Abstract
Purpose
Our laboratory has developed and implanted a novel bioengineered internal anal sphincter (IAS) to treat anal incontinence. Fibroblast growth factor-2 (FGF-2) has been used in mice; however, the optimal growth factor for successful IAS implantation is unclear. This study compares several growth factors in order to optimize IAS viability and functionality.
Methods
Bioengineered IAS rings were implanted subcutaneously into the dorsum of wildtype C57Bl/6 mice, with an osmotic pump dispensing FGF-2, vascular endothelial growth factor (VEGF), or platelet-derived growth factor (PDGF) (n = 4 per group). Control mice received IAS implants but no growth factor. The IAS was harvested approximately 25 days post-implantation. Tissue was subjected to physiologic testing, then histologically analyzed. Muscle phenotype was confirmed by immunofluorescence.
Results
All implants supplemented with growth factors maintained smooth muscle phenotype. Histological scores, blood vessel density and muscle fiber thickness were all markedly better with growth factors. Neovascularization was comparable between the three growth factors. Basal tonic force of the constructs was highest with VEGF or PDGF.
Conclusion
All growth factors demonstrated excellent performance. As our ultimate goal is clinical implantation, our strong results with PDGF, a drug approved for use in the United States and the European Union, pave the way for translating bioengineered IAS implantation to the clinical realm.
Keywords: Internal anal sphincter, Fecal incontinence, Tissue engineering, Growth factors
Introduction
Fecal incontinence (FI) is a condition that affects a myriad of patients ranging from children with anorectal malformations to the elderly with idiopathic weakening of the anal sphincter complex [1, 2]. While there are a large variety of causes of FI, the internal anal sphincter (IAS) plays a major role in the maintenance of continence in many disease processes [3]. The IAS accounts for approximately 70% of the resting anal canal pressure. Any disruption in the IAS mechanism leads to significant loss of anal canal pressure, resulting in FI. While the mainstay of FI treatment is non-surgical, including modalities such as dietary modification, these do not address the root cause of lost IAS functionality [3]. Surgical therapies such as autologous tissue grafts, implantation of prosthetic sphincters and injectable silicone have had limited success in restoring quality of life [4, 5].
Our laboratories have previously described the development and implantation of bioengineered mouse and human IAS constructs in a mouse model [6, 7]. This bioengineered IAS construct offers a novel, and potentially superior approach for the treatment of FI by offering autologous constructs; thereby eliminating the risk of rejection, prosthetic implant extrusion or the need for immunosuppression.
A major challenge facing the implantation of any bioengineered tissue is the vascularization of the construct upon implantation [8]. One commonly used method to improve vascularization is the usage of angiogenic growth factors such as fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [8-13]. However, there appears to be no consensus as to which type of growth factor affords the best growth, function and vascularization of a tissue-engineered construct. When IAS rings were implanted in our mouse model, we found that the histological appearance and vascularization of the implanted construct vastly improved when it was supplemented with FGF-2 compared to no growth factor supplementation [6]. The use of FGF and VEGF has been described in the dental [10] and orthopedic [11] literature, with VEGF affording slightly increased capillary density, but no overall differences in engrafted tissue integrity.
PDGF is a particularly interesting growth factor to study, as it is one of the few angiogenic factors that has approval for human use by both the United States Food and Drug Administration (FDA) and the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP). In some settings its angiogenic effect is weaker than those of VEGF and FGF [14]. However, it has been shown to drive proliferation in cells of mesenchymal origin such as periodontal fibroblasts [12] and colonic smooth muscle cells [15] which makes it an attractive choice as a supplement for our smooth muscle construct in vivo. As our IAS construct is a novel one, the optimal growth factor to use for implantation has not yet been studied.
The objective of this current study is to compare the histologic and basic physiologic outcomes of our implanted IAS construct in a mouse model to determine the most effective growth factor for successful implantation.
Materials and methods
Eight to twelve-week-old male C57Bl/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME), and kept in temperature-, humidity-, and light-controlled conditions. The study conformed to the guidelines for the care and use of laboratory animals established by the university committee on the use and care of animals at the University of Michigan. The study protocol was approved by this University Committee (no. 09714).
Bioengineering of the IAS construct has been described in detail in Raghavan et al. [16]. Three types of growth factor solutions were prepared: 0.1 μg/lL human recombinant FGF-2 (CYT-218, Prospec Bio, Rehovot, Israel), 0.02 μg/μL human recombinant VEGF (CYT-241, Prospec Bio) and 0.02 μg/μL human recombinant platelet-derived growth factor-BB (PDGF-BB, CYT-501, Prospec Bio). One hundred microliters of each growth factor solution (a total of 10 μg of FGF-2, 2 μg of VEGF or 2 μg of PDGF) was inserted into an Alzet model 1004 microosmotic pump (Durect Corp, Cupertino, CA), then the pump was primed as per the manufacturer's instructions, such that the total amount of growth factor was delivered over a 28-day period. A sterile silicone catheter (0.025 in. inner diameter, Dow-Corning, Midland, MI), approximately 5 cm long, was attached to the microosmotic pump.
The mice (n = 4 for each growth factor, and 4 more for IAS implantations without growth factors) were anesthetized using an isoflurane vaporizer, and given a weight-appropriate dose of buprenorphine subcutaneously. Hair on the upper back was clipped, and the skin was prepped with 4% chlorhexidine gluconate solution. The surgical technique is similar to that used by Hashish et al. [6].
A 2 cm transverse skin incision was made in the upper back of the mouse (mid-scapular). If growth factor was being given, a subcutaneous pocket was created caudally, which was made large enough to comfortably fit the pump. The bioengineered IAS ring was placed subcutaneously 0.5 cm away from the skin incision. The silicone catheter was cut so that the tip was pointing directly at the implanted IAS construct. The IAS was then secured and its location was marked using a 5-0 Prolene. The skin incision was closed using a 4-0 Vicryl suture. Animals were returned to an approved facility for recovery in standard fashion.
After an average of 25 days (range 23–28), mice were killed by CO2 asphyxiation. The implanted rings were harvested by re-entering the upper back near the initial skin incision. The harvested construct was placed in phosphate buffered saline, and then assessed immediately for physiologic functionality.
Protocols for measurement of force generation were adapted from previous work on bioengineered IAS [16]. The force transducer set up consisted of a static warmed tissue bath, with provisions for hooking to a harvested tissue. Mean measurement noise was determined and subtracted from total tension recordings with the tissue. Passive tension was calculated by hooking on the tissue with no stretch in zero calcium + 50 mM ethylene glycol tetraacetic acid. The bioengineered IAS post-implantation was hooked on to the force transducer set up with no additional stretch and soaked in basal warmed Dulbecco's modified Eagle's medium. Establishment of spontaneous basal tone was studied by recording the incremental force over a period of time. Passive tension and measurement noise were subtracted from total tension recordings with the tissue to obtain active tension. Values of force reported are only active tension values.
Following measurement of physiologic functionality, the ring was placed in zinc–formalin for fixation. Slides were then prepared and sectioned by the Tissue Core at the University of Michigan Comprehensive Cancer Center. Hematoxylin and eosin (H&E) staining was used for morphological assessment using the scoring scale described by Hashish et al. [6]. The scoring system is shown in Table 1. Blood vessel density and muscle fiber diameter were also assessed on the H&E slides. Statistical comparison between groups was done using one-way ANOVA with Tukey's post-test or the Kruskal–Wallis test with Dunn's post-test depending on the variables being compared.
Table 1.
Histological scoring scale devised to assess the viability of the implanted internal anal sphincter (IAS) construct (adapted from Hashish et al. [6])
| Criteria | Score |
|---|---|
| 1. Morphology of the muscle | |
| Packed polygonal muscle fiber profiles with normal shape | 2 |
| Mild separation of muscle fibers with preservation of shape | 1 |
| Marked separation of the muscle fibers with distortion of shape | 0 |
| 2. Degeneration of muscle fibers | |
| No degeneration (Little variation in muscle fiber size or shape. Cytoplasm is uniform. Small peripherally located nuclei are abundant) | 2 |
| Mild degeneration (Numerous nuclei with round contours are present | 1 |
| Muscle fibers are replaced by fibro-fatty tissue, and abnormal variation in fiber size is due to atrophy of some fibers and the hypertrophy of others) | |
| Marked degeneration (Necrotic muscle fibers seen. Destruction of muscle fibers with the replacement of muscle by fibrous tissue) | 0 |
| 3. Blood vessels | |
| Present and easily seen | 1 |
| Absent or difficult to see | 0 |
Two separate slide sections were scored, giving a total score between 0 and 10 for each IAS construct
Immunofluorescence staining for alpha smooth muscle actin (F3777, Sigma, St. Louis, MO), smooth muscle-specific heavy caldesmon (c-4562, Sigma), and prolyl-4 hydroxylase (MAB2701, Millipore, Billerica, MA) were used to assess tissue phenotype using previously described techniques [16]. Images were viewed using standard settings of a Nikon TS 100 microscope (Nikon Corp., Tokyo, Japan).
Results
There were no premature mouse deaths prior to scheduled killing. All incisions healed well, and there were no external signs of infection (Fig. 1). Upon gross inspection of the tissue, there was no gross evidence of abscess formation, tissue necrosis or rejection.
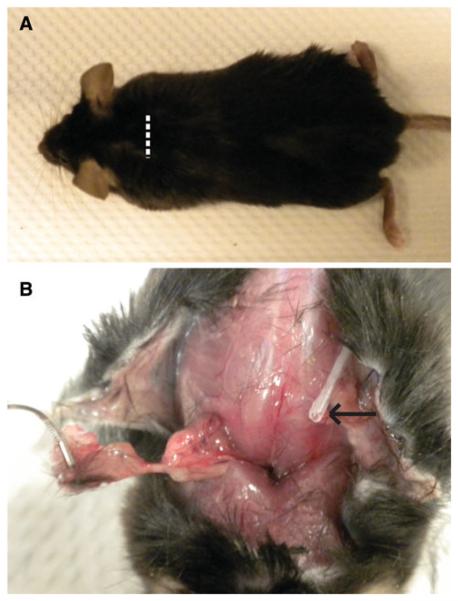
Fig. 1.
a Representative photographs of the mouse 25 days after IAS construct implantation. The white dotted line represents the area of the original incision, which is now healed with hair fully grown over. b The IAS construct (held in forceps) being harvested. The growth factor delivery catheter tip is shown by the black arrow. The tissue in the implantation bed appears healthy without evidence of infection or rejection
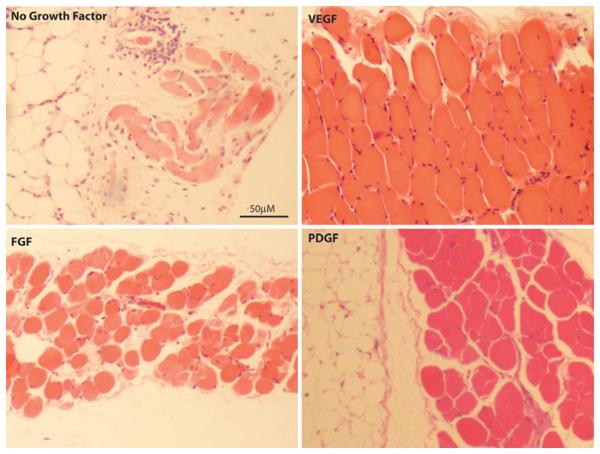
Representative H&E images of implanted IAS with and without growth factors are shown in Fig. 2. The histologic scores of the constructs using the scale developed by Hashish et al. are shown in Table 2. The overall histological scores were markedly higher (better) in the growth factor-treated IAS groups compared to the no growth factor group. The VEGF group attained a significantly higher score, mainly owing to its strong morphology scores.
Fig. 2.
Representative H&E sections of constructs implanted without growth factor, with FGF-2, with VEGF and with PDGF. The VEGF construct has the highest muscle fiber density, leading to the highest histologic scores (see Table 2)
Table 2.
Histological scores of implanted internal anal sphincter constructs
| Morphology score (0–4) |
Degeneration score (0–4) |
Blood vessel score (0–2) |
Total histological score (0–10) |
Blood vessels per HPF |
Muscle fiber diameter (μm) |
|
|---|---|---|---|---|---|---|
| No growth factor | 1.2 ± 0.8 | 2.0 ± 0.7 | 0.2 ± 0.4 | 3.4 ± 1.8 | 1.2 ± 0.4 | 20 ± 5.0 |
| FGF | 3.0 ± 0.7 | 3.4 ± 0.6 | 1.6 ± 0.5 | 8.0 ± 1.6 | 5.6 ± 2.0* | 32.0 ± 5.7 |
| VEGF | 3.5 ± 0.6^ | 3.3 ± 0.5 | 2.0 ± 0.0^ | 8.8 ± 0.5^ | 4.6 ± 1.0* | 31.4 ± 1.0 |
| PDGF | 2.5 ± 1.0 | 3.3 ± 1.0 | 1.8 ± 0.5 | 7.3 ± 1.7 | 4.7 ± 1.3* | 33.8 ± 6.3* |
Results expressed as mean ± standard deviation.
p < 0.05 by one-way ANOVA and Tukey's post-test compared to no growth factor.
p < 0.05 by Kruskal-Wallis test and Dunn's post-test compared to no growth factor
HPF high-powered field
To examine the degree of neovascularization of the construct, the number of blood vessels per high-powered field (HPF) was counted. The results are summarized in Table 2. The blood vessel count was significantly higher in the constructs with growth factor compared to those without growth factor. However, there was no statistically significant difference in blood vessel numbers between the three types of growth factors examined.
Muscle fiber thickness, an indicator of tissue robustness, was also measured as a surrogate for tissue viability. Muscle fiber thickness was markedly larger for tissues receiving growth factor, with the PDGF group achieving significantly better scores compared to other groups (Table 2).
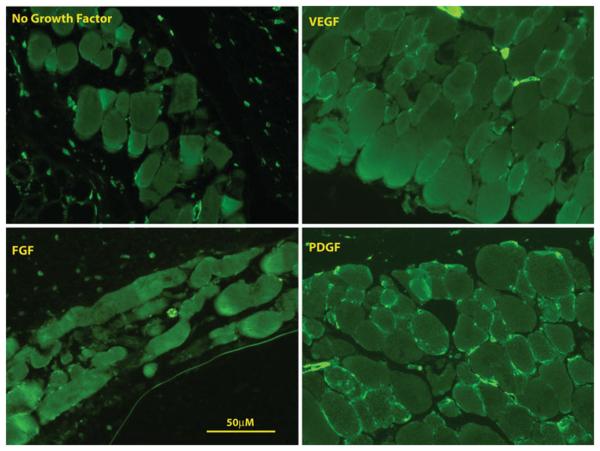
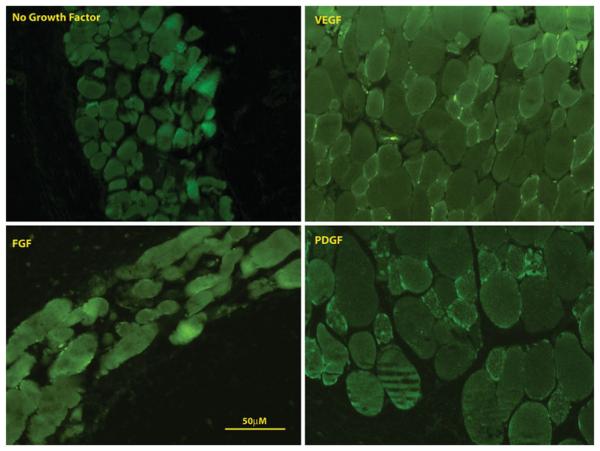
For all implants with growth factor, the IAS phenotype was preserved. Specifically, all implanted constructs stained positive for alpha smooth muscle actin and smooth muscle-specific heavy caldesmon (Figs. 3, 4). None of the constructs stained positive for prolyl-4 hydroxylase (Fig. 5), indicating that the cells within the construct did not de-differentiate into synthetic, myofibroblastic phenotypes.
Fig. 3.
Representative immunofluorescent images staining for α-smooth muscle actin of constructs implanted without growth factor, with FGF, with VEGF and with PDGF. All constructs stained positive for α-smooth muscle actin
Fig. 4.
Representative immunofluorescent images staining for smooth muscle-specific heavy caldesmon of constructs implanted without growth factor, with FGF, with VEGF and with PDGF. All constructs stained positive for smooth muscle specific heavy caldesmon
Fig. 5.
Representative immunofluorescent images staining for prolyl-4 hydroxylase of constructs implanted without growth factor, with FGF, with VEGF and with PDGF. None of the construct stained with prolyl-4-hydroxylase, demonstrating a non-fibroblastic phenotype
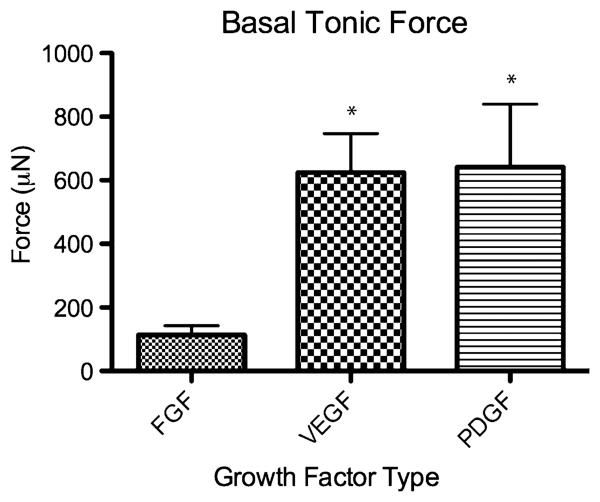
A key feature of IAS physiology is that it has a tonic contractile force at its baseline. Tonic forces for IAS constructs without growth factor supplementation were not measured because of the poor explanted tissue quality (e.g., too friable and weak to be mounted). Basal tonic forces exhibited by IAS constructs implanted with growth factor are summarized in Fig. 6. The VEGF (625 ± 123 lN) and PDGF (641 ± 198 μN) groups had significantly higher basal tonic force than the FGF-2 group (114 ± 29 μN) [16].
Fig. 6.
Bar graph of basal tonic force (in micro Newtons, μN) of the post-implant constructs with growth factor. *p < 0.05 by one-way ANOVA and Tukey's post-test compared to FGF-2 group
Discussion
The impetus for studying the effects of different growth factors was twofold. First was to determine if one growth factor led to superior histologic and/or physiologic status of the implanted construct. The second was to specifically examine PDGF, as PDGF-BB (becaplermin) has FDA- and CHMP-approved indications for the treatment of diabetic foot ulcers [17], thus allowing far greater ease to moving our research to the clinical setting If PDGF has similar efficacy to other growth factors, it would allow for an offuse application when translating bioengineered IAS implantation to a human setting.
Our results confirm our previous findings that the use of growth factors is necessary to maintain the integrity of the construct during the implantation period [6, 16]. More importantly, the histologic and physiologic outcomes of implanting our bioengineered IAS with PDGF were at least as good, if not better, than both FGF and VEGF use. These results suggest the use of PDGF as the preferred growth factor, as its use in humans is already approved.
All three growth factors resulted in histologically confirmed neovascularization. This indicates that these growth factors play a role in the angiogenesis of capillaries within the construct, which leads to in vivo maintenance of the IAS. Despite the different types of growth factor used, there were no significant differences between the degrees of neovascularization as measured by blood vessels per HPF. This is somewhat different from previously reported results where VEGF use led to the higher capillary density over FGF [10, 11]. The effect of these growth factors on IAS cells may be different from that on bone, dental pulp or other mesenchyme-derived tissues. Another explanation is that at an earlier time point (such as 14 days as described by Mullane et al. [10]), there were some differences in microvascular density but these were lost over time.
It was surprising to find that the basal tone of the PDGF and VEGF-treated constructs was significantly higher than the ones treated with FGF-2. One possibility is that the stronger angiogenic potential of VEGF manifested itself led to more robustly surviving muscle tissue, and contributed to a better physiologic response. PDGF is known to be a major mitogen for smooth muscle cells [14, 15]. This property of PDGF may have led to the greater basal tonic force generated by the constructs given this growth factor. The literature is conflicting about the effect of FGF-2 on smooth muscle. In vitro studies have shown that PDGF-BB acts through basic FGF when inducing smooth muscle cell proliferation [18]. However, when FGF was added directly to colonic smooth muscle cell cultures, it did not induce any additional proliferation [15]. Additional work on the in vivo effect of FGF on smooth muscle cells is needed to examine this further.
While this study establishes the need for growth factors for these IAS constructs, there are several important questions that still remain. How much can we lower the dosage of growth factor before we start to affect the integrity of the implanted construct? Perhaps a shorter duration of growth factor administration would be sufficient to establish a vascular bed that allows long-term survival of the construct. In some of our earlier experiments where a more rigid polyethylene deliver catheter was used to deliver FGF, we saw a few cases of the catheter eroding out of the mouse's skin between 1 and 2 weeks post-implantation. One of these constructs was left in for the full 25 days, and despite the early cessation of direct growth factor delivery, the histologic appearance of the tissue was as robust as those constructs that received the full 25–28 days of growth factor (data not shown). Long-term survival of implanted grafts will also need to be addressed in future studies.
Tumorigenic consequences of growth factor usage are still a concern, as even the FDA-approved PDGF has a black box warning for increased risk of malignancy with use of more than three tubes of becaplermin gel. However, the osmotic pumps we used completed delivery of the growth factors by 28 days, which would clearly limit the duration of exposure to the growth factor, lessening the risk of malignancy.
In conclusion, the use of growth factors is essential to promote neovascularization, function and survival of implanted IAS constructs. The type of growth factor does not significantly impact the morphologic outcomes of the implantation; however, VEGF and PDGF use resulted in superior basal muscle tone exhibited by the implanted construct. The strong histological scores and physiologic function of the PDGF-supplemented implants after in vivo implantation paves the way for a more streamlined translation of this concept to the clinical realm.
Acknowledgments
This study was supported by National Institute of Health Grants NIH/NIDDK DK071614, 1RC1DK087151, and the Center for Organogenesis teaching grant T32HD007505.
References
- 1.Nelson R, Norton N, Cautley E, et al. Community-based prevalence of anal incontinence. JAMA. 1995;274:559–561. [PubMed] [Google Scholar]
- 2.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480–484. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharucha AE. Fecal incontinence. Gastroenterology. 2003;124:1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 4.Tjandra JJ, Lim JF, Hiscock R, et al. Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Dis Colon Rectum. 2004;47:2138–2146. doi: 10.1007/s10350-004-0760-3. [DOI] [PubMed] [Google Scholar]
- 5.Thornton MJ, Kennedy ML, Lubowski DZ, et al. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis. 2004;6:470–476. doi: 10.1111/j.1463-1318.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 6.Hashish M, Raghavan S, Somara S, et al. Surgical implantation of a bioengineered internal anal sphincter. J Pediatr Surg. 2010;45:52–58. doi: 10.1016/j.jpedsurg.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somara S, Gilmont RR, Dennis RG, et al. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137:53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marra KG, Defail AJ, Clavijo-Alvarez JA, et al. FGF-2 enhances vascularization for adipose tissue engineering. Plast Reconstr Surg. 2008;121:1153–1164. doi: 10.1097/01.prs.0000305517.93747.72. [DOI] [PubMed] [Google Scholar]
- 10.Mullane EM, Dong Z, Sedgley CM, et al. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J Dent Res. 2008;87:1144–1148. doi: 10.1177/154405910808701204. [DOI] [PubMed] [Google Scholar]
- 11.Larsen M, Willems WF, Pelzer M, et al. Augmentation of surgical angiogenesis in vascularized bone allotransplants with host-derived a/v bundle implantation, fibroblast growth factor-2, and vascular endothelial growth factor administration. J Orthop Res. 2010;28:1015–1021. doi: 10.1002/jor.21098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke JW, Sarment DP, Whitesman LA, et al. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12:1441–1450. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KD, Yamataka A, Kato Y, et al. Basic fibroblast growth factor and granulocyte colony-stimulating factor enhance mucosal surface expansion after adult small bowel transplantation without vascular reconstruction in rats. J Pediatr Surg. 2006;41:737–741. doi: 10.1016/j.jpedsurg.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 15.Stanzel RD, Lourenssen S, Nair DG, et al. Mitogenic factors promoting intestinal smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2010;299(4):C805–C817. doi: 10.1152/ajpcell.00086.2010. [DOI] [PubMed] [Google Scholar]
- 16.Raghavan S, Miyasaka EA, Hashish M, et al. Successful implantation of physiologically functional bioengineered mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2010;299:G430–G439. doi: 10.1152/ajpgi.00269.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117:143S–149S. doi: 10.1097/01.prs.0000222526.21512.4c. discussion 150S–151S, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Millette E, Rauch BH, Defawe O, et al. Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res. 2005;96:172–179. doi: 10.1161/01.RES.0000154595.87608.db. [DOI] [PubMed] [Google Scholar]