Abstract
Previously, we found that male JYD mice developed type 2 diabetes but female mice did not, and that decreased expression levels of caveolin-1 were correlated with the development of a diabetic phenotype in these mice. Therefore, we hypothesized that sex hormones affect the expression of caveolin-1 and contribute to the development of insulin resistance and hyperglycemia in JYD mice. We used glucose and insulin tolerance tests to examine insulin sensitivity in male, female and orchidectomized male JYD mice. Glucose uptake was analyzed by using 18F-fluorodeoxyglucose positron emission tomography. We also examined insulin-signaling molecules and caveolin proteins in various tissues in these mice by Western blotting. In addition, we examined changes of caveolin-1 expression in L6 skeletal muscle cells treated with 17-β estradiol or dihydroxytestosterone. We found that glucose and insulin tolerance were impaired and hyperglycemia developed in male, but not female, JYD mice. Expression of insulin-signaling molecules such as insulin receptor, protein kinase B, and glucose transporter-4 were decreased in male JYD mice compared with female mice. Orchidectomized JYD male mice showed improved glucose and insulin tolerance with a concomitant increase in the expression of insulin-signaling molecules and caveolin-1 in adipose tissue and skeletal muscle. Moreover, 17-β-estradiol treatment increased the expression of caveolin-1 in differentiated skeletal muscle cells. We conclude that sex hormones modulate the expression of caveolin-1 and insulin-signaling molecules, subsequently affecting insulin sensitivity and the development of type 2 diabetes in JYD mice.
INTRODUCTION
Onset of type 2 diabetes and metabolic syndrome in old age has been observed to be more prevalent in men than in women (1), suggesting that sex hormones may affect the development of type 2 diabetes. Circulating levels of sex hormone binding protein, which binds strongly to testosterone and to a lesser extent to estradiol, is a predictor of the risk of type 2 diabetes (2–4). It was previously reported that high serum levels of testosterone in polycystic ovary syndrome is a risk factor for type 2 diabetes and impaired glucose tolerance (5,6). Sex hormones such as estrogen and testosterone are known to modulate glucose and lipid metabolism (7), and administration of testosterone to women impaired glucose tolerance and induced hyperinsulinemia (8). In animal studies, insulin sensitivity was reduced in skeletal muscle of ovarectomized female rats, and treatment of these rats with estradiol restored insulin sensitivity and glucose uptake (9).
Caveolin-1, a 21–24-kDa integral membrane protein, is a major component of the caveolae, which are flask-shaped membrane invaginations enriched with cholesterol, glycosphin-golipid and lipid-modified signaling proteins (10). Caveolae function in vesicular transport, endocytosis and transcytosis (11), and also play a role in signal transduction (12). Caveolin-1 and steroid hormones appear to have reciprocal interactions. Caveolin-1 regulates steroid hormone signaling by directly interacting with hormone receptors in the membrane (13,14), and estrogens modulate caveolin-1 expression in both MCF breast cancer cells and vascular smooth muscle cells (13,15). In addition, caveolin-1 regulates insulin signaling by the glucose uptake system (16,17).
We previously found that male, but not female, JYD mice developed type 2 diabetes in old age, and that reduced levels of caveolin-1 were correlated with the development of diabetes in male mice (18). In the present study, we investigated whether sex hormones affect the development of insulin resistance and diabetes in JYD mice, and if so, whether this effect is correlated with modulation of caveolin-1 expression.
MATERIALS AND METHODS
Animals
JYD F1 mice were obtained from mating female C57BL/6 and male DBA/2 mice, as previously described (18). Mice were given free access to food and water, except when noted otherwise, and were kept on a cycle of 12-h light/12-h dark at 23°C and 65% humidity. Animals used for this study were 25–30 wks old. Mice with blood glucose levels higher than 250 mg/dL were considered to be diabetic. All protocols for animal use and euthanization were reviewed and approved by the animal care committee of the Gachon University of Medicine and Science and Seoul National University College of Medicine.
Orchidectomy
When male mice were 20–25 wks old (nonfasting blood glucose level ≥200 mg/dL), we performed surgical orchidectomy while mice were under light avertin anesthesia, as previously described (19). Sham-operated animals received a ventral incision, but the testes remained intact.
Glucose- and Insulin-Tolerance Tests
Glucose- and insulin-tolerance tests were performed when mice were 30 wks old. For glucose tolerance tests, mice were fasted overnight and then injected with glucose (2 g/kg body weight interperitoneally). A glucometer (Onetouch; Lifescan, Milpitas, CA, USA) was used to measure glucose in the tail vein blood at 0, 15, 30, 60, and 120 min after injection. For insulin tolerance tests, fed mice were injected with insulin (1U/kg body weight interperitoneally) (Humilin; Lilly, Indianapolis, IN, USA), and tail vein blood glucose was measured at 0, 15, 30, 60, and 120 min after injection. Changes in blood glucose were calculated as the percentage of the initial blood glucose level.
Cells and In Vitro Hormone Treatment
L6 cells, a rat skeletal muscle cell line, were obtained from American Type Culture Collection (Manassas, VA, USA). Pre-myocytes were differentiated into myotubules and maintained in DMEM/F12 containing 2% (vol/vol) fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin. Fully differentiated cells were serum starved for 24 h and treated with 25 ng/mL 17β-estradiol (E2) (Sigma, St. Louis, MO, USA) or dihydroxytestosterone (DHT) (Sigma).
Glucose Uptake Measured by Using 18F-Fluorodeoxyglucose Positron Emission Tomography
Positron emission tomography (PET) scanning was performed with a dedicated small animal PET system (microPET-R4; Concorde Microsystems, Knoxville, TN, USA). Mice were starved for 18–20 h and then injected intravenously with 18F-fluorodeoxyglucose (FDG) (7.4 MBq [200 μCi] in 0.1 mL; KIRAMS, Seoul, Korea). Whole-body PET imaging was started 60 min after 18F-FDG injection and continued for 30 min. Mice were kept anesthetized with isoflurane (0.5% [vol/vol] in oxygen) and placed on a heating pad at 30°C for the duration of the procedure. The PET data were transformed into two-dimensional sinograms by using histo-gramming and Fourier rebinning and were reconstructed to tomographic data by using the two-dimensional ordered subset expectation maximization algorithm (18,20,21). The three-dimensional regions of interest (ROIs) were manually drawn around the brain, liver, and hindlimb muscle activity by visual inspection with the nonproprietary Amide’s a Medical Image Data Examiner (AMIDE) software. The mean activities were recorded from the entire ROI. The percentage injected dose per gram (%ID/g) and the glucose standardized uptake values (SUVG) were calculated as follows: %ID/g = ROI activity divided by injected dose; SUVG = (ROI activity × body weight × normalized blood glucose level) divided by injected dose.
Insulin Secretion Assay
Serum samples were collected 30 min after glucose loading (2 g/kg body weight intraperitoneally) of mice that had been fasted overnight. The amounts of secreted insulin were determined by using a mouse insulin ultrasensitive enzyme immunoassay kit (Alpco Diagnostics, Windham, NH, USA) according to the manufacturer’s instructions.
Immunohistochemistry
Tissue sections were deparaffinized in toluene, rehydrated in grades of alcohol, and washed in water. After antigen unmasking, all slides were blocked in blocking solution (2.5% horse serum) for 1 h at room temperature and then incubated at 4°C with primary antibody solution: mouse anti–caveolin-1 (1:100; BD Transduction, Palo Alto, CA, USA). Sections were incubated with biotinylated secondary antibody (Vectastatin Kit; Vector Laboratories, Burlingame, CA, USA), and staining was performed with VIP as a chromogen (Vector VIP kit, Vector Laboratories). Hematoxylin (Sigma, St Louis, MO, USA) was used as a nuclear counterstain for light microscopy.
Western Blot
When the mice were 30 wks old, adipose tissue (epididymal fat), muscle (hindlimb), liver and pancreas were removed and homogenized. Differentiated skeletal muscle cells treated with E2 or DHT were lysed in 1% sodium dodecyl sulfate (SDS) buffer (1% SDS [wt/vol], 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L phenylmethysulfonyl fluoride, 1 mmol/L NaF). Proteins (50 μg) were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% separating gel. After electrophoresis, membranes were blocked with 3% skim milk in Tris-buffered saline with Tween buffer (0.1 % Tween 20, 200 mmol/L Tris-HCl, 500 mmol/L NaCl, pH 7.4) for 1 h at room temperature. After blocking, membranes were incubated overnight at 4°C with one of the following primary antibodies: anti–insulin receptor (IR)-β antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), antitubulin antibody (Santa Cruz Biotechnology), anti–glucose transporter-4 (GLUT-4) antibody, anti–Akt/protein kinase B (Akt/PKB) antibody (BD Transduction, Palo Alto, CA, USA) and anti–caveolin-1 and -3 antibodies (BD Transduction). Immuno-reactive proteins were detected with an enhanced chemiluminescence Western blot detection kit (Pierce, Rockford, IL, USA), and the relative abundance of each band was estimated by densitometric analysis.
Statistics
Results are presented as mean ± SEM. Results were evaluated by ANOVA, and pairwise comparisons were made by using the Duncan multiple range test. A level of P < 0.05 was considered to be significant.
RESULTS
Body Weight, Diabetic Incidence and Glucose and Insulin Tolerance in Male and Female JYD Mice
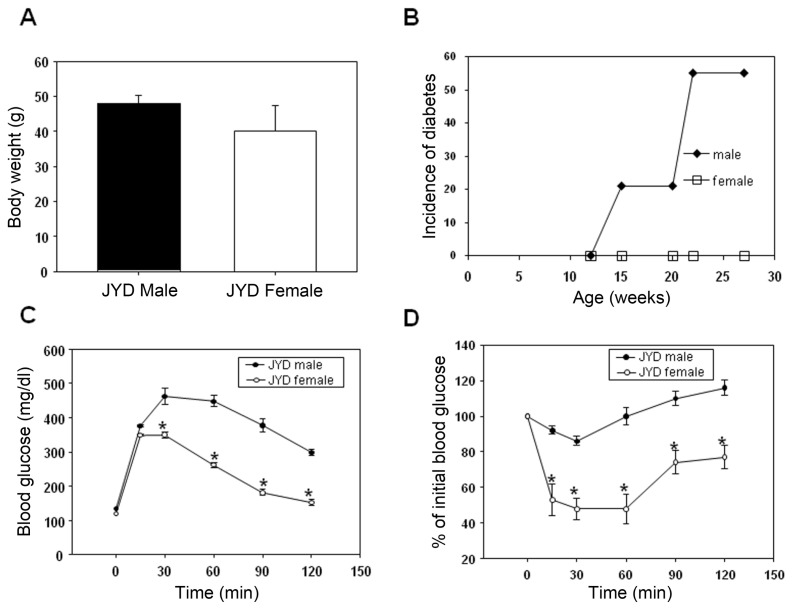
The incidence of diabetes was determined in male and female JYD mice at 30 wks of age. Although body weights were not different (Figure 1A), about 50% of male JYD mice were diabetic at 30 wks of age, whereas diabetes was not detected in female mice (Figure 1B). Fasting glucose levels were significantly higher in male mice compared with female mice (134 ± 5 mg/dL, 116 ± 4 mg/dL). Glucose tolerance tests showed that the blood glucose levels of male mice peaked at 30 min after glucose injection and were significantly higher at all times compared with levels in female mice (Figure 1C). Moreover, results of insulin tolerance tests showed that blood glucose levels in female JYD mice were reduced after insulin injection, whereas blood glucose levels in male JYD mice were not reduced (Figure 1D).
Figure 1.
(A) Body weight (n = 8/group), (B) cumulative incidence of diabetes (n = 15/group), (C) glucose tolerance tests (n = 5/group) and (D) insulin tolerance tests (n = 5/group) of male and female JYD mice at 30 wks of age. *P < 0.05 compared with male JYD mice.
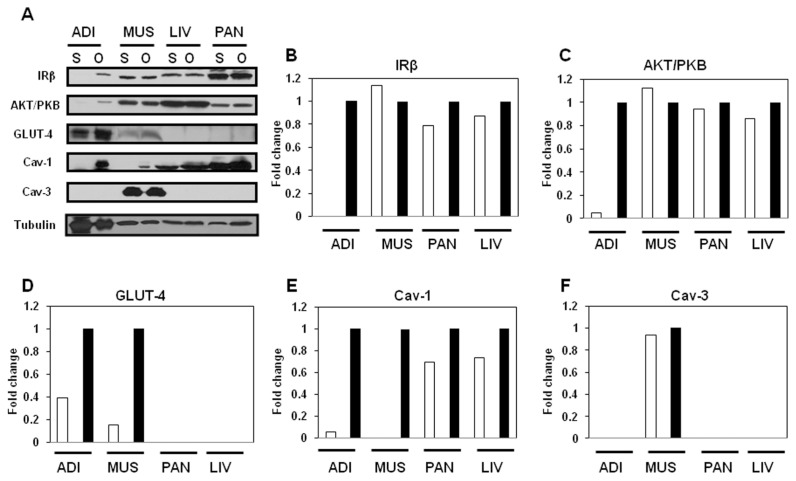
Expression of Insulin-Signaling Molecules and Caveolins in Male and Female JYD Mice
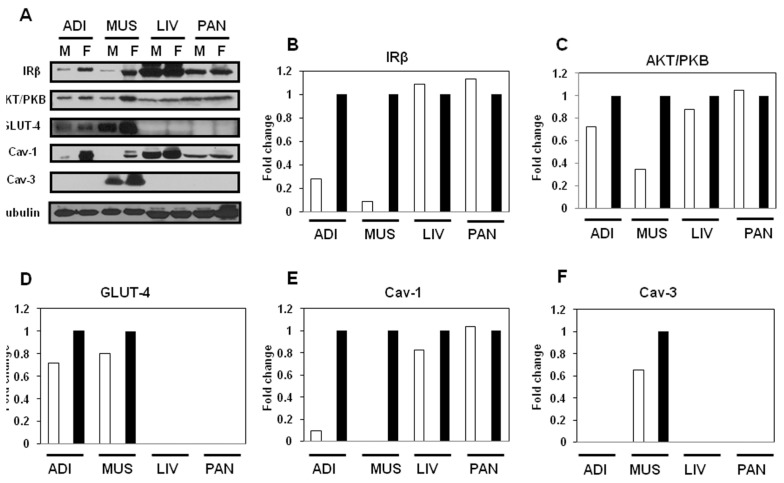
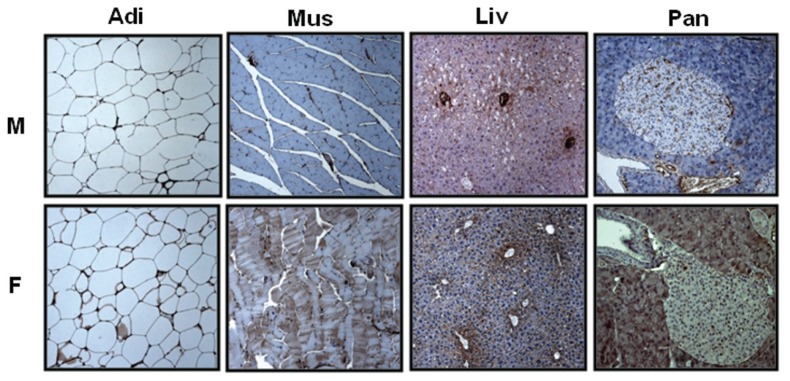
We used Western blot to examine the expression levels of insulin-signaling– related proteins such as IRβ, Akt/PKB and GLUT-4, as well as caveolins in insulin-sensitive tissues, in male and female JYD mice at 30 wks of age (Figure 2A). The expression of IRβ was dramatically decreased in adipose tissue of male JYD mice compared with female mice (Figure 2B). In skeletal muscle, expression levels of IRβ (Figure 2B), Akt/PKB (Figure 2C) and GLUT-4 (Figure 2D) were lowered in male mice compared with females. Expression of these insulin- signaling molecules was not found to be different in the liver and pancreas of male and female JYD mice. Previously, we reported that reduced expression of caveolin-1 in skeletal muscle was correlated with type 2 diabetes in JYD mice (18); therefore, we checked expression of caveolin in male and female mice. Caveolin-1 expression levels were lowered in both adipose tissue and skeletal muscle of JYD male mice compared with female mice (Figure 2E). Levels of caveolin-3, the skeletal muscle–specific isoform, were slightly lowered in male mice compared with female mice (Figure 2F). To determine if caveolin-1 expression is affected in a particular cell type within adipose, skeletal muscle, liver and pancreas tissues, we performed immunohistochemical analysis of caveolin-1 in these tissues (Figure 3). We found that caveolin-1 in adipose tissues showed an increased staining of the cellular membrane in female mice compared with male mice. In skeletal muscle tissue, stained caveolin-1 cells were increased in muscle fibers of female mice. In liver tissues, caveolin-1 was located on the sinusoidal lining cells and bile canaliculi (22), and the expression level of caveolin-1 on both cell types was increased in female mice. Caveolin-1 stained cells were detected both in pancreatic exocrine cells (23) and endocrine cells (24) of female mice, but detected only in endocrine cells of male mice.
Figure 2.
Expression level of insulin-signaling molecules and caveolins in male and female JYD mice. (A) Insulin-signaling molecules and caveolin proteins were analyzed by Western blot in adipose tissue (Adi), skeletal muscle (Mus), liver (Liv) and pancreas (Pan) of male (M) and female (F) JYD mice at 30 wks of age. Tubulin was used to confirm equal loading. (B–F) Quantitative analysis of Western blots in tissues. The relative fold-change in male mice (white bars) to its control band (female mice, black bars) on Western blots was quantified by densitometric analysis.
Figure 3.
Immunohistochemical analysis of caveolin in various tissues. In male (M) and female (F) mice at 30 wks of age, sections of adipose tissue (Adi), skeletal muscle (Mus), liver (Liv) and pancreas (Pan) were stained with anti–caveolin-1 antibody (100×).
Glucose and Insulin Tolerance in Orchidectomized Male JYD Mice
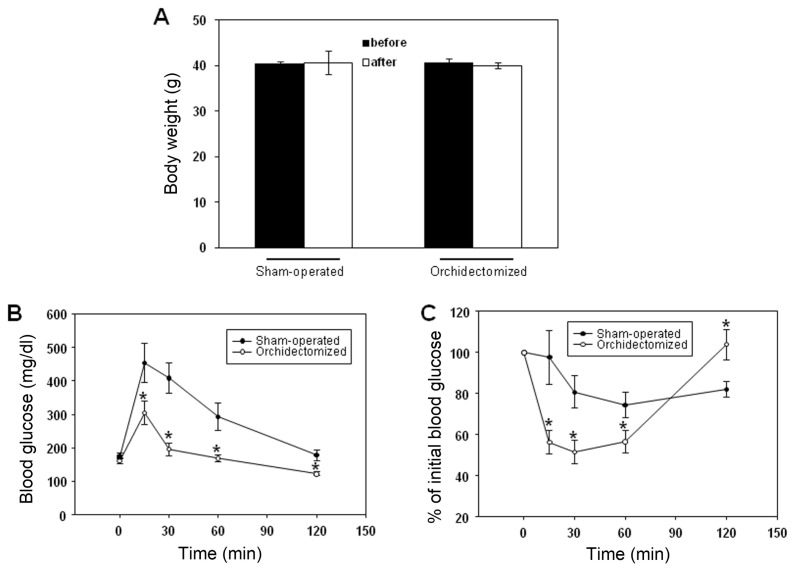
To determine whether sex hormones play a role in the development of type 2 diabetes in male JYD mice, we examined glucose and insulin tolerance in orchidectomized male JYD mice at 30 wks of age, 5 wks after the orchidectomy. Sham-operated or orchidectomized male JYD mice showed no significant weight changes after surgery (Figure 4A). After glucose loading, exogenous glucose was more efficiently cleared in orchidectomized mice than in sham-operated mice (Figure 4B). Also, exogeneously injected insulin significantly reduced glucose levels in orchidectomized mice compared with sham-operated mice (Figure 4C).
Figure 4.
Glucose and insulin tolerance in orchidectomized male JYD mice. (A) Body weights before and 5 wk after orchidectomy for sham-operated and orchidectomized mice. (B) Glucose and (C) insulin-tolerance tests were performed in orchidectomized and sham-operated male mice at 5 wk after orchidectomy; n = 5/group, *P < 0.05, compared with sham-operated mice.
Insulin Secretion and Glucose Uptake in Orchidectomized Male JYD Mice
Both insulin secretion from the pancreas and glucose uptake in peripheral tissues play major roles in the development of type 2 diabetes. To test whether sex hormones affect insulin secretion, we checked the serum insulin levels of male, female, and orchidectomized male mice in both fed and fasted conditions. We found that both fed and fasted orchidectomized male mice had higher serum insulin levels compared with nonorchidectomized male mice, but this finding was not statistically significant (Figure 5A). Next, we compared overall glucose uptake among female, male and orchidecetomized male JYD mice by using FDG-PET methods (18). FDG uptake was highest in orchidectomized male mice, and uptake was higher in female mice compared with intact male mice at 30 wks of age, especially in brain and lung tissue (Figure 5B). Because the major organs responsible for glucose uptake are the liver, muscle and brain, we performed ROI analysis of FDG-PET images. The mean glucose standardized uptake values (SUVG) of brain (21.273) and liver (5.454) in orchidectomized male mice were higher than those of brain (18.348) and liver (4.304) in male mice, but were lower than those of brain (24.508) and liver (5.947) in female mice. The SUVG of muscle (2.94) in female mice was higher than in male and orchidectomized male mice (2.36 and 2.66, respectively) (Figure 5C).
Figure 5.
Insulin secretion and glucose uptake in orchidectomized male JYD mice. (A) Serum insulin levels in fasting (overnight fasting) and nonfasting (at 30 min after glucose loading) male (M), female (F) and orchidectomized male (OM) JYD mice. (B) Sagittal sections through male (M), female (F) and orchidectomized male (OM) JYD mice. %ID/g indicates percent injected dose per gram. (C) Glucose standardized uptake values (SUVG ) of brain, liver and muscle in male (M), female (F), and orchidectomized male (OM) JYD mice. SUVG = ROI activity × body weight × normalized blood glucose level.
Expression of Insulin-Signaling Molecules and Caveolins in Orchidectomized Male JYD Mice
To determine whether sex hormones affect the expression of insulin-signaling proteins and caveolins, we compared the levels of IRβ, Akt/PKB and GLUT-4 in insulin-sensitive tissues of orchidectomized and sham-operated JYD male mice at 30 wks of age (Figure 6A). Levels of IRβ (Figure 6B) and Akt/PKB (Figure 6C) were dramatically increased only in adipose tissue, whereas GLUT-4 was increased in both adipose tissue and skeletal muscle of orchidectomized mice compared with sham-operated mice (Figure 6D). No differences in protein levels were observed in the pancreas or liver. Levels of caveolin-1 were increased in adipose tissue and skeletal muscle in orchidectomized mice compared with sham-operated mice (Figure 6E), whereas the level of caveolin-3, the muscle-specific caveolin isoform, was not different between two groups (Figure 6F).
Figure 6.
Expression of insulin-signaling molecules and caveolins in orchidectomized male JYD mice. (A) Insulin-signaling molecules and caveolin proteins were analyzed by Western blot in adipose tissue (Adi), skeletal muscle (Mus), liver (Liv) and pancreas (Pan) of sham-operated (S) or orchidectomized (O) male JYD mice at 5 wks after orchidectomy. Tubulin was used to confirm equal loading. (B–F) Quantitative analysis of Western blots in tissues. The relative fold-change in sham-operated mice (white bars) compared to the control band (orchidectomized mice, black bars) on Western blots was quantified by densitometric analysis.
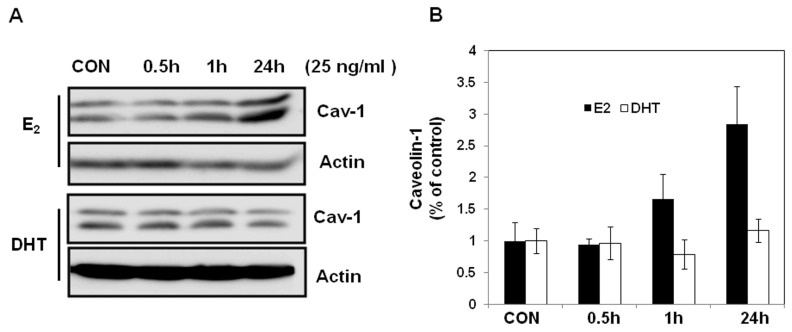
Caveolin-1 Expression after Estradiol or Dihydroxytestosterone Treatment In Vitro
Previously, we observed that overexpression of caveolin-1 in skeletal muscle of JYD mice increased insulin sensitivity (18). To determine whether the increased sensitivity seen in orchidectomized male JYD mice might be an effect of sex hormones on caveolin-1 expression, we treated differentiated skeletal muscle cells with E2 or DHT in vitro and examined the expression level of caveolin-1. The expression of caveolin-1 was significantly increased by incubation with E2 for 24 h, but treatment with DHT had no effect on expression of caveolin-1 (Figure 7).
Figure 7.
Caveolin-1 expression after in vitro E2 and DHT treatment. Differentiated L6 skeletal muscle cells were incubated in the absence (CON) or presence of 25 ng/mL of E2 or DHT for various periods of time under serum-free conditions and then harvested. (A) Total protein was extracted, separated by SDS-PAGE and probed with antibodies against caveolin-1. Actin was used to confirm equal loading. (B) Quantitative analysis of Western blots. Relative abundance of each band was estimated by densitometric analysis. Each bar is the mean ± SEM of three experiments.
DISCUSSION
Type 2 diabetes is the most common form of diabetes, which is characterized by both insulin resistance and insulin deficiency (25). It was recently reported that type 2 diabetes is significantly more common in women, but the prevalence increases with old age in men whereas it remains unchanged in women (1). In addition, some animal models of type 2 diabetes such as OLEFT rats and Wistar, TalleyHo, TSOD and JYD mice develop diabetes specifically in males (18,26). In the present study, we investigated whether sex hormones affect the development of insulin resistance and diabetes in JYD mice, and if so, whether this effect is correlated with modulation of caveolin-1 expression.
We first compared the incidence of diabetes between male and female JYD mice and confirmed that the development of diabetes was male specific. Consistent with this result, only male mice showed impaired glucose and insulin tolerance. It was previously reported that orchidectomy before puberty had protective effects against the development of hyperglycemia in male, but not in female, spontaneously hypertensive rats injected with streptozotocin (27)—a finding that suggests that sex hormones might be involved in the development of diabetes. To determine whether the male-specific diabetic phenotype in JYD mice is affected by sex hormones, we performed orchidectomies in male JYD mice. Orchidectomized male JYD mice had markedly attenuated severity of hyperglycemia, improved insulin sensitivity and increased whole-body glucose uptake compared with sham-operated male JYD mice. These results indicate that sex hormones may influence insulin sensitivity in JYD mice.
Although our results suggest that male sex hormone deficiency is associated with improved glucose and insulin tolerance in male JYD mice, evidence from other studies has indicated that testosterone deficiency is associated with metabolic syndrome, including type 2 diabetes and insulin resistance (28). Testosterone levels are significantly lower in men with type 2 diabetes compared with age-matched controls (4), and low testosterone levels can predict the development of metabolic syndrome (29). However, conflicting results have been obtained in investigations of the effects of testosterone on insulin sensitivity in humans, depending on age, fat mass and basal testosterone levels. For example, insulin sensitivity was not changed by increasing doses of testosterone in young and healthy men (30), and testosterone had no effect on insulin action in normal young men (31) or in men with hypogonadism (32, 33). In animals, administration of testosterone to castrated male rats has been shown to induce insulin resistance (34). We consider it possible that the development of male-specific diabetes in our particular polygenic animal model was not simply a matter of testosterone levels, but the mechanism might be more complex and depend, in part, on the relative proportions of male and female sex hormones.
We next examined the expression of insulin-signaling molecules in various tissues and found that the expression levels of IR, Akt/PKB and GLUT-4 in muscle and adipose tissues were lower in male mice compared with female JYD mice. However, the expression of these molecules was not different in the pancreas and liver in males compared with females, which suggests that tissue-specific changes in the expression of insulin signaling molecules are associated with the sex of the mice. Similarly, E2 had no effect on the amount of hepatic IR protein, but significantly increased the expression of IR protein in the uterus of ovarectomized rats (35). In orchidectomized male JYD mice, we observed increased expression levels of IR, Akt/PKB, and GLUT-4 in adipose tissue and GLUT-4 in skeletal muscle, which suggests that changes in insulin-signaling molecules and GLUT-4 contribute to the improvement of insulin sensitivity after orchidectomy. Moreover, these results suggest that adipose tissue may be a major determinant for insulin sensitivity in orchidectomized JYD mice, because orchidectomy-induced changes in the expression of insulin-signaling molecules were most prevalent in adipose tissue.
Caveolins, integral plasma membrane proteins present in caveolae, along with scaffolding, transport and signaling-cascade proteins (36), have emerged as key players in obesity and insulin resistance (16,37). Recently, it was reported that caveolin-1 was associated with sex hormone sensitivity (38) as well as insulin sensitivity (39). We previously found that decreased levels of skeletal muscle caveolin-1 were correlated with the progression of type 2 diabetes in male JYD mice and that caveolin-1 plays a determining role in the senescent phenotype: upregulation of caveolin-1 in male JYD mice significantly improved insulin sensitivity, with a concomitant increase of glucose uptake (16,18). Therefore, we investigated whether sex hormones affect caveolin-1 expression and insulin sensitivity. We confirmed that expression levels of caveolin-1 were downregulated in male mice compared with female mice, but were similar between orchidectomized male mice and female mice. Moreover, orchidectomy affected glucose uptake, particularly in insulin-sensitive tissues, but did not affect insulin secretion. The addition of E2 in vitro increased the expression of caveolin-1 in differentiated muscle cells, whereas the addition of DHT did not affect caveolin-1 expression. Therefore, it might be presumed that reduction of male sex hormones and upregulation of female sex hormones by orchidectomy (27) would upregulate caveolin-1 expression in skeletal muscle and adipose tissue, and this might lead to enhanced glucose uptake.
The mechanism by which estrogen regulates cytoplasmic signaling is not yet clear. It was reported that E2 rapidly stimulates the association of the estrogen receptor and caveolin-1 in vascular smooth muscle cells, and this complex influences PI3K/Akt activity (40). Similarly, we found that in orchidectomized JYD mice the level of Akt/PKB was increased in adipose tissue, with a concomitant increase of caveolin-1 expression. These results indicate that at least E2 appears to affect insulin sensitivity by regulating caveolin-1 expression in orchidectomized JYD mice. The effect of hormones other than E2 on caveolin expression and insulin sensitivity in JYD mice remains to be elucidated.
Taken together, our data lead us to conclude that sex hormones play an important role in the development of diabetes in male JYD mice, probably by modulating the expression of caveolin-1, which in turn upregulates insulin-related signaling molecules and enhances insulin sensitivity, contributing to the prevention of diabetes in female and orchidectomized male JYD mice.
ACKNOWLEDGMENTS
This study was supported by grants from the Aging and Apoptosis Research Center of the Korea Science and Engineering Foundation (RII-2002-097-05001-0, RII-2002-097-00001-0), National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (MEST) (M20702010002-08N0201-00200), and the Innovative Research Institute for Cell Therapy (A062260), Korea. We thank S-K Woo for technical assistance with imaging analysis and A Kyle for editorial assistance.
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Meneilly GS, Tessier D. Diabetes in elderly adults. J Gerontol A Biol Sci Med Sci. 2001;56:M5–13. doi: 10.1093/gerona/56.1.m5. [DOI] [PubMed] [Google Scholar]
- 2.Ding EL, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–63. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076–84. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]
- 4.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 5.Dunaif A. Insulin resistance in polycystic ovarian syndrome. Ann N Y Acad Sci. 1993;687:60–4. doi: 10.1111/j.1749-6632.1993.tb43854.x. [DOI] [PubMed] [Google Scholar]
- 6.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 7.Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend. Med. 2007;4(Suppl B):S162–77. doi: 10.1016/s1550-8579(07)80056-8. [DOI] [PubMed] [Google Scholar]
- 8.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyl-testosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83:4420–5. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai S, Holmang A, Bjorntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149:91–7. doi: 10.1111/j.1748-1716.1993.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 10.Sargiacomo M, et al. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci U S A. 1995;92:9407–11. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnitzer JE, Allard J, Oh P. NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia. Am J Physiol. 1995;268:H48–55. doi: 10.1152/ajpheart.1995.268.1.H48. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 13.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–15. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 14.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–51. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 15.Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News Physiol Sci. 2001;16:251–5. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 16.Oh YS, et al. Regulation of insulin response in skeletal muscle cell by caveolin status. J Cell Biochem. 2006;99:747–58. doi: 10.1002/jcb.20943. [DOI] [PubMed] [Google Scholar]
- 17.Oh YS, et al. Exercise type and muscle fiber specific induction of caveolin-1 expression for insulin sensitivity of skeletal muscle. Exp Mol Med. 2007;39:395–401. doi: 10.1038/emm.2007.44. [DOI] [PubMed] [Google Scholar]
- 18.Oh YS, et al. A potential role for skeletal muscle caveolin-1 as an insulin sensitivity modulator in ageing-dependent non-obese type 2 diabetes: studies in a new mouse model. Diabetologia. 2008;51:1025–34. doi: 10.1007/s00125-008-0993-0. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe AM, Wray S, Westphal H, Radovick S. Cell-specific expression of the human gonadotropin-releasing hormone gene in transgenic animals. J Biol Chem. 1996;271:20018–23. doi: 10.1074/jbc.271.33.20018. [DOI] [PubMed] [Google Scholar]
- 20.Torizuka T, Fisher SJ, Brown RS, Wahl RL. Effect of insulin on uptake of FDG by experimental mammary carcinoma in diabetic rats. Radiology. 1998;208:499–504. doi: 10.1148/radiology.208.2.9680582. [DOI] [PubMed] [Google Scholar]
- 21.Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology. 1992;183:643–7. doi: 10.1148/radiology.183.3.1584912. [DOI] [PubMed] [Google Scholar]
- 22.Ogi M, et al. Distribution and localization of caveolin-1 in sinusoidal cells in rat liver. Med Electron Microsc. 2003;36:33–40. doi: 10.1007/s007950300004. [DOI] [PubMed] [Google Scholar]
- 23.Denker SP, McCaffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of alpha subunits and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–40. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nevins AK, Thurmond DC. Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J Biol Chem. 2006;281:18961–72. doi: 10.1074/jbc.M603604200. [DOI] [PubMed] [Google Scholar]
- 25.Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–29. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, et al. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics. 2001;74:273–86. doi: 10.1006/geno.2001.6569. [DOI] [PubMed] [Google Scholar]
- 27.Iwase M, et al. Effect of gonadectomy on the development of diabetes mellitus, hypertension, and albuminuria in the rat model. Metabolism. 1996;45:155–61. doi: 10.1016/s0026-0495(96)90046-3. [DOI] [PubMed] [Google Scholar]
- 28.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 29.Haring R, et al. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58:2027–31. doi: 10.2337/db09-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh AB, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87:136–43. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 31.Friedl KE, Jones RE, Hannan CJ, Jr, Plymate SR. The administration of pharmacological doses of testosterone or 19-nortestosterone to normal men is not associated with increased insulin secretion or impaired glucose tolerance. J Clin Endocrinol Metab. 1989;68:971–5. doi: 10.1210/jcem-68-5-971. [DOI] [PubMed] [Google Scholar]
- 32.Basu R, et al. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care. 2007;30:1972–8. doi: 10.2337/dc07-0359. [DOI] [PubMed] [Google Scholar]
- 33.Jedrzejuk D, Medras M, Milewicz A, Demissie M. Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male. 2003;6:151–6. [PubMed] [Google Scholar]
- 34.Holmang A, Bjorntorp P. The effects of testosterone on insulin sensitivity in male rats. Acta Physiol Scand. 1992;146:505–10. doi: 10.1111/j.1748-1716.1992.tb09452.x. [DOI] [PubMed] [Google Scholar]
- 35.Koricanac G, Milosavljevic T, Stojiljkovic M, Zakula Z, Ribarac-Stepic N, Isenovic ER. Insulin signaling in the liver and uterus of ovariectomized rats treated with estradiol. J Steroid Biochem Mol Biol. 2008;108:109–16. doi: 10.1016/j.jsbmb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000;275:21605–17. doi: 10.1074/jbc.M002558200. [DOI] [PubMed] [Google Scholar]
- 37.Cohen AW, Combs TP, Scherer PE, Lisanti MP. Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1151–60. doi: 10.1152/ajpendo.00324.2003. [DOI] [PubMed] [Google Scholar]
- 38.Nasu Y, et al. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nat Med. 1998;4:1062–4. doi: 10.1038/2048. [DOI] [PubMed] [Google Scholar]
- 39.Cohen AW, et al. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285:C222–35. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- 40.Haynes MP, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–82. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]