Abstract
Background
Pancreatic adenosquamous carcinoma has historically been characterized as having a more aggressive clinical course than ductal adenocarcinoma. The natural history of this disease, however, is essentially unknown.
Methods
We evaluated the clinical characteristics of all patients with pancreatic adenosquamous carcinoma recorded in the California Cancer Registry 2000–2007 and compared them to those of patients with ductal adenocarcinoma.
Results
Ninety-five patients with pancreatic adenosquamous carcinoma and 14,746 patients with ductal adenocarcinoma were identified. Demographics were similar between subtypes (p > 0.05). Disease stage at presentation was also similar; over 50% of each diagnostic group presented with metastatic disease (p = 0.62). Surgical resection was more common among patients with locoregional adenosquamous carcinoma than adenocarcinoma (p = 0.0004), but rates of adjuvant therapy administration were similar (p > 0.05). The cohorts’ median overall survival durations were similar in a Cox proportional hazards model (p = 0.45); overall survival was also similar when only patients with resected disease were considered (p = 0.65). Early stage, resection and receipt of radiation or chemotherapy were favorable independent prognostic factors among patients with adenosquamous carcinoma. The median overall survival duration of patients with resected adenosquamous carcinoma was 12 months (95% CI, 8–52).
Conclusions
Adenosquamous carcinoma has a natural history similar to that of ductal adenocarcinoma when treated with prevalent clinical patterns of care.
Keywords: Pancreatic cancer, Adenosquamous cancer, Pancreaticoduodenectomy, Adenocarcinoma, Pancreas
Introduction
Adenosquamous carcinoma (ASC) is a rare pancreatic cancer that is has been suggested to be distinct from pancreatic ductal adenocarcinoma (AC) both histopathologically and clinically.1, 2 Histologically, ASC is distinguished from AC by the presence of both adenocarcinomatous and squamous components.3 Clinically, the disease has been characterized by an extremely poor prognosis, even relative to that of AC—which itself is associated with median overall survival durations as low as 3–6 months among patients with metastatic disease and as high as 24 months among patients with resectable cancers.4, 5 Indeed, the median overall survival duration of patients with localized ASC has been reported to be as low as 6 months following radical tumor resection, with 2-year survival an infrequent event. Patients with advanced ASC treated with palliative intent have fared even worse.5–7
The histopathologic phenotype of ASC is well defined and thus ASC remains a unique diagnostic entity. The clinical significance of this diagnosis is unclear, however, because its natural history is poorly understood. Indeed, the demographics, treatment patterns, and oncologic outcomes of patients with ASC are essentially unknown because all clinical knowledge of the disease has been accumulated from case studies 8–26 and small, single-institution anecdotes—reporting patients compiled over a period of decades—the overwhelming majority of whom had localized disease and were treated with surgery alone.2, 5, 7, 27–31 Given the time, stage, and treatment biases inherent in these previous reports, we hypothesized that the natural history of ASC has been mischaracterized and its clinical significance overstated. We sought to more completely establish the clinical profile of ASC relative to AC and to elucidate any unique characteristics that might influence the design of rational treatment strategies. To these ends, we examined a consecutive series of patients with ASC recorded in a large state cancer registry over a recent 8-year time period. We evaluated demographic and clinical features of ASC, including survival estimates after treatment with prevalent patterns of care, and compared these clinical parameters to those of patients with AC treated in the same recent time period.
Patients and Methods
Cancer Registry
We performed a historical analysis of cases in the California Cancer Registry database (CCR). The CCR is the largest contiguous area, population-based cancer registry in the world, collecting more than 130,000 new cases yearly. Standardized data collection and quality control procedures have been in place since 1988.32, 33 The CCR is part of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program. Case reporting is estimated at 99% for the state, and follow-up completion rates exceed 95%.34, 35 The CCR has received the highest level of certification from the North American Association of Central Cancer Registries.36 Data were abstracted from medical records by trained registrars according to standardized protocols.32, 33 Tumor site and histology were coded according to standardized criteria.37
Study Population
Histopathologic diagnoses recorded in the CCR were ascertained by examination of fine needle aspiration or surgical specimens by local pathologists. Pancreatic tumors were identified using the SEER primary site recode 21100. Pancreatic ductal adenocarcinomas were identified by ICDO (third edition) histology codes 8140, 8141, 8142, 8144, 8490, 8500, 8501, 8503, 8504, 8507.37 Adenosquamous carcinomas were identified by histology code 8560. Other non-ductal cancers were expressly excluded. All incident cases recorded between January 2000 and November 2007 for whom complete follow-up data were available through November 2007 were included for analysis.
Recorded data included demographic information, histology, burden of disease at presentation, first treatment history, socioeconomic status, and vital status. Socioeconomic status is denoted as a single index variable using statewide measures of education, income, and occupation from census data, as described previously.38, 39 Quintiles for the socioeconomic status score were used for analysis, with socioeconomic status 1 and 5 denoting the lowest and highest quintiles, respectively.
The criteria used for American Joint Commission on Cancer (AJCC) staging of pancreatic cancer underwent a dramatic revision between the fifth and sixth editions.40 In the CCR, AJCC staging per seventh edition guidelines is available only for cases diagnosed in or after the year 2004. We therefore allocated cases by the SEER summary stage into cohorts with “localized” (no tumor extension or malignant regional lymphadenopathy regardless of tumor size), “regional” (based on the presence of either tumor extension to adjacent viscera or lymph nodes), or “metastatic” disease. Patients with localized or regional disease in whom pancreaticoduodenectomy, distal pancreatectomy, or total pancreatectomy were performed were considered to have undergone an oncologic resection; patients who underwent an oncologic resection who received either chemotherapy or radiation therapy in the first course of treatment were considered to have undergone adjuvant therapy. Hospital registrars contacted cases annually, and CCR staff annually reviewed state death certificates to identify deceased registry cases.
Statistical Analysis
Clinical characteristics were analyzed with Pearson’s chi-square test or Fisher’s exact test for categorical and dichotomous variables and the Student’s t test for comparison of continuous variables. The overall survival duration (in months) was calculated using dates of diagnosis and either death from any cause or last contact. The Kaplan–Meier method was used to generate survival curves. The log-rank test was used to assess differences between survival curves. Multivariate survival analyses were performed using Cox proportional hazards ratios. Fifty-five patients (all of whom had AC) in whom a diagnosis of cancer was made by review of an autopsy report or death certificate were excluded from all survival analyses. All analyses were conducted using SAS 9.2 (SAS Institute, Inc., Cary, NC). Statistical significance was assumed for a two-tailed p value < 0.05.
Results
Demographics of Patients with ASC and AC
Between 2000 and 2007, 24,604 incident cases of pancreatic neoplasm were recorded in the CCR. Of these, 14,746 (59.9%) patients with AC and 95 (0.38%) patients with ASC were included in this analysis. Demographic data for these patients are reported in Table 1. The median age at diagnosis, sex, race, socioeconomic status, and clinical stage of patients with ASC and AC were similar (p > 0.05). The majority of patients with each diagnosis were Caucasian; sex and socioeconomic status were evenly distributed. Over 50% of both groups were found to have metastatic disease upon presentation. In contrast, localized disease was identified in less than 10% of incident cases of each histopathologic subtype.
Table 1.
Demographics and treatment of patients with pancreatic adenosquamous carcinoma and ductal adenocarcinoma reported in California, 2000–2007
| Adenosquamous | Adenocarcinoma | p | |
|---|---|---|---|
| N, % | 95 (0.39) | 14,746 (59.9) | |
| Demographic variables | |||
| Age, mean (SD) | 68.5 (11.8) | 68.6 (11.8) | 0.9188 |
| Sex, n (%) | 0.1565 | ||
| Male | 55 (57.9) | 7,462 (50.6) | |
| Female | 40 (42.1) | 7,284 (49.4) | |
| Ethnicity, n (%) | 0.3740 | ||
| White | 72 (75.8) | 9,760 (66.2) | |
| Black | 5 (5.3) | 1,108 (7.5) | |
| Hispanic | 11 (11.6) | 2,425 (16.5) | |
| Asian | 7 (7.4) | 1,365 (9.3) | |
| Other | 0 (0) | 88 (0.6) | |
| SES quintile, n (%) | 0.3013 | ||
| Lowest | 10 (10.5) | 2,083 (14.1) | |
| Second lowest | 22 (23.2) | 2,622 (17.8) | |
| Middle | 14 (14.7) | 3,153 (21.4) | |
| High | 23 (24.2) | 3,322 (22.5) | |
| Highest | 26 (27.4) | 3,566 (24.2) | |
| Clinical stage, n (%)a | 0.6242 | ||
| Localized | 8 (8.9) | 976 (7.0) | |
| Regional | 34 (37.8) | 4,864 (35.1) | |
| Metastatic | 48 (53.3) | 8,029 (57.9) | |
| Missing data, n (%)b | 5 (5.3) | 877 (5.9) | |
| Treatment variables | |||
| Any surgery, n (%)a | <0.0001 a | ||
| Yes | 31 (32.6) | 2,428 (16.5) | |
| No | 64 (67.4) | 12,300 (83.5) | |
| Missing data, n (%)b | 0 (0) | 18 (0.1) | |
| Any radiation, n (%)a | 0.1515a | ||
| Yes | 20 (21.1) | 2,310 (15.7) | |
| No | 75 | 12,422 (84.3) | |
| Missing data, n (%)b | 0 (0) | 14 (0.1) | |
| Any chemotherapy, n (%)a | 0.6786a | ||
| Yes | 42 (46.2) | 6,296 (44.0) | |
| No | 49 (53.8) | 8,016 (56.0) | |
| Missing data, n (%)b | 4 (4.2) | 434 (2.9) | |
| Locoregional patients, n evaluated | 42 | 5,838 | |
| Onc. resection, n (%) | 0.0004 | ||
| Yes | 26 (61.9) | 2,071 (35.6) | |
| No | 16 (38.1)c | 3,750 (64.4) | |
| Type of resection, n (%) | 0.1084 | ||
| PD | 18 (69.2) | 1,690 (81.6) | |
| Distal panc. | 5 (19.2) | 181 (8.7) | |
| Total panc. | 3 (11.5) | 200 (9.7) | |
| Tumor diam. (mm); mean (SD)a | 46.3 (19.0) | 33.5 (15.1) | 0.0001 |
| Missing data, n (%)b | 0 (0) | 117 (5.7) | |
| Lymph nodes positive, n (%)a | 0.8562 | ||
| Yes | 15 (57.7) | 1,236 (60.2) | |
| No | 11 (42.3) | 816 (39.8) | |
| Missing data, n (%)b | 0 (0) | 19 (0.9) | |
| Adj. chemotherapy, n (%)a | 0.9902 | ||
| Yes | 13 (52.0) | 1,037 (51.9) | |
| No | 12 (48) | 962 (48.1) | |
| Missing data, n (%)b | 1 (3.8) | 72 (3.5) | |
| Adj. radiation, n (%) | 0.2876 | ||
| Yes | 12 (46.2) | 747 (36.1) | |
| No | 14 (53.8) | 1,324 (63.9) | |
| Metastatic patients, n evaluated | 46 | 7,832 | |
| Pall. chemotherapy, n (%) | 0.2397 | ||
| Yes | 24 (52.2) | 3,411 (43.6) | |
| No | 22 (47.8) | 4,421 (56.4) | |
The numbers in bold are those which are statistically significant, i.e. p < 0.05
For each variable, data was complete unless otherwise specified
SES socioeconomic status, Onc. oncologic, panc. pancreatectomy, Adj. adjuvant, Pall. palliative, diam. diameter, PD pancreaticoduodenectomy
aPercentage and p values refer to patients with complete data
bPercentage of total patients
cAmong patients with locoregional ASC in whom the reason an oncologic resection was not performed was recorded, surgery was not recommended in 12 (two with localized cancers and ten regional), and one patient refused an operation
Treatment Patterns and Pathologic Variables of ASC and AC
Surgery was utilized more frequently for patients with ASC than those with AC, both overall (32.6% vs 16.5%, p < 0.0001) and among patients with locoregional cancers (61.9% vs 35.6%, p = 0.0004) (Table 1). Oncologic procedures performed for patients with ASC included pancreaticoduodenectomy (n = 18), distal (n = 5), and total pancreatectomy (n = 3); the distribution of these operations was similar to that performed for AC (p = 0.11). The mean tumor diameter in resected ASC specimens was larger than that in AC specimens (46.3 vs 33.5 mm, p = 0.0001), but the frequency of positive lymph nodes was similar (57.7 vs 60.2%, p = 0.86).
Overall, radiation (p = 0.15) and chemotherapy (p = 0.68) were administered to similar proportions of patients with ASC and AC. Twelve (46.2%) patients with ASC who underwent an oncologic resection were treated with adjuvant radiation and 13 (52.0%) received chemotherapy. Rates of administration of adjuvant radiation (p = 0.29) and adjuvant chemotherapy (p = 0.99) following resection for locoregional disease did not differ between groups. Likewise, among patients with metastatic disease, the rate of administration of palliative chemotherapy did not differ between patients with ASC and AC (p = 0.24).
Overall Survival of ASC and AC
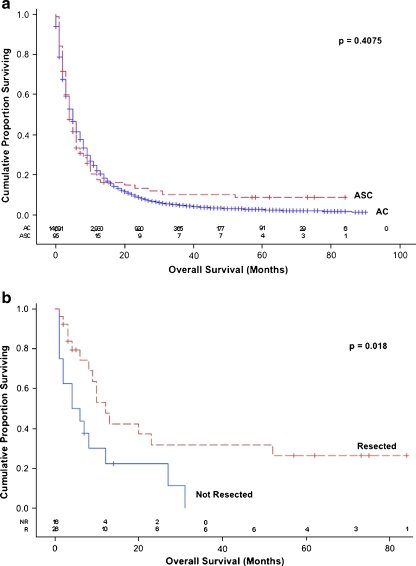
As a group, the median overall survival duration of all patients with ASC was 4 months (95% CI, 3–6) and was similar to that of all patients with AC (p = 0.41, Fig. 1a). The median overall survival duration of patients with ASC was also similar to that of patients with AC in subpopulations of patients stratified by age, sex, ethnicity, socioeconomic status, clinical stage, and the use of oncologic resection, radiation therapy, and chemotherapy on univariate analysis (p > 0.05, data not shown). Furthermore, the median overall survival duration of all patients with ASC was similar to that of all patients with AC in a Cox proportional hazards model after adjustment for age, gender, ethnicity, socioeconomic status, stage of disease, and first treatment strategy [hazard ratio (HR), 1.091; 95% CI, 0.870–1.367; p = 0.45] (Table 2). Finally, when only patients with locoregional cancers who underwent resection were considered, the median overall survival duration of patients with each histopathologic diagnosis were similar after adjustment for age, gender, ethnicity, socioeconomic status, clinical stage, tumor size, lymphatic involvement, and the receipt of adjuvant therapy (HR, 0.886; 95% CI, 0.530–1.482; p = 0.65) (Table 3).
Fig. 1.
a Overall survival of all patients with pancreatic adenosquamous carcinoma and ductal adenocarcinoma reported in California, 2000–2007. Dashed line, adenosquamous carcinoma (ASC); solid line, pancreatic ductal adenocarcinoma (AC). b Overall survival of patients with localized or regional adenosquamous carcinoma stratified by resection status. Dashed line, resected (R); solid line, not resected (NR)
Table 2.
Cox proportional hazards model for overall survival of all patients with pancreatic adenosquamous carcinoma and ductal adenocarcinoma reported in California, 2000–2007
| HR | 95% CI | p | |
|---|---|---|---|
| Histologic subtype | |||
| AC | 1.000 (referent) | ||
| ASC | 1.091 | 0.870–1.367 | 0.4509 |
| Age | 1.010 | 1.009–1.012 | <0.0001 |
| Gender | |||
| Male | 1.000 (referent) | ||
| Female | 0.951 | 0.918–0.986 | 0.006 |
| Ethnicity | |||
| Caucasian | 1.000 (referent) | ||
| Black | 1.035 | 0.966–1.108 | 0.3290 |
| Hispanic | 0.955 | 0.906–1.006 | 0.0802 |
| Asian | 0.922 | 0.866–0.981 | 0.0108 |
| Socioeconomic status | 0.966 | 0.953–0.980 | <0.0001 |
| Clinical stage | |||
| Localized | 1.000 (referent) | ||
| Regional | 1.275 | 1.177–1.382 | <0.0001 |
| Metastatic | 2.293 | 2.117–2.484 | <0.0001 |
| Oncologic resection | |||
| No | 1.000 (referent) | ||
| Yes | 0.444 | 0.419–0.472 | <0.0001 |
| Any radiation | |||
| No | 1.000 (referent) | ||
| Yes | 0.887 | 0.840–0.936 | <0.0001 |
| Any chemotherapy | |||
| No | 1.000 (referent) | ||
| Yes | 0.508 | 0.488–0.528 | <0.0001 |
The numbers in bold are those which are statistically significant, i.e. p < 0.05
HR hazard ratio for death, 95% CI 95% confidence interval
Table 3.
Cox proportional hazards model for overall survival of patients with resected locoregional pancreatic adenosquamous carcinoma and ductal adenocarcinoma reported in California, 2000–2007
| HR | 95% CI | p | |
|---|---|---|---|
| Histologic subtype | |||
| AC | 1.000 (referent) | ||
| ASC | 0.886 | 0.530–1.482 | 0.6454 |
| Age | 1.008 | 1.003–1.014 | 0.0043 |
| Gender | |||
| Male | 1.000 (referent) | ||
| Female | 0.998 | 0.893–1.116 | 0.9740 |
| Ethnicity | |||
| Caucasian | 1.000 (referent) | ||
| Black | 1.095 | 0.865–1.387 | 0.4514 |
| Hispanic | 1.043 | 0.884–1.231 | 0.6150 |
| Asian | 1.007 | 0.818–1.241 | 0.9445 |
| Socioeconomic status | 0.945 | 0.905–0.987 | 0.0112 |
| Clinical stage | |||
| Localized | 1.000 (referent) | ||
| Regional | 1.300 | 1.067–1.583 | 0.0091 |
| Tumor diameter | 1.009 | 1.005–1.012 | <0.0001 |
| Lymphatic involvement | |||
| No | 1.000 (referent) | ||
| Yes | 1.386 | 1.212–1.585 | <0.0001 |
| Adjuvant radiation | |||
| No | 1.000 (referent) | ||
| Yes | 0.791 | 0.679–0.922 | 0.0027 |
| Adjuvant chemotherapy | |||
| No | 1.000 (referent) | ||
| Yes | 0.651 | 0.561–0.756 | <0.0001 |
The numbers in bold are those which are statistically significant, i.e. p < 0.05
HR hazard ratio for death, 95% CI 95% confidence interval
Favorable Prognostic Factors among Patients with ASC
Among all patients with ASC, favorable prognostic factors on univariate analysis included early clinical stage (p < 0.0001), oncologic resection (p < 0.0001), receipt of radiation (p < 0.0001), and receipt of chemotherapy (p < 0.0233). In a Cox proportional hazards model, each of these factors remained independently significant (Table 4).
Table 4.
Cox proportional hazards model for overall survival of all patients with pancreatic adenosquamous carcinoma reported in California, 2000–2007
| HR | 95% CI | p | |
|---|---|---|---|
| Age | 1.012 | 0.986–1.038 | 0.3730 |
| Gender | |||
| Male | 1.000 (referent) | ||
| Female | 0.905 | 0.536–1.528 | 0.7088 |
| Ethnicity | |||
| Caucasian | 1.000 (referent) | ||
| Black | 0.703 | 0.244–2.023 | 0.5135 |
| Hispanic | 0.890 | 0.390–2.032 | 0.7815 |
| Asian | 0.655 | 0.244–1.755 | 0.3998 |
| Socioeconomic status | 0.936 | 0.765–1.145 | 0.5915 |
| Clinical stage | |||
| Localized | 1.000 (referent) | ||
| Regional | 2.717 | 0.781–9.451 | 0.1161 |
| Metastatic | 4.690 | 1.445–15.216 | 0.0101 |
| Oncologic resection | |||
| No | 1.000 (referent) | ||
| Yes | 0.369 | 0.183–0.747 | 0.0056 |
| Any radiation | |||
| No | 1.000 (referent) | ||
| Yes | 0.474 | 0.242–0.927 | 0.0292 |
| Any chemotherapy | |||
| No | 1.000 (referent) | ||
| Yes | 0.530 | 0.300–0.935 | 0.0285 |
The numbers in bold are those which are statistically significant, i.e. p < 0.05
HR hazard ratio for death, 95% CI 95% confidence interval
Separate multivariate models were not constructed for patients with locoregional or metastatic ASC due to relatively small numbers in each of these subgroups. Among patients with locoregional ASC, however, those who underwent an oncologic resection had a median survival duration of 12 months (95% CI, 8–52) compared with 5 months (95% CI, 1–12) for those who did not, and the survival curves were significantly different (p = 0.018) (Fig. 1b). A significant difference in survival could not be demonstrated between patients with resected locoregional ASC who did and did not receive adjuvant therapy (p = 0.09 overall). Eight patients with locoregional ASC survived longer than 2 years, four of whom survived over 5 years. Each of these 5-year survivors underwent surgery and received adjuvant therapy.
Among patients with metastatic ASC, patients who received chemotherapy had a more favorable median survival duration (4.5 months; 95% CI, 3–6 months) than patients who did not (2 months; 95% CI, 1–3 months; p = 0.04).
Discussion
ASC and AC share a similar histologic 3 and molecular 41 profile. ASC, however, has long been characterized as having a natural history distinctly more aggressive than that of AC. This has led some to question the role of aggressive treatment strategies for patients with this disease.2, 5, 7, 27 The clinical significance of this rare diagnosis relative to AC is unclear, however, because the oncologic behavior of ASC has been described only by case studies and small, retrospective surgical series reporting patients with early stage cancers (Table 5). Moreover, no prior case–control studies or population-based analyses have been performed to definitively establish clinical differences between ASC and AC. In this, the largest study of ASC reported to date, we used a large cancer registry to evaluate the clinical features and oncologic outcomes of patients with this diagnosis. Using a relatively unbiased dataset, we characterize the natural history of ASC and show that ASC is no more inherently aggressive than AC. Indeed, we demonstrate that patients with these two diagnoses have a similar natural history when treated using prevalent patterns of modern clinical practice.
Table 5.
Published case reports and clinical series of patients with pancreatic adenosquamous carcinoma, 1990–2010
| Author (ref.) | Number | Resected, n (%) | Median age (years) | Adjuvant treatment, n | Median OS resected, months | Median OS unresected, months |
|---|---|---|---|---|---|---|
| Skafida8 | 1 | 1 (100) | 70 | 1 CTX | 6 | NA |
| Lampropoulos9 | 1 | 1 (100) | 72 | 1 CXRT | 24 | NA |
| Voong27 | 38 | 38 (100) | 68 | 19 CTX 19 CXRT | 10.9 | NA |
| Kobayashi10 | 1 | 0 (0) | 72 | NA | NA | 3 |
| Smoot7 | 23 | 12 (52) | 67a | 5 CXRT | 13.1 | 4.8 |
| Hsu5 | 12 | 7 (58) | 71 | 5 CTX | 6.51 | NR |
| Jamali11 | 1 | 1 (100) | 75 | 1 CTX | 6 | NA |
| Alwaheeb12 | 1 | 1 (100) | 45 | NR | NR | NA |
| Inoue13 | 1 | 0 (0) | 61 | NA | NA | 0.83 |
| Murakami14 | 2 | 2 (100) | 54 | 1 CTX 1 CXRT | 4.5 | NA |
| Rahemtullah28 | 14 | 2 (14) | 70a | NR | 13 | 4 |
| Kardon29 | 25 | 13 (52) | 65a | 5 CTX | 11.3 | 3.0 |
| Yamaue15 | 1 | 1 (100) | 63 | 1 CXRT | 40 | NA |
| Yavuz16 | 2 | 2 (100) | 50 | NR | 36, NR | NA |
| Komatsuda17 | 1 | 1 (100) | 67 | 0 | 6 | NA |
| Aranha18 | 2 | 2 (100) | 57 | 2 CXRT | 13.5 | NA |
| Madura2 | 6 | 6 (100) | 64a | 3 CXRT | 5 | NA |
| Nabae19 | 2 | 2 (100) | 67 |
1 RT 1 NR |
6.5 | NA |
| Lozano20 | 3 | 2 (67) | 59a | 3 CXRTb | NR | NR |
| Myung21 | 1 | 1 (100) | 64 | 0 | 4 | NA |
| Kuji22 | 1 | 1 (100) | 73 | 0 | 2 | NA |
| Campman23 | 1 | 1 (100) | 65 | NR | NR | NA |
| Onoda24 | 1 | 1 (100) | 64 | 1 CTX | 3 | NA |
| Makiyama25 | 1 | 1 (100) | 58 | 0 | 18 | NA |
| Tanaka26 | 1 | 1 (100) | 48 | 1 CTX | 7 | NA |
| Motojima30 | 6 | 3 (50) | 67c | NR | 7 | NR |
| Yamaguchi31 | 8 | 8 (100) | 56a | 0 | 5.5 | NA |
NR not recorded, NA not applicable, CTX chemotherapy, CXRT chemoradiation, RT radiation, OS overall survival
aMean
bNeoadjuvant chemoradiation
cResected only
ASC has been reported to represent up to 4% of pancreatic neoplasms, but in the largest series of specimens analyzed at autopsy, ASC was identified in only 0.9%.42, 43 In this analysis of a large tumor registry, we found a diagnosis of ASC in approximately 0.4% of 24,604 patients with newly documented pancreatic malignancies recorded between 2000 and 2007. This is remarkably similar to the rate of 0.5% identified in a recent 16-year survey of the State of Michigan Tumor Registry.44
Like patients with AC, most of the patients with ASC presented late in their natural history. Indeed, over 50% of patients analyzed in this study initially presented with synchronous distant metastases. Among patients treated surgically, those with ASC had larger tumors than those with AC; however, a larger proportion of patients with locoregional ASC underwent resection than that with AC, and resected ASC specimens were associated with a similar high rate of regional lymphatic involvement—approximately 60%—as AC tumors. Together, these findings reveal that—although considerably rarer—ASC presents at a similar (albeit advanced) stage as AC and suggest that the two diagnoses share a common biologic behavior prior to diagnosis and treatment.
Stage-specific treatment algorithms for patients with AC are reasonably well-established.45 In contrast, the absolute infrequency of ASC has prohibited the development of standardized treatment protocols for this disease. Indeed, even the treatment of patients with early stage ASC remains controversial, due to reportedly dismal survival rates seemingly regardless of intervention.5, 7 In a recent systematic review of prior reports, 39 patients with ASC who underwent surgery for non-metastatic disease had a median survival duration of 6.8 months (range, 4.6–9) and a 1-year survival rate of 25.5%.6 In two recent single-institution series, overall survival of resected patients was somewhat more favorable. Among 38 resected patients from Johns Hopkins, the median overall survival duration was 10.9 months from diagnosis.27 In another series from the Mayo Clinic, patients who underwent R0 or R1 resection had a median survival duration of 14.4 months and 8 months, respectively, compared to 4.8 months among patients treated without an operation.7 The patients in each group were not described, however, suggesting that patients who did not undergo resection had advanced disease, prior comorbidities, a depressed performance status, or a combination of these factors.
The efficacy of non-operative therapies among patients with ASC has not been rigorously evaluated. Only one prior study has examined the utility of adjuvant chemoradiation for patients with this disease. In that small, retrospective series, 19 (50%) patients who underwent postoperative chemoradiation had a more favorable median overall survival than 19 (50%) patients who did not (13.6 months v. 8.6 months, p = 0.005).27 Although adjuvant chemoradiation was found to be the only significant prognostic factor with respect to overall survival on univariate analysis, the analysis suffered from clear selection bias. No studies have specifically studied the effects of systemic chemotherapy when administered in the adjuvant setting, nor its role as palliative therapy for patients with metastatic disease.
In this study, treatment of patients with ASC by surgical resection was associated with a more favorable overall survival relative to no resection, after adjustment for multiple clinical factors including disease stage. Moreover, the overall survival duration of patients with locoregional ASC who underwent surgery was similar to that of patients with locoregional AC who underwent resection in the same time period. Together with the recent single-institution data from high-volume pancreatic treatment centers,7, 27 these data suggest that resection is a reasonable therapeutic approach for patients with ASC in whom a margin-negative resection can be performed safely.
The role of non-operative therapies for patients with ASC is less clear. Although we could demonstrate no association between the administration of adjuvant radiation or chemotherapy on the survival of patients with locoregional ASC following resection, it is interesting that of the only six 5-year survivors with ASC reported to date (four in this series and two in the Johns Hopkins series 27), all received surgery and adjuvant therapy. Among patients with metastatic ASC, patients who received chemotherapy had a longer overall survival duration (4.5 vs 2 months) than patients who did not. The significance of this finding is uncertain, however, because individual performance status—the most influential factor with regard to the administration of anticancer therapy among patients with advanced pancreatic malignancy—was not recorded in the CCR.46 The absence of recorded performance status represents a fundamental limitation of this and other analyses of pancreatic malignancies using large, population-based datasets.
Two other limitations of this study are particularly noteworthy. Although attempts have been made to identify characteristic molecular fingerprints that may effectively distinguish between ASC and AC, the molecular profile of these two tumors are similar.41 Therefore, ASC must be distinguished from AC histopathologically. A strict diagnosis of ASC requires that a malignant squamous component represent at least 30% of a routinely sectioned adenocarcinoma.3, 29 This arbitrary cutoff has introduced ambiguity to the diagnosis of ASC that reflects both the absence of standardization in histopathologic methods used to process surgical specimens and the subjectivity with which they are evaluated. Indeed, when 38 surgical specimens initially diagnosed as ASC at Johns Hopkins were re-evaluated by a single pathologist, 12 (32%) failed to meet strict criteria for the disease.27 Significantly, although the presence of any squamous component was associated with poor prognosis in the Johns Hopkins study relative to a historic control group of patients with AC, the proportion of the squamous component was not associated with overall survival. The rationale for the strict 30% cutoff is therefore unclear, and several investigators have proposed eliminating this criterion altogether.29
It is also possible that some diagnoses were coded incorrectly in the CCR; however, all diagnoses recorded therein were validated by histopathologic or cytopathologic analysis. Moreover, accuracy of the histopathologic diagnoses recorded in large databases has been evaluated and compared with independent histologic review, with favorable results.47, 48 Nonetheless, the accuracy associated with the diagnosis of ASC may not be as favorable due to the stringent diagnostic requirements for this disease. A further potential for misclassification may exist among patients with advanced cancer treated non-operatively, for whom a large surgical specimen for histopathologic evaluation is absent. The extent to which our conclusions are influenced by this issue is unknown.
In summary, we conclude that ASC is an extremely rare subtype of pancreatic cancer that shares many clinical characteristics—including biologic behavior and overall prognosis—with AC. In this population, the overall survival duration of all patients with ASC and AC were similar after adjustment for multiple clinical factors, including stage at presentation and first treatment strategy. These data therefore refute prior suggestions that ASC is inherently more aggressive than AC and imply that a nihilistic view toward patients with ASC must be avoided. Absent the ability to perform prospective studies to determine the response of ASC to individual therapies, and given the molecular, histopathologic and clinical similarity of these diseases, we recommend the use of aggressive, stage-specific, multidisciplinary treatment protocols developed for AC.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Cihak RW, Kawashima T, Steer A. Adenoacanthoma (adenosquamous carcinoma) of the pancreas. Cancer. 1972;29(5):1133–40. doi: 10.1002/1097-0142(197205)29:5<1133::AID-CNCR2820290503>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Madura JA, Jarman BT, Doherty MG, et al. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134(6):599–603. doi: 10.1001/archsurg.134.6.599. [DOI] [PubMed] [Google Scholar]
- 3.Hruban R, Pitman M, Klimstra D. Tumors of the pancreas. In: Silverberg S, Sobin L, editors. AFIP atlas of tumor pathology. Washington, DC: American Registry of Pathology; 2007. pp. 177–81. [Google Scholar]
- 4.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–47. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu JT, Chen HM, Wu RC, et al. Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J Surg Oncol. 2008;6:95. doi: 10.1186/1477-7819-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okabayashi T, Hanazaki K. Surgical outcome of adenosquamous carcinoma of the pancreas. World J Gastroenterol. 2008;14(44):6765–70. doi: 10.3748/wjg.14.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smoot RL, Zhang L, Sebo TJ, Que FG. Adenosquamous carcinoma of the pancreas: a single-institution experience comparing resection and palliative care. J Am Coll Surg. 2008;207(3):368–70. doi: 10.1016/j.jamcollsurg.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Skafida E, Grammatoglou X, Glava C, et al. Adenosquamous carcinoma of the pancreas: a case report. Cases J. 2010;3:41. doi: 10.1186/1757-1626-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampropoulos P, Filippou G, Skafida E, et al. Adenosquamous carcinoma of the pancreas, a rare tumor entity: a case report. Cases J. 2009;2:9129. doi: 10.1186/1757-1626-2-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi N, Higurashi T, Iida H, et al. Adenosquamous carcinoma of the pancreas associated with humoral hypercalcemia of malignancy (HHM) J Hepatobiliary Pancreat Surg. 2008;15(5):531–5. doi: 10.1007/s00534-007-1258-x. [DOI] [PubMed] [Google Scholar]
- 11.Jamali M, Serra S, Chetty R. Adenosquamous carcinoma of the pancreas with clear cell and rhabdoid components. A case report. JOP. 2007;8(3):330–4. [PubMed] [Google Scholar]
- 12.Alwaheeb S, Chetty R. Adenosquamous carcinoma of the pancreas with an acantholytic pattern together with osteoclast-like and pleomorphic giant cells. J Clin Pathol. 2005;58(9):987–90. doi: 10.1136/jcp.2004.025221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, Nagao S, Tajima H, et al. Adenosquamous pancreatic cancer producing parathyroid hormone-related protein. J Gastroenterol. 2004;39(2):176–80. doi: 10.1007/s00535-003-1270-0. [DOI] [PubMed] [Google Scholar]
- 14.Murakami Y, Yokoyama T, Yokoyama Y, et al. Adenosquamous carcinoma of the pancreas: preoperative diagnosis and molecular alterations. J Gastroenterol. 2003;38(12):1171–5. doi: 10.1007/s00535-003-1226-4. [DOI] [PubMed] [Google Scholar]
- 15.Yamaue H, Tanimura H, Onishi H, et al. Adenosquamous carcinoma of the pancreas: successful treatment with extended radical surgery, intraoperative radiation therapy, and locoregional chemotherapy. Int J Gastrointest Cancer. 2001;29(1):53–58. doi: 10.1385/IJGC:29:1:53. [DOI] [PubMed] [Google Scholar]
- 16.Yavuz E, Kapran Y, Ozden I, et al. Pancreatobiliary adenosquamous carcinoma (report of two cases) Pathologica. 2000;92(5):323–6. [PubMed] [Google Scholar]
- 17.Komatsuda T, Ishida H, Konno K, et al. Adenosquamous carcinoma of the pancreas: report of two cases. Abdom Imaging. 2000;25(4):420–3. doi: 10.1007/s002610000059. [DOI] [PubMed] [Google Scholar]
- 18.Aranha GV, Yong S, Olson M. Adenosquamous carcinoma of the pancreas. Int J Pancreatol. 1999;26(2):85–91. doi: 10.1007/BF02781735. [DOI] [PubMed] [Google Scholar]
- 19.Nabae T, Yamaguchi K, Takahata S, et al. Adenosquamous carcinoma of the pancreas: report of two cases. Am J Gastroenterol. 1998;93(7):1167–70. doi: 10.1111/j.1572-0241.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 20.Lozano MD, Panizo A, Sola IJ, Pardo-Mindan FJ. FNAC guided by computed tomography in the diagnosis of primary pancreatic adenosquamous carcinoma. A report of three cases. Acta Cytol. 1998;42(6):1451–4. doi: 10.1159/000332185. [DOI] [PubMed] [Google Scholar]
- 21.Myung SJ, Kim MH, Lee SK, et al. Adenosquamous carcinoma of the pancreas: differentiation from pancreatic pseudocyst. Gastrointest Endosc. 1998;47(5):410–3. doi: 10.1016/S0016-5107(98)70230-5. [DOI] [PubMed] [Google Scholar]
- 22.Kuji I, Sumiya H, Taki J, et al. Intense Ga-67 uptake in adenosquamous carcinoma of the pancreas. Ann Nucl Med. 1997;11(1):41–3. doi: 10.1007/BF03164758. [DOI] [PubMed] [Google Scholar]
- 23.Campman SC, Fajardo MA, Rippon MB, et al. Adenosquamous carcinoma arising in a mucinous cystadenoma of the pancreas. J Surg Oncol. 1997;64(2):159–62. doi: 10.1002/(SICI)1096-9098(199702)64:2<159::AID-JSO13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Onoda N, Kang SM, Sugano S, et al. Mucoepidermoid carcinoma of the pancreas: report of a case. Surg Today. 1995;25(9):843–7. doi: 10.1007/BF00311465. [DOI] [PubMed] [Google Scholar]
- 25.Makiyama K, Takuma K, Zea-Iriarte WL, et al. Adenosquamous carcinoma of the pancreas. J Gastroenterol. 1995;30(6):798–802. doi: 10.1007/BF02349652. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka N, Ohoida J, Matuno T, et al. Response of adenosquamous carcinoma of the pancreas to interferon-alpha, tumor necrosis factor-alpha and 5-fluorouracil combined treatment. Anticancer Res. 1994;14(6):2739–42. [PubMed] [Google Scholar]
- 27.Voong KR, Davison J, Pawlik TM, et al. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2008;41(1):113–22. doi: 10.1016/j.humpath.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahemtullah A, Misdraji J, Pitman MB. Adenosquamous carcinoma of the pancreas: cytologic features in 14 cases. Cancer. 2003;99(6):372–8. doi: 10.1002/cncr.11855. [DOI] [PubMed] [Google Scholar]
- 29.Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14(5):443–51. doi: 10.1038/modpathol.3880332. [DOI] [PubMed] [Google Scholar]
- 30.Motojima K, Tomioka T, Kohara N, et al. Immunohistochemical characteristics of adenosquamous carcinoma of the pancreas. J Surg Oncol. 1992;49(1):58–62. doi: 10.1002/jso.2930490114. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi K, Enjoji M. Adenosquamous carcinoma of the pancreas: a clinicopathologic study. J Surg Oncol. 1991;47(2):109–16. doi: 10.1002/jso.2930470210. [DOI] [PubMed] [Google Scholar]
- 32.California Department of Health Services . Cancer reporting in California: standards for automated reporting volume II. Sacramento: California Department of Health Services; 1997. [Google Scholar]
- 33.California Department of Health Services . Cancer reporting in California: standards for automated reporting volume III. Sacramento: California Department of Health Services; 1997. [Google Scholar]
- 34.Seiffert JE, Price WT, Gordon B. The California tumor registry: a state-of-the-art model for a regionalized, automated, population-based registry. Top Health Rec Manage. 1990;11(2):59–73. [PubMed] [Google Scholar]
- 35.How complete are California Cancer Registry data? Retrieved March 9, 2010 from: http://www.ccrcal.org/questions.html#how%20complete%20is%20ccr%20data. The entry was published in 2007.
- 36.Tucker T, Howe H, Weir H. Certification for population-based cancer registries. J Registry Manage. 1999;26:24–27. [Google Scholar]
- 37.Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology (ICD-O) Geneva: World Health Organization; 2000. [Google Scholar]
- 38.Zell JA, Rhee JM, Ziogas A, et al. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev. 2007;16(3):546–52. doi: 10.1158/1055-9965.EPI-06-0893. [DOI] [PubMed] [Google Scholar]
- 39.Yost K, Perkins C, Cohen R, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. doi: 10.1023/A:1011240019516. [DOI] [PubMed] [Google Scholar]
- 40.Katz MH, Hwang R, Fleming JB, Evans DB. Tumor–node–metastasis staging of pancreatic adenocarcinoma. CA Cancer J Clin. 2008;58(2):111–25. doi: 10.3322/CA.2007.0012. [DOI] [PubMed] [Google Scholar]
- 41.Brody JR, Costantino CL, Potoczek M, et al. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22(5):651–9. doi: 10.1038/modpathol.2009.15. [DOI] [PubMed] [Google Scholar]
- 42.Hsu JT, Yeh CN, Chen YR, et al. Adenosquamous carcinoma of the pancreas. Digestion. 2005;72(2–3):104–8. doi: 10.1159/000088364. [DOI] [PubMed] [Google Scholar]
- 43.Baylor SM, Berg JW. Cross-classification and survival characteristics of 5, 000 cases of cancer of the pancreas. J Surg Oncol. 1973;5(4):335–58. doi: 10.1002/jso.2930050410. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37(2):134–8. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 45.NCCN Clinical Practice Guidelines in Oncology: Pancreatic adenocarcinoma. Retrieved March 9, 2010 from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. The entry was published in 2009.
- 46.Krishnan S, Rana V, Janjan NA, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer. 2006;107(11):2589–96. doi: 10.1002/cncr.22328. [DOI] [PubMed] [Google Scholar]
- 47.Field RW, Smith BJ, Platz CE, et al. Lung cancer histologic type in the surveillance, epidemiology, and end results registry versus independent review. J Natl Cancer Inst. 2004;96(14):1105–7. doi: 10.1093/jnci/djh189. [DOI] [PubMed] [Google Scholar]
- 48.Henson DE, Albores-Saavedra J. Checking up on the surveillance, epidemiology, and end results program. J Natl Cancer Inst. 2004;96(14):1050–1. doi: 10.1093/jnci/djh161. [DOI] [PubMed] [Google Scholar]