Abstract
A recent study of two widely used angiotensin receptor blockers reported a reduced risk of cardiovascular events (−14.4%) when using candesartan compared with losartan in the primary treatment of hypertension. In addition to clinical benefits, costs associated with treatment strategies must be considered when allocating scarce health-care resources. The aim of this study was to assess resource use and costs of losartan and candesartan in hypertensive patients. Resource use (drugs, outpatient contacts, hospitalizations and laboratory tests) associated with losartan and candesartan treatment was estimated in 14 100 patients in a real-life clinical setting. We electronically extracted patient data from primary care records and mandatory Swedish national registers for death and hospitalization. Patients treated with losartan had more outpatient contacts (+15.6%), laboratory tests (+13.8%) and hospitalizations (+13.8%) compared with the candesartan group. During a maximum observation time of 9 years, the mean total costs per patient were 10 369 Swedish kronor (95% confidence interval: 3109–17 629) higher in the losartan group. In conclusion, prescribing candesartan for the primary treatment of hypertension results in lower long-term health-care costs compared with losartan.
Keywords: cardiovascular disease, cost analysis, economic evaluation, blood pressure
Introduction
The prevalence of hypertension is approximately 40% in Europe,1 and it is estimated that the risk of becoming hypertensive during a lifetime exceeds 90%.2 Hypertension is one of the most important risk factors in the development of cardiovascular disease and blood pressure lowering treatment is essential to improve patient health and limit the burden on the health-care system. Although it has been shown that the angiotensin receptor blockers losartan3, 4 and candesartan5, 6 are effective antihypertensives, they have only recently been compared in a real-life clinical situation for the primary treatment of hypertension.7 The study showed that candesartan lowered the risk of cardiovascular events compared with losartan (hazard ratio: 0.86, 95% confidence interval, CI: 0.77–0.96) during a median follow-up of 2.0 years.7 Although this study showed that prescribing candesartan is associated with fewer clinical events compared with losartan, health-care decision makers also need to consider the costs associated with different treatment strategies to determine value for money when prioritizing scarce health-care resources.8 When evaluating the economic consequences of different treatments in this context it is not only the cost of the administered drugs that is important, but also the costs associated with the development of clinical conditions requiring contacts with primary care and hospitalizations.9
In this study, data on resource use associated with losartan and candesartan in a real-life clinical setting were electronically extracted from primary care records and mandatory Swedish national registers for death and hospitalization. Using data on hospitalizations, use of study drugs and contacts with primary health care, the objective of this study was to estimate and compare resource use and costs of losartan vs candesartan in adult patients who were originally prescribed candesartan or losartan for hypertension from 1999 to 2007.
Methods
The design of the study, including the process of extracting data from primary care records and registers, has been described in detail elsewhere.7 In brief, patients with no cardiovascular disease except hypertension and/or type II diabetes were included in the study if losartan or candesartan were prescribed during the years 1999–2007. Patients were followed up until death, no further prescriptions of initial angiotensin receptor blocker, switch to another angiotensin receptor blocker or end of study period (31 December 2007).
Hospitalizations before index prescription
As reported earlier, there were small differences in baseline characteristics between the treatment groups, although the losartan group had a higher prevalence of diabetes (+2.8%), were less frequently treated with thiazides (−2.3%) and β-blockers (−2.0%), and more frequently treated with glucose lowering drugs (+1.7%), statins (+1.3%) and antithrombotics (+0.8%) compared with the candesartan group.7 Further analyses were undertaken in this study to ensure that the treatment groups were similar at baseline. All hospital admissions for non-cardiovascular causes before the index prescription were assessed with data from the National Discharge Registry, covering every hospital admission for any reason in Sweden from year 1984. It should be noted that hospitalizations for cardiovascular causes were excluded in this analysis by design.
Resource use
The use of study drugs, outpatient contacts and laboratory tests were extracted from the electronic primary care records.7 The total number of days and doses of candesartan or losartan prescription were recorded. Outpatient contacts were categorized into actual visits to the primary care centre or telephone calls. Furthermore, contacts were categorized into physician, nurse or other (for example a physiotherapist). A number of different sets of laboratory tests were recorded (listed in Table 1). Hospitalizations, procedures and diagnosis-related groups (DRG) codes were extracted from the National Discharge Registry by the National Board of Health and Welfare in Sweden.10, 11
Table 1. Unit costs applied in the analysis.
| Cost item | Unit cost (SEK) |
|---|---|
| Study drugs | Per day |
| Candesartan 4 mg | 5.9 |
| Candesartan 8 mg | 5.4 |
| Candesartan 16 mg | 7.1 |
| Candesartan 16 mg/12.5 mg | 7.1 |
| Losartan 50 mg | 6.0 |
| Losartan 100 mg | 9.9 |
| Losartan 50 mg/12.5 mg | 7.0 |
| Losartan 100 mg/25 mg | 10.9 |
| Outpatient contacts | Per contact |
| Physician visit | 1 715 |
| Physician phone contact | 572 |
| Nurse visit | 570 |
| Nurse phone contact | 190 |
| Other visit | 570 |
| Other phone contact | 190 |
| Laboratory tests | Per test |
| Total serum cholesterol | 11 |
| HDL and LDL cholesterol | 32 |
| Triglycerides | 11 |
| Blood glucose | 27 |
| HbA1C | 74 |
| Creatinine | 11 |
| Micro-albumine | 37 |
| Potassium | 11 |
| Hospitalisations | Per eventa |
| Heart failure | 33 919 |
| Cardiac arrhythmias | 29 146 |
| Peripheral artery diseaseb | 63 260 |
| Chronic ischaemic heart disease | 34 079 |
| Myocardial infarction | 47 748 |
| Strokec | 43 830 |
| Unstable angina | 42 001 |
| Elective coronary revascularization | 41 387 |
Abbreviations: DRG, diagnosis-related groups; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SEK, Swedish kronor.
Mean cost per ICD-coded cardiovascular event according to DRG.
Includes aortic aneurysms.
Ischaemic, haemorrhagic stroke and transient ischaemic attacks.
Unit costs
All unit costs applied in the study are presented in Table 1. The prices of study drugs were Merck Sharp & Dome (losartan) and AstraZeneca (candesartan) manufactured versions.12 The lowest price per pill for all pack sizes was used to determine the daily cost for each drug and dose. Unit costs applied for outpatient visits were based on the South East County Council region in Sweden.13 Costs for laboratory tests were based on prices from the Centre for Laboratory Medicine in Östergötland, Sweden.14 Unit costs for hospitalizations were determined from the DRG codes using the most recent Nord-DRG.15 Although these costs are from 2007, we did not inflate them to 2008/2009 prices. More than 6000 inpatient visits with 400 different DRG codes were identified in the study. Most common DRG codes were chest pain/angina pectoris (DRGs 140 and 143; 5% of total hospitalizations), hospitalization with PCI procedure (DRGs 112A–112D; 3% of all hospitalizations), arrhythmias (DRGs 138 and 139; 3% of all hospitalizations) and myocardial infarctions (DRG codes 121–123; 2.5% of all hospitalizations). Each hospitalization was, therefore, associated with an ICD code that was used to determine clinical outcome and a DRG code that was used for costing. The same primary ICD diagnosis could, therefore, result in different DRG codes (and thus different costs) because of secondary ICD diagnoses. This merely reflects the fact that the same primary ICD diagnosis may lead to hospitalizations with different length of stay and different procedures. In Table 1, the mean DRG costs associated with hospitalizations for cardiovascular causes according to primary ICD diagnoses are presented.
Statistical analysis
To analyse total costs, unit costs were multiplied by the observed resource use, which provided a total cost per patient. Mean costs for candesartan and losartan were then calculated and compared using data on all patients in the study. A large proportion of patients were subjected to administrative censoring at the end of follow-up (31 December 2007). To account for this, it has been proposed to weight the costs in different time intervals by the inverse of the probability of being observed in an interval.16, 17 In this study, the first interval was 0–6 months with annual intervals thereafter. The accumulated total health-care costs over time are presented together with the number of patients contributing with data in each interval. Given the relatively large sample size, t-tests and standard OLS regression were performed to test for statistical significance.18 Re-sampling bootstrap techniques were used to check the robustness of the parametric t-test, as the observed cost data was substantially skewed.17, 18 Costs were discounted by 3% and are based on a Swedish health-care setting and are presented in 2008/2009 Swedish kronor (SEK); 10 SEK is approximately 1 Euro or 1.40 US dollars in February 2010.
As the patent of branded losartan (Cozaar) will soon expire in many countries, we performed separate analyses to account for the expected decrease in costs of generic losartan compared with branded Cozaar. Although it is difficult to precisely predict the magnitude of this price reduction, previous experience from Sweden suggests that a 70–90% reduction is a likely scenario. Therefore, analyses of 70, 80 and 90% reductions of the branded Cozaar price were applied. More patients on losartan were diabetic at inclusion in the study. To assess whether this had any impact on the results, a separate analysis was performed excluding patients with diabetes at baseline.
All statistical analyses were performed using Stata version 8 (Stata Statistical Software: Release 8, Stata Corporation, College Station, TX, USA).
Results
Hospitalizations before index prescription
The number of patients hospitalized for any reason excluding cardiovascular disease before index prescription were similar in both groups; 3286 (48.5%) in the losartan group and 3560 (48.6%) in the candesartan group. Furthermore, no differences in the mean number of days in hospital before the index prescription were observed; 5.9 days per patient in both the losartan and the candesartan group.
Resource use
The observed resource use is presented in Table 2. Patients treated with losartan had on an average 3.5 (95% CI: 2.4–4.6) more outpatient contacts and 1.6 (95% CI: 1.12–2.17) more laboratory tests compared with candesartan patients. The losartan group had slightly more hospitalizations (mean difference: 0.06, 95% CI: 0.01–0.10) resulting in 0.40 (95% CI: 0.01–0.80) more mean number of days in hospital for the losartan group. There was no difference between the treatment groups in the mean number of days on study medication.
Table 2. Mean number of resource use by treatment group.
| Losartan (n=6771) | Candesartan (n=7329) | Difference (95% confidence interval) | |
|---|---|---|---|
| Study drugs | |||
| Days with prescription (s.e.) | 1220.9 (11.4) | 1221.3 (11.2) | −0.4 (−31.8–31.0) |
| Outpatient contacts | |||
| Physician visits (s.e.) | 10.9 (0.17) | 9.5 (0.14) | – |
| Physician phone contacts (s.e.) | 3.3 (0.06) | 3.1 (0.06) | – |
| Nurse visits (s.e.) | 7.8 (0.23) | 6.4 (0.2) | – |
| Nurse phone contacts (s.e.) | 3.0 (0.08) | 2.6 (0.07) | – |
| Other visits (s.e.) | 1.0 (0.06) | 0.8 (0.06) | – |
| Other phone contacts (s.e.) | 0.07 (0.01) | 0.09 (0.01) | – |
| Any visit (s.e.) | 26.0 (0.43) | 22.5 (0.38) | 3.5 (2.4–4.6) |
| Laboratory tests | |||
| Any laboratory tests (s.e.) | 13.3 (0.19) | 11.6 (0.18) | 1.6 (1.1–2.2) |
| Hospitalizations | |||
| Number of hospitalizations (s.e.) | 0.5 (0.02) | 0.4 (0.01) | 0.06 (0.01–0.10) |
Abbreviation: s.e., standard error.
Costs
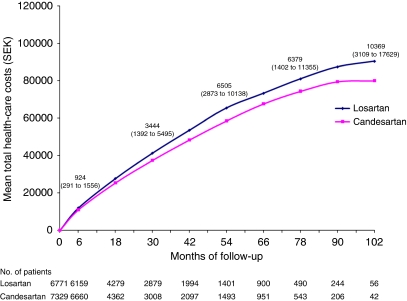
The estimated discounted mean health-care costs per patient by treatment group are presented in Table 3. The cost of study drugs was SEK 790 higher for losartan compared with candesartan. Costs for laboratory tests were small compared with total costs and did not differ between the two treatment groups. The increased number of outpatient contacts for the losartan group resulted in SEK 3463 higher costs compared with candesartan. Costs associated with hospitalization resulted in SEK 6101 higher costs with losartan compared with candesartan. Of the difference in costs for hospitalizations, SEK 2675 (95% CI: 861–4489) were due to hospitalizations with ICD diagnoses defined as cardiovascular, and SEK 3426 (95% CI: −1931–8786) were due to hospitalizations with ICD codes not primarily defined as cardiovascular. The mean total health-care costs with losartan were SEK 10 369 (95% CI: 3109–17 629) higher than that of candesartan over a follow-up of maximum 9 years. The bootstrap analysis showed similar results to the parametric analysis (mean difference SEK: 10 240, 95% CI: 3912–19 222). When controlling for diabetes, age and gender, the difference in total health-care costs was SEK 9280 (95% CI: 2024–16 535). The mean total costs per patient over time, by treatment group, are presented in Figure 1.
Table 3. Mean total discounted health-care costs by treatment group.
| Losartan (n=6771) | Candesartan (n=7329) | Difference (95% confidence interval) | P-value | |
|---|---|---|---|---|
| Study drugs | 13 211 | 12 422 | 790 (209–1370) | 0.008 |
| Outpatients contacts | 43 365 | 39 902 | 3 463 (874–6051) | 0.009 |
| Laboratory tests | 659 | 644 | 15 (−28–58) | 0.500 |
| Hospitalizations | 33 080 | 26 979 | 6 101 (255–11 950) | 0.041 |
| Total | 90 316 | 79 947 | 10 369 (3 109–17 629) | 0.005 |
Figure 1.
Mean total discounted health-care costs over time by treatment group. Numbers show the difference in mean total health-care costs (losartan compared with candesartan) with 95% CIs in brackets. SEK=Swedish kronor.
Sensitivity analyses
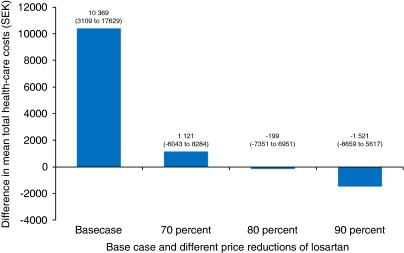
The results of running different scenario analyses assessing the effect of a price reduction of losartan are presented in Figure 2. With a price reduction of losartan of 70%, the mean total health-care cost of losartan is still numerically higher compared with candesartan, but this was not significant. A price reduction of 80 or 90% results in lower mean total health-care cost of losartan compared with candesartan, although these differences were also not statistically significant. Patients with diabetes had higher costs regardless of treatment group. Excluding patients with diabetes at baseline, the mean total costs with losartan was SEK 9332 (95% CI: 1136–17 529, P-value: 0.03) higher compared with candesartan, based on 11 773 patients (5556 with losartan and 6217 with candesartan). Corresponding mean difference for patients with diabetes at baseline was 11 855 (95% CI: −2854–26 564, P-value: 0.11), based on 2327 patients (1215 with losartan and 1112 with candesartan).
Figure 2.
Mean differences in total discounted health-care costs with various price reductions of generic losartan. Numbers show the difference in mean health-care costs (losartan compared with candesartan) with 95% confidence intervals in brackets. SEK=Swedish kronor.
Discussion
This study has shown that prescribing candesartan for the primary treatment of hypertension is associated with a reduced use of health-care resources in the long term compared with losartan. At current prices of losartan, this reduction in health-care resources translated into a reduction in total mean health-care costs of SEK 10 369 per patient prescribed candesartan compared with losartan over a follow-up of 9 years. Owing to patent expiry of branded losartan (Cozaar) in many countries in the near future, the impact of a reduced price of losartan for the results of the present study was investigated. Clearly, with a large reduction in the price of losartan, the total drug costs of candesartan will be higher compared with losartan. A price reduction of losartan of 80% or more of the current price will lead to numerically higher total health-care costs for candesartan compared with losartan. However, the difference in mean total health-care costs was not statistically significant with a 90% reduction in the price of losartan. These results should be interpreted in the light of the observed 14.4% reduction in cardiovascular events with candesartan compared with losartan.7 At current prices of losartan, prescribing candesartan will reduce total health-care cost and improve health outcomes, indicating that candesartan should be preferred to losartan from a clinical and a health-policy perspective. With a large reduction of the price of generic losartan, total health-care costs may be numerically higher with candesartan compared with losartan. In such a scenario, a more formal evaluation of cost-effectiveness may be warranted. Such an analysis should consider health-related quality of life and long-term survival prognosis of patients with and without cardiovascular events.
It should be noted that we did not include the costs of other drugs in this study. The use of most other drugs was similar between the treatment groups, with the exception of a more frequent use of thiazides in the losartan group.7 The inclusion of other drugs in our analyses could, therefore, have slightly strengthened the findings of this study.
The design of this study has strengths and weaknesses of which most have been discussed in a recent publication.7 One of the strengths with the methods used in this study is that resource use data was collected from a real-life clinical setting by extracting data from patient records and mandatory nationwide registers. This reduces the risk of protocol-driven costs and implies that it should be possible to extrapolate the results to clinical practice. Furthermore, it should be emphasized that this methodology enabled a comparison of health-care resource use and costs between candesartan and losartan using >36 000 patient years of follow-up. Such an analysis is highly unlikely to have been initiated prospectively. A possible limitation of this study is that selection bias can never be eliminated entirely in a retrospective study. However, the method enabled us to extract detailed clinical baseline data and patient history. In the recent publication reporting on cardiovascular end points on the same patient material, it was shown that the differences of cardiovascular risk were robust despite several adjustment methods for baseline differences.7 In the current analysis, we have additionally assessed any hospitalizations before the index prescription without disclosing any differences between the treatment groups. When considering hospitalizations before the index prescription as an expression of baseline disease burden (co-morbidity), this strongly suggests that the groups are comparable at baseline. The results on total health-care costs also seemed robust when controlling for the same covariates as in the clinical study. It should also be pointed out that the results are based on a Swedish patient material, and thus reflect a Swedish health-care system. Extrapolating the results to other health-care systems should always be accompanied by a careful assessment whether a Swedish health-care setting can plausibly be assumed representative.
Conclusions
The study shows that the use of candesartan for the primary treatment of hypertension results in lower mean total health-care costs compared with losartan. Although an expected reduction in the cost of losartan because of patent expiry will lead to lower drug costs for losartan compared with candesartan, the total costs for the health-care system are expected to be similar for the two treatment strategies as candesartan is associated with a reduction in costly clinical events.

Acknowledgments
We are very thankful for the ideas, encouragement and scientific support provided by Anders Ljunggren, AstraZeneca Mölndal. The study was funded by AstraZeneca AS Norway.
Professor David Russell, Professor Sverre E Kjeldsen and Jan Stålhammar did not receive any financial compensation for their work in this study and declare that they have no conflict of interest. Johan Bodegard, Paal Hasvold and Martin Henriksson are employed by AstraZeneca. Urban Olsson was responsible for database managing and is employed by an independent statistical company who was invoiced by AstraZeneca. Associate professor Lars-Åke Levin was responsible for the health economic analysis as a salaried consultant.
References
- Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289 (18:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370 (9587:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359 (9311:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Jonsson B, Carides GW, Burke TA, Dasbach EJ, Lindholm LH, Dahlof B. Cost effectiveness of losartan in patients with hypertension and LVH: an economic evaluation for Sweden of the LIFE trial. J Hypertens. 2005;23 (7:1425–1431. doi: 10.1097/01.hjh.0000173527.73179.f5. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362 (9386:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Andersson FL, Stewart S, Svensson K, Solal AC, Dietz R, et al. Resource utilization and costs in the candesartan in Heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2006;27 (12:1447–1458. doi: 10.1093/eurheartj/ehl016. [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Stålhammar J, Hasv P, Bodegard J, Olsson U, Russell D. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension—an observational study of 14 100 patients. J Hum Hypertens. 2009;24 (4:263–273. doi: 10.1038/jhh.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmgren J, Berggren F, Andersson F. Health economic guidelines—similarities, differences and some implications. Value Health. 2001;4 (3:225–250. doi: 10.1046/j.1524-4733.2001.43040.x. [DOI] [PubMed] [Google Scholar]
- Drummond M, Sculpher M, Torrance G, O'Brien B, Stoddart G.Methods for the Economic Evaluation of Health Care Programmes3rd (edn.)Oxford University Press: Oxford; 2005 [Google Scholar]
- Björkenstam C. The Causes of Death Register. The Centre for Epidemiology, The National Board of Health and Welfare: Stockholm; 2008. [Google Scholar]
- Edberg A, Forsberg L, Jacobsson A, Nykvist K. The National Patient Register. The Centre for Epidemiology, The National Board of Health and Welfare: Stockholm; 2008. [Google Scholar]
- The Dental and Pharmaceutical Benefits Agency (TLV)Price database (accessed on October 2009); Available at: http://www.tlv.se/in-english/price-database/ .
- Regionvårdsnämnden Prices for the South East County Council Region 2008 (accessed on October 2009); Available at: http://www.lio.se/upload/16047/20071218Prislista2008totversion2.pdf .
- Laboratory Medicine Östergötland, Sweden Prices of laboratory medicine services (accessed on October 2009); Available at: http://lio.se/upload/88036/Ny_PL%2008_2009_0615%20(2).pdf .
- National Board of Health and Welfare and SKL Cost of care 2007 for Nord DRG (accessed on October 2009); Available at: http://www.skl.se/artikeldokument.asp?C=473&A=58810&FileID=247583&NAME=Rappport%5Fkostnadsdata%5F2007.pdf .
- Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrica. 2000;87:329–343. [Google Scholar]
- Glick H, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford University Press: Oxford; 2007. [Google Scholar]
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed. BMJ. 2000;320:1197–1200. doi: 10.1136/bmj.320.7243.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]