Abstract
High-mobility group box 1 (HMGB1) is a nuclear and cytosolic protein that is increasingly recognized as an important proinflammatory mediator actively secreted from monocytes and macrophages and passively released from necrotic cells. In antineutrophilic cytoplasmatic antibody (ANCA)-associated vasculitis (AAV), the kidneys are commonly affected vital organs, characterized by focal necrotizing and/or crescentic pauci-immune glomerulonephritis. The aim of the study was to determine whether HMGB1 serum levels are elevated in AAV with renal manifestations. A total of 30 AAV patients (16 female and 14 male; median age 59 years, range 17–82) with Wegener granulomatosis, microscopic polyangiitis and Churg-Strauss syndrome with available renal biopsies and serum samples were included. In seven cases, serum was also obtained at rebiopsy in remission. HMGB1 was analyzed with Western blot. Birmingham Vasculitis Activity Score (BVAS, version 2003), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), urinanalysis, creatinine, estimated glomerular filtration rate, sex and age were included in the analysis. Twenty-five episodes of biopsy-proven active disease with BVAS 17.9 ± 4.6 and 13 cases with inactive biopsies and BVAS 2.3 ± 3.7 (P = 0.0001) were identified. CRP, ESR, hematuria and proteinuria were significantly higher in active cases. HMGB1 was significantly elevated (P = 0.01) comparing active with inactive cases (120 ± 48 versus 78 ± 46 ng/mL) and significantly lower in the seven control patients (P = 0.03) at rebiopsy in remission. HMGB1 remained higher in inactive cases compared with historic healthy controls (10.9 ± 10.5 ng/mL). HMGB1 levels did not differ significantly between AAV subgroups. CRP and ESR did not correlate with HMGB1. HMGB1 is significantly increased in AAV with renal involvement. Residual HMGB1 elevation in remission could possibly reflect low-grade inflammatory activity or tissue damage. Future studies may further reveal whether HMGB1 is useful as a marker of disease activity and a predictor of outcome in AAV.
INTRODUCTION
The primary small-vessel systemic vasculitic disorders associated with anti-neutrophilic cytoplasmatic antibodies (ANCAs) include Wegener granulomatosis (WG), microscopic polyangiitis (MPA) and Churg-Strauss syndrome (CSS) and are categorized as ANCA- associated vasculitides (1).
Disease severity and prognosis varies primarily because of the heterogeneity in organ involvement. The most commonly affected vital organs are the kidneys, characterized by a focal necrotizing and/or crescentic pauci-immune glomerulonephritis in 70% of ANCA- associated vasculitis (AAV) patients, with important implications for therapy and long-term outcome (2).
A number of cell types have been implicated in the development of AAV. These include neutrophils, monocytes and T and B lymphocytes (3). Animal models have confirmed a pathogenic role for ANCAs specific for myeloperoxidase and cytokines, such as tumor necrosis factor (TNF) and interleukin (IL)-6, are important in the disease development in AAV (4–6).
HMGB1, a 30-kDa ubiquitous nuclear protein, is a DNA-binding protein known as a transcription and growth factor (7,8). This protein has also been identified as acting as a proinflammatory mediator when found extracellularly in animal models and human disease. The translocation from the nucleus to the extracellular milieu transforms HMGB1 into an “alarmin,” a danger signal with the ability to activate the immune system. HMGB1 is actively secreted by innate immune cells such as macrophages and monocytes upon endotoxin stimulation, is passively released by injured and necrotic cells, and has been shown to stimulate necrosis- induced inflammation (9–12). Moreover, HMGB1 induces other cytokines such as TNF, IL-1, IL-6 and IL-8 and is also an activator of endothelial cells (human umbilical vein endothelial cells) leading to the upregulation of adhesion molecules (13,14). HMGB1 has been shown to interact with toll-like receptor (TLR)-2, TLR-4 and the receptor for advanced glycation end products (RAGE) in established cell lines and animal models, leading to a downstream translocation of nuclear factor (NF)-κB, inducing immunostimulatory and chemotactic responses (15–17). In animal models of arthritis, a strict nuclear HMGB-1 pattern was observed in synovial cells of healthy mice and rats. In contrast, a distinct pattern of extracellular expression in the cytoplasm of macrophages and synoviocytes has been identified with immunohistochemical staining in animals with arthritis (18,19). Targeting HMGB1 has been demonstrated to confer protection in animal models of sepsis, endotoxemia and arthritis (10,20,21). Elevated HMGB1 levels in serum have been documented in clinical inflammatory conditions such as sepsis and rheumatoid arthritis (RA) as well as chronic kidney disease (22–25).
The aim of the study was to investigate the role of HMGB1 in AAV with renal involvement and to determine whether the serum levels may correspond to clinical and histopathological disease activity.
MATERIALS AND METHODS
Patients
A total of 30 patients, 16 female and 14 male with a median age of 59 years (range 17–82), with a diagnosis of AAV were included in the study. They were all investigated and treated at the Unit of Rheumatology at Karolinska University Hospital between 1998 and 2008. A summary of patient characteristics including age, sex, diagnosis, organ involvement, disease activity score, renal biopsy result, renal function and ANCA antibody is shown in Table 1.
Table 1.
Patient characteristics.
| Patient | Age | Sex | Diagnosisa | Organ involvement other than kidney at presentationb | ANCA at presentationc | Biopsy findingd | CRPe | Creatinine/eGFRf | BVAS |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 30 | F | MPA | Joints | MPO | FN/CGNd | 17 | 137/41.7 | 13 |
| 4ag | 61 | M | CSS | Skin, lung, neuropathy | MPO | FN/CGN | 108 | 97/53.8 | 22 |
| 4bg | 69 | Joints, lung, neuropathy | MPO | FN/CGN | 10 | 906/4 | 27 | ||
| 5 | 47 | M | WG | Joints, skin, mouth ulcers, ENT | PR3 | FN/CGN | 202 | 198/25 | 20 |
| 6 | 40 | M | WG | Joints, skin, ENT, lung, neuropathy | PR3 | FN/CGN | 58 | 81/72.2 | 21 |
| 7 | 70 | F | WG | Joints, skin, lung | PR3 | CGN | 75 | 73/72.7 | 19 |
| 9 | 82 | M | WG | Joints | PR3 | FN/CGN | 97 | 206/21.3 | 12 |
| 11 | 74 | M | MPA | Joints | MPO | FN | 30 | 136/35 | 15 |
| 12 | 63 | F | MPA | Joints | MPO | FN/CGN | 12 | 100/51.6 | 11 |
| 15 | 66 | F | WG | Lung | PR3 | FN/CGN | <4 | 206/22.2 | 14 |
| 18 | 67 | M | MPA | Myalgia | MPO | FN/CGN | 176 | 174/27 | 14 |
| 21 | 53 | F | WG | Joints, skin | PR3 | FN/CGN | 101 | 59/98.3 | 17 |
| 22 | 75 | F | WG | Joints, myalgia, ENT | PR3/MPO | FN/CGN | 28 | 160/29 | 21 |
| 25 | 41 | M | WG | Joints, ENT, lung | PR3 | FN/CGN | 115 | 76/77.3 | 19 |
| 28 | 17 | F | WG | ENT, lung | PR3 | FN/CGN | 95 | 66/109 | 14 |
| 29 | 81 | F | MPA | Myalgia, lung | MPO | FN | 204 | 86/21.6 | 21 |
| 30 | 41 | M | WG | Joints, skin, ENT, pericarditis | PR3 | FN/CGN | 9 | 66/91 | 18 |
| 31 | 60 | M | WG | ENT, spleen infarction | PR3 | FN/CGN | 43 | 55/104 | 24 |
| Patients with repeated renal biopsy after induction treatment | |||||||||
| 8a | 53 | M | WG | Joints, eyes | PR3 | FN/CGN | <7 | 104/51.1 | 12 |
| 8bh | RR | <4 | 99/54.1 | 0 | |||||
| 10a | 52 | M | CSS | Lung, pericarditis | MPO | FN/CGN | 14 | 78/71.5 | 11 |
| 10bh | RR | <7 | 83/66.6 | 0 | |||||
| 14a | 57 | M | WG | Joints, ENT, lung, neuropathy | PR3 | FN/CGN | 70 | 73/75.8 | 25 |
| 14bh | RR | <4 | 74/74.6 | 0 | |||||
| 17a | 59 | F | WG | Joints, ENT, neuropathy | PR3 | FN/CGN | 95 | 79/68.7 | 19 |
| 17bh | ENT, lung | RR | 58 | 88/60.6 | 6 | ||||
| 19a | 64 | F | MPA | Joints, lung | MPO | FN/CGN | 16 | 200/23.1 | 17 |
| 19bh | RR | <4 | 116/43.4 | 0 | |||||
| 26a | 48 | F | MPA | Myalgia, joints, skin | MPO | FN/CGN | 9 | 109/49.4 | 17 |
| 26bh | RR | 3 | 73/78.4 | 0 | |||||
| 27a | 43 | F | WG | Joints, skin, ENT | PR3 | CGN | 57 | 69/85.6 | 24 |
| 27bh | Joints, ENT | RR | 2 | 98/57.1 | 8 | ||||
| Patients with inactive renal biopsy after induction therapy or workup for suspected AAV | |||||||||
| 13h | 70 | F | MPA | Joints | MPO | RR | 13 | 69/77.5 | 0 |
| 16h | 59 | M | WG | Joints, myalgia, intestinal granuloma | PR3 | RR | <4 | 79/68.7 | 0 |
| 20h | 59 | F | MPA | Lung, neuropathy | MPO | RR | <5 | 89/59.9 | 10 |
| 23h | 79 | F | MPA | Myalgia | MPO | RR | <4 | 116/41.5 | 0 |
| 24h | 78 | F | MPA | Myalgia | MPO | RR | 5 | 199/22.3 | 0 |
| 32h | 55 | M | WG | Lung | PR3 | RR | 8 | 82/66.7 | 6 |
MPA, microscopic polyangiitis; CSS, Churg-Strauss syndrome; WG, Wegener granulomatosis.
Joints (arthralgia or arthritis); ENT (ear, nose and throat).
ANCA antibodies were directed against myeloperoxidase (MPO) or proteinase 3 (PR3).
FN/CGN, focal necrotizing/crescentic glomerulonephritis; RR, renal remission.
CRP reference was <3–7 mg/L depending on local laboratory.
Creatinine reference was <100 μmol/L for males and <90 μmol/L for females, eGFR using the MDRD formula.
Same patient at disease onset and new renal flare 8 years after primary disease.
Patient samples during renal remission.
Induction therapy for active vasculitis in patients was typically standard induction treatment with corticosteroids and cyclophosphamide either intravenously or orally, followed by remission therapy with low-dose corticosteroids and either azathioprin or methotrexate. Plasma exchange was used in one case on renal relapse with dialysis dependence 8 years after primary disease (case 4b, see Table 1). During the induction therapy phase (3–6 months), the patients were followed on average six times until remission was established. After that, follow-up would be approximately every third month unless clinically required.
Methods
All patients were included in the study at the time of renal biopsy with simultaneous collection of serum samples and evaluation of disease activity. Serum was frozen within 4 h and stored at −70°C for future analysis. In seven of the cases, biopsies were performed during the active phase of the disease followed by repeat biopsies in remission 6–9 months later. Paired serum samples were available at these two time points. In four patients, biopsy was performed in inactive renal disease with no samples available during active disease. These patients had had induction treatment as described above, and the remaining two (cases 13 and 32) were biopsied and sampled during workup for suspected AAV with renal involvement.
HMGB1 was analyzed by Western blot in the sera of patients by a method previously described (24,25). Birmingham Vasculitis Activity Score (BVAS, version 2003) (a clinical evaluation tool standardized for the management of patients with systemic vasculitis where complete remission is defined as BVAS = 0), CRP, ESR, urinalysis (dip stick procedure), 24-h albumin excretion in urine, creatinine, estimated glomerular filtration rate (eGFR) using the modification of diet in renal disease (MDRD) formula (26), sex and age were included in the analysis. Biochemical analyses and ANCA serology by enzyme-linked immunosorbent assay (ELISA) were carried out using routine methods at the Department of Clinical Chemistry and Department of Clinical Immunology at Karolinska University Hospital.
Active kidney disease was defined as ongoing focal necrotizing and/or crescentic pauci-immune glomerulonephritis, whereas renal remission was labeled as the absence of these lesions. All renal biopsies were processed for light microscopy, immunofluorescence, and in some cases, electron microscopy according to standard techniques.
The study protocol was approved by the local ethics committee, and informed consent was obtained from each subject.
Immunhistochemistry
Immunohistochemical stainings were performed on cryosectioned 4-μm formaldehyde-fixed sections of renal biopsies. For detection of HMGB1 expression, sections were stained according to protocols previously described (27). Briefly, to reduce background staining due to unspecific binding, sections were incubated with 2% normal goat sera for 30 min. Thereafter, the slides were incubated overnight with an affinity-purified monoclonal mouse IgG2b anti-HMGB1 antibody (concentration 2 μg/mL, 2G7, received as a hybridoma from Critical Therapeutics, Lexington, KY, USA). Cells were thereafter incubated with Alexa Fluor© 488 conjugated goat antimouse IgG2b antibody (Molecular Probes, Invitrogen, Eugene, OR, USA) for 30 min, and nuclei were counterstained with Hoechst 33342. Phosphate-buffered saline supplemented with 0.1% saponin was used in all subsequent washes and incubation steps to permeabilize the cells. In each assay, controls for staining specificity were included, based on parallel staining studies omitting the primary antibody and using a primary isotype-matched immunoglobulin of irrelevant antigen specificity (negative mouse IgG2b control, DAKO Cytomation, Glostrup, Denmark).
Statistics
Continuous variables are presented as the median (10th to 90th percentile). Group differences were determined by nonparametric Wilcoxon two-sample rank sum test and χ2 test for nominal variables, and univariate correlations between variables were assessed using Spearman rank correlation (ρ). Statistical significance was set at the level of P < 0.05. A multivariate general linear model was used to assess the relationship between HMGB1 and various subgroups of vasculitis adjusting for eGFR (MDRD formula). Statistical computations were carried out using SAS 9.2 (SAS Campus Drive, Cary, NC, USA).
RESULTS
A total of 25 episodes of biopsy-proven active renal disease featuring focal necrotizing and/or crescentic pauci-immune were identified in 24 patients, whereas 13 biopsies featuring resolution with fibrous or sclerotic lesions were regarded as inactive. There was no difference between active and inactive cases with regard to age, sex (data not shown) and creatinine, whereas CRP, ESR, serum albumin, dip-stick hematuria and proteinuria differed significantly (Table 2). HMGB1 was significantly elevated in active cases (120 ± 48 ng/mL) compared with inactive ones (78 ± 46 ng/mL) as shown in Figure 1, whereas BVAS was 17.9 ± 4.6 compared with 2.3 ± 3.7 (P = 0.0001). Prednisone doses (mg/day) and 24-h urine albumin did not differ significantly between groups (see Table 2). However, the prednisone dose was, in many cases, not increased until the result of the kidney biopsy was available, which would be the same day or the following day after sampling. Therefore, the prednisone doses in active and inactive patients do not quite reflect actual doses given later during induction treatment.
Table 2.
Characteristics of patients with active renal disease and in remission.
| Active, n = 25 | Remission, n = 13 | P value | |
|---|---|---|---|
| CRP (mg/L) | 58 (9–151) | 7 (2–58) | 0.004 |
| ESR (mm/h) | 70 (17–102) | 21 (8–46) | 0.008 |
| Creatinine (μmol/L) | 100 (63–206) | 88 (70–165) | 0.59 |
| S-Albumin (g/L) | 29 (22–38) | 37 (28–44) | 0.007 |
| U-protein, >2+ (%) | 48 | 15 | 0.002 |
| U-erythrocytes, >2+ (%) | 90 | 37 | 0.001 |
| U-albumin (g/day) | 0.46 (0.06–1) | 0.25 (0–1.4) | 0.46 |
| Prednisone (mg/day) | 15 (0–60) | 13.7 (2.2–22) | 0.79 |
Data are expressed as median and 10th to 90th percentile. Urine dipstick 0–3 for U-protein and U-erythrocytes is expressed as a percentage. S, serum; U, urine.
Figure 1.
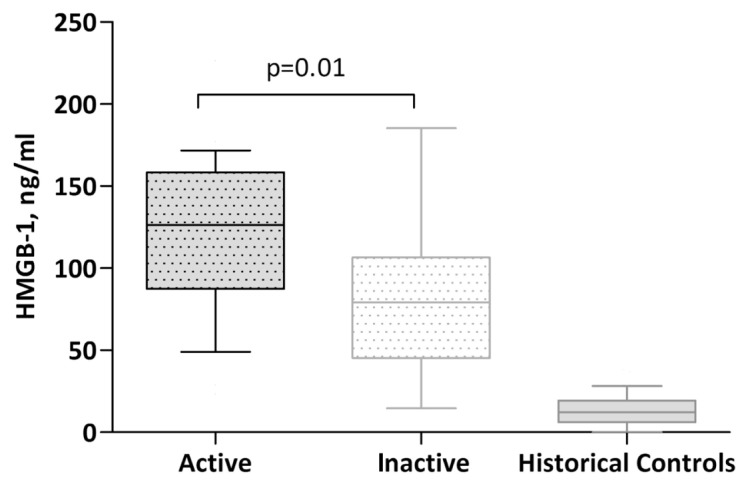
HMGB1 levels in active versus inactive renal disease. Data are expressed as median and 10th to 90th percentile.
In the seven patients who were their own controls, HMGB1 and BVAS were significantly lower (P = 0.03) at rebiopsy when the patients were in clinical and histopathological renal remission (Figure 2). However, two of these patients (17b and 27b) had signs of low-active nonrenal vasculitic disease at this time point with a BVAS score >0. In addition, two patients (20 and 32) had nonrenal vasculitis activity specified in Table 1, while having an inactive renal histology report. Neither CRP (ρ = 0.31, P = 0.06) nor ESR (ρ = 0.23, NS) correlated significantly with HMGB1. HMGB1 remained higher in inactive cases compared with historic controls (10.9 ± 10.5), as shown in Figure 1. These controls (n = 48) were from a previous study comparing HMGB1 levels in chronic kidney disease and healthy controls (25).
Figure 2.
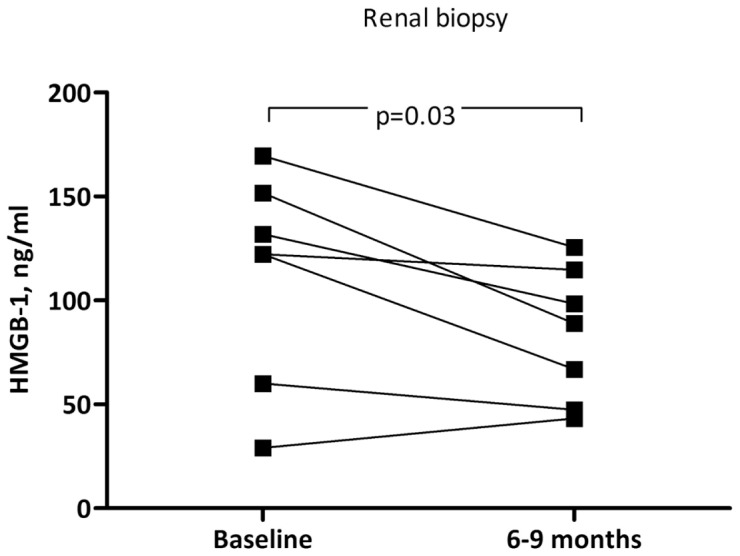
HMGB1 levels in paired serum samples (n = 7). Biopsies at baseline with active renal disease and at remission 6–9 months after first biopsy are shown.
Median HMGB1 levels in WG were 95.8 (10th to 90th percentile, 22.6–186.3), in MPA, 127.7 (22–183.7), and in CSS, 112.9 (93.9–131.9); but they did not differ significantly between groups (P = 0.53). When comparing the vasculitis subgroup patients with regard to renal function using the MDRD formula, MPA patients had lower GFR (P = 0.05) than the CSS and WG groups. By excluding the two CSS cases, the difference became significant (P = 0.03). However, when controlling for eGFR with MDRD, a statistically significant difference in HMGB1 levels between subgroups was not reached. There was no significant difference for HMGB1 with regard to ANCA pattern, since all WG patients were proteinase 3 (PR3) positive and all MPA and CSS patients were myeloperoxidase positive (MPO), with the exception of one WG case, who was double positive.
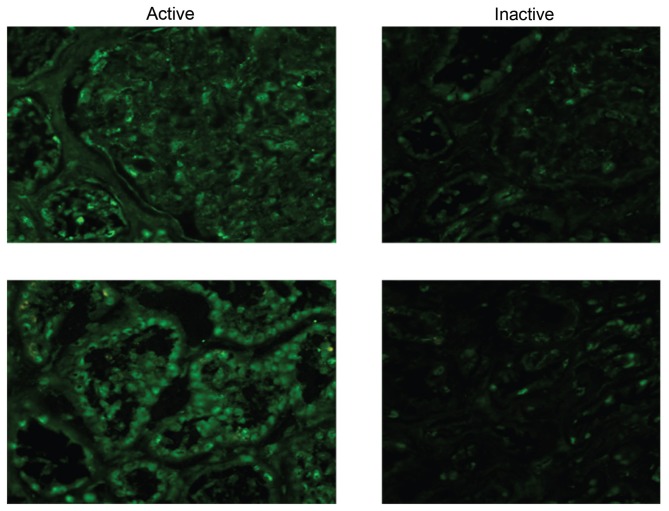
Immunohistochemistry of a renal biopsy revealed a stronger staining for HMGB1 in active disease compared with the same patient at rebiopsy 6 months later (Figure 3).
Figure 3.
HMGB1 staining by immunohistochemistry of paired kidney biopsies (active disease versus remission). Expression of HMGB1 (green Alexa 488) appears stronger with a more distinct extranuclear staining pattern in active disease than in remission.
DISCUSSION
In our present report, we show significantly elevated serum HMGB1 in AAV cases with active renal manifestations compared with patients with inactive renal disease. Patients with available serum as well as renal biopsies both in active and inactive disease, thus serving as their own controls, had significantly lower serum HMGB1 in renal remission phase. Tissue staining with immunohistochemistry was also consistent with a more distinct pattern with extranuclear HMGB1 expression in active renal disease.
ANCA-associated vasculitides are autoimmune systemic inflammatory diseases associated with frequent kidney involvement and the presence of ANCA. The introduction of immuno-suppression and glucocorticoids has greatly improved outcomes, but kidney involvement increases the risk for renal dysfunction and end-stage renal disease (28). Relapses are frequent with risk of further organ damage, and some patients have persistent low disease activity, called grumbling disease (29). There is a need for improved biomarkers to predict drug responsiveness, relapse risk, as well as detection of subclinical disease activity. Recently, IL-18 was found to be upregulated in renal biopsies from patients with ANCA-associated vasculitis. In vitro experiments have shown that IL-18 primes ANCA-induced neutrophils. The use of anti-TNF antibody did not block this effect, indicating that separate pathways and cytokines other than TNF may be of importance for the pathogenesis in these diseases (30). Furthermore, it was recently shown that synovial HMGB1 protein and mRNA expression did not change in any consistent manner after 9 weeks of TNF-blocking therapy with infliximab in rheumatoid synovitis, suggesting that HMGB1 expression may not depend on TNF activity in patients with RA (31). In AAV, TNF blockade with etanercept was found to not be effective for the maintenance of remission in patients with WG when added to standard therapy (32).
HMGB1 is an interesting biomarker candidate in AAV, since it is actively secreted by immune cells and also passively released by injured and necrotic cells and has been shown to stimulate necrosis-induced inflammation (9–12). In a rat model of adenine-induced nephropathy, it was shown that HMGB1 promotes granulomatous nephritis and that the HMGB1 receptors RAGE and TLR4 were expressed in granulomatous nephritis tissue (33). Sato et al. (34) detected HMGB1 in the sera of patients with active renal diseases who underwent renal biopsies, among them AAV, Henoch-Schönlein purpura and IgA nephropathy with crescentic formation. However, these cases were all active. Recently, Wibisono et al. (35) reported increased serum HMGB1 levels in active WG. Furthermore, HMGB1 levels were only elevated in active Wegener cases but not in MPA, and the authors therefore suggested that HMGB1 serum levels could vary with the amount of necrotic tissue, which could be more abundant in WG. However, renal involvement could not be assessed, since organ manifestations were not specified in the report. With regard to the Wibisono study, our results contradict their suggestion that serum HMGB1 differentiates between active forms of AAV, since we could not find any significant difference in HMGB1 levels between subgroups. Although we cannot say with certainty that in the current study renal involvement reflects HMGB1 activity, it is possible that the difference could be due to fewer patients with necrotizing and/or crescentic glomerulonephritis in the Wibisono study, hence the conflicting results regarding MPA. Another factor that could affect the diverging results is the method for HMGB1 detection. We analyzed our samples with a commonly used Western blot method, whereas Wibisono used a commercial ELISA. However, Urbonaviciute et al. (36) found a discrepancy between Western blot and ELISA results, suggesting that serum/plasma components bind to HMGB1 and interfere with its detection by ELISA systems (36).
It is likely that HMGB1 with its potential as a proinflammatory cytokine emanates from systemic inflammation in AAV in addition to local necrotizing or granulomatous processes. Our study, to our knowledge the largest one in HMGB1 and vasculitis with renal manifestations, includes both active cases and cases without active renal lesions and may thus add more insight into the role of HMGB1 regulation during different disease stages. However, it would be important to study AAV patients with different disease manifestations to clarify the difference between localized disease and systemic disease, as well as the importance of kidney disease for HMGB1 expression.
The definition of renal involvement in this report was based on the histological report. Because a kidney biopsy is a snapshot, it is possible that some minor activity was missed even though the quality of the biopsy and the expertise of the pathologist was excellent. Persistence of low-grade hematuria and/or proteinuria could also correlate to some remaining activity. However, the four patients with a BVAS >0 and renal remission according to the pathology report presented mainly with other disease manifestations such as joints; ear, nose and throat; lung; and neuropathy either as grumbling disease or new-onset symptoms.
Nevertheless, our results indicate that patients with biopsy-proven renal remission, albeit not always in complete disease remission according to BVAS, had lower circulating HMGB1 levels than patients with active renal vasculitis, suggesting a role for HMGB1 in kidney disease associated with AAV.
Interestingly, HMGB1 remained elevated in remission when compared with historical controls, which may reflect remaining low-grade inflammatory activity or tissue damage. Considering the lack of correlation with common inflammatory markers such as CRP and ESR in the current study, HMGB1 merits further research as a marker for both active disease and possible subclinical activity in AAV.
We have previously shown that HMGB1 correlates with renal function in chronic kidney disease (25). In the current study, we could not find such a correlation. One possible explanation is the limited patient number; another explanation is that the renal function was significantly higher and the range smaller compared with the previous study, which included patients with a wide range of glomerular filtration rates. When comparing the vasculitis subgroups, MPA patients had significantly lower GFR than WG patients, which is a common finding and has implications for worse outcome in AAV (37). Again, although HMGB1 levels did not differ significantly between AAV groups in the current study, HMGB1 seems to be a relevant factor with regard to renal function and would be worthwhile to follow over time in AAV with renal involvement.
Therapeutic targeting of HMGB1 using antagonistic, truncated HMGB1 A box protein or neutralizing HMGB1-specific antibodies has been demonstrated to confer protection in animal models of sepsis, endotoxemia and collagen-induced arthritis (10,20,21). If HMGB1 is proven to be an important inflammatory mediator in vasculitis (in particular, if anti-HMGB1 antibodies will diminish disease activity in AAV models), reducing HMGB1 expression may be an interesting new drug target in AAV.
In summary, we have demonstrated increased serum HMGB1 levels in active AAV with renal involvement with significantly lower levels in the remission phase. However, serum levels of HMGB1 in the remission phase still remained elevated compared with healthy controls. These findings may reflect a persistent low-grade inflammatory activity or tissue damage despite an appearance of clinical and histopathological renal remission. Further studies may reveal whether HMGB1 is a predictor of disease activity and outcome in AAV and a possible target for pharmacological modulation.
ACKNOWLEDGMENTS
We thank Westman Research Fund, Karolinska Institutet Funds, The Swedish League Against Rheumatism, King Gustav V:S Memorial Fund, the Swedish Research Council, The Swedish Society of Medicine and the Fund for Renal Research.
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Jennette JC, et al. Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 2.Jayne D. Progress of treatment in ANCA-associated vasculitis (Review) Nephrology (Carlton) 2009;14:42–8. doi: 10.1111/j.1440-1797.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 3.Voswinkel J, Muller A, Lamprecht P. Is PR3-ANCA formation initiated in Wegener’s granulomatosis lesions? Granulomas as potential lymphoid tissue maintaining autoantibody production. Ann N Y Acad Sci. 2005;1051:12–9. doi: 10.1196/annals.1361.042. [DOI] [PubMed] [Google Scholar]
- 4.Xiao H, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little MA, et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;15:2050–8. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- 6.Hattar K, et al. Wegener’s granulomatosis: antiproteinase 3 antibodies induce monocyte cytokine and prostanoid release-role of autocrine cell activation. J Leukoc Biol. 2002;71:996–1004. [PubMed] [Google Scholar]
- 7.Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989;243:1056–9. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi ME, Beltrame M. Upwardly mobile proteins. Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO Rep. 2000;1:109–14. doi: 10.1093/embo-reports/kvd030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yang H, Tracey KJ. Extra cellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–31. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 12.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 13.Andersson U, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treutiger CJ, et al. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–85. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Kokkola R, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 17.Yu M, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–9. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 18.Kokkola R, et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002;46:2598–603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- 19.Pullerits R, et al. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 2003;48:1693–700. doi: 10.1002/art.11028. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokkola R, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–8. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 22.Sunden-Cullberg J, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–73. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 23.Hatada T, et al. Plasma concentrations and importance of high mobility group box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94:975–9. doi: 10.1160/TH05-05-0316. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein RS, et al. Cholinergic anti-inflammatory pathway activity and high mobility group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–5. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruchfeld A, et al. High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD) Mol Med. 2008;14:109–15. doi: 10.2119/2007-00107.Bruchfeld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ostberg T, et al. Protective targeting of HMGB1 in a spontaneous arthritis model. Arthritis Rheum. 2010;62:2963–72. doi: 10.1002/art.27590. [DOI] [PubMed] [Google Scholar]
- 28.Booth AD, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–84. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 29.Hogan SL, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–31. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Hewins P, et al. IL-18 is upregulated in the kidney and primes neutrophil responsiveness in ANCA-associated vasculitis. Kidney Int. 2006;69:605–15. doi: 10.1038/sj.ki.5000167. [DOI] [PubMed] [Google Scholar]
- 31.Sundberg E, et al. Systemic TNF blockade does not modulate synovial expression of the pro-inflammatory mediator HMGB1 in rheumatoid arthritis patients: a prospective clinical study. Arthritis Res. Ther. 2008;10:R33. doi: 10.1186/ar2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 33.Oyama Y, et al. High-mobility group box-1 protein promotes granulomatous nephritis in adenine-induced nephropathy. Lab Invest. 2010;90:853–66. doi: 10.1038/labinvest.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato F, et al. High mobility group box chromosomal protein 1 in patients with renal diseases. Nephron. Clin. Pract. 2008;108:c194–201. doi: 10.1159/000118942. [DOI] [PubMed] [Google Scholar]
- 35.Wibisono D, et al. Serum HMGB1 levels are increased in active Wegener’s granulomatosis and differentiate between active forms of ANCA-associated vasculitis. Ann Rheum Dis. 2010;69:1888–9. doi: 10.1136/ard.2009.119172. [DOI] [PubMed] [Google Scholar]
- 36.Urbonaviciute V, Fürnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007;81:67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- 37.Mukhtyar C, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67:1004–10. doi: 10.1136/ard.2007.071936. [DOI] [PubMed] [Google Scholar]