Abstract
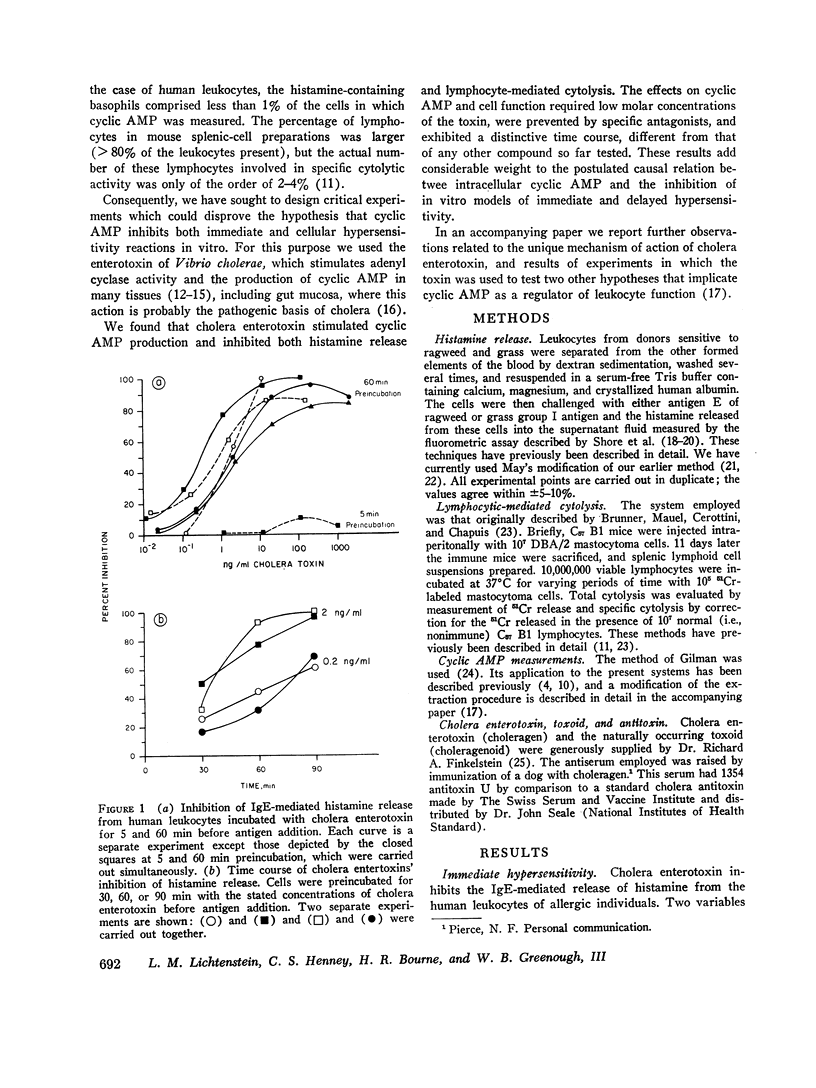
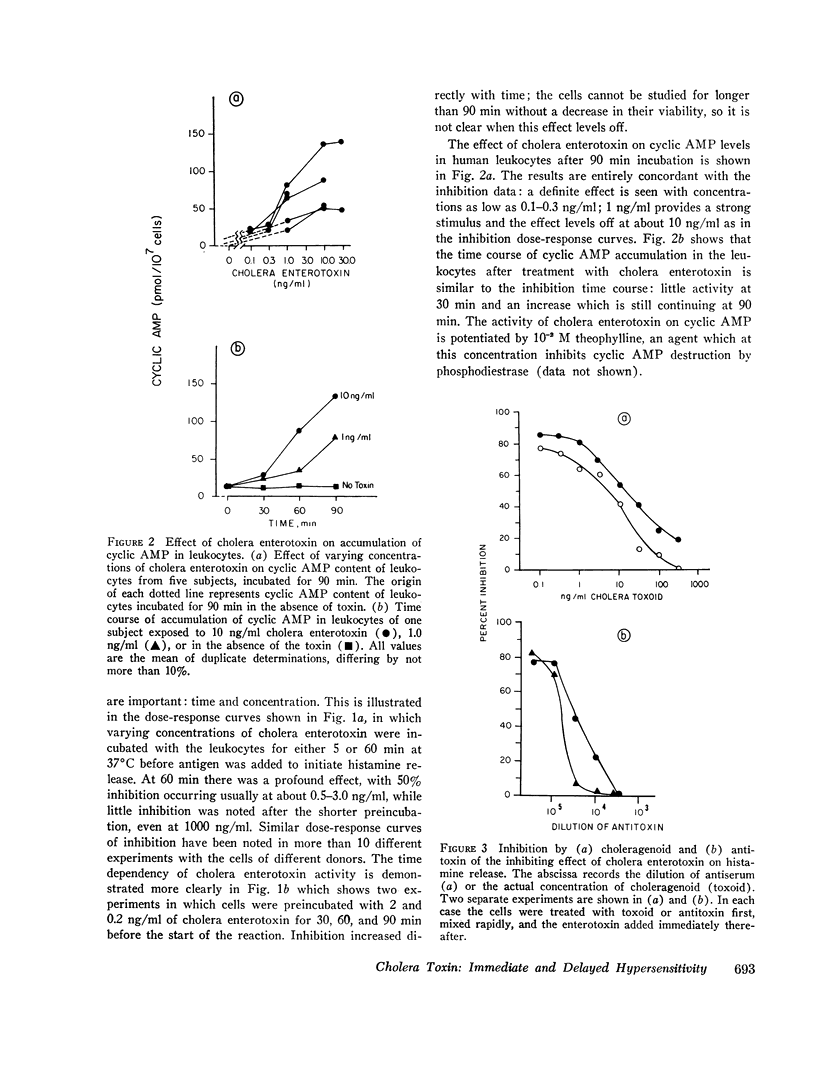
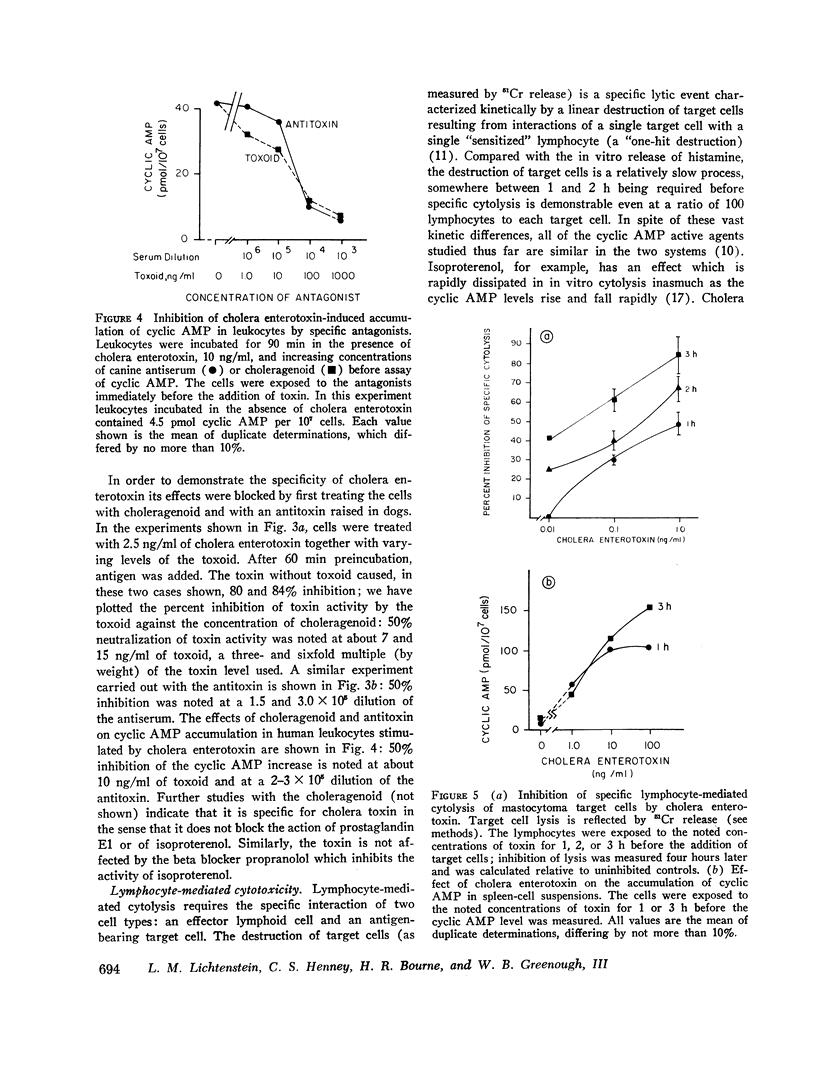
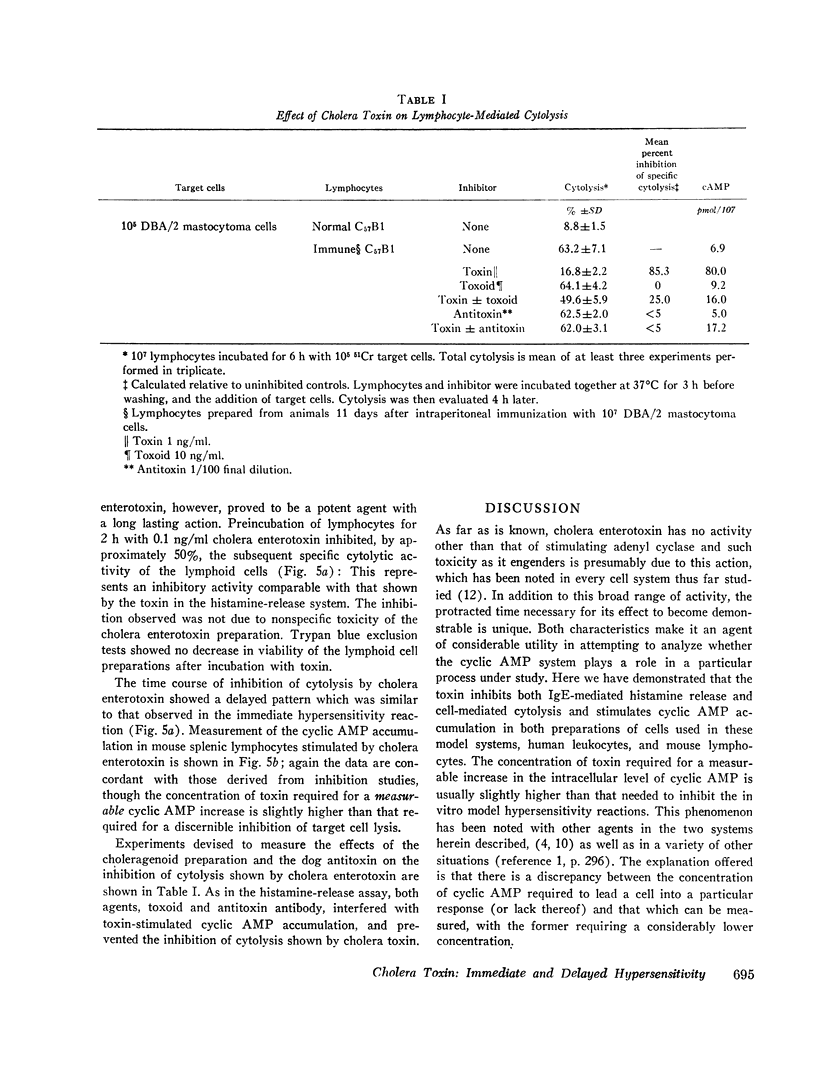
Cholera enterotoxin inhibits the antigen-induced. IgE-mediated release of histamine from human leukocytes and the lysis of allogeneic mastocytoma cells by splenic lymphocytes from specifically immunized mice. This effect requires a prolonged preincubation time of the toxin with the lymphocyte/leukocyte preparations: a demonstrable inhibition requires about 30 min of pre-incubation and the toxin activity is still increasing at 90-180 min. Cholera enterotoxin also stimulates adenyl cyclase and leads to increased levels of cyclic AMP in the lymphocyte/leukocyte preparations. The concentration of toxin required for both cyclic AMP accumulation and inhibition of the biologic responses is about the same (ca. 1 ng/ml), and the time course of cyclic AMP accumulation parallels the development of inhibitory activity. Both activities, inhibition of the in vitro hypersensitivity reactions and cyclic AMP accumulation, are blocked by cholera antitoxin and by a toxoid prepared from the toxin (choleragenoid). These are specific antagonists in that they do not block the inhibiting activity or rise in cyclic AMP levels caused by other adenyl cyclase stimulators. Because cholera enterotoxin has no known activity other than the stimulation of adenyl cyclase and because of its unusual time course and the availability of specific antagonists, this data considerably strengthens the hypothesis that the cyclic AMP system influences the expression of these two forms of hypersensitivity phenomena.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assem E. S., Schild H. O. Inhibition by sympathomimetic amines of histamine release by antigen in passively sensitized human lung. Nature. 1969 Dec 6;224(5223):1028–1029. doi: 10.1038/2241028a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Lichtenstein L. M., Weissmann G., Zurier R. Effects of cholera enterotoxin on adenosine 3',5'-monophosphate and neutrophil function. Comparison with other compounds which stimulate leukocyte adenyl cyclase. J Clin Invest. 1973 Mar;52(3):698–708. doi: 10.1172/JCI107231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L. Pharmacologic control of allergic histamine release in vitro: evidence for an inhibitory role of 3',5'-adenosine monophosphate in human leukocytes. J Immunol. 1972 Mar;108(3):695–705. [PubMed] [Google Scholar]
- Bourne H. R., Melmon K. L., Lichtenstein L. M. Histamine augments leukocyte adenosine 3',5'-monophosphate and blocks antigenic histamine release. Science. 1971 Aug 20;173(3998):743–745. doi: 10.1126/science.173.3998.743. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Henney C. S., Lichtenstein L. M. The role of cyclic AMP in the cytolytic activity of lymphocytes. J Immunol. 1971 Aug;107(2):610–612. [PubMed] [Google Scholar]
- Henney C. S. Quantitation of the cell-mediated immune response. I. The number of cytolytically active mouse lymphoid cells induced by immunization with allogeneic mastocytoma cells. J Immunol. 1971 Dec;107(6):1558–1566. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Orange R. P., Austen K. F. Pharmacologic inhibition of the antigen-induced release of histamine and slow reacting substance of anaphylaxis (SRS-A) from monkey lung tissues mediated by human IgE. J Immunol. 1971 May;106(5):1267–1273. [PubMed] [Google Scholar]
- KING T. P., NORMAN P. S. Isolation studies of allergens from regweed pollen. Biochemistry. 1962 Jul;1:709–720. doi: 10.1021/bi00910a027. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M., DeBernardo R. The immediate allergic response: in vitro action of cyclic AMP-active and other drugs on the two stages of histamine release. J Immunol. 1971 Oct;107(4):1131–1136. [PubMed] [Google Scholar]
- Lichtenstein L. M., Margolis S. Histamine release in vitro: inhibition by catecholamines and methylxanthines. Science. 1968 Aug 30;161(3844):902–903. doi: 10.1126/science.161.3844.902. [DOI] [PubMed] [Google Scholar]
- Marsh D. G., Milner F. H., Johnson P. The allergenic activity and stability of purified allergens from the pollen of common rye grass (lolium perenne). Int Arch Allergy Appl Immunol. 1966;29(6):521–535. doi: 10.1159/000229739. [DOI] [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R., Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy. 1970 Jul;46(1):12–20. doi: 10.1016/0021-8707(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Vaughan M., Pierce N. F., Greenough W. B., 3rd Stimulation of glycerol production in fat cells by cholera toxin. Nature. 1970 May 16;226(5246):658–659. doi: 10.1038/226658a0. [DOI] [PubMed] [Google Scholar]
- Zieve P. D., Pierce N. F., Greenough W. B., 3rd Stimulation of glycogenolysis by purified cholera exotoxin in disrupted cells. Johns Hopkins Med J. 1971 Dec;129(6):299–303. [PubMed] [Google Scholar]


