Abstract
Purpose
Several studies have reported aberrant expression of MUC4 in pancreatic cancer (PC) which is associated with tumorigenecity and metastasis. Mechanisms through which MUC4 promote metastasis of PC cells to distant organs are poorly defined.
Experimental design
Identification of MUC4-galectin-3 interaction and its effect on the adhesion of cancer cells to endothelial cells were done by immunoprecipitation and cell-cell adhesion assays, respectively. Serum galectin-3 level for normal and PC patients were evaluated through enzyme-linked immunosorbent assay (ELISA).
Results
In the present study, we have provided clinical evidence that the level of galectin-3 is significantly elevated in the sera of PC patients with metastatic disease compared to patients without metastasis (p=0.04) and healthy controls (p= 0.00001). Importantly, for the first time, we demonstrate that MUC4 present on the surface of circulating PC cells plays a significant role in the transient and reversible attachment (docking) of circulating tumor cells to the surface of endothelial cells. Further, exogenous galectin-3 at concentrations similar to that found in the sera of PC patients interacts with MUC4 via surface glycans like T antigens, which results in the clustering of MUC4 on the cell surface and a stronger attachment (locking) of circulating tumor cells to the endothelium.
Conclusions
Altogether, these findings suggest that PC cell-associated MUC4 helps in the docking of tumor cells on the endothelial surface. During cancer progression, galectin-3-MUC4 interaction mediated clustering of MUC4 may expose the surface adhesion molecules, which in turn promotes a stronger attachment (locking) of tumor cells to the endothelial surface.
Keywords: Mucin, MUC4, galectin-3, pancreatic cancer, metastasis
Introduction
Pancreatic cancer (PC) is the fifth leading cause of adult cancer death in the United States, with a five-year survival rate of only 1-4% [1]. PC is an extremely aggressive tumor, with early metastases to both lymph nodes and distant organs [2]. The invasive properties PC cells lead to the growth of the tumor in major abdominal vessels, the neighboring organs, and the retroperitoneal bed, which makes curative resection often impossible [2, 3]. During the processes of invasion and metastasis, tumor cells leave their primary site, invade the surrounding extracellular matrix and the endothelium, penetrate the blood and lymph vessels, and finally attach and proliferate at a secondary site [2]. However, little is known of the molecular and cellular mechanisms that contribute to this cascade of events that leads to local tumor invasion and the formation of distant metastases. A better understanding of the molecular mechanisms behind PC metastasis will help to develop newer, more efficacious anti-cancer therapies. The normal cell expresses a variety of cell adhesion molecules on its surface. These receptor molecules are involved in cell-to-cell communication and characterize the cell's position and function in the community with other cells and the extracellular matrix [2, 4]. During malignant cell transformation, the pattern of surface molecules and their activity can be dynamically changed. Thus, the cancer cells develop the ability to disrupt and invade normal tissue structures and finally form metastases in distant organs [5, 6].
Haematogenous metastasis is a multi-step process and includes the detachment and release of tumor cells into the circulation, their adhesion to the endothelial wall of target tissues and either local growth or invasion through the microvascular wall and proliferation in the target organ parenchyma [7]. It is a well established phenomenon that the arrest of circulating cancer cells is a key rate-limiting step in their emigration from the circulation to the metastatic sites. In this regard, accumulating evidence suggests that within the systemic circulation, circulating tumor cells interact with both humoral and cellular constituents of blood and that this interaction promotes attachment of tumor cells with the endothelial cells for further metastasis. Several studies have shown that blood-borne tumor cells mediate their adhesion to the endothelium by using mechanisms similar to those adopted by leukocytes. Initial weak or transient contacts between cancer cells and the endothelium (docking) are likely to be mediated by carbohydrate-carbohydrate reorganization [8, 9]. This transient attachment further induces molecular changes in both the attached tumor cells and the endothelium by altering the expression and/or the localization of various permanent adhesion receptors (e.g. integrins and cadherins) and their corresponding ligands, leading to subsequent strong attachment (locking) of tumor cells to the endothelial surface [10].
MUC4 mucin is a high-molecular-weight glycoprotein which is aberrantly expressed by PC cells but not by the non-neoplastic ducts. Structurally, MUC4 consists of two subunits: the large extra-cellular subunit, MUC4α, and the transmembrane subunit, MUC4β. Specifically, the mucin-like MUC4α subunit is heavily O-glycosylated [11]. Previous studies from our laboratory have shown that MUC4 potentiates PC cell proliferation, survival, invasion, and distant organ metastasis [11-14]. Due to the presence of many carbohydrate moieties on its surface, MUC4 is a potential binding partner for different carbohydrate binding proteins including galectin-3. galectin-3, a member of the β-galactoside-binding family of lectins has emerged as a major player in cancer metastasis in general and PC in particular [15, 16]. Lurisci et al. had earlier reported that serum galectin-3 levels were elevated in patients with metastatic cancer (particularly gastrointestinal malignancies) compared to that in patients with localized disease. This suggested that galectin-3 levels may play a role in the progression of malignant gastrointestinal tumors including PC [17]. Galectin-3 binds to several extracellular and membrane bound ligands including laminin, fibronectin, tenascin and various integrins. This interaction, which is dependent on the glycosylation status of these ligands, has been demonstrated to either promote or inhibit cell adhesion depending upon the concentration of galectin-3 and upon the concentration and level of glycosylation of its ligands. Further, due to its oligomer forming nature, extracellular galectin-3 may facilitate cell-cell interactions by acting as a cross linker [18, 8]. Secreted galectin-3 (present in serum) has previously been demonstrated to interact with another membrane bound mucin MUC1, and this interaction promoted cancer cell adhesion to the endothelium by revealing epithelial adhesion molecules that were otherwise concealed by MUC1 overexpression on the cell surface [19]. Given the extensive O-glycosylation present in the extracellular portion of MUC4 and its association with metastasis in PC, we hypothesized that galectin-3 (either cell surface bound or secreted) could be a potential binding partner for MUC4 present on the surface of the circulating PC tumor cells and that this interaction modulates the adhesion of MUC4 expressing PC cells with the endothelium.
The results of our experiments reveal, for the first time, a novel interaction of MUC4 (present on PC cells) with secreted galectin-3. We also demonstrate that this interaction is mediated by the carbohydrate structures (T-antigens) present on MUC4. We also observed that serum galectin-3 levels were significantly higher in patients with metastatic PC than in those with localized PC or healthy controls. We also observe that MUC4 is important for the transient adhesion of circulating tumor cells to the endothelial cells in vitro. Finally, we report that extra-cellular galectin-3 binding to cancer-associated MUC4 alters its cell-surface localization, which could expose other cell-surface adhesion molecules and thus, helps with the strong adhesion of tumor cells to the endothelial cells. The identification of the MUC4-galectin-3 interaction as a modulator of PC cell adhesion to the endothelium indicates that this interaction could serve as an important target to develop novel anti-adhesive therapies for advanced PC.
Material and Methods
Galectin-3 recombinant protein (rGal-3) and anti-galectin-3 antibody
Human Galectin-3 cDNA was obtained from the American Type Culture Collection (ATCC). A double-digested with EcoRI and Pst I fragment was sub-cloned into pUC19 and transformed into E.coli mrf' cells. The insertion was confirmed by analysis by the UNMC DNA sequence core facility. Log-phase cultures in LB broth at 37°C were incubated with 0.1mM Isopropyl thio-galactopyranoside (Stratagene, La Jolla, CA) for five hr. Cells were isolated by centrifugation and suspended in PBS containing 1mg/ml lysozyme (Sigma, St. Louis, MO). Following cell lysis by sonication (4x90sec. at 4°C), cell membranes were precipitated by centrifugation at 10,000xg for 20 min and the supernatant fraction applied to a lactose-sepharose (Vector Labs, Burlingame, CA) column. After extensive washing with PBS, galectin-3 was eluted with 300mM lactose in PBS.
The M3/38 hybridoma secreting rat anti-galectin-3 IgG2a antibody was obtained from the ATCC, and grown in RPMI1640, 37°C. Culture supernatant was used as a source of antibody.
Cell culture
The human PC cell lines CD18/HPAF and Colo357 were cultured as before [16]. Human umbilical vein endothelial cells (HUVEC) were obtained from ATCC and were cultured as described previously [17].
Immunoprecipitation and immunoblot analysis
Immunoprecipitation, SDS-PAGE, and immunoblotting analysis were done as previously described [11, 18]. Lysates from CD18/HPAF and Colo357 cells were utilized for immunoprecipitation. The immunoprecipitants were electrophoretically resolved on 2% agarose (for MUC4) or 15% polyacrylamide gel (for galectin-3). Antibodies, mouse anti-MUC4 monoclonal antibody at a concentration of 1.87μg/ml [18] and rat anti-galectin-3 (described previously) were used for the analysis. For immunoprecipitation, isotype-matched mouse and rat IgG were used as negative controls.
Galectin binding assay
Cells were harvested and resuspended at a density of 2.5 × 105 cells/ml. A total of 100μl of the prepared cells were seeded in triplicate to galectin-1 and -3 protein-coated 96-well plates (Calbiochem, La Jolla, CA) and incubated for 1 h at 37°C in the presence and absence of 50mM lactose and sucrose. After incubation, the cell suspension was discarded and the wells were gently washed twice with PBS. The cells that adhered to the wells were incubated with 100μl of Calcein-AM dye for 1 h at 37°C. The fluorescence of the samples was measured using the fluorescence plate reader at an excitation wavelength of 485nm and the emission wavelength of 520 nm. The significance of each binding assay was evaluated using the t test assuming unequal variances. P-values lower than 0.05 were considered statistically significant. To determine statistical significance between more than two groups, ANOVA was used (n=3).
Determination of serum galectin-3 levels by sandwich ELISA
Galectin-3 levels in serum were measured quantitatively by sandwich ELISA using the DuoSet ELISA kit for human Galectin-3 (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. ELISA plates were read at 450 nm, and data collected was analyzed using the SOFTMAX PRO software (Molecular Devices Corp., Sunnyvale, CA). Data were analyzed by using the MedCalc for Windows version 9.6.4.0 software (MedCalc Software, Mariakerke, Belgium). Variables were compared by using the two-tailed Student's t-test. P value ≤ 0.05 was considered as statistically significant.
Cell adhesion to HUVECs
CD18/HPAF-Scr and CD18/HPAF-siMUC4 cells were labeled with DIO fluorescent cell labeling solution for 30 min. The cells were washed with PBS and treated with non-enzymatic cell dissociation solution and cells were incubated with or without rGal-3 (1μg/ml) for 30 min at 37°C and followed by incubation of these cells (5×104) on HUVEC monolayer cultured on chamber slides. To remove the unbound cells, the chamber slides were then gently washed with PBS and inverted for 10 min at room temperature. The slides were mounted, and fluorescent cells were counted in ten randomly chosen fields using a fluorescent microscope with a 20x objective. Statistical analysis was carried out as described previously for the galectin binding assay.
Immunofluorescence microscopy
With a few modifications, this experiment was carried out in a manner similar to that described previously [11]. After the blocking step, the anti-MUC4 antibody was replaced with PNA-Alexa Fluor 488 conjugates (Molecular Probe, Eugene, Oregon, final concentration 20μg/ml). Then, without any further secondary antibody treatment, cells were washed and processed for confocal laser microscopy.
Cell surface localization of MUC4
CD18/HPAF cells were released from the culture plates using the non-enzymatic cell dissociation solution. After washing, 104 cells were incubated with or without rGal-3 (1-2 μg/ml) for 1 h at 4°C. The cell suspensions were then seeded on polylysine-coated slides for 1 h at 37°C. After gentle washing, the cells were fixed with 2% paraformaldehyde, blocked with 5% normal goat serum, and probed with anti-MUC4 antibody (18.7 μg/ml), followed by fluorescent-labeled secondary antibody. MUC4 localization was visualized using confocal microscopy.
Statistical Analysis
The statistical analyses were performed using the unpaired t test for single comparison, and the one-way analysis of variance (ANOVA) test for multiple comparisons, where appropriate. Differences were considered significant when p<0.05.
Results
Serum galectin-3 levels are elevated in pancreatic cancer patients with metastatic lesions
Elevated serum galectin-3 level has been shown to be associated with metastasis of different cancer. Therefore, we were interested to investigate the status of serum galectin-3 level in pancreatic cancer patients. For this purpose, in a larger cohort of patients, we did a quantitative analysis of serum galectin-3 levels in PC patients (with or without metastasis) and healthy controls. A total of 89 samples were analyzed, of which 22 were from healthy controls, 43 of PC patients with distant metastasis and 24 of PC patients with localized disease. The mean galectin-3 levels were significantly higher in PC patients compared to that in healthy controls (p<0.05, Table 1). Further, among PC patients, serum galectin-3 levels were significantly (p<0.05) higher in those with metastatic PC than in those with localized disease.
Table 1.
Descriptive statistics of galectin-3 levels in serum.
| Group | Diagnosis | Number | Mean ± SEM (ng ml-1) |
95% CI (ng ml-1) |
Range (ng ml-1) |
p-value |
|---|---|---|---|---|---|---|
| A | Normal | 22 | 119± 16 | 61-156 | 19-269 | A and B = 0.020902 A and C = 1.13E-05 B and C = 0.04487 |
| B | Pancreatic cancer (No metastasis) |
24 | 197± 28 | 140-254 | 32-495 | |
| C | Pancreatic cancer (With metastasis) |
43 | 279± 29 | 129-339 | 46-733 |
MUC4 interacts with galectin-3 in PC cells
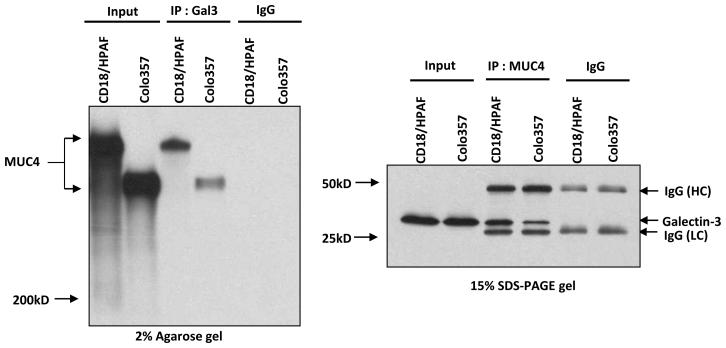
MUC4 is overexpressed in PC cells and has an extensively O-glycosylated extracellular portion that harbors many carbohydrate structures, which might interact with carbohydrate binding proteins like galectin-3. To investigate whether an interaction exists between MUC4 and galectin-3, we analyzed two MUC4+ galectin-3+ cell lines (CD18/HPAF and Colo357) by reciprocal co-immunoprecipitation. Our results clearly demonstrate that MUC4 and galectin-3 form a stable complex in both the PC cell lines (Figure 1).
Figure 1. Binding of galectin-3 to MUC4.
Reciprocal-coimmunoprecipitation studies showed galectin-3-MUC4 interaction in protein lysates obtained from CD18/HPAF and Colo357 cells.
Specific binding of MUC4-expressing cells to galectin-3
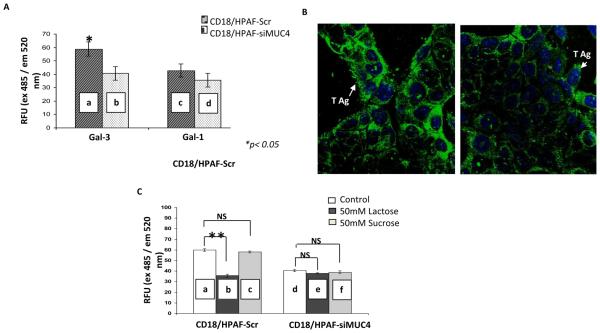
In order to determine the specificity of MUC4-galectin-3 interaction, a binding assay was performed. Recently, we have generated a MUC4-knockdown model in CD18/HPAF, MUC4 overexpressing PC cells [20]. This model is comprised of a scrambled shRNA-expressing pooled population (CD18/HPAF-Scr) and MUC4-target shRNA-expressing pooled population (CD18/HPAF-siMUC4), exhibiting a >80% decrease in the expression of MUC4 as compared to CD18/HPAF-Scr cells [10]. The binding assay was performed on galectin-3 coated plates. The CD18/HPAF-siMUC4 cells showed a significant decrease in binding to galectin-3 coated plates in comparison to CD18/HPAF-Scr cells (p <0.05). However, binding to galectin-1 was not significantly altered (Figure 2A).
Figure 2. MUC4-galectin-3 interaction is glycosylation dependent and MUC4 possesses T antigen structures on its surface.
A, cell adhesion assay showed a significant difference between the binding of CD18/HPAF-SCR and CD18/HPAF-siMUC4 cells (a and b) to galectin-3 coated plates (*P<0.05). But, there was no difference in the case of galectin-1-coated plates (c and d). B, immunofluorescence analysis using T antigen specific Lectin PNA-Alexa Fluor 488 conjugates showed more intense T antigen staining on CD18/HPAF-Scr cells than MUC4 reduced CD18/HPAF-si-MUC4 cells. C, cell adhesion assay in the presence of lactose (a competitive inhibitor) and sucrose (a non-competitive inhibitor) showed involvement of carbohydrate structures in MUC4-gelectin-3 interaction. There is a significant decrease in the number of cells adhered to galectin-3-coated plates (**P=8.66E-11) only in the case of CD18/HPAF-Scr cells, in the presence of lactose (a and b). Micrographs shown are representative of several independent experiments.
MUC4 carries T antigen structures on its surface, and lactose competitively inhibits MUC4-galectin-3 binding
For a direct interaction between MUC4 and galectin-3, MUC4 should carry galectin-3 binding sites on its surface. T antigen, a core 1 mucin glycan (Galβ1-3GalNAcαSer/Thr), is expressed on the outer surface of many cancer cells, including PC [8, 19]. Importantly, studies have shown that galectin-3 interaction with cancer-associated T antigen promotes cancer metastasis by enhancing the adhesion of circulating tumor cells to endothelial cells [17]. Among the proteins that are used to detect T antigen, peanut (Arachis hypogaea) agglutinin (PNA) is most widely used. Therefore, to check whether MUC4 has any T antigen structures on its surface or not, using PNA-Alexa Fluor 488 conjugates, an immunofluorescence experiment was carried out. In this experiment, CD18/HPAF-SCR cells showed more intense staining than CD18/HPAF-siMUC4 cells (Figure 2B). Further, to check the specificity of galectin-3 and MUC4 interaction, a binding experiment was carried out (Figure 2C). We found that in contrast to MUC4 knock-down cells, the binding of scrambled RNA transfected CD18/HPAF cells was significantly inhibited in the presence of 50mM lactose (specific competitive inhibitor of galectin-3; p= 8.66E-11). However, the binding was not affected in the presence of 50mM sucrose (as a negative control; p = 0.2).
MUC4 itself promotes adhesion of PC cells to HUVECs and the adhesion is enhanced in the presence of r-galectin-3
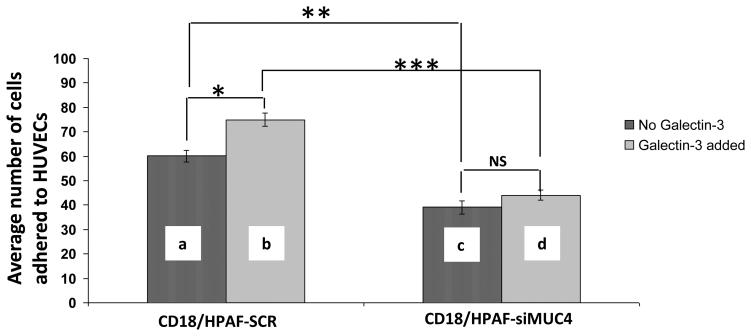
Glycans present on the cell surface glycoproteins, extend away from the cell membrane and put forth a first line of contact with nearby cells and substrates. To determine whether MUC4 itself plays any role in the adhesion of tumor cells to the endothelial cells (un-stimulated) , we performed cell-cell binding assays in the absence of exogenous galectin-3. We observed that the number of CD18/HPAF-SCR cells bound to HUVECs was significantly higher than the number bound to the CD18/HPAF-siMUC4 cells (p=5.89E-05, Figure 3). The addition of exogenous galectin-3 significantly enhanced the binding of CD18/HPAF-SCR cells (but not CD18/HPAF SiMUC4 cells) to the endothelial (p=5.88E-05).
Figure 3. MUC4 increases the adhesion of CD18/HPAF cells to the HUVEC endothelial cells and this binding is enhanced in the presence of rGal-3.
In the cell-cell adhesion assay, (a and c) showed a role of MUC4 itself in the adhesion of PC cells to HUVECs. CD18/HPAF-Scr cells showed that a significantly higher number of cells (**p=1.04E-05) adhere to HUVECs than CD18/HPAF-siMUC4 cells. A similar experiment in the presence of exogenous galectin-3 showed a significant increase in binding of CD18/HPAF-Scr cells to HUVECs than in the absence of gal-3 (a and b, *p=0.00056), but there was no significant difference in the case of CD18/HPAF-siMUC4 cells (c and d, p=0.147). Combined statistical analysis showed that galectin-3 significantly increases the adhesion of CD18/HPAF-Scr cells to HUVECs (b and d, ***p=5.89E-05). The data shown are representative of several independent experiments.
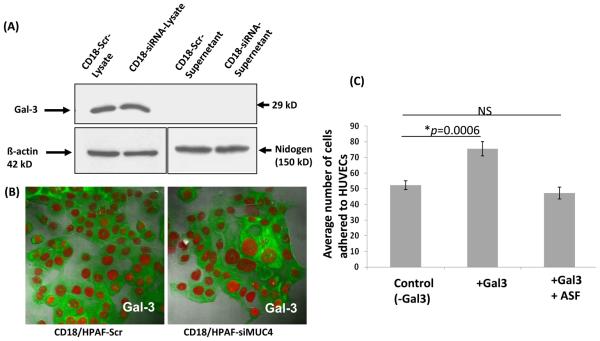
As galectin-3 is a secreted protein, we also checked whether galectin-3 might is secreted into the culture media by the CD18/HPAF cells. However, the enriched culture media collected from these cells did not have a detectable amount of galectin-3 (Figure 4A). However, the total cell from both CD18/HPAF SCR and SiMUC4 cells had an equal amount of galectin-3 expression. Further, the confocal analysis also showed a similar intensity and type of cytoplasmic localization of galectin-3 in both the CD18/HPAF derived cells (Figure 4B). From these results, we concluded that the contribution of endogenous galectin-3 to the rGal-3-mediated cell adhesion was negligible. Further, pretreatment of CD18/HPAF cells with TF antigen-expressing asialofetuin glycoprotein abolished the galectin-3 mediated adhesion of cancer cells to the endothelial cells (Figure 4C).
Figure 4. Cytoplasmic localization of galectin-3 in CD18/HPAF-derived cells, and the effect of asialofetuin on galectin-3-mediated adhesion of CD18/HPAF cells to HUVECs.
A, Immunoblot analysis detected galectin-3 expression in CD18/HPAF-derived cells lysates, but not in the enriched cell culture media. Here β-actin and nidogen were taken as internal controls. B, Immunofluorescence analysis showed the cytoplasmic localization of galectin-3 in CD18/HPAF-derived cells. C, Preincubation of CD18/HPAF cells with asialfetuin at a concentration of 1mg/mL abolished the galectin-3 mediated enhanced adhesion of CD18/HPAF cells to HUVECs.
Galectin-3 alters MUC4 cell surface localization
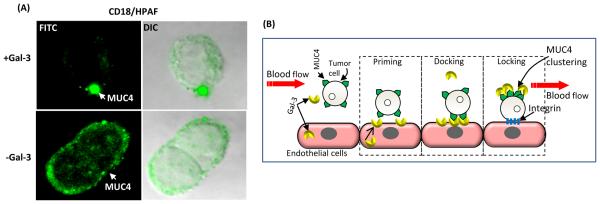
To further understand the mechanisms by which serum-galectin-3-MUC4 interaction promotes tumor cell adhesion to the endothelium, we determined the effect of rGal-3 on cell surface localization of MUC4 (Figure 5A). After incubation of CD18/HPAF cells with rGal-3, the immunofluorescence experiment clearly showed clustering of MUC4 molecules on the surface of cancer cells (Figure 5A).
Figure 5. Mechanisms associated with galectin-3-MUC4 interaction mediated metastasis of PC cells.
A, the capping experiment showed that in the presence of exogenous galectin-3, MUC4 was clustered on the cell surface. B, schematic representation for the proposed action of galectin-3-MUC4 interaction. MUC4-expressing circulating tumor cells helps in priming the endothelial cells by inducing the mobilization of the intracellular galectin-3 to the cell surface of endothelial cells. Further, MUC4 interacts with galectin-3 present on the endothelial cell surface. This temporary reversible interaction aids in docking of tumor cells on the endothelial cells. Binding of circulating galectin-3 causes redistribution of MUC4 on the cell surface leading to exposure of adhesion molecules like integrins, which help for an irreversible adhesion of tumor cells to the endothelial cells.
Discussion
Previous studies in our laboratory have established the role of MUC4 in the metastasis of PC cells [11, 12]. As discussed previously, a possible interaction between galectin-3 and MUC4 may be a promising mechanism behind MUC4-medated PC metastasis. Galectin-3 has been reported to interact with other membrane-bound mucins, MUC1 and MUC16 [21, 22]. Our present study shows that MUC4, a membrane bound mucin specifically interacts with galectin-3 and that MUC4-galectin-3 interaction modulates the adhesion of PC cells to the endothelium in vitro.
Interaction of galectin-3 with MUC4 provides evidence to support the hypothesis that glycans present on the surface of MUC4 may act as binding sites for carbohydrate binding proteins. In order to directly interact with galectin-3, MUC4 should carry galectin-3 binding sites on its surface. Studies have shown that galectin-3 interacts with cancer-associated T antigens to promote cancer cell metastasis (by enhancing the adhesion of circulating tumor cells to endothelial cells) [22]. In the present study, higher binding of Lectin PNA-Alexa Fluor 488 conjugates to CD18/HPAF-SCR cells than to CD18/HPAF-siMUC4 cells suggests the presence of T antigens on the surface of the MUC4 protein (Figure 2B). Further, the inhibition of the MUC4-galectin 3 binding in the presence of lactose (a specific competitive inhibitor of galectin-3) suggests that carbohydrate residues (potentially T antigens) on MUC4 act as ligands for galectin-3 (Figure 2C). Inhibition of galectin-3-mediated adhesion of CD18/HPAF cells to HUVECs by asialofetuin (Figure 4C) further suggests that T antigens are the major galectin-3 binding sites present on these cells, which significantly contributes to the galectin-3-mediated cell-cell adhesion.
The current study suggests that the presence of MUC4 on the tumor cell surface enhances the binding of PC cells to the endothelial cells. This carbohydrate-mediated interaction might be transient and reversible, which is essential for docking of tumor cells on the surface of the endothelial cells. Previous studies have shown that the inflammatory cytokines stimulated HUVECs to express E-selectin as the major carbohydrate binding protein on the cell surface [23]. Indeed, studies have also demonstrated that breast and prostate cancer cell adhesion to the microvascular endothelium (un-stimulated) is mediated by interactions between cancer-associated T antigen and galectin-3 present on the endothelial cell surface [24-26]. Similar to selectins, endothelial galectin-3 is often localized intracellular; however, it translocates to the cell surface in a short period of time (~ 25min) in the presence of cancer cells bearing the T antigen epitopes. Thus, galectin-3 on the surface of endothelial cells modifies the adhesive properties of the endothelial cells and primes them for binding of metastatic cancer cells [27]. In the cell-cell adhesion experiment, the CD18/HPAF-SCR and CD18/HPAF-siMUC4 cells were incubated for 1 hr on the surface of HUVECs. Based on the existing information, activation of HUVECs and translocation of selectins to the cell surface cannot occur within 1 hr [28, 29]. Therefore, we hypothesize that the MUC4 present on the surface of CD18/HPAF-SCR cells might have induced translocation of endothelial galectin-3 to the cell surface and therefore promoted tumor cell to endothelial cell adhesion.
High serum galectin-3 concentrations have been reported in patients with metastatic colorectal, breast and pancreatic cancer [17]. In a large sample cohort, our present study also showed a similar observation of high serum galectin-3 levels in PC patients having metastasis compared to PC patients with localized disease and healthy controls. Taken together, these data suggest the possible importance of galectin-3 as a prognostic marker in PC patients. An earlier study through immunohistochemical analysis has shown that decrease in galectin-3 level in primary pancreatic tissue correlates with advanced stage of pancreatic cancer [30]. However, in the present study, the higher levels of serum galectin-3 that was detected in metastatic PC patients might be due to secretion of high amount of galectin-3 by tumor associated inflammatory and/or stromal cells [31, 17]. Thus, different levels of galecin-3 depending on various tumor microenvironments (primary tumor/ blood) might facilitate cancer metastasis trough different mechanisms.
The rGal-3, which mimics the serum galectin-3 present in PC patients, played an important role in promoting heterotypic interaction between PC tumor cells and endothelial cells (Figure 3). From this, we can hypothesize that high levels of serum galectin-3 could directly facilitate the metastatic process by promoting the interaction of metastatic cancer cells with vascular endothelium.
Observations from the binding experiments indicate that MUC4 promotes the adhesion of PC cells to the endothelial cells, and exogenous galectin-3 positively modulates this process. Interestingly, MUC4 could modulate the attachment of PC cells to the endothelial cells even in the absence of exogenous galectin-3 (rGal-3). The presence of rGal-3 however significantly enhanced binding of MUC4-expressing PC cells to the endothelial cells compared to in the absence of it.
As galectin-3 forms oligomers through its N-terminal domain, it may bind to carbohydrate epitopes present on the surface of MUC4 through the Carbohydrate Recognition Domain (CRD domain). By doing so, galectin-3 would crosslink MUC4 molecules and causes them to cluster on the cell surface. This clustering of MUC4 on the cell surface may expose other adhesion molecules like integrins, which are responsible for a strong attachment (locking) of the tumor cells with the endothelial cells (Figure 5B).
In summary, we have identified a novel interaction of MUC4 mucin with galectin-3 in PC cells. galectin-3 specifically recognizes carbohydrate motifs on MUC4. Also, the T antigens present on the surface of MUC4 are probably the binding sites for galectin-3. To the best of our knowledge, this is the first report describing the role of MUC4-mediated adhesion of tumor cells. Although MUC4 and galectin-3 may also interact with other associated ligands, MUC4-galectin-3 interactions are likely to play a major role in the overall tumor cells adhesion to endothelial cells. Additionally, we showed exogenous galectin-3 mediated re-localization of MUC4 on the tumor cell surface, indicating a possible mechanism through which MUC4 itself and MUC4-galectin-3 together can promote both transient (docking) and later permanent (locking) adhesion of tumor cells to the endothelial cells. Interference of such a binding of galectin-3 to the MUC4 mucin may be a target for novel therapeutic strategies for metastatic PC.
Translational relevance.
In this study, we have provided the experimental evidence that MUC4, a transmembrane oncogenic glycoprotein aberrantly expressed by pancreatic cancer (PC) cells, interacts with galectin-3 present on endothelial cells. This interaction is specific for galectin-3 and involves the T-antigen present on MUC4. We also demonstrate that the adhesion of PC cells to endothelial HUVECs is dependent on MUC4 expression and presence of exogenous galectin-3, but not the intracellular galectin-3. Quantitative analysis of serum galectin-3 levels in samples from PC patients shows that galectin-3 levels are significantly elevated in PC patients with metastatic disease compared to those with localized disease and healthy controls. The interaction between serum galectin-3 and MUC4 might provide the mechanistic basis underlying the metastasis of PC cells to distant organs. Overall, these discoveries have important implications in further our understanding of the molecular mechanisms that underlie cancer metastasis and emphasize the importance of glycosylation in cancer progression.
Acknowledgments
The invaluable technical support of Dr. Sukhwinder Kaur, and Ms. Kavita Mallya is greatly appreciated. We would also like to thank the University of Nebraska Medical Center (UNMC) Confocal and FACS analyses core facilities.
Financial support: This work was supported by grants from the National Institutes of Health (RO1 CA78590, RO1 CA131944, RO1 CA 133774, EDRN UO1 CA 111294, and SPORE P50 CA127297).
Footnotes
Conflict of interests
The authors declare no conflict of interest.
Reference List
- 1.Flanders TY, Foulkes WD. Pancreatic adenocarcinoma: epidemiology and genetics. J Med Genet. 1996;33:889–98. doi: 10.1136/jmg.33.11.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellenrieder V, Adler G, Gress TM. Invasion and metastasis in pancreatic cancer. Ann Oncol. 1999;10(Suppl 4):46–50. 46-50. [PubMed] [Google Scholar]
- 3.Warshaw AL, Fernandez-del CC. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 4.Zetter BR. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993;4:219–29. [PubMed] [Google Scholar]
- 5.Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214–21. doi: 10.1016/s0959-8049(97)10129-0. [DOI] [PubMed] [Google Scholar]
- 6.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–37. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 8.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–7. [PubMed] [Google Scholar]
- 9.Honn KV, Tang DG. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992;11:353–75. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- 10.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–7. [PubMed] [Google Scholar]
- 11.Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–20. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 12.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–30. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 13.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35:236–45. doi: 10.1016/j.tibs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berberat PO, Friess H, Wang L, et al. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem. 2001;49:539–49. doi: 10.1177/002215540104900414. [DOI] [PubMed] [Google Scholar]
- 16.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 17.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6:1389–93. [PubMed] [Google Scholar]
- 18.Ahmad N, Gabius HJ, Andre S, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–7. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q, Guo X, Nash GB, et al. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009;69:6799–806. doi: 10.1158/0008-5472.CAN-09-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295:69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins to galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–45. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu LG, Andrews N, Zhao Q, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282:773–81. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- 23.Nubel T, Dippold W, Kleinert H, Kaina B, Fritz G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18:140–2. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 24.Glinskii OV, Turk JR, Pienta KJ, Huxley VH, Glinsky VV. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumour cells. J Physiol. 2004;554:89–99. doi: 10.1113/jphysiol.2003.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glinsky VV, Glinsky GV, Glinskii OV, et al. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–11. [PubMed] [Google Scholar]
- 26.Glinsky VV. Intravascular cell-to-cell adhesive interactions and bone metastasis. Cancer Metastasis Rev. 2006;25:531–40. doi: 10.1007/s10555-006-9029-8. [DOI] [PubMed] [Google Scholar]
- 27.Glinskii OV, Turk JR, Pienta KJ, Huxley VH, Glinsky VV. Evidence of porcine and human endothelium activation by cancer-associated carbohydrates expressed on glycoproteins and tumour cells. J Physiol. 2004;554:89–99. doi: 10.1113/jphysiol.2003.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59:1356–61. [PubMed] [Google Scholar]
- 29.Biao Z, Zhanggang X, Hao J, Changhong M, Jing C. The in vitro effect of desflurane preconditioning on endothelial adhesion molecules and mRNA expression. Anesth Analg. 2005;100:1007–13. doi: 10.1213/01.ANE.0000146432.39090.D4. [DOI] [PubMed] [Google Scholar]
- 30.Shimamura T, Sakamoto M, Ino Y, et al. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2002;8:2570–5. [PubMed] [Google Scholar]
- 31.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–28. [PMC free article] [PubMed] [Google Scholar]