Abstract
Background
High viral load (VL) setpoint is a marker for rapid HIV progression, but few studies have examined whether use of hormonal contraception (HC) prior to HIV seroconversion affects VL setpoint.
Methods
We determined VL setpoints in 285 HIV seroconverters using blood samples collected six months or more after estimated HIV seroconversion but before disease progression to CD4≤250 or WHO Stage 3 or 4. We used multivariate linear regression to estimate the effect of HC use prior to HIV seroconversion on VL setpoint, and multivariate Cox regression to estimate the hazards ratio of death associated with VL setpoint.
Results
Of 285 women, 42 (15%) reported using HC prior to HIV seroconversion. Mean VL setpoint was 4.49 (SD 0.79) log10 copies/mL among women who used HC prior to HIV seroconversion and 4.47 (SD 0.86) among non-HC users (p=0.88). In multivariate analysis, HC prior to HIV seroconversion was not associated with VL setpoint (+0.11 log10 copies /mL; p=0.47). Higher socioeconomic status was associated with lower VL setpoint (-0.43 log10 copies/mL; p=0.04). VL setpoints above the median were associated with faster time to death (adjHR: 2.54, 95% CI: 1.30-4.98, p-value <0.01).
Conclusions
Use of HC prior to HIV seroconversion was not associated with elevated VL setpoint.
Keywords: hormonal contraception, viral load setpoint, HIV progression, Uganda
Introduction
Women are disproportionately affected by HIV in sub-Saharan Africa, accounting for 60% of all HIV infections in the region.1 Many women living in areas of high HIV prevalence are of childbearing age, and may desire access to safe and effective contraceptive methods. Access to comprehensive family planning services is critical in sub-Saharan Africa, where rates of maternal mortality are high, with 900 maternal deaths per 100,000 live births.2,3 Contraception can provide control over the spacing and limiting of births, reduce pregnancy-related morbidity and mortality, and reduce the demand for abortion, which is particularly crucial in regions where access to safe and legal abortion services are restricted.4,5 Contraceptive use has also been linked to social and economic benefits for women and their families.6
Hormonal contraceptive methods are among the most effective methods of pregnancy prevention, but evidence assessing whether hormonal contraception (HC) affects events in early HIV infection, such as establishment of viral load setpoint, is mixed. A high viral load (VL) setpoint is associated with rapid HIV disease progression.7-10 VL setpoint is thought to reflect the equilibrium between viral replication and immune response. This generally occurs between two and six months after HIV infection and remains relatively steady throughout the asymptomatic latent phase of HIV infection.11 HIV progression can also be affected by other factors, including viral subtype, viral diversity, co-infections such as STIs and malaria, and older age at seroconversion.12-16
Three studies have examined whether HC use near the time of HIV infection affects VL setpoint. One study of 161 sex workers in Kenya found that use of injectable contraception at seroconversion was associated with a higher VL setpoint, relative to non-hormonal contraceptive users.17 However, in a related analysis of these data which controlled for viral diversity, HC use was not independently associated with higher VL setpoint.18 Another study of 27 women being treated for genital infections in Malawi also found that users of injectable contraception had a higher VL setpoint.19 Conversely, a recent prospective study among 175 Ugandan and Zimbabwean women seeking family planning services found no association between HC use and cervical VL in early infection or plasma VL at setpoint.20 No studies have been conducted in a population-based cohort and it may be difficult to generalize findings from studies of high-risk groups such as sex workers.
The objective of our study was to assess the relationship between use of HC prior to HIV seroconversion on VL setpoint in a large, population-based cohort of women with incident HIV infection.
Methods
The Rakai Community Cohort Study
Since 1994, the Rakai Health Sciences Program (RHSP) has followed an open cohort of all consenting adults aged 15-49 years living in 50 communities in Rakai District, southwestern Uganda. The Rakai Community Cohort Study (RCCS) has been described in detail,21,22 but in brief, participants provide informed consent and are privately interviewed by same-sex interviewers every 12-15 months, using a standardized questionnaire. Venous blood is collected at each survey round for HIV-1 and STI serology. Over 90% of eligible individuals have participated in any given survey round. Sexually-experienced female participants who acquired HIV and had VL measurements while under observation in the RCCS were eligible for inclusion in this analysis.
Laboratory methods
Peripheral blood was collected in tubes that contained EDTA (Becton Dickinson), separated within 8h of collection into plasma cells by Megafuge 1.0R centrifugation, and frozen at -80°C until assay. All specimens were tested in batch for HIV-1 RNA at the Rakai Health Sciences Program laboratory in Uganda. We quantified HIV-1 RNA in human plasma using the Amplicor HIV-1 Monitor Test, Version 1.5 (Roche Diagnostics Corporation, Indianapolis IN), which has been validated to monitor HIV-1 VL in patients identified as being HIV-1 positive for all group M subtypes of HIV-1, including subtypes A and D, which predominate in Uganda. We quantified HIV-1 viral RNA using the HIV-1 Quantification Standard Procedure. HIV-1 RNA levels in the test were determined by comparing the HIV-1 signal to the quantification standard signal for each specimen. Test results less than 400 copies/mL were below the lower limit of quantification of the standard test and were reported as “HIV-1 RNA detected less than 400 (2.6 log10) HIV-1 RNA copies/mL”. Such samples were recorded as 399 copies/mL to conservatively calculate VL setpoint. CD4 cell counts were performed with FacsCalibur until 2004, and a FacsCount system thereafter.
Variable definitions
VL setpoint was estimated by averaging all VL measurements that fulfilled each of the following criteria: (1) collected six months or more after the estimated date of seroconversion, (2) collected prior to the date of disease progression to CD4≤250 or WHO Stage 3 or 4 or initiation of antiretroviral therapy, and (3) for individuals who did not experience disease progression to CD4≤250 or WHO Stage 3 or 4 while under observation, collected prior to the median time to CD4≤250 or WHO Stage 3 or 4 or death in this population (4.5 years from estimated date of seroconversion).23 These criteria were chosen to exclude VL measurements from samples taken during acute infection or advanced disease. VL setpoint levels were log10 transformed for analysis.
Date of HIV seroconversion was estimated as the midpoint between the last HIV negative and the first HIV positive antibody test dates. Seroconversion was detected by two independent ELISA tests confirmed by Western Blot. For confirmed seroconverters, we performed PCR tests on the prior negative sample. If the subject had acute infection during her study visit (as indicated by PCR positive and ELISA negative tests), that visit was considered the date of HIV seroconversion. For individuals who had an indeterminate Western Blot test result (with an RNA positive test) between their last negative HIV and first positive HIV antibody test, the date of this indeterminate test was considered the HIV seroconversion date.
For 75% of the 120 women, disease progression was defined as CD4 cell count ≤250 cells/mm3 (when individuals in Rakai become eligible for ART), and the onset date was estimated as the midpoint between the last CD4 count above 250 and first CD4 count ≤250. If the first CD4 count was ≤250, that was considered the onset date. For nine women without available CD4 counts, disease progression was defined as clinical symptoms meeting the WHO clinical stage 3 or 4 criteria,24 with onset date calculated as the date of the symptoms interview. For 21 women with both CD4 information and clinical symptoms information, the earlier of the two dates was considered the onset date.
Date of death was ascertained from information collected during an annual census and surveys, as well as verbal autopsy interviews. A structured verbal autopsy was administered to the individual closest to the deceased at the time of death to obtain information on the date and probable cause of death. We were unable to definitively distinguish between HIV-related and HIV-unrelated deaths, but previous analyses in Rakai have found that over 90% of all deaths among HIV-positive persons are HIV-related.25,26
HC use was defined as self-reported current HC use at the last HIV-negative visit prior to the visit at which HIV seroconversion was identified, given that HC use in the interval prior to HIV seroconversion was most likely to accurately represent HC use at the time of HIV infection. Our definition of HC use included use of oral and injectable contraceptives, and after 1999, also included Norplant.
Socioeconomic status (SES) was determined based upon materials used to construct the floor, walls and roof. “Poor” meant that the floor was either mud, wood, or “other”, walls were either thatch, mud/wattle, “other”, or “mobile” (indicating that the individual’s family does not own a structure in that community), and that the roof was either made of thatch or “other materials” (such as banana fiber or papyrus), or “mobile.” “Above average” indicates that the roof was either made of iron or tiles, that the floor was cement, and that the walls were cement, brick, or stone. Average includes all other categories.
Statistical analysis
We calculated VL setpoint by averaging all VL measurements meeting our criteria for setpoint for each individual. We compared VL setpoints between HC users and non-users using a t-test, and we used univariate and multivariate linear regression to examine the effect of HC use prior to HIV seroconversion on VL setpoint. The following variables, taken from last HIV-negative visit, were considered as potential confounders: age, educational level, marital status, SES, parity, breastfeeding, number of sexual partners in past year, current non-marital relationship, condom use, pregnancy, and genital ulcer disease. In multivariate analyses, we controlled for all variables associated with both HC use and VL setpoint at p=0.20 in univariate regressions (socioeconomic status, non-marital relationship, parity, and breastfeeding), as well as for variables included for comparability with previous research (age, education, number of sexual partners in past year, and current genital ulcer disease).
We first examined use of any HC (oral contraceptive pills, injectables, or implants), then examined each method separately to assess for the possibility of different biological effects. We performed two sensitivity analyses to determine whether risk associated with using HC could have been biased due to initiation or discontinuation of HC by the time of HIV seroconversion. First, we performed an analysis including only women who reported use of HC both before and after HIV seroconversion (i.e., women that were most likely to have been on a method at the time of HIV seroconversion), and compared them against women who reported no use of HC either before or after HIV seroconversion. Second, we excluded women in whom ascertainment of contraceptive use was more than six months or more than one year after the estimated date of seroconversion.
Finally, we dichotomized VL setpoints above or below the median (4.50 log10 copies/mL), and performed univariate and multivariate Cox regression analyses to estimate whether this variable was significantly associated with the hazards ratio for time to death. All analyses were done using STATA.SE, Version 10.1.
Results
We identified 285 seroconverting women who provided 366 blood samples which met our criteria for setpoint estimation. Twenty samples (5%) had below detectable viral loads (≤399 cps/mL). VL setpoint was determined using one measurement for 75% of women (n=214), two measurements for 22% of women (n=62), three measurements for 3% of women (n=8), and four measurements for 0.4% of women (n=1). Among women with one VL measurement, the majority of those VL measurements (66%) were taken between six months and one year after HIV seroconversion. A single VL measurement ascertainment date was not possible among women for whom VL setpoint was determined by averaging multiple measurements. Average VL setpoint was 4.47 log10 copies/mL (standard deviation: 0.85). Average VL setpoints were similar between women with one measurement (4.43 log10copies/mL) and women with multiple measurements (4.59 log10copies/mL) (-0.16 log10copies/mL, 95% CI: -0.38 to 0.07, p=0.18).
Of the 285 women, 42 (15%) reported using HC at the survey round prior to HIV seroconversion. Among the 42 women who reported using a method of HC at the survey prior to HIV seroconversion, 71.4% (30/42) used injectables, 26.2% (11/42) used oral contraceptive pills, and 2.4% (1/42) used implants. Women who reported using HC prior to HIV seroconversion had a higher SES, were less likely to have ever been married, reported less condom use, pregnancy, and genital ulcer disease than HC non-users, and were more likely to have one sexual partner in the past year (whereas non-HC users were more likely to report either zero or multiple partners in the past year) (Table 1). We found no univariate association of HC use with VL setpoint (p=0.88).
Table 1.
Association of demographic, behavioral, and reproductive characteristics with HC use prior to seroconversion
| No HC use (n=243) | HC use (n=42) | p-value | |||
|---|---|---|---|---|---|
| Mean (sd) | Mean (sd) | ||||
| Log viral load setpoint | 4.47 (0.86) | 4.49 (0.79) | 0.882 | ||
| n | % | n | % | ||
| Age | 0.850 | ||||
| 15-24 | 110 | 45% | 21 | 50% | |
| 25-34 | 88 | 36% | 14 | 33% | |
| 35+ | 45 | 19% | 7 | 17% | |
| Socioeconomic status | 0.001 | ||||
| Poor | 24 | 10% | 0 | 0% | |
| Average | 163 | 67% | 22 | 52% | |
| Above average | 56 | 23% | 20 | 48% | |
| Education | 0.197 | ||||
| None | 37 | 15% | 3 | 7% | |
| Primary | 154 | 63% | 25 | 60% | |
| Secondary | 45 | 19% | 11 | 26% | |
| Technical/University | 7 | 3% | 3 | 7% | |
| Marital status | 0.053 | ||||
| Never married | 49 | 20% | 14 | 33% | |
| Previously married | 38 | 16% | 2 | 5% | |
| Currently married | 156 | 64% | 26 | 62% | |
| Non-marital relationship | 56 | 23% | 15 | 36% | 0.080 |
| Number of total births | 0.815 | ||||
| None | 34 | 14% | 4 | 10% | |
| 1 | 38 | 16% | 7 | 17% | |
| 2-4 | 90 | 37% | 18 | 43% | |
| 5+ | 81 | 33% | 13 | 31% | |
| Currently pregnant | 55 | 23% | 0 | 0% | 0.001 |
| Breastfeeding | 38 | 16% | 5 | 12% | 0.533 |
| Condom use | 0.019 | ||||
| No | 194 | 80% | 41 | 98% | |
| Yes | 25 | 10% | 1 | 2% | |
| Not asked b/c pregnant | 24 | 10% | 0 | 0% | |
| Sex partners in past year | 0.029 | ||||
| None | 22 | 9% | 0 | 0% | |
| One | 190 | 78% | 40 | 95% | |
| Two or more | 31 | 13% | 2 | 5% | |
| Current genital ulcer | 0.035 | ||||
| No | 182 | 75% | 39 | 93% | |
| Yes | 5 | 2% | 0 | 0% | |
| Not asked* | 56 | 23% | 3 | 7% | |
Some survey rounds did not ask about current genital ulcer, so responses were not available to all women for this question
No covariates were significantly associated with VL setpoint at p≤0.05 in univariate linear regression, but there was a non-significant difference of lower VL setpoint among women with no previous births (p=0.07) (Table 2).
Table 2.
Univariate associations with viral load setpoint (log10 copies/mL)
| Variable | Change in viral load set point (log10 copies/mL) | 95% CI | p-value |
|---|---|---|---|
| Hormonal contraceptive use | +0.02 | -0.26 to +0.30 | 0.882 |
| Age | 0.277 | ||
| 15-24 | ref | -- | -- |
| 25-34 | +0.17 | -0.05 to +0.39 | -- |
| 35+ | +0.00 | -0.27 to +0.28 | -- |
| Socioeconomic status | 0.164 | ||
| Poor | ref | -- | -- |
| Average | -0.09 | -0.45 to +0.27 | -- |
| Above average | -0.29 | -0.68 to +0.10 | -- |
| Education | 0.664 | ||
| None | ref | -- | -- |
| Primary | +0.06 | -0.23 to +0.35 | -- |
| Secondary | +0.20 | -0.15 to +0.55 | -- |
| Technical/University | +0.07 | -0.53 to +0.66 | -- |
| Marital status | 0.208 | ||
| Never married | ref | -- | -- |
| Previously married | +0.16 | -0.18 to +0.49 | -- |
| Currently married | -0.10 | -0.34 to +0.15 | -- |
| Non-marital relationship | +0.16 | -0.07 to +0.39 | 0.167 |
| Number of total births | 0.068 | ||
| None | ref | -- | -- |
| 1 | +0.47 | +0.11 to +0.84 | -- |
| 2-4 | +0.23 | -0.08 to + 0.54 | -- |
| 5+ | +0.33 | +0.01 to + 0.64 | -- |
| Currently pregnant | -0.03 | -0.28 to +0.22 | 0.811 |
| Breastfeeding | +0.19 | -0.09 to +0.46 | 0.181 |
| Condom use | 0.612 | ||
| No | ref | -- | -- |
| Yes | +0.17 | -0.17 to +0.52 | -- |
| Not asked b/c pregnant | -0.01 | -0.37 to +0.35 | -- |
| Sex partners in past year | 0.217 | ||
| None | ref | -- | -- |
| One | -0.11 | -0.48 to +0.26 | -- |
| Two or more | +0.16 | -0.30 to +0.62 | -- |
| Current genital ulcer | 0.252 | ||
| No | ref | -- | -- |
| Yes | -0.42 | -1.17 to +0.33 | -- |
| Not asked | +0.14 | -0.10 to +0.39 | -- |
In multivariate linear regression (Table 3), HC use was not significantly associated with VL setpoint (+0.11 log10 copies/mL; 95% CI: p=0.47). Above average socioeconomic status was associated with lower VL setpoint as compared with women of poor socioeconomic status (-0.43 log10 copies/mL; p=0.04), and women with one child had a significantly higher VL setpoint (+0.39 log10 copies/mL, p = 0.05) than nulliparous women. Neither OCs nor injectables were significantly associated with VL setpoint in multivariate regression (OC: +0.14 log10 copies/mL, 95% CI: -0.38 to +0.66, p=0.59; injectable: +0.10 log10 copies/mL, 95% CI: -0.23 to +0.43, p=0.56). Since only one woman reported implant use around the time of seroconversion, we did not assess this method separately.
Table 3.
Effect of covariates on viral load setpoint (log10 copies/mL) in multivariate regression
| Variable | Change in viral load set point (log10 copies/mL) | 95% CI | p-value |
|---|---|---|---|
| Hormonal contraceptive use | +0.11 | -0.18 to + 0.40 | 0.469 |
| Age | 0.515 | ||
| 15-24 | ref | -- | -- |
| 25-34 | +0.13 | -0.13 to +0.38 | -- |
| 35+ | +0.00 | -0.34 to +0.33 | -- |
| Socioeconomic status | 0.027 | ||
| Poor | ref | -- | -- |
| Average | -0.11 | -0.48 to +0.26 | -- |
| Above average | -0.43 | -0.85 to -0.02 | -- |
| Education | 0.220 | ||
| None | ref | -- | -- |
| Primary | +0.08 | -0.21 to +0.38 | -- |
| Secondary | +0.34 | -0.03 to +0.70 | -- |
| Technical/University | +0.27 | -0.34 to +0.88 | -- |
| Non-marital relationship | +0.18 | -0.06 to +0.42 | 0.138 |
| Number of total births | 0.224 | ||
| None | ref | -- | -- |
| 1 | +0.39 | +0.01 to +0.77 | -- |
| 2-4 | +0.18 | -0.16 to +0. 51 | -- |
| 5+ | +0.26 | -0.13 to +0. 66 | -- |
| Sex partners in past year | 0.132 | ||
| None | ref | -- | -- |
| One | -0.24 | -0.62 to +0.15 | -- |
| Two or more | +0.04 | -0.43 to +0.52 | -- |
| Current breastfeeding | +0.13 | -0.18 to +0.43 | 0.414 |
| Current genital ulcer | 0.388 | ||
| No | ref | -- | -- |
| Yes | -0.41 | -1.16 to +0.35 | -- |
| Not asked | +0.11 | -0.16 to +0.37 | -- |
Analyses controlled for age, socioeconomic status, education, non-marital relationship, parity, sex partners in past year, current breastfeeding, and genital ulcer disease.
A sensitivity analysis comparing 15 women who reported using HC both before and after HIV seroconversion against 158 women reporting no HC use either before or after HIV seroconversion did not substantially change our findings (-0.08 log10 copies/mL; 95% CI: -0.57 to +0.42, p=0.76). Another sensitivity analysis excluding 42 women in whom ascertainment of contraceptive use was more than one year after estimated date of seroconversion found no association between HC use and VL setpoint (+0.10 log10 copies/mL, 95% CI: -0.22 to +0.41, p=0.55). An analysis restricted to 48 women with less than six months between contraceptive ascertainment and HIV seroconversion suggested that the 3 women reporting HC use had a significantly lower VL setpoint than 45 women reporting non-use of HC (-1.72 log10 copies/mL, 95% CI: -3.09 to -0.34, p=0.02).
Unadjusted analyses suggested that VL setpoints above the median for this population were associated with increased hazards of mortality (Figure 1). After controlling for age at seroconversion, current age, education, SES, marital status, involvement in a non-marital relationship, current pregnancy, current breastfeeding, parity, and number of sexual partners in the past year, having a VL setpoint above the median remained strongly associated with increased hazards of mortality: adjHR: 2.54, 95% CI: 1.30-4.98, p-value <0.01.
Figure 1.
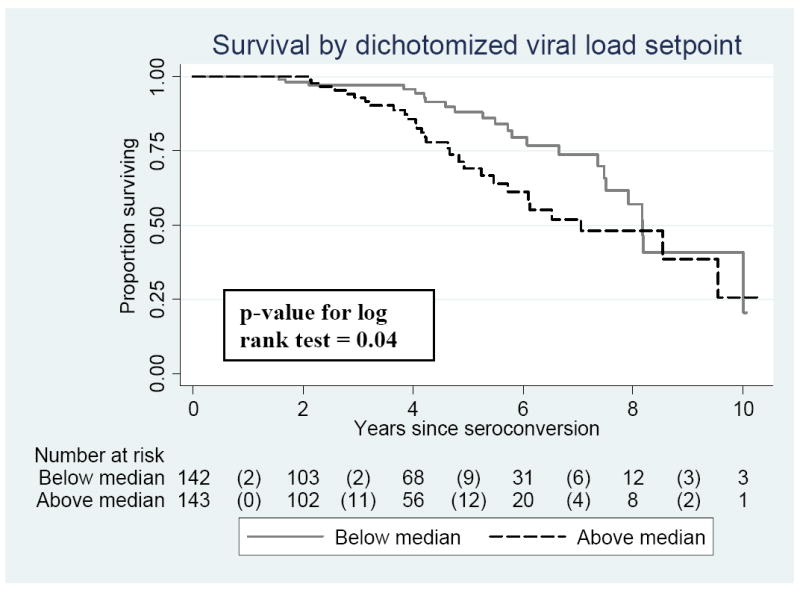
Univariate effect of viral load setpoint on time to death
Discussion
We found no evidence that use of HC prior to HIV seroconversion was associated with an elevated VL setpoint. This observation is consistent with a prior analysis of Rakai data which found no association between HC use and hazards of disease progression or mortality.23 Thus, our findings suggest that HC use does not directly affect VL at setpoint or indirectly affect HIV disease progression via an effect on VL at setpoint.
Our findings differ from two previous studies conducted among sex workers or women being treated for genital tract infections.17,19 Studies involving higher risk women may be confounded by other sexually transmitted infections, such as HSV-2, possibly associated with both HC use and with HIV viremia. Our results from a general community cohort corroborate findings from a study conducted among women seeking reproductive and general health care services from facilities in Uganda and Zimbabwe.20 Unlike the latter study, we did not find that pregnancy or breastfeeding near the time of HIV seroconversion were associated with a higher VL setpoint or that younger age was associated with lower VL setpoint.
Our data suggest that higher VL setpoint is associated with more rapid progression to death, as has been previously reported.
This study has limitations. For 75% of women, VL setpoint was estimated using only one VL measurement. Although the majority of women had VL measurements which occurred between six months and one year after HIV seroconversion, it is possible that for a minority of women, VL measurements were ascertained after the typical time frame used to define VL setpoint. However, VL setpoint is assumed to be relatively stable throughout the asymptomatic latent phase of HIV infection.11 Furthermore, averaging VL measurements may have resulted in a large variance for the VL setpoint estimate, which may have reduced our power to detect a difference in the VL setpoint between HC users and non-users. However, the mean VL setpoint in our data (4.47 log10 copies/mL) was similar to that reported in three other studies of HC and VL setpoint in Kenya (4.46 log10 copies/mL),17 Malawi (4.45 log10 copies/mL),19 and a study in Uganda and Zimbabwe (4.20 log10 copies/mL).20 We were unable to distinguish between different formulations of OCs, which may have changed over time. Additionally, women who reported HC use at the interview prior to HIV seroconversion may have initiated or discontinued use of HC by the HIV seroconversion date. While we cannot rule out misclassification of HC exposure, two sensitivity analyses conducted on women most likely to have been using HC at the time of HIV seroconversion suggested similar results to our main analysis, limiting the concern that HC initiation or discontinuation biased our results. Restricting the sample to include only women with less than six months between HC ascertainment and HIV seroconversion suggested an association between HC use and lower VL setpoint. While these results provide some reassurance that we did not underestimate risk, the sample size for that sensitivity analysis was small and cannot be interpreted as a protective effect of HC.
In summary, this analysis of a population-based cohort contradicts findings of similar analyses based in higher-risk populations, but corroborates findings from a more generalizable population, in that HC use near the time of HIV seroconversion was not associated with a higher VL setpoint. These findings are reassuring given that HC methods are among the most effective methods of pregnancy prevention.
Acknowledgments
The authors wish to acknowledge the contributions of Heena Brahmbhatt, Michael Z. Chen, Homayoon Farzadegan, the Rakai Health Sciences Program study teams, and the RCCS cohort participants.
Sources of support: Funding was provided by UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction. Data collection was supported by grants R01 A134826 and R01 A134265 from the National Institute of Allergy and Infectious Diseases; grant 5P30HD06826 from the National Institute of Child and Health Development; the World Bank STI Project, Uganda; grant 5D43TW00010 from the Fogarty Foundation and grant RO1 A134826, by the Department of the Army, United States Army Medical Research and Material Command Cooperative Agreement DAMD17-98-2-8007 and the Henry M. Jackson Foundation.
Footnotes
Presentation of results: Presented at XVIII International AIDS Conference, July 2010, Vienna, Austria
References
- 1.UNAIDS. 2009 AIDS epidemic update. 2009 [Google Scholar]
- 2.Shah IH, Say L. Maternal mortality and maternity care from 1990 to 2005: uneven but important gains. Reprod Health Matters. 2007;15:17–27. doi: 10.1016/S0968-8080(07)30339-X. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. World Health Statistics, 2010. Geneva, Switzerland: World Health Organization Press; 2010. [Google Scholar]
- 4.Conde-Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ. 2000;321:1255–1259. doi: 10.1136/bmj.321.7271.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DaVanzo J, Razzaque A, Rahman M, et al. The effects of birth spacing on infant and child mortality, pregnancy outcomes, and maternal mortality in Matlab Bangladesh. Santa Monica, California: RAND Corporations Publications Department; 2004. Working Paper 198. [Google Scholar]
- 6.Singh S, Darroch JE, Ashford LS, et al. Adding It Up: The Costs and Benefits of Investing in Family Planning and Maternal and Newborn Health. New York: Guttmacher Institute and United Nations Population Fund; 2009. [Google Scholar]
- 7.Sterling TR, Vlahov D, Astemborski J, et al. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Eng J Med. 2001;344:720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 8.Lefrere JJ, Roudot-Thoraval F, Mariotti M, et al. The risk of disease progression is determined during the first year of human immunodeficiency virus type 1 infection. J Infect Dis. 1998;177:1541–1548. doi: 10.1086/515308. [DOI] [PubMed] [Google Scholar]
- 9.Schacker TW, Hughes JP, Shea T, et al. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Mellors JW, Kingsley LA, Rinaldo CR, et al. Quantitation of Hiv-1 Rna in Plasma Predicts Outcome After Seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Eng J Med. 2007;356:2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 12.Kiwanuka N, Laeyendecker O, Robb M, et al. Effect of HIV-1 subtypes on disease progression in a cohort of incident cases infected with pure subtypes, recombinants, and multiple subtypes, Rakai, Uganda. J Infect Dis. 2008;197:707–713. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
- 13.Lutalo T, Gray RH, Wawer M, et al. Survival of HIV-infected treatment-naive individuals with documented dates of seroconversion in Rakai, Uganda. AIDS. 2007;21(Suppl 6):S15–S19. doi: 10.1097/01.aids.0000299406.44775.de. [DOI] [PubMed] [Google Scholar]
- 14.Collaborative Group on AIDS Incubation and HIV Survival. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- 15.Phair J, Jacobson L, Detels R, et al. Acquired immune deficiency syndrome occurring within 5 years of infection with human immunodeficiency virus type-1: the Multicenter AIDS Cohort Study. JAIDS. 1992;5:490–496. [PubMed] [Google Scholar]
- 16.Sagar M, Lavreys L, Baeten JM, et al. Infection with multiple human immunodeficiency virus type 1 variants is associated with faster disease progression. J Virol. 2003;77:12921–12926. doi: 10.1128/JVI.77.23.12921-12926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavreys L, Baeten JM, Kreiss JK, et al. Injectable contraceptive use and genital ulcer disease during the early phase of HIV-1 infection increase plasma virus load in women. J Infect Dis. 2004;189:303–311. doi: 10.1086/380974. [DOI] [PubMed] [Google Scholar]
- 18.Sagar M, Lavreys L, Baeten JM, et al. Identification of modifiable factors that affect the genetic diversity of the transmitted HIV-1 population. AIDS. 2004;18:615–619. doi: 10.1097/00002030-200403050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kumwenda JJ, Makanani B, Taulo F, et al. Natural history and risk factors associated with early and established HIV type 1 infection among reproductive-age women in Malawi. Clin Infect Dis. 2008;46:1913–1920. doi: 10.1086/588478. [DOI] [PubMed] [Google Scholar]
- 20.Morrison CS, Demers K, Kwok C, et al. Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS. 2010;24:573–582. doi: 10.1097/QAD.0b013e32833433df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wawer MJ, Gray RH, Sewankambo NK, et al. A randomized, community trial of intensive sexually transmitted disease control for AIDS prevention, Rakai, Uganda. AIDS. 1998;12:1211–1225. doi: 10.1097/00002030-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353:525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 23.Polis CB, Wawer MJ, Kiwanuka N, et al. Effect of hormonal contraceptive use on HIV progression in female HIV seroconverters in Rakai, Uganda. AIDS. 2010;24:1937–1944. doi: 10.1097/QAD.0b013e32833b3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Geneva, Swizerland: World Health Organization Press; 2006. [PubMed] [Google Scholar]
- 25.Wawer MJ, Serwadda D, Gray RH, et al. Trends in HIV-1 prevalence may not reflect trends in incidence in mature epidemics: data from the Rakai population-based cohort, Uganda. AIDS. 1997;11:1023–1030. doi: 10.1097/00002030-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Sewankambo NK, Gray RH, Ahmad S, et al. Mortality associated with HIV infection in rural Rakai District, Uganda. AIDS. 2000;14:2391–2400. doi: 10.1097/00002030-200010200-00021. [DOI] [PubMed] [Google Scholar]


