Abstract
Various formulations of selenium and vitamin E, both essential human dietary components, have been shown to possess a therapeutic and preventive effect against prostate cancer. Fortuitous results of clinical trials also implied a risk-reduction effect of selenium and vitamin E supplements. The Selenium and Vitamin E Cancer Prevention Trial (SELECT), using oral selenium and vitamin E supplementation in disease-free volunteers, was designed to test a prostate cancer chemoprevention hypothesis. SELECT was terminated early because of both safety concerns and negative data for the formulations and doses given. Here, we review and discuss the studies done before and since the inception of SELECT, as well as the parameters of the trial itself. We believe that there is a lack of appropriate in vivo preclinical studies on selenium and vitamin E despite many promising in vitro studies on these agents. It seems that the most effective doses and formulations of these agents for prostate cancer chemoprevention have yet to be tested. Also, improved understanding of selenium and vitamin E biology may facilitate the discovery of these doses and formulations.
INTRODUCTION
Selenium and vitamin E are essential components of the human diet and have been studied as antioxidants and/or potential agents for a variety of human diseases. Initiated in 2001, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) was a phase III, randomized, placebo-controlled human trial to investigate the prostate cancer (PCa) chemopreventive effects of selenium, vitamin E or their combination (1). SELECT was among the largest clinical chemoprevention trials ever, with an enrollment of 35,533 men and an intended follow-up of up to 12 years (1). SELECT was predicated on basic and clinical research including secondary endpoint data from cancer prevention studies that implied selenium and vitamin E supplements could be useful in reducing PCa risk. The trial was prematurely terminated in 2008, 18 months before its intended minimum follow-up length (1). The selenium and vitamin E doses and formulations used in SELECT were found to be ineffective, and concern was raised about a possible trend in developing type 2 diabetes mellitus among the study participants taking selenium (1). Further, a statistically nonsignificant increased risk of PCa was also seen in the vitamin E group participants (1). Unfortunately, despite the perceived suitability of PCa (mainly because of its long latency period) for chemoprevention and the considerable evidence suggesting the usefulness of selenium and vitamin E for PCa prevention, SELECT failed to show a positive outcome. This raises several questions: Why was SELECT unsuccessful? What went wrong and what should we learn from it? Can we do something differently in the future, or should vitamin E and/or selenium be abandoned? In this review, we discuss (a) SELECT, (b) the evidence supporting PCa chemoprevention with selenium and/or vitamin E, (c) the possible reasons why SELECT was not successful, and (d) some suggestions that may be useful for the future.
PROSTATE CANCER: A CANDIDATE DISEASE FOR CHEMOPREVENTION
Prostate cancer, next only to lung cancer, is the second leading cause of cancer-related deaths in American males, with similar global trends. In 2010, approximately 217,730 men are expected to be diagnosed with PCa in the United States, and approximately 32,050 PCa-related deaths are predicted (2). The CDC (Centers for Disease Control and Prevention) has estimated that a man’s chance of developing PCa by a certain age are as follows: 1 in 2,500 by age 45; 1 in 476 by age 50; 1 in 120 by age 55; 1 in 43 by age 60; 1 in 21 by age 65; 1 in 13 by age 70; and 1 in 9 by age 75. Further, PCa is a major public health concern in the developed world and was reported to be responsible for approximately 221,000 deaths worldwide in 2002 (3). Although increases in screening efficiency may be finding more men who would have died with latent PCa, PCa remains poised to become an ever-greater relative cause of morbidity and mortality for men in the U.S. for decades to come (4,5). Unfortunately, the existing treatments as well as surgical approaches have not been fully effective either for prevention or for treatment of PCa. Thus, there is a need to develop mechanism-based approaches for the prevention/therapy of PCa.
Chemoprevention is being increasingly appreciated as one such approach. Chemoprevention, by definition, is a means of cancer control where the occurrence of the disease can be entirely prevented, slowed or reversed by the administration of one or more naturally occurring and/or synthetic compounds. The expanded definition of cancer chemoprevention also includes the chemotherapy of precancerous lesions. In the recent past, chemoprevention has gained considerable attention as a potential means of cancer management (6–19).
Mutagenic and epigenetic factors both in the transformation of precancerous cells and in the progression of cancerous lesions could be targeted with chemoprevention agents (20). Chemopreventive approaches may also be used for secondary prevention of new lesions or recurrence of disease in individuals with prior diagnosis and history of cancer. Prevalence of PCa increases with age, and the progression of latent lesions to clinically detectable lesions makes PCa an ideal candidate disease for chemoprevention. In recent years, chemoprevention of PCa has gained considerable attention, and a wide range of chemopreventive agents are being explored for the management of PCa, both in prevention as well as in therapeutic settings (7,11,13,14,18).
Despite similar incidence of small latent carcinomas worldwide, the prevalence of advanced disease and PCa-related mortality is greater in developed nations (21). Ethnic origin and family history have been established as risk factors for the development of PCa, with a clear increased risk among African-American men. Although environmental factors such as high-fat diet may contribute to PCa, research continues to isolate the exact nature of these risks (22,23). Strategies need to be developed that take these factors into account. Chemoprevention is a reasonable strategy against prevalent diseases with long latent periods and a lack of modifiable risk factors, such as PCa.
Finally, because of promising preclinical outcomes, there is willingness on the part of men at risk to try chemoprevention with nontoxic agents. Nam et al. showed between 26% and 80% of 155 Canadian urologic clinic patients and 113 support group patients diagnosed with PCa, or at increased risk for it, endorsed the use of preventive approaches and complementary therapies including low-fat diet, vitamin E or garlic, with the belief that these efforts might slow or prevent disease progression (24).
VITAMIN E: THE DISCOVERY AND THE CHEMISTRY
Evans and Bishop, in 1922, discovered that dietary supplements with alfalfa leaves (rich in vitamin E) prevent placental hemorrhage and reverse dietary sterility in rats (25). Vitamin E is not a single agent but is at least eight “vitamers,” named tocochromanols. The tocochromanols are either tocopherols or tocotrienols (named by number of chiral centers in the molecule) and are further assigned Greek letter prefixes by degree of methyl substitution, that is, α-tocopherol (with three chiral centers) exists naturally as the (2R,4′R,8′R) stereoisomer and is synthesized as all-racemic or (2RS,4′RS,8′RS) product (26). In 1943, Joffe and Harris demonstrated varying biopotencies of the eight forms of vitamin; however, the precise reason for differences remains unclear, and many basic questions have yet to be properly formulated (27). It is known that of the tocopherols, α-tocopherol is taken up in the human liver by hepatic transfer proteins and that some tocopherol stereoisomers penetrate cell membranes more easily (27,28). The 2R isomers of α-tocopherol are putatively used to establish the vitamin E requirement (29). As commercial dietary supplements, both “natural” (RRR) and synthetic (all-rac) α-tocopheryl esters (acetate or succinate) are available and extensively used. Although there is no clear distinction of which of these forms may be more effective, some studies have reported better effectiveness of one particular form over the others. For example, Lee et al. (30) showed that vitamin E succinate has a distinct antiprostaglandin effect in human lung epithelial cells. Studies have suggested that vitamin E is relatively safe for consumption even at high dosages, since a 3,200-IU dose was well tolerated by adults in short-term studies (31).
In a wide range of studies, α-tocopherol, the most well-studied isomer of vitamin E, has been shown to possess an anti-cancer effect. This area has been a topic of intense research for many years (32–34). However, in the recent past, the researchers have also started evaluating γ-tocopherol for its chemopreventive efficacy (32–38). Interestingly, on the basis of recent studies, γ-tocopherol is being increasingly appreciated to have a superior response than α-tocopherol (39). Studies have shown that the in vitro products of γ-tocopherol antioxidant reactions differ from those of α-tocopherol, with the latter alone forming nitrosating agents when exposed to NO2 (39). Interestingly, Christen et al. (40) showed that γ-tocopherol may be required to complement the activity of α-tocopherol, suggesting that supplementation of α-tocopherol alone may suppress γ-tocopherol levels. As of 2010, the majority of available experimental data on vitamin E for suppressing PCa has been obtained with α-tocopherol, leaving the possibility that other tocopherols may possess better chemopreventive and/or anticancer potential against PCa. This potential needs to be carefully explored.
VITAMIN E AND PCA: IN VITRO PRECLINICAL STUDIES
Several in vitro studies have evaluated the antiproliferative effects of vitamin E in human PCa cells. The addition of all-rac α-tocopherol was found to cause modest inhibition of growth in androgen-responsive human PCa cell lines at a dose <10 μmol/L (41). Ni et al. (42) found a cell G1 phase cell cycle arrest in LNCaP cells at 20 μmol/L (RRR) α-tocopheryl succinate (vitamin E succinate [VES]). Chang et al. (43) found cell growth inhibition by VES and its analog (RRR) α-tocopheryloxybutyric acid (TOB) in human PCa cells. Zhang et al. (44) demonstrated that VES effectively suppresses dihydrotestosterone-mediated growth of LNCaP cells at a 10μmol/L concentration. Israel et al. (45) found that (a) VES triggers apoptosis in human PCa cells but not normal prostate cells in vitro, and (b) VES modulates Fas signaling in PCa cells. Crispen et al. (46) found that VES inhibits nuclear factor (NF)-κB and prevents the development of a metastatic phenotype in PCa cells and suggested the role of vitamin E analogs as potential chemopreventative agents against PCa. A study by Jia et al. (47) suggested an involvement of JNK, c-Jun, and Fas/FasL signaling in vitamin E analog-induced apoptosis in human PCa cells. Further, VES was shown to increase transcription of IGFBP-3 mRNA in LNCaP and PC3 cells, whereas α-tocopheryl succinate was found to induce Bcl2 pathway–mediated apoptosis in LNCaP cells (48,49). Zu and Ip demonstrated that α-tocopheryl succinate modulated the MAPK pathway and caspases in PC3 cells (50), whereas Ni et al. (51) found that RRR–α-tocopheroxybutyl sulfonic acid induced apoptosis in LNCaP and PC3 cells. γ-Tocopherol also had an anti-PCa effect and was shown to be more effective than α-tocopherol in its antiproliferative response (52).
VITAMIN E AND PCA: IN VIVO PRECLINICAL STUDIES
Studies have evaluated the antiproliferative effects of vitamin E formulations in several experimental settings. For example, Yin et al. (48) demonstrated that VES imparts therapeutic and preventive effects against PCa, at least partially through upregulating IGFBP-3, which inhibits cell proliferation and promotes cell apoptosis. In this study, VES (100 mg/kg i.p.) was found to have a significant antiproliferative response in LNCaP and PC3 xenografts (48). Further, VES administration was also shown to impart chemopreventive response in TRAMP mice, decreasing progression of prostatic intraepithelial neoplasia (PIN) to invasive carcinoma (48). However, the use of VES in clinical settings is not ideal because it is complicated by in vivo hydrolysis after oral administration. Further, succinate esters are poorly absorbed, resulting in lower tissue concentrations, as shown by Jensen et al. (53), who demonstrated that α-tocopherol acetate was a better vitamin E source than α-tocopherol succinate for broilers when given in their feed. These challenges have led to the development of more stable formulations such as VEBSA (RRR-α-tocopheryloxybutyl sulfonic acid), which has been reported in TRAMP mice to achieve a 7 × higher concentration (compared with VES) after 1 month of administration (51). Further, oral intake of VEBSA for 20 weeks was also found to inhibit prostate tumor growth and progression more efficiently than VES in the TRAMP mice (51).
Given orally, 11.4 mg/kg/d all-rac α-tocopheryl acetate was reported to attenuate dietary fat–exacerbated LNCaP tumor growth in nu/nu mice, but did not have an effect on tumor growth in animals fed a low-fat diet (54). However, when intraperitoneally administered at a high dose (100 mg/kg), VES was found to retard growth of xenografted LNCaP tumors in BALB/c mice fed both high- and low-fat diets (55). A γ-tocopherol–enriched diet fed to TRAMP mice for 24 weeks was reported to cause a significant decrease (60% versus 30%) in high-grade PIN lesions (35). Also, TRAP rats given γ-tocopherol (100 mg/kg) in diet were reported to exhibit decreased cancer progression along with increased activation of caspases 3 and 7 (56).
VITAMIN E AND PCA: CLINICAL STUDIES
On the basis of some clinical human trials, the use of vitamin E for PCa management was initially thought to be justified. However, it is now being increasingly appreciated that more in vivo clinical studies are needed in this direction, to accept or reject the need of further clinical studies.
The α Tocopherol β Carotene (ATBC) study, conducted by the U.S. National Cancer Institute and the National Institute for Health and Welfare of Finland from 1985 to 1993, determined whether certain vitamin supplements would prevent lung cancer and other cancers in a group of 29,133 male smokers in Finland (57). The 50- to 69-year-old participants took α-tocopherol (50 mg) or β-carotene (a precursor of vitamin A), or their combination, or a placebo pill. In this study, α-tocopherol was found to reduce PCa incidence by 32% and mortality by 41% during an 8-year study period (57). Further analysis showed an inverse relationship between serum α-tocopherol concentration and incidence of PCa, with a stronger effect on advanced disease (58). Subsequent studies have not been as clear but have provided data to support a vitamin E chemoprevention hypothesis. The Vitamins and Lifestyle (VITAL) prospective study of 35,342 men in western Washington State showed a trend toward statistical significance of vitamin E supplementation of ≥400 IU/d in reducing risk of advanced PCa, independent of smoking status (59). On the basis of these data, it was thought that it is possible that vitamin E may be effective against advanced, but not latent or early, cases of PCa. In another trial, vitamin E was found to have no PCa–protective effect in a group of 47,780 U.S. men but suggested an inverse association of vitamin E and risk of metastatic disease (60). Analysis of 74,702 U.S. men in the Cancer Prevention Study II Nutrition Cohort found no association between use of vitamin E supplement and reduced risk of PCa (61). The ATBC study and the other available data have led some researchers to question whether vitamin E might have a PCa–protective effect for smokers only (62). It is therefore unclear whether vitamin E has a preventive effect in humans. Without enough preclinical evidence, it is difficult to suggest whether vitamin E could be beneficial for PCa.
SELENIUM: THE DISCOVERY AND THE CHEMISTRY
Selenium is a nonmetallic element and was appreciated to be an essential dietary nutrient by Schwarz in 1957, when brewer’s yeast was found to prevent dietary necrotic degeneration of the liver in rats (63). Selenium is incorporated into at least 25 distinct selenoproteins, including the antioxidant enzyme glutathione per-oxidase (GPX), which acts to prevent lipid peroxidation of membranes and scavenges free radicals (64). While it is an essential nutrient at appropriate levels in the diet, selenium is toxic at higher doses. The safe upper limit of intake and recommended daily allowance for adults are 400 μg and 55 μg/d, respectively. According to the INTERnational collaborative study of MAcronutrients, micronutrients and blood Pressure (INTERMAP) of nutrient intakes, the mean daily dietary intake of selenium of U.S. participants was 153 and 109 μg for men and women, respectively (65). The possible use of selenium for cancer management began to be studied when it was shown to possess an inverse relationship between selenium forage crop content and cancer mortality (66,67). Selenium formulations that have been studied for their anticancer efficacy include inorganic formulations such as sodium selenite as well as organic preparations such as methylselenic acid and selenomethionine.
SELENIUM AND PCA: IN VITRO PRECLINICAL STUDIES
Several studies have evaluated the antiproliferative effects of different formulations of selenium. Webber et al. (68) showed that inorganic selenium (sodium selenite) inhibits the growth of DU145 (androgen-unresponsive) PCa cells at a concentration of 1 μmol/L. Zhong and Oberley (69) found that sodium selenite (2.5 μmol/L) has different effects on LNCaP cells under acute exposure versus chronic exposure conditions. In this study, the authors demonstrated an induction of apoptosis during acute exposure and an intracellular alteration of redox state accompanied with cell cycle blockade on chronic exposure (69). Thompson et al. (70) reported that when organic selenium [1,4-phenylene-bis(methylene)selenocyante (p-XSC)] was added to mouse mammary carcinoma cells in culture, a 3–6× greater intracellular selenium concentration was achieved when compared with sodium selenate. DL-Selenomethionine was reported by Redman et al. (71) to inhibit growth of the DU145 cell line at only 5% of the inhibitory concentration required for normal diploid fibroblasts. Mouse mammary tumor cells exposed to methylselenocysteine (50 μmol/L) showed a cell cycle arrest in the S phase with subsequent apoptosis (72). Exposure to both inorganic (selenite) and organic (L-selenomethionine) selenium led to dose-dependent induction of apoptosis and G2-M cell cycle arrest in multiple PCa cell lines, with an increased effect on androgen-sensitive cells, at doses that did not affect normal human prostate epithelial cells (PrEC) (73).
Generation of selenium metabolites has been suggested to be an important contributor in the mechanism of seleium’s action. Spallholz et al. (74) showed that selenomethionine in the presence of methioninase generated methylselenol in vitro, whereas selenium-methylselenocysteine and methioninase failed to do so. The monomethylated metabolites produced by the selenomethionine-methioninase reaction were reported by Zhao et al. (75) to induce apoptosis in PCa cells specifically via the mitochondrial (intrinsic, p53-dependent) pathway. Methylselenic acid, a preparation for delivery of monomethylated selenium, was reported to induce apoptosis in both LNCaP and DU145 cell lines and to sensitize PCa cells to tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) (76). At concentrations <10 μmol/L, methylselenic acid exposure was found to result in a significant reduced binding of NF-κB to DNA response element in LNCaP cells, suggesting a possible proapoptotic mechanism (77). A study by Huang et al. (78) suggested that blockade of NF-κB activity in human PCa cells is associated with suppression of angiogenesis, invasion and metastasis.
Thus, as outlined above, in vitro studies have suggested that the potential mechanisms of the antiproliferative response of different selenium formulations in PCa cells are induction of apoptosis, alteration of intracellular redox state, cell cycle arrest and blockade of NF-κB signaling pathways.
SELENIUM AND PCA: IN VIVO PRECLINICAL STUDIES
Although not extensively studied, a few studies have assessed the usefulness of selenium against cancer. Ip and Lisk (79) found that a selenium-enriched formulation of garlic inhibited initiation of mammary epithelial cell carcinogenesis in rats. Corcoran et al. (80) evaluated the effectiveness of a different selenium formulation on established orthotopic PC3 tumors in nude mice and found that inorganic selenium (sodium selenate) significantly retarded the growth of primary prostatic tumors and the development of retroperitoneal lymph node metastases, which was associated with a decrease in angiogenesis. Lee et al. (81) found that monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft and decreases the expression of androgen receptor and prostate-specific antigen. Li et al. (82) demonstrated a superior in vivo growth inhibitory efficacy of methylselenic acid over selenomethionine and selenite, against two human PCa xenograft models without the genotoxic property of selenite. In a recent study, Wang et al. (83) showed that methyl-selenium compounds inhibit prostate carcinogenesis in TRAMP mice with survival benefits.
Clearly, several studies support the usefulness of selenium against PCa. However, it appears that more studies are needed to determine the effective in vivo dose and formulation of selenium that produce optimal response in appropriate models.
SELENIUM AND PCA: CLINICAL STUDIES
There are a few, mostly indirect, studies suggesting the usefulness of selenium against PCa in the human population. Sabichi et al. (84) found that selenium taken as oral supplementation accumulates preferentially in the human prostate gland, supporting the hypothesis that selenium supplementation could be useful in chemoprevention of PCa. Clinical studies with selenium supplementation have shown mixed results with respect to overall cancer incidence and mortality. The Linxian Province (China) nutritional studies of 1993 found significantly reduced overall mortality among individuals taking β carotene, inorganic selenium, and vitamin E (85). However, as part of the same group of studies, a population with precancerous lesions (esophageal dysplasia) was not found to benefit from multiple vitamin supplementations (including selenium from yeast source) with respect to overall mortality or incidence of esophageal cancer (86).
One of the most important clinical trials supporting the positive efficacy of selenium against PCa is the Nutritional Prevention of Cancer (NPC) study. This trial used a brewer’s yeast intervention in a group of 1,312 participants (74% male) with a history of non-melanoma skin cancer. Doses of 0.5 g high-selenium brewer’s yeast were taken daily for 4.2 ± 2.8 years with a follow-up maintained for 6.8 ± 2.0 years (87). Although the primary endpoint in this study was non-melanoma skin cancer, on which the brewer’s yeast was found to have no effect, a relative risk of 0.37 for the development of PCa was found among the selenium group as a secondary endpoint. Additional analysis found an inverse relationship between baseline selenium level and magnitude of treatment effect. Although slightly attenuated, the treatment effect was maintained over 7.5 years of follow-up (88,89). The authors postulated that a difference in p53 mutations might explain the apparently protective effect of selenium on incidence of PCa but not on non-melanoma skin cancer, since 69% of actinic keratoses (premalignant lesions with a potential to become squamous cell carcinoma) and 90% of squamous cell carcinoma lesions demonstrated ultraviolet-induced p53 mutations. This was relevant, since a loss of p53 was seen as a late event in most other carcinomas, including PCa. By this rationale, early PCa lesions with intact p53 signaling might be more susceptible to an anticarcinogenic pathway promoted by selenium. However, data obtained since the NPC study indicate that up to one-third of early PCa lesions may have p53 mutations, making this explanation less likely (90). Furthermore, whether an intact p53 system is necessary for any anticarcinogenic effects of selenium or vitamin E remains a contentious issue. While Lanfear et al. (91) reported that selenium metabolite selenodiglutathione caused apoptosis in cells with wild-type and mutated p53 status (murine erythroleukemia and human ovarian carcinoma), Jiang et al. (92) reported that selenite-induced apoptosis of LNCaP cells depends on intact p53 activity. The specific role of p53 in selenium-induced PCa cell apoptosis, and perhaps in turn whether selenium acts preferentially via the intrinsic or extrinsic pathways, remains an active area of research. In addition to p53, the specific effects on the NF-κB transcription factor and Bcl-2 family members in PCa cells exposed to selenium, vitamin E and the combination of agents remains to be elucidated. A recent study demonstrated differential rates of excretion in human subjects ingesting DL-selenomethionine, L-selenomethionine, or selenite, suggesting that selenium excretion may depend on its specific formulation (93). Because many factors may confound excretion rates, these results do not support use of one selenium supplement over another, but they suggest potential differences in selenium metabolite plasma concentrations that may be formulation dependent.
SELENIUM AND VITAMIN E COMBINATION: POTENTIAL SYNERGISTIC RESPONSES
While both vitamin E and selenium have been individually shown to have in vitro and in vivo efficacy against prostate and other cancers, some studies have also suggested a potential synergistic effect when the two agents were used in combination. The interrelation of selenium and vitamin E deficiency has been reported to manifest in several animal diseases (94). Combs and Scott (95) reported that lipid peroxidation in hepatic microsomes of vitamin E and selenium-deficient chicks is reversed only with repletion of both nutrients. It is possible that the antioxidant actions of both selenium-containing glutathione peroxidase and vitamin E synergize to retard formation of cytosolic lipid peroxides, as postulated by Diplock (96). Induction of PCa cell apoptosis by both agents could also be due to complementary activation of multiple caspases or modulation of the Bcl-2/Bax proteins. This result was supported by our studies as well as by other laboratories, where we found that VES and methylselenic acid have a synergistic proapoptotic response in PC3 cells, which was characterized by the modulation of initiator and executioner caspases involved in both the mitochondrial and cytokine-signaling pathways (50,97). Although limited by several factors including a small sample size and short duration of therapy, a recent study showed that the SELECT study agents, when given for 3–6 weeks before prostatectomy, created a greater overall difference in gene expression in tumor epithelial cells than administration of vitamin E or selenium alone, including specific increases in p53 gene transcription and translation (98). Using the male Lady version of the TRAMP mice, Venkateswaran et al. (99) showed that administration of antioxidants (vitamin E, selenium and lycopene) in the diet dramatically inhibits PCa development and increases the disease-free survival. A strong correlation between disease-free state and increased levels of the prognostic marker p27(Kip1) and a marked decrease in proliferating cell nuclear antigen expression was also seen by the authors (99). However, a subsequent study by the same group suggested that lycopene is an essential component of the combination (100). Further, a recent study suggested that combined micronutrients (vitamin E, selenium and lycopene) effectively promotes tumor dormancy in early PCa, after initiation mutations that may drive the angiogenesis-dependent response of the tumor, by inducing platelet factor-4 expression and concentrating it at the tumor endothelium through enhanced platelet binding (101). These results support the hypothesis that a combination of antioxidants including selenium and vitamin E may be useful in the management of PCa.
SELECT: THE DETAILS AND THE RISE AND FALL OF THE TRIAL
On the basis of the outcome of several epidemiological and laboratory studies, selenium and vitamin E supplements received attention from scientists and clinicians. This interest was reflected in the decision of the National Cancer Institute to sponsor SELECT, one of the largest human trials specifically aimed at PCa prevention in the human population. In this phase III trial, a total of 35,533 healthy volunteers from 427 participating sites were recruited. SELECT was a phase III randomized, placebo-controlled trial of selenium (200 μg/d from L-selenomethionine), vitamin E (400 IU/d of α-tocopheryl acetate) or both (planned follow-up of minimum of 7 years and maximum of 12 years) for PCa prevention (1). The major eligibility requirements of SELECT included an age of 50 years or older for African-American men and 55 years or older for all other men, no prior PCa diagnosis, ≤4 ng/mL of prostate-specific antigen in serum and a digital rectal examination not suspicious for cancer (1).
Recruitment took place at 428 clinical sites throughout all 50 United States, Puerto Rico, the District of Columbia, and Alberta, British Columbia, Nova Scotia, Ontario and Quebec in Canada. Accrual was brisk and, in 2004, enrollment was closed, 2 years ahead of schedule. This was attributed in part to intense media coverage and an existing infrastructure of recruitment sites from a previous large-scale trial. To prevent “drop-in” use of multivitamins that might confound the study results, bottles of multivitamins containing neither selenium nor vitamin E were given to all trial participants.
In the design of SELECT, selection of the agents was based on the recommendation by a panel of experts. For selenium formulation, inorganic selenium was deemed unacceptable for long-term use because of safety, and monomethylated selenium was rejected because of practical considerations including poor commercial availability. By a majority vote, the panel decided on L-selenomethionine, a major component of high-selenium yeast that showed efficacy in other trials. Though there were several other known selenium species present in yeast, these molecules were thought to be of lesser biologic importance. The use of high-selenium yeast itself was rejected for concerns about the consistency of the product obtained from the manufacturers (102). With respect to vitamin E, rather than using the 50-mg dose of α-tocopheryl acetate that was used in the ATBC study, the SELECT investigators decided to use a dose of 400 IU. Natural (RRR) α-tocopherol formulations were rejected, since they had not been previously part of a controlled trial, and succinate ester was found to have no biologic activity when given orally.
SELECT was activated in July 2001, but was discontinued prematurely (in October 2008) because of adverse findings in an initial independent review of data in September 2008. This initial data from SELECT demonstrated that selenium and vitamin E supplements, taken either alone or together for an average of 5 years, did not prevent PCa (1). In fact, the data also showed two concerning trends: (1) a statistically nonsignificant increase in type 2 diabetes mellitus (P = 0.08) was observed in the group of men receiving 200 μg selenium daily, and (2) a statistically nonsignificant (P = 0.09) increase in the incidence of PCa was also found among men taking only 400 IU vitamin E daily (1). On the basis of this outcome, it was concluded that SELECT failed (1).
As a unique and bold chemoprevention trial in the normal human population, SELECT was viewed as an outstanding effort of its kind. During the experimental design procedure, it was acknowledged that more data on selenium and vitamin E would have been helpful, but it was felt the time was right to proceed with the study, and regardless of limited data, selenium and vitamin E represented the best possible options among promising PCa chemoprevention agents (103). However, several investigators were skeptical and voiced a concern that preclinical studies do not support the rationale and design of SELECT (104).
WHY SELECT FAILED: POTENTIAL REASONS
SELECT was initiated on the basis of reasonably strong evidence suggesting the potential efficacy of selenium and vitamin E in reducing the risk of PCa. Therefore, it is difficult to accept that vitamin E and/or selenium, when used smartly and appropriately, will be harmful rather than beneficial. We believe it is worth considering to try to explain the possible reasons why SELECT failed. A careful analysis of possible reasons may be useful for future studies in this direction. Below, we provide important reasons that could be useful in designing future studies.
First, the use of L-selenomethionine rather than high-selenium brewer’s yeast in SELECT was influenced by logistic factors, although the SELECT formulation was reasonably thought to be an adequate yeast surrogate. L-selenomethionine is only one of >20 selenium-containing species in brewer’s yeast (105). The oral doses or formulations required to deliver selenium metabolites to prostate cells in vivo have yet to be firmly established, and this may explain why SELECT did not duplicate the NPC results.
Second, the NPC study participants who clearly benefited from selenium administration had lower baseline selenium levels, but the relevance of this finding to SELECT is unclear. Of 51,529 men in the Health Professionals Follow-Up Study, an odds ratio of 0.35 for individuals in the highest versus the lowest toenail selenium concentration quintiles was found for risk of advanced PCa (106). It has been suggested that it might have been more beneficial to conduct a trial such as SELECT in a seleno-deficient region, to better replicate the conditions under which brewer’s yeast provided a benefit in NPC. However, a case-control study of 656 British men found no association between nail selenium concentration and PCa risk (107,108). These issues should be thoroughly considered for future studies.
Third, the higher dose of vitamin E used in SELECT did not match the dose of vitamin E used in the ATBC study. Referring to the NPC study on selenium, analysis of a higher-dose arm of the trial (400 μg rather than 200 μg) showed no increased PCa preventive effect (109). Therefore, in at least one case, increasing the dose of the study agent did not have an increased effect. As previously outlined, not all of the stereoisomers of vitamin E have the same biologic activity, and it is not known how administration of all-rac α-tocopherol may affect overall vitamin E bioactivity. Further, as discussed above, in recent preclinical studies, another formulation of vitamin E, γ-tocopherol, has been found to be effective. It is possible that high levels of supplemental vitamin E may reduce serum levels of γ-tocopherol, an effective agent.
It will be worthwhile to conduct well-designed preclinical studies to evaluate the different formulations of vitamin E, especially γ-tocopherol, for their possible PCa preventive effects. Then, on the basis of the outcome of these preclinical studies, a smaller clinical study should be undertaken.
Fourth, smoking status may also be important in considering the ATBC results, since in vitro studies have shown that cigarette smoke depletes α-tocopherol in human plasma (110). Analysis of the screening arm of the ongoing Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial showed a reduction in relative risk for both advanced and non-advanced PCa among current and former (within 10 years) smokers who supplemented their diets with vitamin E (111). The data indicate a statistically significant benefit associated with two factors: dose of at least 400 IU/d and adherence to supplementation for at least 10 years. These findings support the use of the approximately eight-fold higher dose in SELECT; however, as previously discussed, the biology of the tocopherols is incompletely understood, and the use of the higher dose in SELECT may have been a confounding factor, if the ATBC study was to be used as a preliminary study. Further, as discussed by Moyad (104), men with the highest serum levels of vitamin E had significantly lower testosterone, androstenedione, sex hormone–binding globulin and estrone levels. It is possible that vitamin E prevents PCa in smokers by lowering androgen levels. In this scenario, the smoker population will benefit most. This can be confirmed by a well-designed clinical trial in smokers.
Fifth, the ATBC study suggested obesity as a confounding factor, since the data from this trial demonstrated that the negative impact of extreme obesity was greater than the positive impact (32% decrease in risk) of taking the vitamin E supplement (104). Thus, obesity should also be taken into consideration when conducting future PCa trials.
Finally, there is a lack of understanding of proper biology of these agents. This is especially important for selenium because a proper understanding of selenium biology is far from complete (107). As suggested by Hatfield and Gladyshev (107), better understanding of selenium biology needs to be pursued that may be informative in designing future clinical studies for elucidating specific roles of selenium in human biology. Then, supplementation with selenium could be better targeted to a subset of the human population that can benefit most from this micronutrient (107). The same is true for vitamin E as well.
SELENIUM AND VITAMIN E: POST-SELECT SCENARIO
Although the formulations and doses of selenium and vitamin E used in SELECT were carefully considered at the time of its design, new data have become available since 2001. SELECT failed to show an effect in a human population. However, pre-SELECT studies as well as continuing post-SELECT studies are still supporting the potential usefulness of selenium and/or vitamin E for prevention of PCa and possibly other conditions as well. Much remains to be understood about the absorption, metabolism and physiologic chemistry of the NPC and ATBC study agents. In the case of selenium, it has yet to be shown what particular substance in brewer’s yeast may have led to the NPC results. In the case of vitamin E, the dose-dependent effects are not clear and there is insufficient data regarding the specific roles of the tocopherols in arrest or retardation of carcinogenesis. More studies need to be conducted to elucidate the proper doses and formulations at which the tocopherols and selenium produce maximum effects. Additionally, the most appropriate populations for interventions with both agents or with either agent are still being debated. The SELECT findings were negative in the face of numerous prior and subsequent preclinical studies, demonstrating anticarcinogenic effects of vitamin E and selenium both alone and in combination. Deficiencies in the understanding of the mechanisms of vitamin E and selenium may be to blame for the failure of SELECT. A better understanding of these mechanisms and the best way to deliver effective chemopreventive regimens will come only with more preclinical data on these substances. Nonetheless, the existing evidence supporting selenium and vitamin E as potential PCa chemopreventive agents is possibly enough to justify further efforts in this direction.
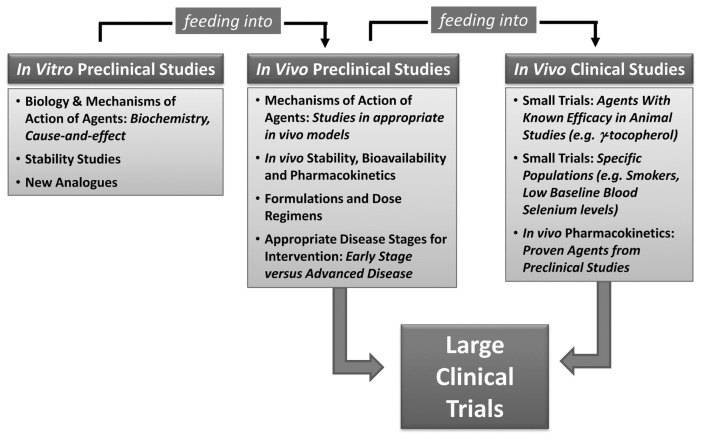
An active engagement of expert researchers, including the SELECT team, from various laboratories is needed to address important questions in this post-SELECT era. In this direction, as shown in Figure 1, we need a concerted effort to conduct useful multidirectional and simultaneous in vitro, in vivo pre-clinical and in vivo clinical studies. The results from these studies should form the basis of a large clinical trial that may ultimately become a success story and enable us to better manage PCa and possible other cancers and diseases.
Figure 1.
A rational approach to design a future SELECT. A concerted multidirectional effort with simultaneous in vitro, in vivo preclinical and in vivo clinical studies is needed to design an ultimate final clinical trial with vitamin E and/or selenium. The in vitro studies need to be conducted to understand the biology and mechanism(s) of vitamin E and selenium. In vivo preclinical studies in appropriate animal models with relevance to human PCa should be carried out to study the best formulations, most effective doses and most appropriate disease stages. These studies should also be directed to study the mechanism of action of the agents and their in vivo pharmacokinetics and bioavailability. The outcome of these studies should feed into novel but initially smaller clinical trials. The small trial, either arising from the known knowledge or from the new preclinical studies, should be conducted with specific agents and in specific populations. The results from these studies should form the basis of a large clinical trial with best agent(s).
ACKNOWLEDGMENTS
This work was partially supported by National Institutes of Health grants R01CA114060 and 1R01AR059130 (to N Ahmad) and T32ES007015 (training grant; predoctoral traineeship to TL Schmit).
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Konety BR, Bird VY, Deorah S, Dahmoush L. Comparison of the incidence of latent prostate cancer detected at autopsy before and after the prostate specific antigen era. J Urol. 2005;174:1785–8. doi: 10.1097/01.ju.0000177470.84735.55. [DOI] [PubMed] [Google Scholar]
- 5.Chan JM, Jou RM, Carroll PR. The relative impact and future burden of prostate cancer in the United States. J Urol. 2004;172:S13–6. [PubMed] [Google Scholar]
- 6.Wada S. Chemoprevention of tocotrienols: the mechanism of antiproliferative effects. Forum Nutr. 2009;61:204–16. doi: 10.1159/000212752. [DOI] [PubMed] [Google Scholar]
- 7.Kramer BS, et al. Use of 5alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Urol. 2009;181:1642–57. doi: 10.1016/j.juro.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 8.William WN, Jr, Heymach JV, Kim ES, Lippman SM. Molecular targets for cancer chemo-prevention. Nat Rev Drug Discov. 2009;8:213–25. doi: 10.1038/nrd2663. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui IA, et al. Introducing nanochemo-prevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 11.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 12.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–21. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–41. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 15.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease. FASEB J. 2005;19:1193–5. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 16.Reagan-Shaw S, Afaq F, Aziz MH, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23:5151–60. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 17.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (Review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 18.Sarkar F, Li Y, Wang Z, Kong D. Novel targets for prostate cancer chemoprevention. Endocr. Relat. Cancer. 2010;17:R195–212. doi: 10.1677/ERC-10-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar FH. Current trends in the chemoprevention of cancer. Pharm Res. 2010;27:945–9. doi: 10.1007/s11095-010-0146-2. [DOI] [PubMed] [Google Scholar]
- 20.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–30. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 21.Breslow N, et al. Latent carcinoma of prostate at autopsy in seven areas: The International Agency for Research on Cancer, Lyons, France. Int. J. Cancer. 1977;20:680–8. doi: 10.1002/ijc.2910200506. [DOI] [PubMed] [Google Scholar]
- 22.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 23.Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl. 1990;201:3–23. [PubMed] [Google Scholar]
- 24.Nam RK, et al. Prevalence and patterns of the use of complementary therapies among prostate cancer patients: an epidemiological analysis. J Urol. 1999;161:1521–4. [PubMed] [Google Scholar]
- 25.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650–1. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 26.Jensen SK, Lauridsen C. Alpha-tocopherol stereoisomers. Vitam Horm. 2007;76:281–308. doi: 10.1016/S0083-6729(07)76010-7. [DOI] [PubMed] [Google Scholar]
- 27.Joffe M, Harris P. The biological potency of the natural tocopherols and certain derivatives. J Am Chem Soc. 1943;65:925–7. [Google Scholar]
- 28.Burton G, Ingold K. The antioxidant activity of vitamin E and relative chain-breaking phenolic antioxidants in vitro. J Am Chem Soc. 1981;103:6472–7. [Google Scholar]
- 29.Hosomi A, et al. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997;409:105–8. doi: 10.1016/s0014-5793(97)00499-7. [DOI] [PubMed] [Google Scholar]
- 30.Lee E, et al. Alpha-tocopheryl succinate, in contrast to alpha-tocopherol and alpha-tocopheryl acetate, inhibits prostaglandin E2 production in human lung epithelial cells. Carcinogenesis. 2006;27:2308–15. doi: 10.1093/carcin/bgl073. [DOI] [PubMed] [Google Scholar]
- 31.Bendich A, Machlin LJ. Safety of oral intake of vitamin E. Am J Clin Nutr. 1988;48:612–9. doi: 10.1093/ajcn/48.3.612. [DOI] [PubMed] [Google Scholar]
- 32.Galli F, Azzi A. Present trends in vitamin E research. Biofactors. 2010;36:33–42. doi: 10.1002/biof.75. [DOI] [PubMed] [Google Scholar]
- 33.Ju J, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31:533–42. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: an insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer. 2008;123:739–52. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 35.Barve A, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int. J. Cancer. 2009;124:1693–9. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu G, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth. Carcinogenesis. 2010;31:687–94. doi: 10.1093/carcin/bgp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu W, et al. Anticancer actions of natural and synthetic vitamin E forms: RRR-alpha-tocopherol blocks the anticancer actions of gamma-tocopherol. Mol Nutr Food Res. 2009;53:1573–81. doi: 10.1002/mnfr.200900011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Q, Moreland M, Ames BN, Yin X. A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. J Nutr Biochem. 2009;20:894–900. doi: 10.1016/j.jnutbio.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooney RV, et al. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A. 1993;90:1771–5. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christen S, Woodall AA, Shigenaga MK, South-well-Keely PT, Duncan MW, Ames BN. Gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–22. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunawardena K, Murray DK, Meikle AW. Vitamin E and other antioxidants inhibit human prostate cancer cells through apoptosis. Prostate. 2000;44:287–95. doi: 10.1002/1097-0045(20000901)44:4<287::aid-pros5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 42.Ni J, Chen M, Zhang Y, Li R, Huang J, Yeh S. Vitamin E succinate inhibits human prostate cancer cell growth via modulating cell cycle regulatory machinery. Biochem Biophys Res Commun. 2003;300:357–63. doi: 10.1016/s0006-291x(02)02851-6. [DOI] [PubMed] [Google Scholar]
- 43.Chang E, et al. Alpha-vitamin E derivative, RRR-alpha-tocopheryloxybutyric acid inhibits the proliferation of prostate cancer cells. Asian J Androl. 2007;9:31–9. doi: 10.1111/j.1745-7262.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. Proc Natl Acad Sci U S A. 2002;99:7408–13. doi: 10.1073/pnas.102014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Israel K, Yu W, Sanders BG, Kline K. Vitamin E succinate induces apoptosis in human prostate cancer cells: role for Fas in vitamin E succinate-triggered apoptosis. Nutr. Cancer. 2000;36:90–100. doi: 10.1207/S15327914NC3601_13. [DOI] [PubMed] [Google Scholar]
- 46.Crispen PL, et al. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: implications for chemoprevention. Prostate. 2007;67:582–90. doi: 10.1002/pros.20468. [DOI] [PubMed] [Google Scholar]
- 47.Jia L, et al. Critical roles for JNK, c-Jun, and Fas/FasL-signaling in vitamin E analog-induced apoptosis in human prostate cancer cells. Prostate. 2008;68:427–41. doi: 10.1002/pros.20716. [DOI] [PubMed] [Google Scholar]
- 48.Yin Y, et al. The therapeutic and preventive effect of RRR-alpha-vitamin E succinate on prostate cancer via induction of insulin-like growth factor binding protein-3. Clin Cancer Res. 2007;13:2271–80. doi: 10.1158/1078-0432.CCR-06-1217. [DOI] [PubMed] [Google Scholar]
- 49.Shiau CW, et al. Alpha-tocopheryl succinate induces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. J Biol Chem. 2006;281:11819–25. doi: 10.1074/jbc.M511015200. [DOI] [PubMed] [Google Scholar]
- 50.Zu K, Ip C. Synergy between selenium and vitamin E in apoptosis induction is associated with activation of distinctive initiator caspases in human prostate cancer cells. Cancer Res. 2003;63:6988–95. [PubMed] [Google Scholar]
- 51.Ni J, et al. In vitro and in vivo anticancer effects of the novel vitamin E ether analogue RRR-alpha-tocopheryloxybutyl sulfonic acid in prostate cancer. Clin Cancer Res. 2009;15:898–906. doi: 10.1158/1078-0432.CCR-08-1087. [DOI] [PubMed] [Google Scholar]
- 52.Galli F, et al. The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch Biochem Biophys. 2004;423:97–102. doi: 10.1016/j.abb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Jensen SK, Engberg RM, Hedemann MS. All-rac-alpha-tocopherol acetate is a better vitamin E source than all-rac-alpha-tocopherol succinate for broilers. J Nutr. 1999;129:1355–60. doi: 10.1093/jn/129.7.1355. [DOI] [PubMed] [Google Scholar]
- 54.Fleshner N, Fair WR, Huryk R, Heston WD. Vitamin E inhibits the high-fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J Urol. 1999;161:1651–4. [PubMed] [Google Scholar]
- 55.Basu A, Grossie B, Bennett M, Mills N, Imrhan V. Alpha-tocopheryl succinate (alpha-TOS) modulates human prostate LNCaP xenograft growth and gene expression in BALB/c nude mice fed two levels of dietary soybean oil. Eur J Nutr. 2007;46:34–43. doi: 10.1007/s00394-006-0629-4. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi S, et al. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Prostate. 2009;69:644–51. doi: 10.1002/pros.20915. [DOI] [PubMed] [Google Scholar]
- 57.Heinonen OP, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–6. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 58.Weinstein SJ, et al. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1253–9. doi: 10.1158/1055-9965.EPI-06-1084. [DOI] [PubMed] [Google Scholar]
- 59.Peters U, et al. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and Lifestyle (VITAL) study cohort. Cancer Causes Control. 2008;19:75–87. doi: 10.1007/s10552-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 60.Chan JM, et al. Supplemental vitamin E intake and prostate cancer risk in a large cohort of men in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8:893–9. [PubMed] [Google Scholar]
- 61.Rodriguez C, et al. Vitamin E supplements and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2004;13:378–82. [PubMed] [Google Scholar]
- 62.Gann PH, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–30. [PubMed] [Google Scholar]
- 63.Schwarz K, Foltz CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. 1951. Nutrition. 1999;15:255. [PubMed] [Google Scholar]
- 64.Kryukov GV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–43. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 65.Bialostosky K, Wright JD, Kennedy-Stephenson J, McDowell M, Johnson CL. Dietary intake of macronutrients, micronutrients, and other dietary constituents: United States 1988–94. Vital Health Stat. 2002;11:1–158. [PubMed] [Google Scholar]
- 66.Shamberger RJ, Frost DV. Possible protective effect of selenium against human cancer. Can. Med. Assoc. J. 1969;100:682. [PMC free article] [PubMed] [Google Scholar]
- 67.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–54. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 68.Webber MM, Perez-Ripoll EA, James GT. Inhibitory effects of selenium on the growth of DU-145 human prostate carcinoma cells in vitro. Biochem Biophys Res Commun. 1985;130:603–9. doi: 10.1016/0006-291x(85)90459-0. [DOI] [PubMed] [Google Scholar]
- 69.Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001;61:7071–8. [PubMed] [Google Scholar]
- 70.Thompson HJ, et al. Comparison of the effects of an organic and an inorganic form of selenium on a mammary carcinoma cell line. Carcinogenesis. 1994;15:183–6. doi: 10.1093/carcin/15.2.183. [DOI] [PubMed] [Google Scholar]
- 71.Redman C, et al. Inhibitory effect of se-lenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998;125:103–10. doi: 10.1016/s0304-3835(97)00497-7. [DOI] [PubMed] [Google Scholar]
- 72.Sinha R, Medina D. Inhibition of cdk2 kinase activity by methylselenocysteine in synchronized mouse mammary epithelial tumor cells. Carcinogenesis. 1997;18:1541–7. doi: 10.1093/carcin/18.8.1541. [DOI] [PubMed] [Google Scholar]
- 73.Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–82. [PubMed] [Google Scholar]
- 74.Spallholz JE, Palace VP, Reid TW. Methioninase and selenomethionine but not Se-methylselenocysteine generate methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritional carcinostatic activity of selenoamino acids. Biochem Pharmacol. 2004;67:547–54. doi: 10.1016/j.bcp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol Cancer Ther. 2006;5:3275–84. doi: 10.1158/1535-7163.MCT-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamaguchi K, et al. Methylseleninic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Oncogene. 2005;24:5868–77. doi: 10.1038/sj.onc.1208742. [DOI] [PubMed] [Google Scholar]
- 77.Christensen MJ, Nartey ET, Hada AL, Legg RL, Barzee BR. High selenium reduces NF-kappaB-regulated gene expression in uninduced human prostate cancer cells. Nutr Cancer. 2007;58:197–204. doi: 10.1080/01635580701328701. [DOI] [PubMed] [Google Scholar]
- 78.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–97. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 79.Ip C, Lisk DJ. Enrichment of selenium in allium vegetables for cancer prevention. Carcinogenesis. 1994;15:1881–5. doi: 10.1093/carcin/15.9.1881. [DOI] [PubMed] [Google Scholar]
- 80.Corcoran NM, Najdovska M, Costello AJ. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004;171:907–10. doi: 10.1097/01.ju.0000092859.16817.8e. [DOI] [PubMed] [Google Scholar]
- 81.Lee SO, et al. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA) Prostate. 2006;66:1070–5. doi: 10.1002/pros.20329. [DOI] [PubMed] [Google Scholar]
- 82.Li GX, et al. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis. 2008;29:1005–12. doi: 10.1093/carcin/bgn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, et al. Methyl-selenium compounds inhibit prostate carcinogenesis in the transgenic adenocarcinoma of mouse prostate model with survival benefit. Cancer Prev. Res. (Phila.) 2009;2:484–95. doi: 10.1158/1940-6207.CAPR-08-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sabichi AL, et al. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of l-selenomethionine: a Southwest Oncology Group Study. Clin Cancer Res. 2006;12:2178–84. doi: 10.1158/1078-0432.CCR-05-0937. [DOI] [PubMed] [Google Scholar]
- 85.Blot WJ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 86.Li JY, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993;85:1492–8. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 87.Clark LC, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 88.Clark LC, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–4. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 89.Duffield-Lillico AJ, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–12. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 90.Downing SR, Jackson P, Russell PJ. Mutations within the tumour suppressor gene p53 are not confined to a late event in prostate cancer progression: a review of the evidence. Urol Oncol. 2001;6:103–10. doi: 10.1016/s1078-1439(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 91.Lanfear J, Fleming J, Wu L, Webster G, Harrison PR. The selenium metabolite selenodiglutathione induces p53 and apoptosis: relevance to the chemopreventive effects of selenium? Carcinogenesis. 1994;15:1387–92. doi: 10.1093/carcin/15.7.1387. [DOI] [PubMed] [Google Scholar]
- 92.Jiang C, Hu H, Malewicz B, Wang Z, Lu J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004;3:877–84. [PubMed] [Google Scholar]
- 93.Kuehnelt D, et al. Selenium metabolites in human urine after ingestion of selenite, L-selenomethionine, or DL-selenomethionine: a quantitative case study by HPLC/ICPMS. Anal Bioanal Chem. 2005;383:235–46. doi: 10.1007/s00216-005-0007-8. [DOI] [PubMed] [Google Scholar]
- 94.Combs GF, Jr, Scott ML. Nutritional interrelationships of vitamin E and selenium. Bioscience. 1977;27:467–73. [Google Scholar]
- 95.Combs GF, Jr, Scott ML. Dietary requirements for vitamin E and selenium measured at the cellular level in the chick. J Nutr. 1974;104:1292–6. doi: 10.1093/jn/104.10.1292. [DOI] [PubMed] [Google Scholar]
- 96.Diplock AT. The biological function of vitamin E and the nature of the interaction of the vitamin with selenium. World Rev Nutr Diet. 1978;31:178–83. doi: 10.1159/000401322. [DOI] [PubMed] [Google Scholar]
- 97.Reagan-Shaw S, Nihal M, Ahsan H, Mukhtar H, Ahmad N. Combination of vitamin E and selenium causes an induction of apoptosis of human prostate cancer cells by enhancing Bax/Bcl-2 ratio. Prostate. 2008;68:1624–34. doi: 10.1002/pros.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsavachidou D, et al. Selenium and vitamin E: cell type- and intervention-specific tissue effects in prostate cancer. J Natl Cancer Inst. 2009;101:306–20. doi: 10.1093/jnci/djn512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Venkateswaran V, Fleshner NE, Sugar LM, Klotz LH. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004;64:5891–6. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 100.Venkateswaran V, Klotz LH, Ramani M, Sugar LM, Jacob LE, Nam RK, Fleshner NE. A combination of micronutrients is beneficial in reducing the incidence of prostate cancer and increasing survival in the Lady transgenic model. Cancer Prev. Res. (Phila.) 2009;2:473–83. doi: 10.1158/1940-6207.CAPR-08-0124. [DOI] [PubMed] [Google Scholar]
- 101.Cervi D, et al. Micronutrients attenuate progression of prostate cancer by elevating the endogenous inhibitor of angiogenesis, platelet factor-4. BMC Cancer. 2010;10:258. doi: 10.1186/1471-2407-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lippman SM, et al. Designing the selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 103.Taylor PR, Albanes D. Selenium, vitamin E, and prostate cancer: ready for prime time. J Natl Cancer Inst. 1998;90:1184–5. doi: 10.1093/jnci/90.16.1184. [DOI] [PubMed] [Google Scholar]
- 104.Moyad MA. Selenium and vitamin E supplements for prostate cancer: evidence or embellishment? Urology. 2002;59:9–19. doi: 10.1016/s0090-4295(01)01190-6. [DOI] [PubMed] [Google Scholar]
- 105.Bird SM, Block E, Denoyer E. High-performance liquid chromatography of selenoamino acids and organo selenium compounds: speciation by inductively coupled plasma mass spectrometry. J Chromatogr A. 1997;789:349–59. doi: 10.1016/s0021-9673(97)00657-2. [DOI] [PubMed] [Google Scholar]
- 106.Yoshizawa K, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90:1219–24. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 107.Hatfield DL, Gladyshev VN. The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9:18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allen NE, Morris JS, Ngwenyama RA, Key TJ. A case-control study of selenium in nails and prostate cancer risk in British men. Br J Cancer. 2004;90:1392–6. doi: 10.1038/sj.bjc.6601701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peters U, Takata Y. Selenium and the prevention of prostate and colorectal cancer. Mol Nutr Food Res. 2008;52:1261–72. doi: 10.1002/mnfr.200800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Handelman GJ, Packer L, Cross CE. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am J Clin Nutr. 1996;63:559–65. doi: 10.1093/ajcn/63.4.559. [DOI] [PubMed] [Google Scholar]
- 111.Kirsh VA, et al. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J Natl Cancer Inst. 2006;98:245–54. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]