Abstract
Gemtuzumab ozogamicin (GO), an anti-CD33 immunoconjugate, was combined with high dose cytarabine (HiDAC; cytarabine 3 g/m2 over 3 hours daily for 5 days) for adults with relapsed or refractory AML. HiDAC plus GO 9 mg/m2 on day 7 and 4.5 mg/m2 on day 14 was not tolerated, but HiDAC followed by GO 9 mg/m2 on day 7 was safe: 12/37 (32%) patients with relapsed AML achieved complete remission. Median overall survival was 8.9 months. No grade 4 hepatic veno-occlusive disease was observed. This regimen merits further study, both in this setting and as a remission consolidation therapy.
Keywords: gemtuzumab ozogamicin, acute myeloid leukemia, cytarabine, relapse
Introduction
Therapy for acute myeloid leukemia (AML) in adults remains disappointing. Approximately one-third of adults between the ages of 18-60 years can expect long-term disease-free survival (DFS) with anthracycline plus cytarabine chemotherapy for remission induction, followed by consolidation with intensive chemotherapy or hematopoietic stem cell transplantation (HCT) [1]. The situation for older adults is worse; even among those who are treated aggressively, only 5-10% will be long-term survivors [2]. While rarely cured solely by additional chemotherapy, patients with relapsed AML can sometimes be rendered into a minimal disease state following reinduction therapy [3]. Such patients can often proceed to a curative HCT either from an allogeneic[4] or autologous[5,6] source.
The optimal therapy for patients with relapsed or refractory AML in unclear. High-dose cytarabine (HiDAC), either alone[7] or in combination with other agents[8] is commonly used. However, increasingly routine use of this therapy during induction[9] and especially during post-remission treatment[10] makes subsequent success less likely. Other agents used to treat patients with relapsed AML include gemtuzumab ozogamicin (GO)[11] etoposide/mitoxantrone[12], novel nucleoside analogs cladribine[13] or fludarabine[14] and non-cytotoxic agents such as flavopiridol[15] or sirolimus[16]. The wide variation in remission rates (10-50%) after these therapies reflects intrinsic differences among these agents and combinations as well as host factors, such as age, the amount of prior of therapy, and most importantly, the length of the disease-free interval preceding the relapse [17].
The most recently approved agent for the treatment of relapsed AML in adults is GO[18-20]. GO is a humanized monoclonal antibody directed against the CD33 antigen, expressed on blast cells from 80% - 90% of patients with AML. The antibody is conjugated to the toxin calicheamicin. When this molecule binds to a CD33-expressing cell, internalization occurs and the calicheamicin toxin is liberated in the acidic microsomal environment. When released, calicheamicin induces double strand DNA breaks and cell death. Pivotal studies were performed in 142 patients with relapsed AML whose first complete remission (CR1) lasted for at least 3 months and generally more than 6 months[18-20]. A 30% CR rate was reported, although half of these responders had incomplete platelet recovery to <100,000/μl (CRp). These data led to approval by the FDA for patients over age 60 with relapsed AML whose blasts expressed CD33. Major side effects were limited to infusion-related toxicities, reversible hepatic toxicity, and prolonged myelosuppression. Subsequent studies have described severe hepatotoxicity when GO was given alone or in combination with chemotherapy[21], or if an allogeneic HCT was done within 3 months after exposure[22]. GO has been investigated alone or in combination as frontline therapy in patients with AML[23.24] including large randomized (MRC-15[25] and SWOG 0106[26]) trials, and/or as a post-remission strategy (ECOG 1900[27] and SWOG 0106 trials). The MRC 15 trial used GO at 3 mg/m2 on day 1 of induction and consolidation chemotherapy and the SWOG 0106 trial used 6 mg/m2 on day 4 of induction therapy and then 5 mg/m2 for 3 monthly doses during maintenance.
The clinical trial reported here combined GO and HiDAC. These two drugs have different mechanism of actions and toxicities. We hypothesized that GO could be given safely immediately after cytarabine because it does not cause mucositis and that initial cytoreduction with HiDAC would yield a low number of resdiual target cells, thus allowing more concentrated binding of the anti-CD33 monoclonal antibody. Our study determined a tolerable dose of GO that could be given following a standard 5-day regimen of HiDAC. We originally hoped to employ a novel schedule wherein 2 doses of GO were given 7 days apart in contrast to the standard 14-day interval, but this did not prove to be feasible. We now report the Phase I component of the trial as well as the results obtained in 37 patients with relapsed AML who were treated at the recommended Phase II doses (RPTD) of cytarabine at 3 gm/m2 per day for 5 days plus GO at 9 mg/m2 on day 7.
Methods
Trial Design
The objective of CALGB study 19902 was to define a tolerable combination of HiDAC and GO in patients with relapsed or refractory AML. One objective was to explore a novel schedule of GO given on day 1 and day 8 instead of the approved schedule that uses day 1 and day 15. Another objective was to determine the response rate of a tolerable schedule of HiDAC + GO in patients with advanced AML. The initial trial design required that patients have relapsed or primary refractory AML. CD33 expression was required on greater than 20% of the blasts measured by flow cytometry in clinical labs. Patients could not have had a hematopoietic stem cell transplant within 6 months or exposure to HiDAC within 3 months and could have no history of prior hepatic disease. The bilirubin level had to be less than 2 mg/dl. Primary refractory AML was defined as 10% residual AML blasts in the bone marrow or blood following two cycles of standard cytarabine plus anthracycline-based induction chemotherapy. Relapsed AML required that patients have had a prior documented remission lasting at least 30 days, followed by recurrence of greater than 10% AML blasts in the bone marrow or blood. Although more than one prior relapse was permitted, eligible patients could not have had treatment for the current relapse. No active central nervous system (CNS) involvement was allowed. After experience with the first 9 patients, the eligibility criteria were amended to require an ECOG performance status of ≤ 2, no serious active infection, and no cytotoxic therapy within 2 months for relapsed patients. Each participant signed an IRB-approved, protocol specific informed consent in accordance with federal and institutional guidelines.
The study was designed to have a Phase I component which would establish safety by defining a maximum total dose (MTD) for both the novel day 1 and day 8 GO schedule and the combination of HiDAC followed by GO. A subsequent Phase II component was designed to establish efficacy in the relapsed patients. Patients with primary refractory AML were not considered for determination of the number of patients required for the phase II efficacy study. During the first 9 patient cohort, dose limiting toxicity (DLT) was defined by grade 4 myelosuppression lasting longer than 35 days or grade 3 or greater non-hematological toxicity. However, due to the frequent lack of blast clearance, the determination of DLT was difficult. Therefore, when the eligibility criteria were amended, the definition of DLT was changed to be based on the number of treatment-related deaths. More than 2 deaths probably or definitely related to the chemotherapy in any cohort of 9 or fewer patients was considered unacceptable.
Patient Cohorts
The first group of 9 patients (cohort I) received a dose of GO 9 mg/m2 IV over 2 hours on day 1 and 4.5 mg/m2 on day 8. Hydroxyurea was used to lower the white blood cell count to <30,000/ul prior to GO (Premedications were acetaminophen and diphenhydramine). The second group of 9 patients (cohort IA) received HiDAC at 3 gm/m2 IV over 3 hours daily for 5 days plus GO at 9 mg/m2 on day 7. The third cohort (II) received the same dose and schedule of HiDAC plus GO at 9 mg/m2 on day 7 and 4.5 mg/m2 on day 14. This regimen was deemed intolerable. Therefore, the Phase II dose was considered to be the cohort IA dose (Table 1).
Table 1.
Dosing Schedules for gemtuzumab ozogamicin (GO) and high-dose cytarabine (HiDAC) including the number of relapsed and refractory patients in each cohort.
| Refractory | Relapsed | ||||
|---|---|---|---|---|---|
| Phase I | 1 | GO | Day 1: GO 9.0 mg/m2 | ||
| Day 8: GO 4.5 mg/m2 | 3 | 6 | |||
| 1A | HiDAC + GO | Day 1: Cytarabine 3.0 g/m2/day × 5 days | |||
| Day 7: GO 9.0 mg/m2 | 2 | 10 | |||
| Cohort 2 | HiDAC + GO | Day 1: Cytarabine 3.0 g/m2/day × 5 days | |||
| Day 7: GO 9.0 mg/m2 | |||||
| Day 14: GO 4.5 mg/m2 | 0 | 7 | |||
| Phase II | RPTD | HiDAC + GO | Day 1: Cytarabine 3.0 g/m2/day × 5 days | ||
| Day 7: GO 9.0 mg/m2 | 5 | 27 |
Statistical Considerations
The statistical design for the initial Phase I component of the trial stated that the study would be stopped if 2 or more of the first 9 patients had a DLT (when DLT was defined as grade 4 hematological toxicity lasting longer than 35 days after the last dose of GO or irreversible nonhematological toxicity ≥ grade 3). The probability of 2 or more of the first 9 patients having such DLT was approximately 0.86 if the true rate of a DLT were 0.33, with a 0.77 probability of continuing treatment on the arm if the true DLT rate were 10%. When the study was amended to re-define DLT as treatment-related death, the statistical plan was changed. Because HiDAC therapy for patients with relapsed/refractory AML has an anticipated treatment related mortality rate of 30%, we decided the study could continue to higher dose cohorts if 2 or fewer of the 9 patients in the cohort experienced treatment-related mortality (not due to progressive AML) within 60 days. The probability of observing 3 or more treatment-related deaths assessing 9 patients was approximately 0.62 if the true rate of treatment related death were 33%. The probability of fewer than 3 treatment related deaths in the first 9 patients was 0.74 if the true treatment related mortality rate were no more than 20%.
A two stage design was used to monitor the response rate in relapsed patients. The number of total patients includes the relapsed patients receiving the dose established as the MTD in the Phase I portion of the study (cohort IA as noted above) plus those in the Phase II portion. The design stated that if ≤ 3 of the first 19 patients achieved a complete response (CR + CRp), accrual to the phase II study would be stopped and the combination therapy rejected for further research. However, if 4 or more of the first 19 patients had a complete response, another 14 patients would be accrued at the established MTD. If ≤ 7 of the 33 total patients achieved a response, the therapy would be rejected. If 8 or more of these patients achieved a response, the therapy could be considered for further research. The probability of having a complete response gives type 1 and type 2 error rates at 0.10 for the test of the null hypothesis that the response rate is ≤ 0.15 versus the alternative hypothesis that the probability is > 0.35. If the null hypothesis were true, there was a 68% probability of early termination of accrual with the expected sample size of 23 patients. We expected that the number of primary refractory patients would be around 10% of the total patients.
Patient registration, data collection and all analyses were performed by the CALGB Statistical Center. Data quality was ensured by careful review of the data by Statistical Center staff and the study chairperson following standard CALGB policies. The CALGB Audit Committee visits all participating institutions at least once every 3 years to verify compliance with federal regulations and protocol requirements for CALGB studies, including those pertaining to eligibility, treatment, response, and follow-up. Such on-site review of medical records was performed for a subgroup of 25 patients (42%) of the 60 patients under this study.
Results
Study Conduct and Phase I Results
60 patients were enrolled and began study treatment. The patient demographics are depicted in Table 1 (including relapsed or refractory status per cohort) and Table 2. As expected, this was an older group of patients, most of whom were experiencing their first relapse. Only about one-third had received prior high dose ara-C; but the median duration of remission was well under one year. Institutionally-derived pretreatment karyotyping data was available for 38 patients: 22 had normal cytogenetics, 1 had inv16, 13 had unfavorable karyotypes and 2 had other intermediate risk abnormalities.
Table 2. Baseline Characteristics, all patients (n=60).
| Age at study entry | median in years (IQR) | 62.6 (52.7, 69.3) |
|---|---|---|
| Disease status | refractory | 10 (17%) |
| first relapse | 47 (78%) | |
| 2 or more relapses | 3 (5%) | |
| Prior HiDAC | Yes | 21 (35%) |
| no | 39 (65%) | |
| Duration of CR1 | median in months (IQR) | 9.0 (4.0, 13.0) |
| FAB Classifications | n/a | 3 (5%) |
| AML | 3 (5%) | |
| M0 | 5 (8%) | |
| M1 | 10 (16%) | |
| M2 | 15 (25%) | |
| M4 | 14 (23%) | |
| M5B | 1 (2%) | |
| M6 | 3 (5%) | |
| M7 | 1 (2%) | |
| MDS (RAEB) | 2 (3%) | |
| Multilineage | 2 (3%) | |
| t-AML | 1 (2%) |
The first 9 patients were treated at a dose schedule of GO 9 mg/m2 on day 1 and 4.5 mg/m2 on day 8. One patient achieved a CRp one month after GO, and experienced a CNS relapse 6.5 months after treatment ended. Many of these patients, however, could not have an accurate assessment of their toxicities due to the presence of persistent leukemia. Therefore, it was decided not to pursue the GO alone dosing but rather to move forward to HiDAC plus GO.
Nine patients were treated in cohort IA which was HiDAC at 3 gm/m2 over 3 hours daily for 5 days plus GO 9 mg/m2 on day 7. There were no treatment-related deaths. As this was eventually determined to represent the MTD, we subsequently analyzed these patients as a group along with those treated at the same doses in the Phase II portion of the study.
After cohort 1A was deemed tolerable, we assigned 10 patients to cohort II. HiDAC was given over 3 hours on days 1-5 plus GO 9 mg/m2 on day 7 and 4.5 mg2 on day 14. Four of the first 7 patients were deemed to have had a treatment-related death and therefore this dose schedule was rejected for further study. Two patients died of sepsis, at 11 and 23 days after initiation of therapy. Another patient died of a gemtuzumab-associated allergic reaction on day 7. A fourth patient died of an apparent pulmonary embolism 32 days after the start of therapy. The patient's death was conservatively attributed to protocol therapy. Although >2 deaths mandated stopping accrual to a cohort, the last two deaths occurred at almost the same date. There were three patients who were originally assigned to cohort II, but were treated on cohort 1A. This occurred because they had started therapy just prior to the determination that cohort II was too toxic, and we were able to delete the planned dose of GO 4.5 mg/m2 on day 14. These patients are also considered in the analysis of the patients treated at the RPTD.
Subsequently, all patients (including three originally assigned to cohort II) were treated with the cohort 1A doses. Including the 9 original patients in cohort 1A, a total of 44 patients were treated at this dose, 37 of whom had relapsed AML; thus meeting the accrual goal for the two stage design.
Patient characteristics (Phase II study)
The 37 patients with relapsed AML treated at the RPTD had a median age of 64 years (intraquartile range, 55-70 years), and 43% were female. Two were African American, 1 was Asian, 33 were Caucasian, and one unknown. The baseline performance status was 0 in 54%, 1 in 35%, and 2 in 11%.
Toxicity (Phase II study)
The treatment-related adverse events observed for the 37 patients with relapsed AML treated at the Phase II dose (see appendix 1) included the following severe, life threatening, or fatal toxicities which occurred in at least 10% of patients: fatigue 14% (3% were grade 4); elevated transaminase in 29% (5% grade 4); hyperbilirubinemia in 27% (3% grade 4); hypoalbuminemia in 11% (none grade 4), nausea in 11% (none grade 4), hemorrhage in 11% (none grade 4); neutropenic fever in 67% (8% grade 4); infection/fever of any type in 92% (11% grade 4; 8% grade 5); documented infections in 57% (5% grade 4), hypoxia or dyspnea in 19% (8% grade 4); and hypotension in 19% (5% grade 4; 3% grade 5). Only 2 patients (6%) had grade 3 or 4 oral mucositis. The maximum non-hematologic treatment-related adverse events were grade 3 in 59%, grade 4 in 19%, and grade 5 in 19%. All 37 patients had grade 4 neutropenia and 36 had grade 4 thrombocytopenia, as expected. Of the 37 patients, 31 have since died, 7 from treatment-related causes, 19 from disease-related causes, and 5 from unknown or unrelated causes.
Clinical responses
Refractory patients fared poorly: 0/3 (cohort 1), 0/2 (cohort 1A) and 0/5 at the phase II dose (one patient at this dose achieved a PR) achieved a CR or CRp. Twelve of the 37 patients (32%) with relapsed AML treated at the Phase II dose achieved CR and one additional patient (3%) had a CRp. Based on the original design requiring at least 8/33 CRs, this regimen can be considered for further research. Median overall survival was 8.9 months (95% CI: 3.8 - 19.9 months) (Figure 1). The response rate did not vary based on age, CR1 duration or the prior use of hgh dose arac (Table 3). There are six long term survivors among the 37 relapsed patients treated at the RPTD. Five for whom complete data are available underwent an allogeneic HCT; three of which were from HLA-matched unrelated donors. All other transplanted patients (n=6) died from transplant or disease-related causes. The median age of those undergoing HCT was 55 (range 22-65), younger than the median age of all patients in the study.
Figure 1.
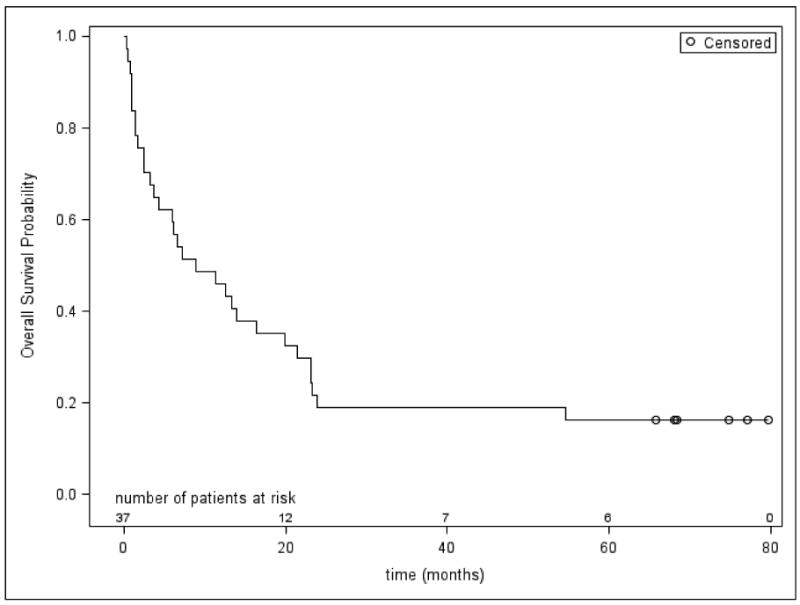
Overall (Kaplan-Meier) Survival for the 37 Patients with Relapsed AML treated at the Recommended Phase II Dose of HiDAC + GO.
Table 3. Response by age, CR1 duration, and prior HiDAC therapy.
Response by age, CR1 duration, and prior high dose ara-C in patients with relapsed AML treated at the phase II dose (n=37
| N | CR or CRp | PR or nr | Fisher's Exact test, two-sided | ||
|---|---|---|---|---|---|
| Age at study entry | < 60 | 15 | 6 | 9 | p=0.7304 |
| ≥ 60 | 22 | 7 | 15 | ||
| CR1 duration | < median | 14 | 4 | 10 | p=0.7245 |
| ≥ median | 21 | 9 | 14 | ||
| Prior HiDAC therapy | Yes | 13 | 6 | 7 | p=0.4719 |
| no | 24 | 7 | 17 | ||
| all patients | 37 | 13 | 24 |
Discussion
We sought to develop a novel schedule of GO in which patients could receive two doses of the drug only seven days apart as a way of shortening the overall period of myelosuppression commonly observed with the standard administration given 14 days apart[11, 18-20]. However, in our initial cohort of patients with advanced AML, we found the degree of leukemic reduction was insufficient to allow delineation of treatment-related hematological toxicity. Thus, the study was amended to incorporate a standard 5-day course of HiDAC for rapid cytoreduction prior to GO. It was then straightforward to assign DLT based on an expected number of treatment-related deaths commonly observed when HiDAC chemotherapy is given for relapse. A 33% toxic death rate is not unexpected for this difficult group of patients. Thus, the problems of lack of treatment efficacy and treatment toxicity could be summed together which is highly relevant for AML.
A single dose of GO on day 7 after 5 days of HiDAC appears to be feasible. A preliminary study in untreated AML patients[23] documented tolerability of full doses of standard cytarabine plus daunorubicin induction chemotherapy plus a single dose of GO at 6 mg/m2. A randomized trial conducted in the United Kingdom[25] suggested a benefit when GO was added to the induction chemotherapy; however, a SWOG trial failed to demonstrate a superiority for chemotherapy + GO during induction[26]. Based on the results from the SWOG trial, the manufacturer recently responded to a request from the United States Food and Drug Administration (FDA) and withdrew GO from commercial availability.
The 32% CR rate seen in the Phase II portion of our trial with 37 patients with relapsed AML is similar to what might be expected from other cytotoxic chemotherapy regimens. It is difficult to compare one study with another because of the marked heterogeneity of the relapsed AML population. More than half of our patients were older than 60 years. An important prognostic factor is the duration of CR1, which was quite short in some of our patients. We did not see an excess of hepatic toxicity in this patient population. This was encouraging given the concerns[21.22] about veno-occlusive disease after GO administration either alone, in combination with chemotherapy, or prior to allogeneic HCT. At least 5 of our patients who achieved a CR were subsequently able to complete a successful allogeneic HCT.
In summary, the combination of HiDAC and GO is reasonably well tolerated and yields complete responses in relapsed AML. Other studies[14.28] have also demonstrated remissions in relapsed patient who received chemotherapy plus GO. This therapy could also be used as a consolidation after induction chemotherapy or in patients with underlying cardiac disease who could not tolerate anthracyclines. However, given the lack of commercial availability as of October 15, 2010, any clinical research with such combinations will require the submission of an Investigational New Drug application to the FDA regarding the use of GO.
Supplementary Material
Acknowledgments
We thank the patients who enrolled in this study and the physicians, nurses, and clinical research associates at the CALGB institutions that participated. We also thank our protocol editor Michael Kelly and data coordinator Tracey Adams.
The research for CALGB 19902 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The following institutions participated in this study: Christiana Care Health Services, Inc. CCOP, Wilmington, DE–Stephen Grubbs, MD, supported by CA45418; Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY–Jeffrey Kirshner, MD, supported by CA45389; Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO–Rakesh Gaur, MD; Dana-Farber Cancer Institute, Boston, MA–Harold J. Burstein, MD, supported by CA32291; North Shore-Long Island Jewish Health System, New Hyde Park, NY - Daniel Budman, MD, supported by CA35279; Rhode Island Hospital, Providence, RI–William Sikov, MD, supported by CA08025; The Ohio State University Medical Center, Columbus, OH–Clara D. Bloomfield, MD, supported by CA77658; University of Chicago, Chicago, IL–Hedy L Kindler, MD, supported by CA41287; University of Illinois MBCCOP, Chicago, IL–David J. Peace, MD, supported by CA74811; University of Iowa, Iowa City, IA–Daniel A. Vaena, MD, supported by CA47642; University of Vermont, Burlington, VT–Hyman B. Muss, MD, supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC–David D. Hurd, MD, supported by CA03927; Washington University School of Medicine, St. Louis, MO–Nancy Bartlett, MD, supported by CA77440; and Western Pennsylvania Cancer Institute, Pittsburgh, PA–Richard K. Shadduck, MD.
Footnotes
Authors' Contributions: RMS: designed study, conducted study, entered patients, analyzed data, wrote manuscript; BM: designed study, analyzed data, edited manuscript; BS: Analyzed data, edited manuscript; PS: designed study, co-conducted study, entered patients, edited manuscript; JEK, SA, WS, GM and DH: Entered patients, edited manuscript; IG: analyzed and collected data, edited manuscript and RAL: designed study, entered patients, analyzed data, edited manuscript
Conflict of Interest: No authors have conflict of interest to report except for DH who owns Pfizer stock.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kohrt HE, Coutre SE. Optimizing Therapy for Acute Myeloid Leukemia. JNCCN. 2008;6:1003–1016. doi: 10.6004/jnccn.2008.0076. [DOI] [PubMed] [Google Scholar]
- 2.Klepin HD, Balducci L. Acute Myelogenous Leukemia in Older Adults. The Oncologist. 2009;14:222–232. doi: 10.1634/theoncologist.2008-0224. [DOI] [PubMed] [Google Scholar]
- 3.Mato AR, Morgans A, Luger SMA. Novel strategies for relapsed and refractory acute myeloid leukemia. Current Opinion in Hematology. 2008;15:108–14. doi: 10.1097/MOH.0b013e3282f463d2. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR. Incorporating hematopoietic cell transplantation (HCT) into the management of adults aged under 60 years with acute myeloid leukemia (AML) Bailliere's Best Practice in Clinical Haematology. 2008;21:85–92. doi: 10.1016/j.beha.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Breems DA, Lowenberg B. Acute myeloid leukemia and the position of autologous stem cell transplantation. Seminars in Hematology. 2007;44:259–266. doi: 10.1053/j.seminhematol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Linker CA, Owzar K, Powell B, Hurd D, Damon LE, Archer LE. Larson RA and the Cancer and Leukemia Group B. Auto-SCT for AML in second remission: CALGB study 9620. Bone Marrow Transplant. 2009;44:353–359. doi: 10.1038/bmt.2009.36. [DOI] [PubMed] [Google Scholar]
- 7.Bolwell BJ, Cassileth PA, Gale RP. High dose cytarabine: a review. Leukemia. 1988;2:253–60. [PubMed] [Google Scholar]
- 8.Faderl S, Gandhi V, O'Brien S, Bonate P, Cortes J, Estey E, Beran M, Wierda W, Garcia-Manero G, Ferrajoli A, Estrov Z, Giles FJ, Du M, Kwari M, Keating M, Plunkett W, Kantarjian H. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 9.Mitus AJ, Miller KB, Schenkein DP, Ryan HF, Parsons SK, Wheeler C, Antin JH. Improved survival for patients with acute myelogenous leukemia. J Clin Oncol. 1995;13:560–569. doi: 10.1200/JCO.1995.13.3.560. [DOI] [PubMed] [Google Scholar]
- 10.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei E., 3rd Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 11.Sievers EL. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. Expert Opinion on Biological Therapy. 2001;1:893–901. doi: 10.1517/14712598.1.5.893. [DOI] [PubMed] [Google Scholar]
- 12.Ho AD, Lipp T, Ehninger G, Illiger HJ, Meyer P, Freund M, Hunstein W. Combination of mitoxantrone and etoposide in refractory acute myelogenous leukemia--an active and well-tolerated regimen. J Clin Oncol. 1988;6:213–217. doi: 10.1200/JCO.1988.6.2.213. [DOI] [PubMed] [Google Scholar]
- 13.Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Holowiecki J, Kyrcz-Krzemien S, Grosicki S, Giebel S, Skotnicki AB, Piatkowska-Jakubas B, Kuliczkowski K, Kielbinski M, Zawilska K, Kloczko J, Wrzesien-Kus A. Polish Adult Leukemia Group. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Europ J Haem. 2008;80:115–26. doi: 10.1111/j.1600-0609.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin MG, Augustin KM, Uy GL, Welch JS, Hladnik L, Goyal S, Tiwari D, Monahan RS, Reichley RM, Cashen AF, Stockerl-Goldstein K, Westervelt P, Abboud CN, Dipersio JF, Vij R. Salvage therapy for acute myeloid leukemia with fludarabine, cytarabine, and idarubicin with or without gemtuzumab ozogamicin and with concurrent or sequential G-CSF. Am J Hematol. 2009;84:733–737. doi: 10.1002/ajh.21545. [DOI] [PubMed] [Google Scholar]
- 15.Karp JE, Passaniti A, Gojo I, Kaufmann S, Bible K, Garimella TS, Greer J, Briel J, Smith BD, Gore SD, Tidwell ML, Ross DD, Wright JJ, Colevas AD, Bauer KS. Phase I and pharmacokinetic study of flavopiridol followed by 1-β-D-Arabinofuranosylcytosine and mitoxantrone in relapsed and refractory acute leukemias. Clin Cancer Res. 2005;11:8403–8412. doi: 10.1158/1078-0432.CCR-05-1201. [DOI] [PubMed] [Google Scholar]
- 16.Perl AE, Kasner MT, Tsai DE, Vogl DT, Loren AW, Schuster SJ, Porter DL, Stadtmauer EA, Goldstein SC, Frey NV, Nasta SD, Hexner EO, Dierov JK, Swider CR, Bagg A, Gewirtz AM, Carroll M, Luger SM. A phase I study of the mammalian target of rapamycin inhibits sirolimus and MEC chemotherapy in relapsed and refractory myelogenous leukemia. Clin Cancer Res. 2009;15:6732–9. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian HM, Keating MJ, Walters RS, McCredie KB, Freireich EJ. The characteristics and outcome of patients with late relapse acute myelogenous leukemia. J Clin Oncol. 1988;6:232–238. doi: 10.1200/JCO.1988.6.2.232. [DOI] [PubMed] [Google Scholar]
- 18.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia [erratum appears in Clin Cancer Res 2002 Jan; 8(1): 300] Clin Cancer Res. 2001;7:1490–6. [PubMed] [Google Scholar]
- 19.Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO, Shannon-Dorcy K, Berger MS, Bernstein ID. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood. 1999;93:3678–3684. [PubMed] [Google Scholar]
- 20.Larson RA, Sievers EL, Stadtmauer EA, Lowenberg B, Estey EH, Dombret H, Theobald M, Voliotis D, Bennett JM, Richie M, Leopold LH, Berger MS, Sherman ML, Loken MR, van Dongen JJ, Bernstein ID, Appelbaum FR. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 21.Giles F, Estey E, O'Brien S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer. 2003;98:2095–104. doi: 10.1002/cncr.11791. [DOI] [PubMed] [Google Scholar]
- 22.Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, Alyea EP, Antin JH, Stone RM, Soiffer RJ, DeAngelo DJ. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102:1578–82. doi: 10.1182/blood-2003-01-0255. [DOI] [PubMed] [Google Scholar]
- 23.DeAngelo D, Stone R, Durrant S, Liu D, Baccarani M, Schiffer CA, Amrein PC, Sherman ML. Gemtuzumab ozogamicin (Mylotarg®) in combination with induction chemotherapy for the treatment of patients with de novo acute myeloid leukemia: two age-specific phase II trials. Blood. 2003;102:146a. abstr 502. [Google Scholar]
- 24.Kell WJ, Burnett AK, Chopra R, Yin JA, Clark RE, Rohatiner A, Culligan D, Hunter A, Prentice AG, Milligan DW. A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood. 2003;102:4277–4283. doi: 10.1182/blood-2003-05-1620. [DOI] [PubMed] [Google Scholar]
- 25.Burnett AK, Kell WJ, Goldstone AH, Milligan D, Hunter A, Prentice AG, Russell NR, Gibson B, Wheatley K, Hills RK. The addition of gemtuzumab ozogamicin to induction chemotherapy for AML improves disease free survival without extra toxicity: preliminary analysis of 1115 patients in the MRC AML 15 Trial. Blood. 2006;108:8a. abstr 13. [Google Scholar]
- 26.Petersdorf S, Kopecky K, Stuart RK, Larson RA, Nevill TJ, Stenke L, Slovak ML, Tallman MS, Willman CL, Erba H. Appelbaum FR Preliminary results of Southwest Oncology Group Study S0106: An international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114:327a. abstr 790. [Google Scholar]
- 27.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM, Lazarus HM, Tallman MS. Anthracycline dose intensification in acute myeloid leukemia. New Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chevalier P, Delaunay J, Turlure P, Pigneux A, Hunault M, Garand R, Guillaume T, Avet-Loiseau H, Dmytruk N, Girault S, Milpied N, Ifrah N, Mohty M, Harousseau JL. Long-term disease-free survival after gemtuzumab, intermediate dose cytarabine, and mitoxantrone in patients with CD33+ primary resistant or relapsed acute myeloid leukemia. J Clin Oncol. 2008;26:5192–5197. doi: 10.1200/JCO.2007.15.9764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


