in vitamin D receptor-null mice (42). Stimulation of FGF23 by 1,25-dihydroxyvitamin D might be viewed as a counter-regulatory effect to increase renal phosphate clearance in the setting of enhanced gastrointestinal phosphate absorption stimulated by 1,25-dihydroxyvitamin D. In addition, the 1,25-dihydroxyvitamin D-stimulated increase in FGF23 inhibits further 1,25-dihydroxyvitamin D production in a classic negative endocrine feedback loop. The mechanism and sensing pathway through which a high dietary phosphorus intake stimulates FGF23 is not clear, but interestingly, does not appear to be mediated by changes in serum phosphate levels as might be expected (43, 44). Indeed, no studies have proved that serum phosphate directly regulates FGF23.
Role of FGF23 in disordered mineral metabolism in chronic kidney disease
Levels of FGF23 increase in CKD as early as when the GFR drops below 90 ml/min/1.73m2 (45–47). While it had been controversial whether FGF23 or PTH rise first in CKD, recent animal models of progressive CKD suggest that increased FGF23 is first (48, 49). The increased FGF23 helped the animals maintain a normal serum phosphate level by increasing their urinary phosphate excretion but the high FGF23 levels led to reduced 1,25-dihydroxyvitamin D, a modest reduction in serum calcium, and then, an increase in PTH. Investigators confirmed the primacy of FGF23 by demonstrating that anti-FGF23 neutralizing antibodies reduced phosphaturia and normalized serum phosphate, 1,25-dihydroxyvitamin D, calcium and PTH levels in normal animals, Hyp mice and in the animal model of progressive CKD (49–51).
Early increases in FGF23 explain the predominant phenotype of mineral metabolism in early CKD: normal serum phosphate levels, high fractional excretion of phosphate, deficiency of 1,25-dihydroxyvitamin D and elevated PTH levels. As GFR progressively declines, FGF23 levels increase sharply to the point that dialysis patients can manifest 100- to 1000- fold elevations above the normal range (47). Importantly, most if not all of the circulating FGF23 is biologically intact even in dialysis patients (52). The extreme elevations of biologically intact FGF23 in dialysis patients sets the stage for tertiary FGF23 excess in the event of a kidney transplant.
FGF23 and hypophosphatemia following kidney transplantation
Several independent groups validated the hypothesis that post-transplant hypophosphatemia was a syndrome of tertiary FGF23 excess. In the first report, Bhan et al studied 27 recipients of living donor kidney transplants and followed them prospectively for 3 months during which time the patients had repeated blood and urine collections (7). Living donors were chosen to ensure a high likelihood of immediate allograft function that would minimize potential confounding by delayed graft function. Sample collections were discontinued if a recipient developed allograft dysfunction. A similar immune suppression strategy was used in virtually all recipients in this single center study, which further minimized confounding.
Hypophosphatemia (defined as serum phosphate < 2.6 mg/dL) occurred in 85% of patients and 37% developed severe hypophosphatemia (defined as serum phosphate < 1.5 mg/dL). Only one recipient had previously undergone parathyroidectomy but that patient nevertheless developed hypophosphatemia. There were no episodes of hypercalcemia in any recipient. Each of these simple observations argued against a primary role for PTH in the hypophosphatemia.
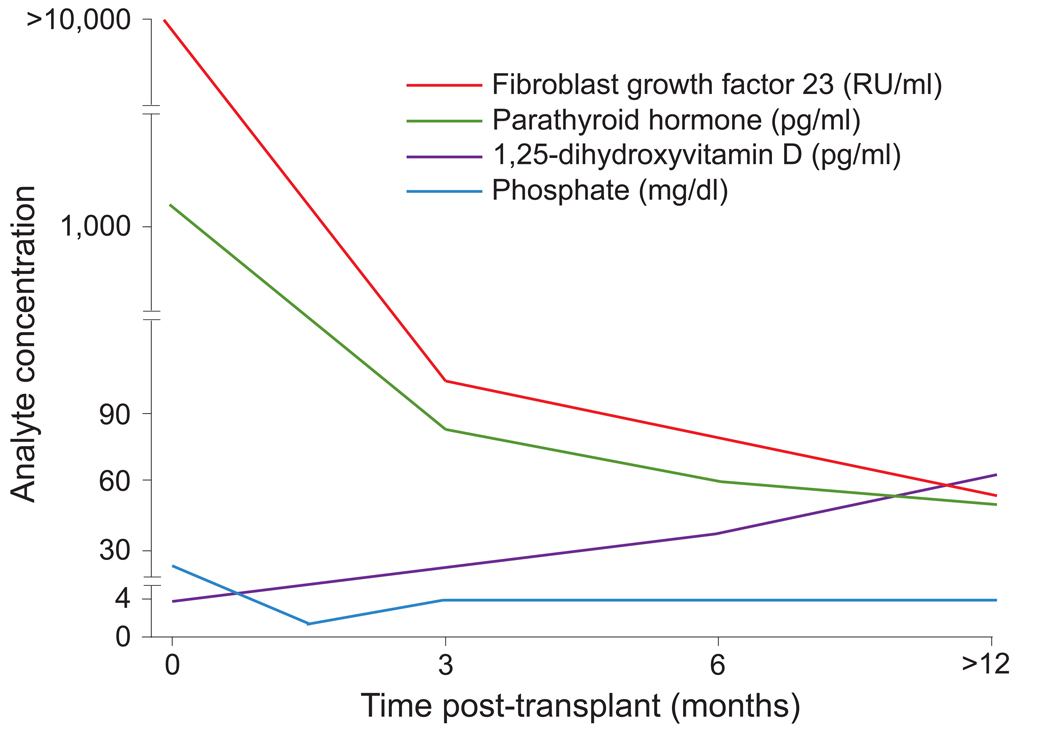
Both FGF23 and PTH levels decreased substantially during the first week following transplant, but FGF23 levels remained 10 times above normal. A schematic of the serial change in serum phosphate, PTH and FGF23 is shown in Figure 1. In multivariable-adjusted analyses, FGF-23 was independently associated with post-transplant serum phosphate levels, urinary fractional excretion of phosphate and 1,25-dihydroxyvitamin D levels (inversely). In contrast, PTH was not independently associated with any of these parameters. A persistently elevated FGF23 level in the early post-transplant period was independently associated with a 5.3-fold relative risk (P = 0.02) of developing severe hypophosphatemia when comparing recipients whose early post-transplant area under the FGF23 curve was greater than the median compared to those below the median. The area under the PTH curve was not associated with hypophosphatemia.
Figure 1. Temporal changes in serum phosphate and its regulatory hormones following successful kidney transplantation.
The X axis represents time after kidney transplantation in months. The Y axis represents the circulating concentration of fibroblast growth factor 23, parathyroid hormone, 1,25-dihydroxyvitamin D, and serum phosphate (color coded).
Dissociating the effects of FGF23 on urinary and serum phosphate from the similar effects of PTH is challenging in human studies, which cannot use parathyroidectomy to isolate FGF23. Thus, the most important finding in this study was the strong inverse association between FGF23 excess and decreased levels of 1,25-dihydroxyvitamin D, which persisted following transplantation despite excessive PTH, a healthy allograft and hypophosphatemia, each of which should have stimulated calcitriol production. This finding supports the overall central role of FGF23 in post-transplant hypophosphatemia.
In another observational study, mineral metabolism was analyzed prior to and 3 months post-transplant in 41 recipients of deceased donor kidneys (8). 90% of patients developed hypophosphatemia (defined as serum phosphate < 2.3 mg/dL), and 66% were severe (serum phosphate <1.5 mg/dL). Serum phosphate levels reached a nadir of 1.5 ± 0.5 mg/dL between 2 – 4 weeks after transplant. An increased pre-transplant FGF23 level was the only independent predictor of the post-transplant nadir phosphate. Likewise, post-transplant FGF23 was the only independent predictor of post-transplant phosphaturia but the most severe phosphaturia was noted in patients who had high levels of both FGF23 and PTH. Both FGF23 (inversely) and PTH (directly) were independent predictors of 1,25-dihydroxyvitamin D concentrations. These results are consistent with known physiological mechanisms and previous studies: both FGF23 and PTH promote phosphaturia but FGF23 inhibits calcitriol production even in the setting of stimulation by PTH. In addition, the contribution of PTH to ongoing phosphaturia suggests that therapies that target PTH reduction might attenuate urinary phosphate wasting even if they do not have activity against high FGF23 levels.
In a subsequent longitudinal study, transplant recipients were followed for 12 months following transplantation and were compared with GFR-matched CKD patients (10). Fourteen percent of recipients had persistent hypophosphatemia at one year following transplant, and mean serum phosphate was significantly lower and PTH persistently higher among the transplant recipients compared with the CKD patients. In contrast, FGF23 levels and urinary fractional excretion of phosphate had returned to similar levels as in CKD patients by one year post-transplant, although these remained significantly elevated compared to normal volunteers (10). Along with similar findings from an additional study (11), the data collectively suggest that although FGF23 levels decline to a range that is comparable to CKD patients, ongoing elevations above the normal range contribute to changes in serum phosphate and urinary phosphate handling. Whether these changes have an adverse impact on long-term transplant outcomes is unknown.
FGF23 in pediatric kidney transplant recipients
Although hypophosphatemia following kidney transplantation is common in children (9, 53), there are scant data on the impact of FGF23 in this population. Batchetta et al recently reported increased serum levels of FGF23 in pediatric kidney transplant recipients compared to children with comparable renal function and serum phosphate concentrations (54). Since markedly elevated FGF23 levels were also noted in children with CKD and ESRD (55, 56), it is likely that tertiary FGF23 excess plays a similarly important role in mineral metabolism in the post-transplant period in children as in adults. Dedicated longitudinal studies of children are needed to determine the magnitude and duration of persistent FGF23 excess during the post-transplant period, and to assess the impact on phosphorus and vitamin D metabolism and clinical outcomes.
Potential impact of FGF23 excess in patient post kidney transplant
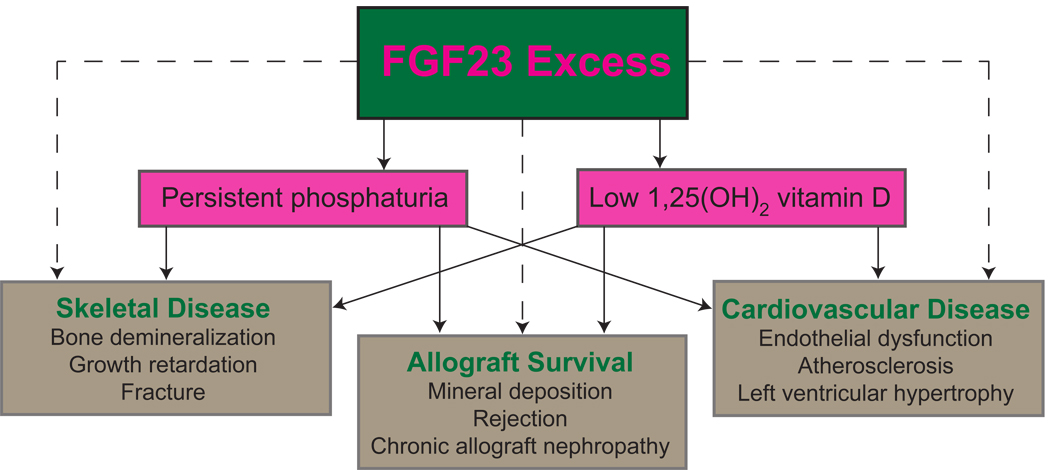
Tertiary FGF23 excess accounts for many of the abnormalities in phosphorus handling that follow kidney transplantation but the impact on hard clinical outcomes of persistently elevated FGF23 remains completely unknown in children or adults. Outcomes studies from patients with ESRD, pre-dialysis CKD and the general population, in which FGF23 was associated with mortality, cardiovascular disease and progressive CKD, highlight the importance of understanding the role of FGF23 in kidney transplant recipients. In the absence of transplant data, we speculate on potential effects of FGF23 that are worthy of study (Figure 2).
Figure 2. Potential adverse effects of fibroblast growth factor 23 (FGF23) on the skeleton, allograft, and cardiovascular system in patients after kidney transplantation.
Solid lines represent known physiological effects of FGF23 excess and dashed lines represent speculation regarding potential adverse clinical effects of FGF23.
FGF23 and bone disease post-transplant
Persistently high urinary phosphate excretion induced by high FGF23 and PTH could reflect an appropriate purge of phosphate that accumulated during the period of ESRD. However, persistently elevated fractional excretion of phosphate could be inappropriate, particularly in the setting of hypophosphatemia or even a normal serum phosphate if skeletal phosphate release balances urinary phosphate loss. Thus, one adverse effect of persistent FGF23-induced urinary phosphate loss could be gradual bone demineralization, which in combination with steroids, high frequency of vitamin D deficiency, and FGF23-mediated inhibition of 1,25-dihydroxyvitamin D synthesis could predispose patients to osteomalacia, osteoporosis and increased risk of fracture. Indeed, the prevalence of mineralization defects was high in the one bone histomorphometric study of pediatric renal transplant recipients (57).
The risk of fracture among kidney transplant recipients is significantly greater than in the general population (58), with hip fracture rates actually higher among transplant recipients than dialysis patients (59). In children, the incidence of vertebral fracture after kidney transplantation was approximately 140 times higher than the general population (60). Although, high FGF23 was associated with increased bone mineralization in a cross-sectional study of children with ESRD (61), analysis of the relationships between FGF23, bone histology and fracture risk after transplantation is lacking in children and adults. In addition to addressing these important questions, a uniquely important question in the area of pediatric kidney transplantation is whether tertiary FGF23 excess might exert adverse effects on growth similar to the growth retarding effects of primary FGF23 excess.
FGF23 and long-term allograft function
Increased FGF23 is associated with more rapid progression of CKD in adults but it is unknown if this represents a direct effect of FGF23 or if the high FGF23 is a biomarker of phosphorus-related or other toxicity (62, 63). A direct effect of phosphorus or FGF23 is supported by animal and human studies in which dietary phosphorus restriction, which is known to lower FGF23 levels (64), was associated with slower progression of CKD (48, 65). Some investigators have proposed nephrocalcinosis as a mechanism of phosphorus-associated renal toxicity (66–68), and post-transplant hypophosphatemia and hypercalcemia were associated with calcium and phosphate deposition in renal allografts (69). Human data are conflicting on whether renal calcifications contribute to worse allograft outcomes with some studies reporting an association with future chronic allograft nephropathy (66, 67) while a pediatric study found no association (68).
Since deficiencies in the vitamin D axis are associated with altered immune regulation, another mechanism through which tertiary FGF23 excess might be harmful to allografts is reduction of 1,25-dihydroxyvitamin D levels. Vitamin D receptors and 1-α hydroxylase are expressed by multiple cells of the immune system (70, 71). In vitro and in vivo studies have shown that 1,25-dihydroxyvitamin D decreases T cell activation and proliferation, and inhibits dendritic cell differentiation and maturation (72–74) Deficiencies in the vitamin D axis have been linked to several autoimmune diseases including multiple sclerosis and type 1 diabetes (75). Although data on the immunological impact of vitamin D deficiency in kidney transplant recipients are scant, supplementation of calcitriol was associated with decreased expression of several co-stimulatory molecules in one study (76) and improved renal function in others (77–79). Animal studies also suggest that calcitriol and vitamin D analogs may protect renal allografts (80). Combined with the high prevalence of vitamin D deficiency in renal transplant recipients (81–83), low 1,25-dihydroxyvitamin D concentrations due to tertiary FGF23 excess could theoretically contribute to worse allograft outcomes via immunological mechanisms but this has not yet been explored.
FGF23 and cardiovascular disease post-transplant
Cardiovascular disease is the leading cause of mortality in adult renal transplant recipients (84). Although major cardiovascular events are rare in children, left ventricular hypertrophy and sub-clinical atherosclerosis are often present even in young kidney transplant recipients (85, 86). Several observational studies in adults suggest that increased FGF23 is independently associated with mortality, left ventricular hypertrophy, endothelial dysfunction and atherosclerosis in patients with high or even normal serum phosphate levels (87–91). In a preliminary study of pediatric hemodialysis patients from our group, increased FGF23 was associated with elevated left ventricular mass index (92). It is uncertain if the links between FGF23 excess, cardiovascular disease and death are causal or if FGF23 is a biomarker of other pathological mechanisms. However, if FGF23 is mechanistically linked to adverse outcomes independent of serum phosphate, then it would be a novel therapeutic target for improving outcomes in the post-transplant period. Of course, as allograft function declines over time, typical risk factors in pre-dialysis CKD patients, such as hyperphosphatemia, FGF23 excess and deficiency of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D (87, 91, 93–97) will also become relevant to transplant recipients.
Treatment
Unlike post-transplant hypercalcemia due to tertiary hyperparathyroidism for which parathyroidectomy reverses hypercalcemia (98, 99), the management of post-transplant hypophosphatemia due to tertiary FGF23 excess presents different challenges. Fortunately, hypophosphatemia resolves on its own in most patients. However, when hypophosphatemia is severe and protracted, standard therapies such as oral phosphate replacement and administration of calcitriol may be ineffective because these are the primary stimuli of FGF23 secretion and would be expected to raise FGF23 levels further. In addition, these therapies could contribute to diarrhea and hypercalcemia and, theoretically, to nephrocalcinosis by markedly increasing phosphaturia.
Cinacalcet lowers levels of PTH, 1,25-dihydroxyvitamin D and thus FGF23, and increases serum phosphate levels (100). A recent report suggested that it might improve the metabolic parameters and reduce the doses of phosphate and calcitriol required to treat hypophosphatemia in patients with X-linked hypophosphatemia (101). In hypercalcemic kidney transplant recipients, cinacalcet lowered serum calcium and raised serum phosphate levels (102–105). By minimizing PTH-mediated phosphaturia and modestly reducing FGF23, cinacalcet might be an option in certain kidney transplant recipients. However, additional studies are needed to determine the long-term efficacy and safety of cinacalcet in kidney transplant recipients given the population’s high rate of low turnover bone disease that could be exacerbated by cinacalcet (105, 106).
Anti-FGF23 antibodies improve hypophosphatemia and calcitriol deficiency in animal studies of Hyp mice (50, 51). Post-transplant hypophosphatemia would be another interesting application of these antibodies. Whether FGF23 blockade should be a target of therapy even in transplant recipients with normal serum phosphate levels would become an important question if FGF23 excess is found to contribute directly to bone loss, long-term allograft dysfunction or cardiovascular disease, as we hypothesized above.
Conclusions
Hypophosphatemia in the early period following kidney transplantation is extremely common but is often paid little clinical attention. At that time, it is, justifiably, no competition for the greatest clinical priorities which include post-operative surgical management, and optimizing immune suppression and infection prophylaxis regimens. Hypophosphatemia fades further into the background in the next few months as it usually resolves on its own. Recent data that highlight the role of tertiary FGF23 excess in the pathogenesis of post-transplant hypophosphatemia and its strong association with adverse clinical outcomes in dialysis, pre-dialysis and non-CKD populations suggest that perhaps greater attention to disordered phosphate metabolism might be warranted in kidney transplant recipients. Whether tertiary FGF23 excess might be linked to adverse outcomes in transplant recipients is unknown but worthy of dedicated studies in both adults and pediatric populations. Of course, studying potential effects of FGF23 on growth, bone histology, fracture risk, cardiovascular parameters and long-term outcomes in children is challenging given small numbers of patients at individual transplant centers. Like the Chronic Kidney Disease in Children Study, “CKiD” (107), a network of collaborative sites to recruit and retain a sufficient sample of pediatric renal allograft recipients for long-term evaluation is urgently needed.
Acknowledgments
Supported by grants RO1DK076116 (MW) and R01DK081374 (MW) from the National Institutes of Health.
References
- 1.United States Renal Data System. [Access 1 June 2010];Annual data report 2009: Volume 2. Chapter 8: Pediatric ESRD. http://wwwusrdsorg/2009/pdf/V2_08_09PDF.
- 2.Muneeruddin S, Chandar J, Abitbol CA, et al. Two decades of pediatric kidney transplantation in a multi-ethnic cohort. Pediatr Transplantation. 2010 doi: 10.1111/j.1399-3046.2010.01323.x. (in press) [DOI] [PubMed] [Google Scholar]
- 3.Shroff R, Ledermann S. Long-term outcome of chronic dialysis in children. Pediatr Nephrol. 2009;24:463–474. doi: 10.1007/s00467-007-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) [Access 10 June 2010];Annual report 2008. https://webemmescom/study/ped/annlrept/Annual%20Report%20-2008pdf.
- 5.Shapiro R, Sarwal MM. Pediatric kidney transplantation. Pediatr Clin North Am. 2010;57:393–400. doi: 10.1016/j.pcl.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Ambuhl PM, Meier D, Wolf B, Dydak U, Boesiger P, Binswanger U. Metabolic aspects of phosphate replacement therapy for hypophosphatemia after renal transplantation: impact on muscular phosphate content, mineral metabolism, and acid/base homeostasis. Am J Kidney Dis. 1999;34:875–883. doi: 10.1016/S0272-6386(99)70045-4. [DOI] [PubMed] [Google Scholar]
- 7.Bhan I, Shah A, Holmes J, et al. Post-transplant hypophosphatemia: Tertiary 'Hyper-Phosphatoninism'? Kidney Int. 2006;70:1486–1494. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 8.Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y. Tertiary 'hyperphosphatoninism' accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant. 2007;7:1193–1200. doi: 10.1111/j.1600-6143.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanjad SA, Ibrahim A, Al Shorafa S, et al. Renal tubular dysfunction following kidney transplantation: a prospective study in 31 children. Transplant Proc. 2001;33:2830–2831. doi: 10.1016/s0041-1345(01)02208-4. [DOI] [PubMed] [Google Scholar]
- 10.Evenepoel P, Meijers BK, De Jonge H, et al. Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol. 2008;3:1829–1836. doi: 10.2215/CJN.01310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Economidou D, Dovas S, Papagianni A, Pateinakis P, Memmos D. FGF-23 Levels before and after Renal Transplantation. J Transplant. 2009;2009:379–382. doi: 10.1155/2009/379082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins RM, Richardson AJ, Endre ZH, Frostick SP, Morris PJ. Hypophosphataemia after renal transplantation: relationship to immunosuppressive drug therapy and effects on muscle detected by 31P nuclear magnetic resonance spectroscopy. Nephrol Dial Transplant. 1990;5:62–68. doi: 10.1093/ndt/5.1.62. [DOI] [PubMed] [Google Scholar]
- 13.Julian BA, Quarles LD, Niemann KM. Musculoskeletal complications after renal transplantation: pathogenesis and treatment. Am J Kidney Dis. 1992;19:99–120. doi: 10.1016/s0272-6386(12)70118-x. [DOI] [PubMed] [Google Scholar]
- 14.Cruz EA, Lugon JR, Jorgetti V, Draibe SA, Carvalho AB. Histologic evolution of bone disease 6 months after successful kidney transplantation. Am J Kidney Dis. 2004;44:747–756. [PubMed] [Google Scholar]
- 15.Tyden G, Fehrman I, Siden A, Persson A. Hypophosphataemia and reversible neurological dysfunction in a patient subjected to combined renal and pancreatic transplantation. Nephrol Dial Transplant. 1988;3:823–825. [PubMed] [Google Scholar]
- 16.Levi M. Post-transplant hypophosphatemia. Kidney Int. 2001;59:2377–2387. doi: 10.1046/j.1523-1755.2001.00755.x. [DOI] [PubMed] [Google Scholar]
- 17.Green J, Debby H, Lederer E, Levi M, Zajicek HK, Bick T. Evidence for a PTH-independent humoral mechanism in post-transplant hypophosphatemia and phosphaturia. Kidney Int. 2001;60:1182–1196. doi: 10.1046/j.1523-1755.2001.0600031182.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum RW, Hruska KA, Korkor A, Anderson C, Slatopolsky E. Decreased phosphate reabsorption after renal transplantation: Evidence for a mechanism independent of calcium and parathyroid hormone. Kidney Int. 1981;19:568–578. doi: 10.1038/ki.1981.54. [DOI] [PubMed] [Google Scholar]
- 19.Graf H, Kovarik J, Stummvoll HK, Wolf A, Pinggera WF. Handling of phosphate by the transplanted kidney. Proc Eur Dial Transplant Assoc. 1979;16:624–629. [PubMed] [Google Scholar]
- 20.Parfitt AM, Kleerekoper M, Cruz C. Reduced phosphate reabsorption unrelated to parathyroid hormone after renal transplantation: implications for the pathogenesis of hyperparathyroidism in chronic renal failure. Miner Electrolyte Metab. 1986;12:356–362. [PubMed] [Google Scholar]
- 21.Steiner RW, Ziegler M, Halasz NA, Catherwood BD, Manolagas S, Deftos LJ. Effect of daily oral vitamin D and calcium therapy, hypophosphatemia, and endogenous 1–25 dihydroxycholecalciferol on parathyroid hormone and phosphate wasting in renal transplant recipients. Transplantation. 1993;56:843–846. doi: 10.1097/00007890-199310000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Loffing J, Lotscher M, Kaissling B, et al. Renal Na/H exchanger NHE-3 and Na-PO4 cotransporter NaPi-2 protein expression in glucocorticoid excess and deficient states. J Am Soc Nephrol. 1998;9:1560–1567. doi: 10.1681/asn.v991560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falkiewicz K, Nahaczewska W, Boratynska M, et al. Tacrolimus decreases tubular phosphate wasting in renal allograft recipients. Transplant Proc. 2003;35:2213–2215. doi: 10.1016/s0041-1345(03)00765-6. [DOI] [PubMed] [Google Scholar]
- 24.Shane E, Rivas M, Staron RB, et al. Fracture after cardiac transplantation: a prospective longitudinal study. J Clin Endocrinol Metab. 1996;81:1740–1746. doi: 10.1210/jcem.81.5.8626827. [DOI] [PubMed] [Google Scholar]
- 25.Ninkovic M, Skingle SJ, Bearcroft PW, Bishop N, Alexander GJ, Compston JE. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2000;12:931–935. doi: 10.1097/00042737-200012080-00013. [DOI] [PubMed] [Google Scholar]
- 26.Kempe DS, Dermaku-Sopjani M, Frohlich H. Rapamycin-induced phosphaturia. Nephrol Dial Transplant. 2010 Apr 5; doi: 10.1093/ndt/gfq172. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Rowe PS. Molecular biology of hypophosphataemic rickets and oncogenic osteomalacia. Hum Genet. 1994;94:457–467. doi: 10.1007/BF00211008. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 29.Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 30.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki Y, Okazaki R, Shibata M, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab. 2007;293:E1636–E1644. doi: 10.1152/ajpendo.00396.2007. [DOI] [PubMed] [Google Scholar]
- 34.Riminucci M, Collins MT, Fedarko NS, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 36.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 38.Saito H, Kusano K, Kinosaki M, et al. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- 39.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 40.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito H, Maeda A, Ohtomo S, et al. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 43.Ito N, Fukumoto S, Takeuchi Y, et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25:419–422. doi: 10.1007/s00774-007-0779-3. [DOI] [PubMed] [Google Scholar]
- 44.Nishida Y, Taketani Y, Yamanaka-Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 45.Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant. 2010;25:993–997. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 47.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 48.Moe SM, Chen NX, Seifert MF, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75:176–184. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010 doi: 10.1038/ki.2010.313. In press. [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki Y, Tamada T, Kasai N, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 51.Aono Y, Yamazaki Y, Yasutake J, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009;24:1879–1888. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 52.Shimada T, Urakawa I, Isakova T, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578–585. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nogueira PC, Rey N, Said MH, Cochat P. Evolution of hyperparathyroidism after renal transplantation in children--effect of pre-emptive transplantation and duration of dialysis. Nephrol Dial Transplant. 1997;12:984–987. doi: 10.1093/ndt/12.5.984. [DOI] [PubMed] [Google Scholar]
- 54.Bacchetta J, Dubourg L, Harambat J, et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741–1748. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 55.Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44:250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 56.Van Husen M, Fischer AK, Lehnhardt A, et al. Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int. 2010 Apr 21; doi: 10.1038/ki.2010.107. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Sanchez CP, Salusky IB, Kuizon BD, et al. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int. 1998;53:1358–1364. doi: 10.1046/j.1523-1755.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- 58.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 59.Ball AM, Gillen DL, Sherrard D, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288:3014–3018. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 60.Helenius I, Remes V, Salminen S, et al. Incidence and predictors of fractures in children after solid organ transplantation: a 5-year prospective, population-based study. J Bone Miner Res. 2006;21:380–387. doi: 10.1359/JBMR.051107. [DOI] [PubMed] [Google Scholar]
- 61.Wesseling-Perry K, Pereira RC, Wang H, et al. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94:511–517. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 63.Wolf M. Forging Forward with 10 Burning Questions on FGF23 in Kidney Disease. J Am Soc Nephrol. 2010 May 27; doi: 10.1681/ASN.2009121293. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barsotti G, Cupisti A, Moriconi L, et al. Effects of reduced protein intake in rats with congenital polycystic kidney without renal failure. Contrib Nephrol. 1995;115:134–136. doi: 10.1159/000424410. [DOI] [PubMed] [Google Scholar]
- 66.Schwarz A, Mengel M, Gwinner W, et al. Risk factors for chronic allograft nephropathy after renal transplantation: a protocol biopsy study. Kidney Int. 2005;67:341–348. doi: 10.1111/j.1523-1755.2005.00087.x. [DOI] [PubMed] [Google Scholar]
- 67.Pinheiro HS, Camara NO, Osaki KS, De Moura LA, Pacheco-Silva A. Early presence of calcium oxalate deposition in kidney graft biopsies is associated with poor long-term graft survival. Am J Transplant. 2005;5:323–329. doi: 10.1111/j.1600-6143.2004.00684.x. [DOI] [PubMed] [Google Scholar]
- 68.Habbig S, Beck BB, Feldkotter M, et al. Renal allograft calcification -- prevalence and etiology in pediatric patients. Am J Nephrol. 2009;30:194–200. doi: 10.1159/000217585. [DOI] [PubMed] [Google Scholar]
- 69.Evenepoel P, Lerut E, Naesens M, et al. Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am J Transplant. 2009;9:2470–2478. doi: 10.1111/j.1600-6143.2009.02792.x. [DOI] [PubMed] [Google Scholar]
- 70.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 71.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 72.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 74.Adorini L, Penna G, Giarratana N, et al. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004;89–90:437–441. doi: 10.1016/j.jsbmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 76.Ahmadpoor P, Ilkhanizadeh B, Ghasemmahdi L, Makhdoomi K, Ghafari A. Effect of active vitamin D on expression of co-stimulatory molecules and HLA-DR in renal transplant recipients. Exp Clin Transplant. 2009;7:99–103. [PubMed] [Google Scholar]
- 77.Tanaci N, Karakose H, Guvener N, Tutuncu NB, Colak T, Haberal M. Influence of 1,25-dihydroxyvitamin D3 as an immunomodulator in renal transplant recipients: a retrospective cohort study. Transplant Proc. 2003;35:2885–2887. doi: 10.1016/j.transproceed.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 78.Sezer S, Uyar M, Arat Z, Ozdemir FN, Haberal M. Potential effects of 1,25-dihydroxyvitamin D3 in renal transplant recipients. Transplant Proc. 2005;37:3109–3111. doi: 10.1016/j.transproceed.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 79.Mathieu C, Jafari M. Immunomodulation by 1,25-dihydroxyvitamin D3: therapeutic implications in hemodialysis and renal transplantation. Clin Nephrol. 2006;66:275–283. doi: 10.5414/cnp66275. [DOI] [PubMed] [Google Scholar]
- 80.Amuchastegui S, Daniel KC, Adorini L. Inhibition of acute and chronic allograft rejection in mouse models by BXL-628, a nonhypercalcemic vitamin D receptor agonist. Transplantation. 2005;80:81–87. doi: 10.1097/01.tp.0000164619.49828.7a. [DOI] [PubMed] [Google Scholar]
- 81.Marcen R, Ponte B, Rodriguez-Mendiola N, et al. Vitamin D deficiency in kidney transplant recipients: risk factors and effects of vitamin D3 supplements. Transplant Proc. 2009;41:2388–2390. doi: 10.1016/j.transproceed.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 82.Tripathi SS, Gibney EM, Gehr TW, King AL, Beckman MJ. High prevalence of vitamin D deficiency in African American kidney transplant recipients. Transplantation. 2008;85:767–770. doi: 10.1097/TP.0b013e3181613fb5. [DOI] [PubMed] [Google Scholar]
- 83.Ewers B, Gasbjerg A, Moelgaard C, Frederiksen AM, Marckmann P. Vitamin D status in kidney transplant patients: need for intensified routine supplementation. Am J Clin Nutr. 2008;87:431–437. doi: 10.1093/ajcn/87.2.431. [DOI] [PubMed] [Google Scholar]
- 84.United States Renal Data System. [Access 1 June 2010];Annual data report. 2009. Volume 2. Chapter 7: Transplantation. http://wwwusrdsorg/2009/pdf/V2_07_09PDF.
- 85.Basiratnia M, Fazel M, Lotfi M, et al. Subclinical atherosclerosis and related risk factors in renal transplant recipients. Pediatr Nephrol. 2010;25:343–348. doi: 10.1007/s00467-009-1345-0. [DOI] [PubMed] [Google Scholar]
- 86.Wilson AC, Greenbaum LA, Barletta GM, et al. High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatr Transplant. 2010;14:52–60. doi: 10.1111/j.1399-3046.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 87.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mirza MA, Hansen T, Johansson L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24:3125–3131. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 89.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 90.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci. 2009;337:116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- 91.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seeherunvong W, Abitbol C, Chandar J, Rusconi P, Zilleruelo G, Freundlich M. Fibroblast Growth Factor 23 (FGF23) and Left Ventricular Hypertrophy (LVH) in Children on Dialysis. (Abstract accepted to presented at The Annual Meeting of American Society of Nephrology 2010) [Google Scholar]
- 93.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 94.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 95.Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 96.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 97.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168:397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 98.Zeidan B, Shabtai M, Waltzer WC, Wadhwa NK, Siddarth P, Rapaport FT. Improvement in renal transplant function after subtotal parathyroidectomy in a hypercalcemic kidney allograft recipient. Transplant Proc. 1991;23:2285–2286. [PubMed] [Google Scholar]
- 99.Wheatley TJ, Doughman T, Veitch PS, Nicholson ML. Clinical and physiological responses to total parathyroidectomy after renal transplantation. Transplant Proc. 1997;29:3054–3055. doi: 10.1016/s0041-1345(97)00780-x. [DOI] [PubMed] [Google Scholar]
- 100.Finch JL, Tokumoto M, Nakamura H, et al. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1315–F1322. doi: 10.1152/ajprenal.00552.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3:658–664. doi: 10.2215/CJN.04981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serra AL, Wuhrmann C, Wuthrich RP. Phosphatemic effect of cinacalcet in kidney transplant recipients with persistent hyperparathyroidism. Am J Kidney Dis. 2008;52:1151–1157. doi: 10.1053/j.ajkd.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 103.Lopez V, Toledo R, Sola E, et al. Treatment with cinacalcet in 29 kidney transplant patients with persistent hyperparathyroidism. Transplant Proc. 2009;41:2394–2395. doi: 10.1016/j.transproceed.2009.06.055. [DOI] [PubMed] [Google Scholar]
- 104.Guerra R, Auyanet I, Fernandez EJ, et al. Hypercalcemia secondary to persistent hyperparathyroidism in kidney transplant patients: analysis after a year with cinacalcet. J Nephrol. 2010 May 2; doi: 10.5301/jn.2010.293. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 105.Borchhardt KA, Diarra D, Sulzbacher I, Benesch T, Haas M, Sunder-Plassmann G. Cinacalcet decreases bone formation rate in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Nephrol. 2010;31:482–489. doi: 10.1159/000304180. [DOI] [PubMed] [Google Scholar]
- 106.Borchhardt K, Sulzbacher I, Benesch T, Fodinger M, Sunder-Plassmann G, Haas M. Low-turnover bone disease in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Transplant. 2007;7:2515–2521. doi: 10.1111/j.1600-6143.2007.01950.x. [DOI] [PubMed] [Google Scholar]
- 107.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]