Abstract
Sepsis and ischemia-reperfusion (I/R) injury are among the leading causes of death in critically ill patients at the surgical intensive care unit setting. Both conditions are marked by the excessive inflammatory response which leads to a lethal disease complex such as acute lung injury, systemic inflammatory response syndrome and multiple organ dysfunction syndrome. Despite the advances in the understanding of the pathophysiology of those conditions, very little progress has been made toward therapeutic interventions. One of the key aspects of these conditions is the accumulation of apoptotic cells that have the potential to release toxic and proinflammatory contents due to secondary necrosis without appropriate clearance by phagocytes. Along with the prevention of apoptosis, that is reported to be beneficial in sepsis and I/R injury, thwarting the development of secondary necrosis through the active removal of apoptotic cells via phagocytosis may offer a novel therapy. Milk fat globule-EGF factor VIII (MFG-E8), which is mainly produced by macrophages and dendritic cells, is an opsonin for apoptotic cells and acts as a bridging protein between apoptotic cells and phagocytes. Recently, we have shown that MFG-E8 expression is decreased in experimental sepsis and I/R injury models. Exogenous administration of MFG-E8 attenuated the inflammatory response as well as tissue injury and mortality through the promotion of phagocytosis of apoptotic cells. In this review, we describe novel information available about the involvement of MFG-E8 in the pathophysiology of sepsis and I/R injury, and the therapeutic potential of exogenous MFG-E8 treatment for those conditions.
INTRODUCTION
The critically ill patient frequently develops a complex disease spectrum that may include acute lung injury (ALI), systemic inflammatory response syndrome (SIRS), sepsis and/or septic shock (1). It has been estimated that in the United States alone more than 750,000 patients per year develop sepsis and septic shock with an overall mortality of 28.6% (2). The incidence of sepsis and septic shock has increased significantly over the past two decades (3–5) and the economic burden of severe sepsis is becoming alarmingly high (2). Current wisdom implies that after severe injury or infectious challenge, some patients respond by overexpressing inflammatory mediators that lead to a systemic inflammatory response that culminates in severe shock, multiple organ dysfunction syndrome (MODS) and death (1).
Ischemia-reperfusion (I/R) injury is also one of the major clinical conditions that induces systemic inflammatory response. Ischemia causes tissue damage, which is exacerbated when reperfusion, restoration of blood flow, occurs (6,7). I/R injury happens commonly in cases of shock, tissue transplantation, myocardial infarction, stroke, certain infections and arterial disease and trauma. The intense inflammation triggered by I/R injury may precipitate inflammatory damage in organs not involved in the initial ischemic insult. These remote effects of I/R injury are observed mostly in the lungs and cardiovascular system, and may result in the development of SIRS and MODS, both of which account for 30% to 40% mortality in tertiary referral intensive care unit (8). Despite extensive investigations, the cellular and molecular mechanisms that are involved in the initiation and propagation of sepsis and I/R injury have not been understood fully. This lack in the fundamental knowledge has made attempts at developing effective therapies for sepsis and I/R injury exceedingly difficult.
Milk fat globule-EGF factor VIII (MFG-E8, the lactadherin homolog in humans) is a membrane-associated glycoprotein originally found in milk and mammary epithelial cells (9). Previous investigators have shown that MFG-E8 participates in multiple physiological processes associated with tissue remodeling (10–15). Among these, its role for the clearance of apoptotic cells has made a huge impact on subsequent research. This review discusses the role of MFG-E8, focusing on the antiinflammatory property that is derived from the enhancement of phagocytotic capacity, in the pathogenesis of sepsis and I/R injury and explores the therapeutic potential of MFG-E8 in those conditions.
MILK-FAT GLOBULE-EGF FACTOR VIII (MFG-E8)
MFG-E8 Structure and Production
MFG-E8, a 66 kDa glycoprotein, initially was identified as one of the major protein components associated with milk fat globule membrane in the mouse (9). It has since been isolated independently from the mammary gland of several other mammalian species such as bovine and human (16–19). Hanayama et al. (14) and Aziz et al. (20) investigated MFG-E8 gene expression in various tissues of normal mice and reported that MFG-E8 gene is expressed ubiquitously in almost all organs, and several organs, such as mammary glands, spleen, lymph nodes, brain and lung, express it abundantly. However, some vital organs including intestine and liver have lower expression of MFG-E8. MFG-E8 contains a signal sequence for secretion, two N-terminal epidermal growth factor (EGF) domains and two C-terminal discoidin domains with homology to the C1 and C2 domains found in blood-clotting factors V and VIII (9,16). The second EGF domain contains an arginine-glycine-aspartic (RGD) integrin-binding motif that engages αvβ 3/αvβ5 integrins to facilitate cell adhesion as well as induce integrin-mediated signal transduction (21–23). Oshima et al. (24) found the presence of two mRNA variants for MFG-E8 in mouse mammary gland, which were formed by alternative splicing of the same premature mRNA. The 66 kDa long form of MFG-E8 (MFG-E8L) contains a 37 amino acid proline/threonine rich (P/T-rich) domain between the second EGF domain and the first dicoidin domain (24,25). The 53 kDa short form of MFG-E8 (MFG-E8S) lacks the P/T-rich domain (24,25). The expression of the two splice variants shows spatial and temporal specificity. MFG-E8S is distributed widely, whereas MFG-E8L has been found in activated mouse macrophages (14,26), as well as in immature dendritic cells (27), Langerhans cells of the skin (27) and in epidermal keratinocytes (28). Recently, MFG-E8L has been identified in a species other than the mice (24,25). It also is shown that both forms seem to have a similar biological activity (13,15).
The functional roles of MFG-E8–associated tissue remodeling have been investigated widely, they include promoting removal of apoptotic lymphocytes by macrophages (13,14), clearance of mammary epithelial cells in involution (12), mediating sperm-egg bindings (11), maintenance of intestinal epithelium (10) and facilitating neovascularization as a downstream effector of vascular endothelial growth factor (VEGF) signaling (15). The MFG-E8 production from macrophages is increased by granulocyte/monocyte colony-stimulating factor (27,29) and fractalkine (CX3CL1) (30, 31). Furthermore, it is reported that MFG-E8 expression, which is evaluated in human and animal models, is downregulated in some disease conditions such as autoimmune disease (14), Alzheimer disease (32), atherosclerosis (33), acute colitis (20), sepsis (10,31,34–36) and I/R injury (37,38). However, in contrast, MFG-E8 is highly expressed in systemic lupus erythematosus (39), lung fibrosis (40), breast cancer (19) and melanoma (41).
MFG-E8 Contributes to Phagocytic Removal of Apoptotic Cells
Phagocytic removal of apoptotic cells occurs quite efficiently in vivo such that, even in tissues with significant apoptosis, very few apoptotic cells are detectable (42). This is thought to be due to the release of so called “eat-me” signals by apoptotic cells that recruit motile phagocytes such as monocytes, macrophages and dendritic cells, leading to the prompt clearance of the dying cells (43,44). So far, several “eat-me” signals have been discovered. The redistribution of phosphatidylserine (PS) to the external surface of the plasma membrane is a key element of apoptotic cell recognition and is a molecular cue that dying cells should be engulfed (45). Some receptor molecules, including PS receptor (46), LDL receptor (47) and scavenger receptors (48), directly recognize PS on apoptotic cells. In addition, soluble proteins that bind to PS on apoptotic cells for phagocytosis also were reported (49). How these receptors are involved in the recognition and engulfment of apoptotic cells has not been fully understood. Hanayama et al. (13) were first to show that activated peritoneal macrophage strongly produces MFG-E8 and the C-terminal discoidin domains mediate attachment to PS on apoptotic lymphocytes and the RGD motif of N-terminal domains engages αvβ3/αvβ5 integrins expressed on the advancing phagocytes. Through this process, MFG-E8 plays a role as “bridging molecule” and enhances the engulfment of apoptotic cells by phagocytes. Additionally, MFG-E8 deficient mice showed various characteristics of autoimmunity that are specially due to defects in apoptotic cell engulfment by tingible-body macrophages in germinal centers (14). Analogously, the human homolog of MFG-E8 (hMFG-E8) also has a role for “bridging molecule” and low levels of hMFG-E8 enhance phagocytosis, however, at high concentrations, engulfment is inhibited in a dose- dependent manner (39). This biphasic function of hMFG-E8 indicates the complicated involvement of MFG-E8 for disease pathogenesis and progression.
INAPPROPRIATE CLEARANCE OF APOPTOTIC CELLS INDUCES INFLAMMATORY RESPONSES
Historically, apoptosis has been seen as an ordinary process of cell suicide that, unlike necrosis, does not elicit inflammation (50). Recently, it has been shown that if the removal process of apoptotic cells fails, apoptotic cells undergo post-apoptotic secondary necrosis and release potentially cytotoxic and antigenic intra-cellular contents such as heat shock proteins and high mobility group box 1 protein (HMGB1), which can elicit an inflammatory response (51–54). In a study by Hotchkiss et al. (55), pretreatment of animals with apoptotic splenocytes worsens the outcome in a mouse model of sepsis, pointing out the detrimental effect of accumulated apoptotic cells in the body. Furthermore, it is well known that one of the key biological features of apoptotic cell clearance itself is the non-inflammatory and non-immunogenic nature of this uptake process (56). In contrast to the uptake of pathogens or FcR-mediated phagocytosis, the engulfment of apoptotic cells does not lead to proinflammatory cytokine production by macrophages (56). In this context, the ability of apoptotic cells to induce the secretion of antiinflammatory cytokines may be of relevance. Macrophages that engulf apoptotic cells have been shown to secrete antiinflammatory cytokines, such as transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), which could potentially dampen inflammation (57,58). In another study, lipopolysaccharide (LPS)-stimulated macrophages inhibited the production of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IL-1 and IL-12 after phagocytosis of apoptotic cells as macrophages that did not phagocytose before (58,59). We have shown that mature resident peritoneal macrophages (RPMs) which are major sources of inflammatory cytokines, while losing their efficacy for phagocytosis of apoptotic cells, express lower MFG-E8 levels and show higher TNF-α response to LPS compared with immature thioglycolate-elicited peritoneal macrophages (TGPM) (60). After LPS-stimulation, the capability of RPMs to phagocytose is decreased along with further downregulation of MFG-E8 and RPMs also lack phagocytosis-induced inhibition of TNF-α release (60). Furthermore, MFG-E8-mediated apoptotic cell phagocytosis results in an inhibition of mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB signaling pathways (60). Asano et al. (26) reported that a dominant-negative form of MFG-E8, designated as D89E, carrying a point mutation in an RGD motif, inhibited not only the phagocytosis of apoptotic cells by a wide variety of phagocytes, but also inhibited the enhanced production of IL-10 by TGPM phagocytizing apoptotic cells.
Taken together, these results indicate that MFG-E8 plays a pivotal role in controlling excess inflammatory responses through the inhibition of apoptotic cell accumulation, which otherwise results in subsequent secondary necrosis, the antiinflammatory cytokine production and the inhibition of inflammatory cytokine production by activated phagocytes.
MFG-E8 MEDIATES SEPSIS-INDUCED SYSTEMIC INFLAMMATION
MFG-E8 Expression and Apoptotic Cell Clearance Are Suppressed in Sepsis
Under septic conditions, it has been shown that widespread and profound apoptosis of crucial immune cells occurs and this excessive apoptosis induces the impairment of immune function and proinflammatory cytokine upregulation (61–63). Hence, focusing on MFG-E8, which promotes appropriate clearance of apoptotic cells by phagocytes before the secondary necrotic cell development, is considered to be promising therapeutic approach for sepsis to avoid further tissue injury along with the antiapoptotic strategies (57,62).
We were the first to show that MFG-E8 protein expression dramatically decreased (by 48% in the spleen and even by 70% in the liver) at 20 hours after septic insult induced by rat cecal ligation and puncture (CLP) model (35). The blood level also was decreased by 45%, indicating the systemic scale of MFG-E8 depletion under septic condition (36). We also showed that LPS from Gram- negative bacteria, which play a pivotal role for the pathogenesis of CLP sepsis, downregulate this protein production from cultured RAW 264.7 macrophages (murine macrophage cell line) and peritoneal macrophages in vitro (31,35). We pursued this MFG-E8 inhibition by LPS and showed that in vivo LPS injection in mice also reduced splenic MFG-E8 mRNA expression in a dose-dependent manner and the downregulation of splenic MFG-E8 mRNA expression in mice CLP sepsis was attenuated by polymyxin B administration, which neutralizes LPS activity (34). Furthermore, this CLP-induced MFG-E8 inhibition was not observed in either CD14−/− or Toll-like receptor (TLR)4−/− mice, and we concluded that sepsis-induced downregulation of MFG-E8 production is mainly LPS-CD14-TLR4 pathway dependent (34). Besides LPS-CD14-TLR4 pathway, sepsis-derived apoptosis of immune cells as a source of MFG-E8 is suggested to be responsible for the depletion of MFG-E8 in sepsis (36). It also is reported that MFG-E8 is expressed constitutively in intestinal lamina propria macrophages of mice and CLP sepsis downregulates MFG-E8 production (10).
We have demonstrated a decreased phagocytic activity of macrophages after CLP sepsis which was evaluated by coincubation with isolated peritoneal macrophages and autologous dexam-ethasone-induced apoptotic thymocytes ex vivo (35). Then we developed a novel phagocytosis assay using pHrodo succinimidyl ester-labeled apoptotic lymphocytes as targets and tissue macrophages as phagocytes, which is quick and reliable to evaluate the internalization of apoptotic cells, and is able to clearly distinguish the engulfment, rather than the attachment, of apoptotic cells by phagocytes (64). Using this method, the decreased phagocytic activity of isolated splenic and peritoneal macrophages was reconfirmed in CLP sepsis models (34,36).
We have demonstrated extensively in this model that exogenous administration of MFG-E8 with recombinant murine protein (rmMFG-E8) and immature dendritic cell-derived exosome that contains MFG-E8 robustly promotes the engulfment of apoptotic cells and a decrease in apoptotic cell number. In this regard, MFG-E8 deficient mice showed a decreased phagocytic activity and the promotion for phagocytic activity by MFG-E8–containing exosomes administration was abrogated in MFG-E8 inhibition study (35,36). Hence, the sepsis- associated decrease of MFG-E8 expression contributes to the impairment of clearance of apoptotic cells even under septic conditions. Additionally, from the evidence of in vitro study, we have shown also that MFG-E8 did not inhibit apoptosis directly; MFG-E8–containing exosomes do not decrease the presence of apoptotic cells in sepsis through the direct modulation of apoptotic pathways, but rather through the increased clearance of apoptotic cells (35,36).
Exogenous Administration of MFG-E8 Attenuates Sepsis-Induced Inflammation and Improves Survival
Sepsis is marked by a systemic inflammatory response caused by an overwhelming infection. Although this inflammatory response is helpful in minor infections, it becomes overzealous in sepsis, causing more harm than good to the organism. Among a number of sepsis models that have been used to study the pathophysiology of sepsis, the model of CLP mimics many features, such as cytokine profiles, of clinical sepsis- peritonitis (65–67). Sepsis-induced over-stimulation of inflammatory cytokines such as TNF-α, IL-1β, IL-6 and HMGB1, which act as a late proinflammatory cytokine by a substantial neuroendocrine and immune activation, plays a central role in the morbidity and mortality in experimental sepsis as well as in septic patients (68–72). Studies using inhibitors of these cytokines demonstrated increased survival of septic mice treated with TNF-α or HMGB1 blocking antibodies (71,73). Therefore, we investigated the alterations of inflammatory response after exogenous administration of MFG-E8 in a rat model of CLP-sepsis. MFG-E8–containing exosome administration dramatically suppressed CLP-induced systemic TNF-α, IL-6 and HMGB1 responses (35,36). Interestingly, MFG-E8–containing exosomes failed to suppress TNF-α release from LPS-stimulated macrophage in vitro in the absence of apoptotic cells, suggesting that the antiinflammatory effect of MFG-E8 is derived from an indirect immunosuppressive effect (36). In this regard, we demonstrated that administration of fractalkine (CX3CL1) in mice, reduced the CLP sepsis-induced increases in blood lactate levels, liver injury, and proinflammatory markers—which was associated with a complete reversal of decreased plasma MFG-E8 levels (31).
We also showed the protective effect of exogenous MFG-E8 administration for sepsis-associated mortality using a consistent septic model with an approximate lethality of LD50, which is provided by CLP, and excision of necrotic cecum after 20 hours after CLP (35,36). Both MFG-E8–containing exosomes and rmMFG-E8 administration dramatically increased the survival rate to approximately 80% in a rat model (35,36). In addition, MFG-E8 deficient mice showed a reduced survival rate compared with wild-type mice (36). As mentioned above, although only a limited amount of research has been done to study the role of MFG-E8 in sepsis, MFG-E8 has been reported to be of crucial importance in this prophagocytic and secondary immunosuppressive effect, which finally leads to a survival benefit, even under septic conditions.
MFG-E8 IN ISCHEMIA-REPERFUSION INJURY
The hallmarks of I/R injury are the formation of reactive oxygen species (74), cytokine release (75), complement activation (6,76), the production of eicosanoids (77,78), recruitment of activated leukocytes (79,80), mitochondrial dysfunction (81) and a combination of cell necrosis and apoptosis (82,83). These I/R injury-induced events can trigger intense and detrimental inflammatory responses which lead to organ damages. Hence, similar to sepsis, mediating inflammatory responses after I/R injury is considered to be a possible therapeutic approach (75,84–86).
It is recognized that I/R injury induces the increased occurrence of apoptotic cell death of not only targeting but also remote organs (87–89), and deficient clearance of apoptotic cells potentially leads to increased inflammation and impaired tissue repair (90,91). Recently, we have shown the clinical importance of MFG-E8 in the pathogenesis and the therapeutic potentials of ALI after intestinal I/R injury (37). Mesenteric ischemia remains a critical problem, resulting in mortality as high as 60% to 80% (92). Multiple organ failure, including ALI, is a common complication of intestinal I/R injuries and contribute to its high mortality rate (93). Using a mouse intestinal I/R injury model, which consists of a mesenteric artery occlusion for 90 min followed by reperfusion for 4 h, we showed dramatic decrease of MFG-E8 mRNA and protein levels in the spleen and lungs by 50% to 60% (37). Administration of rmMFG-E8 markedly suppressed the intestinal I/R injury-induced local and systemic hyper-inflammation (gut TNF-α and IL-1β; lung IL-1β, IL-6 and myeloperoxidase [MPO] activity; plasma TNF-α, IL-1β and IL-6) and organ injury (histological injury scores of intestine and lung; plasma lactate, LDH, ALT, AST, creatinine), and finally improved the survival rate from 0% to 47% during 24-hour observation (37). In addition, apoptosis of lung epithelium and endothelium, as well as the accumulation of neutrophils, play a major role in the development and progression of ALI (94,95). We showed that treatment with rmMFG-E8 improved intestinal I/R injury–induced apoptotic cells accumulations in the lungs (37). Although the direct data showing the alterations of clearance of apoptotic cells after intestinal I/R injury was not demonstrated in our work, previous studies (35,36) strongly support the scenario that the decreased MFG-E8 level is associated with impaired phagocytosis which result in accumulation of apoptotic cells. Additionally, the MFG-E8’s effect on decreasing apoptosis is not mediated by a direct antiapoptotic effect but through the stimulation of apoptotic cell clearance. These studies collectively suggest that MFG-E8 is indeed beneficial in downregulating local and remote detrimental inflammatory response through the stimulation of apoptotic cell clearance even under I/R injury.
Recently, we have shown the involvement of MFG-E8 in the pathophysiology not only of intestinal but also of renal I/R injury (38). This study revealed that renal I/R injury reduced MFG-E8 expressions in the spleen and kidneys. Exogenous administration of rmMFG-E8 improved deteriorated renal functions (BUN, creatinine) and histological tubular injuries, and increased inflammatory markers (kidney IL-6, IL-1β, macrophage inflammatory protein-2 [MIP-2] and MPO) after renal I/R injury. These findings suggested to us a novel potential of MFG-E8 as treatment for broad-spectrum I/R injuries.
POTENTIAL MECHANISM OF MFG-E8 IN SEPSIS AND I/R INJURY
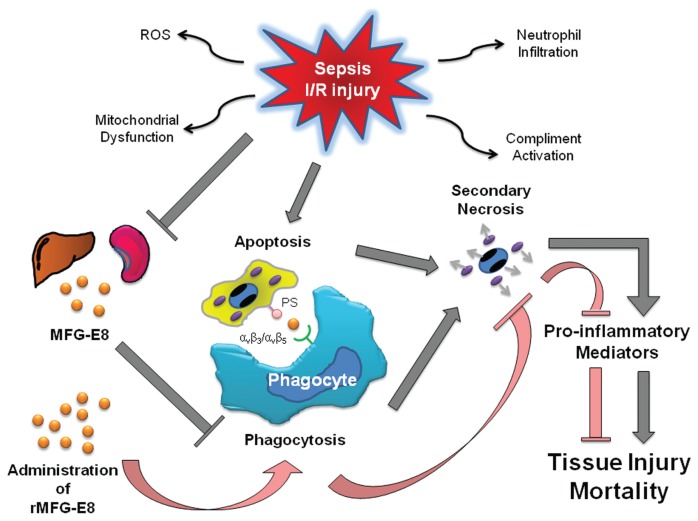
Sepsis and I/R injury share some similar pathophysiological features, which induce a detrimental inflammatory response that causes tissue injury. Among these, increased accumulation of apoptotic cells and inappropriate phagocytotic removal of these cells, have the potential to release toxic and proinflammatory contents owing to secondary necrosis (51–54). The synergistic effect of increased apoptotic cell death, and decreased clearance of apoptotic cells by phagocytes presumably due to the downregulation of MFG-E8 emphasize the importance of mediating apoptosis in sepsis and I/R injury. Administration of MFG-E8 enhances the clearance of apoptotic cells and inhibits the detrimental proinflammatory cascade which is induced by apoptotic cells (Figure 1).
Figure 1.
Schematic representation of pathophysiology of sepsis and I/R injury. Sepsis and I/R injury induce the increased apoptotic cell death in various cells and tissues and decrease apoptotic cell clearance by phagocytes, such as macrophages and dendritic cells, through the downregulation of milk fat globule-EGF factor VIII (MFG-E8). Apoptotic cells, without appropriate phagocytotic removal, have the potential to release toxic and proinflammatory contents due to secondary necrosis, and potentiate tissue injury and mortality. Administration of MFG-E8 enhances phagocytotic activity and therefore attenuates detrimental inflammation, thereby decreasing tissue injury and mortality.
FUTURE STUDIES AND PERSPECTIVES
The data described above provide a rationale for continued investigation of MFG-E8 as an antiinflammatory agent that serves to ameliorate sepsis or I/R injury. MFG-E8 is crucial in enhancing engulfment of apoptotic cells by phagocytes. Phagocytosis prevents the release of potentially harmful or immunogenic materials from dying cells. We have shown that MFG-E8 expressions are downregulated in sepsis and I/R injury, and that this downregulation causes the impairment of clearance of apoptotic cells. Recently, however, Swan et al. (96) reported that during mice CLP-sepsis the capacity of splenic macrophages to engulf apoptotic cells was enhanced in the later stage of sepsis (at over 24 hours after CLP). The author suggests that this could lead to an antiinflammatory macrophage phenotype shift, thus potentially contributing to the immunosuppression observed in late sepsis. The divergence of phagocytotic activity of macrophages in sepsis between our results and Swan et al. (96) may be derived from the difference of the phase of sepsis (inflammatory and antiinflammatory) and this may be associated with the pathophysiological complexity and the therapeutic difficulty of sepsis. We have made further significant progress toward understanding the molecular mechanisms associated with the downregulation of MFG-E8 in sepsis, which involves the LPS-CD14-TLR4 pathway. However, the corresponding mechanism of the down-regulation of MFG-E8 in I/R injury has not been elucidated. It is reported that diverse TLR agonists, besides LPS, and necrotic cells downregulated the expression of MFG-E8 on antigen presenting cells in vitro (29). Future studies are required to investigate such issues.
On the basis of the available animal studies and clinical patients findings observed, cell death caused by apoptosis, necrosis and pyroptosis plays a significant role in the pathogenesis of sepsis and I/R injury. Direct or indirect inhibition of apoptotic cell death and/or enhancement of apoptotic cell clearance seem to be promising and interesting therapeutic strategies to prevent death of immune cells and cells that make up living organisms. However, plenty of factors including the severity, timing and types of damaged organs affect the contribution of cell death for the pathophysiology of these disease conditions. Although MFG-E8 has no direct effect on altering apoptosis, its role in the enhancement of phagocytosis of apoptotic cells and the subsequent antiinflammatory property, could be promising strategies for sepsis and I/R injury treatment in the clinical settings, but various optimizations or combinations with other molecules should be considered for the clinical applications.
Since its discovery, MFG-E8 has proven to be an essential factor for multiple physiologic systems. In the past decade, major emphasis has focused on its role for clearance of apoptotic cells in various organ systems to maintain homeostatic balance. More recently, however, MFG-E8-mediated findings of a variety of clinical, immunological, physiological and pharmacological events have been reported, where it may serve as a major player for various cell signaling events. Therefore, further studies are needed to explore other mechanisms involving the beneficial effect of MFG-E8 treatment in sepsis and I/R injury.
In conclusion, these data taken together strongly indicate that treatment with MFG-E8 reduces the morbidity and mortality associated with sepsis and I/R injury and would enrich our view for the MFG-E8-mediated therapeutic potential in several life-threatening disease conditions.
ACKNOWLEDGMENTS
This work is supported by the National Institutes of Health (NIH) grants, R01 GM057468 and R01 GM053008 (PW).
Footnotes
DISCLOSURE
One of the authors (P Wang) is an inventor of the pending PCT application #WO/2006/122327: “Milk fat globule epidermal growth factor-factor VIII and sepsis” and PCT application #WO/2009/064448: “Prevention and treatment of inflammation and organ injury after ischemia/reperfusion using MFG-E8.” These patent applications cover the fundamental concept of using MFG-E8 for the treatment of sepsis and ischemia/reperfusion injury. All other authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–92. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 4.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 5.van den Berghe G, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 6.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–9. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Neary P, Redmond HP. Ischaemia-reperfusion injury and the systemic inflammatory response syndrome. In: Grace PA, Mathie RT, editors. Ischaemia-Reperfusion Injury. Blackwell Science; London: 1999. pp. 123–6. [Google Scholar]
- 9.Stubbs JD, et al. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc Natl Acad Sci U S A. 1990;87:8417–21. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bu HF, et al. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117:3673–83. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405–17. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 12.Hanayama R, Nagata S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc Natl Acad Sci U S A. 2005;102:16886–91. doi: 10.1073/pnas.0508599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 14.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 15.Silvestre JS, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 16.Aoki N, et al. Molecular cloning of glycoprotein antigens MGP57/53 recognized by monoclonal antibodies raised against bovine milk fat globule membrane. Biochim Biophys Acta. 1995;1245:385–91. doi: 10.1016/0304-4165(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 17.Couto JR, Taylor MR, Godwin SG, Ceriani RL, Peterson JA. Cloning and sequence analysis of human breast epithelial antigen BA46 reveals an RGD cell adhesion sequence presented on an epidermal growth factor-like domain. DNA Cell Biol. 1996;15:281–6. doi: 10.1089/dna.1996.15.281. [DOI] [PubMed] [Google Scholar]
- 18.Hvarregaard J, Andersen MH, Berglund L, Rasmussen JT, Petersen TE. Characterization of glycoprotein PAS-6/7 from membranes of bovine milk fat globules. Eur J Biochem. 1996;240:628–36. doi: 10.1111/j.1432-1033.1996.0628h.x. [DOI] [PubMed] [Google Scholar]
- 19.Larocca D, et al. A Mr 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains factor VIII-like domains. Cancer Res. 1991;51:4994–8. [PubMed] [Google Scholar]
- 20.Aziz MM, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphav-beta3 integrin signaling. J Immunol. 2009;182:7222–32. doi: 10.4049/jimmunol.0803711. [DOI] [PubMed] [Google Scholar]
- 21.Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry. 2000;39:6200–6. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- 22.Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106:957–66. doi: 10.1002/jcb.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 1997;16:861–9. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- 24.Oshima K, et al. Lactation-dependent expression of an mRNA splice variant with an exon for a multiply O-glycosylated domain of mouse milk fat globule glycoprotein MFG-E8. Biochem Biophys Res Commun. 1999;254:522–8. doi: 10.1006/bbrc.1998.0107. [DOI] [PubMed] [Google Scholar]
- 25.Burgess BL, Abrams TA, Nagata S, Hall MO. MFG-E8 in the retina and retinal pigment epithelium of rat and mouse. Mol Vis. 2006;12:1437–47. [PubMed] [Google Scholar]
- 26.Asano K, et al. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 2004;200:459–67. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyasaka K, Hanayama R, Tanaka M, Nagata S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur J Immunol. 2004;34:1414–22. doi: 10.1002/eji.200424930. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, et al. Production of the long and short forms of MFG-E8 by epidermal keratinocytes. Cell Tissue Res. 2005;321:185–93. doi: 10.1007/s00441-005-1148-y. [DOI] [PubMed] [Google Scholar]
- 29.Jinushi M, et al. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117:1902–13. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonardi-Essmann F, Emig M, Kitamura Y, Spanagel R, Gebicke-Haerter PJ. Fractalkine-upregulated milk-fat globule EGF factor-8 protein in cultured rat microglia. J Neuroimmunol. 2005;160:92–101. doi: 10.1016/j.jneuroim.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Miksa M, Amin D, Wu R, Ravikumar TS, Wang P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol Med. 2007;13:553–60. doi: 10.2119/2007-00019.Miksa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boddaert J, et al. Evidence of a role for lactadherin in Alzheimer’s disease. Am J Pathol. 2007;170:921–9. doi: 10.2353/ajpath.2007.060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ait-Oufella H, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–77. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 34.Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is downregulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009;182:581–7. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miksa M, et al. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586–93. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- 36.Miksa M, et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII. J Immunol. 2009;183:5983–90. doi: 10.4049/jimmunol.0802994. erratum 2009;183:8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui T, et al. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am J Respir Crit Care Med. 2010;181:238–46. doi: 10.1164/rccm.200804-625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuda A, et al. The renoprotective effect of Milk Fat Globule EGF-Factor VIII after ischemia and reperfusion injury in mice. Shock. 2010;33(Suppl 1):53. [Google Scholar]
- 39.Yamaguchi H, et al. Milk fat globule EGF factor 8 in the serum of human patients of systemic lupus erythematosus. J Leukoc Biol. 2008;83:1300–7. doi: 10.1189/jlb.1107730. [DOI] [PubMed] [Google Scholar]
- 40.Atabai K, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–22. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jinushi M, et al. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008;68:8889–98. doi: 10.1158/0008-5472.CAN-08-2147. [DOI] [PubMed] [Google Scholar]
- 42.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–50. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 44.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189–97. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 47.Oka K, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9535–40. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawasaki Y, Nakagawa A, Nagaosa K, Shiratsuchi A, Nakanishi Y. Phosphatidylserine binding of class B scavenger receptor type I, a phagocytosis receptor of testicular sertoli cells. J Biol Chem. 2002;277:27559–66. doi: 10.1074/jbc.M202879200. [DOI] [PubMed] [Google Scholar]
- 49.Anderson HA, et al. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 50.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–25. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 52.Munoz LE, et al. SLE—a disease of clearance deficiency. Rheumatology (Oxford) 2005;44:1101–7. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 53.Silva MT, do Vale A, dos Santos NM. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:463–82. doi: 10.1007/s10495-008-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–26. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 55.Hotchkiss RS, et al. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci U S A. 2003;100:6724–9. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–81. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 57.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 59.Monks J, et al. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005;12:107–14. doi: 10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- 60.Miksa M, et al. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 2008;22:743–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Chung CS, Xu YX, Chaudry IH, Ayala A. Sepsis induces increased apoptosis in lamina propria mononuclear cells which is associated with altered cytokine gene expression. J Surg Res. 1998;77:63–70. doi: 10.1006/jsre.1998.5339. [DOI] [PubMed] [Google Scholar]
- 62.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 63.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–21. [PubMed] [Google Scholar]
- 64.Miksa M, Komura H, Wu R, Shah KG, Wang P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J Immunol Methods. 2009;342:71–7. doi: 10.1016/j.jim.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaudry IH. Sepsis: lessons learned in the last century and future directions. Arch Surg. 1999;134:922–9. doi: 10.1001/archsurg.134.9.922. [DOI] [PubMed] [Google Scholar]
- 66.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Echtenacher B, Freudenberg MA, Jack RS, Man-nel DN. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect Immun. 2001;69:7271–6. doi: 10.1128/IAI.69.12.7271-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 69.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 70.Meyer TA, et al. Sepsis and endotoxemia stimulate intestinal interleukin-6 production. Surgery. 1995;118:336–42. doi: 10.1016/s0039-6060(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 71.Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–4. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 72.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 73.Qin S, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–42. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levonen AL, Vahakangas E, Koponen JK, Yla-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation. 2008;117:2142–50. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 75.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arumugam TV, et al. Complement mediators in ischemia-reperfusion injury. Clin Chim Acta. 2006;374:33–45. doi: 10.1016/j.cca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Huk I, et al. Prostaglandin E1 reduces ischemia/reperfusion injury by normalizing nitric oxide and superoxide release. Shock. 2000;14:234–42. doi: 10.1097/00024382-200014020-00026. [DOI] [PubMed] [Google Scholar]
- 78.Zingarelli B, et al. Diverse cardioprotective signaling mechanisms of peroxisome proliferator-activated receptor-gamma ligands, 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone, in reperfusion injury: role of nuclear factor-kappaB, heat shock factor 1, and Akt. Shock. 2007;28:554–63. doi: 10.1097/shk.0b013e31804f56b9. [DOI] [PubMed] [Google Scholar]
- 79.Vedder NB, et al. Inhibition of leukocyte adherence by anti-CD18 monoclonal antibody attenuates reperfusion injury in the rabbit ear. Proc Natl Acad Sci U S A. 1990;87:2643–6. doi: 10.1073/pnas.87.7.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winn RK, Ramamoorthy C, Vedder NB, Sharar SR, Harlan JM. Leukocyte-endothelial cell interactions in ischemia-reperfusion injury. Ann N Y Acad Sci. 1997;832:311–21. doi: 10.1111/j.1749-6632.1997.tb46259.x. [DOI] [PubMed] [Google Scholar]
- 81.Chen CH, Liu K, Chan JY. Anesthetic preconditioning confers acute cardioprotection via up-regulation of manganese superoxide dismutase and preservation of mitochondrial respiratory enzyme activity. Shock. 2008;29:300–8. doi: 10.1097/SHK.0b013e3181454295. [DOI] [PubMed] [Google Scholar]
- 82.Rivo J, et al. Attenuation of reperfusion lung injury and apoptosis by A2A adenosine receptor activation is associated with modulation of Bcl-2 and Bax expression and activation of extra-cellular signal-regulated kinases. Shock. 2007;27:266–73. doi: 10.1097/01.shk.0000235137.13152.44. [DOI] [PubMed] [Google Scholar]
- 83.Wei Q, Yin XM, Wang MH, Dong Z. Bid deficiency ameliorates ischemic renal failure and delays animal death in C57BL/6 mice. Am J Physiol Renal Physiol. 2006;290:F35–42. doi: 10.1152/ajprenal.00184.2005. [DOI] [PubMed] [Google Scholar]
- 84.Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102:240–7. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 85.Walsh KB, Toledo AH, Rivera-Chavez FA, Lopez-Neblina F, Toledo-Pereyra LH. Inflammatory mediators of liver ischemia-reperfusion injury. Exp Clin Transplant. 2009;7:78–93. [PubMed] [Google Scholar]
- 86.Wanderer AA. Ischemic-reperfusion syndromes: biochemical and immunologic rationale for IL-1 targeted therapy. Clin Immunol. 2008;128:127–32. doi: 10.1016/j.clim.2008.03.514. [DOI] [PubMed] [Google Scholar]
- 87.An S, Hishikawa Y, Liu J, Koji T. Lung injury after ischemia-reperfusion of small intestine in rats involves apoptosis of type II alveolar epithelial cells mediated by TNF-alpha and activation of Bid pathway. Apoptosis. 2007;12:1989–2001. doi: 10.1007/s10495-007-0125-1. [DOI] [PubMed] [Google Scholar]
- 88.Collange O, et al. Pulmonary apoptosis after supraceliac aorta clamping in a rat model. J Surg Res. 2005;129:190–5. doi: 10.1016/j.jss.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 89.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–58. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 90.Hanayama R, Miyasaka K, Nakaya M, Nagata S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr Dir Autoimmun. 2006;9:162–72. doi: 10.1159/000090780. [DOI] [PubMed] [Google Scholar]
- 91.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods. 2008;44:280–5. doi: 10.1016/j.ymeth.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Tendler DA. Acute intestinal ischemia and infarction. Semin Gastrointest Dis. 2003;14:66–76. [PubMed] [Google Scholar]
- 93.Matthay MA, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–35. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 94.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31:S184–8. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- 95.Perl M, Lomas-Neira J, Chung CS, Ayala A. Epithelial cell apoptosis and neutrophil recruitment in acute lung injury – a unifying hypothesis? What we have learned from small interfering RNAs. Mol Med. 2008;14:465–75. doi: 10.2119/2008-00011.Perl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swan R, et al. Polymicrobial sepsis enhances clearance of apoptotic immune cells by splenic macrophages. Surgery. 2007;142:253–61. doi: 10.1016/j.surg.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]