Abstract
Aggressive behavior is common during adolescence. Although aggression-related functional changes in the ventromedial prefrontal cortex (vmPFC) and frontopolar cortex (FPC) have been reported in adults, the neural correlates of aggressive behavior in adolescents, particularly in the context of structural neurodevelopment, are obscure. We used functional and structural magnetic resonance imaging (MRI) to measure the blood oxygenation level-depended signal and cortical thickness. In a block-designed experiment, 14–17-year old adolescents imagined aggressive and non-aggressive interactions with a peer. We show reduced vmPFC activation associated with imagined aggressive behavior as well as enhanced aggression-related activation and cortical thinning in the FPC with increasing age. Changes in FPC activation were also associated with judgments of the severity of aggressive acts. Reduced vmPFC activation was associated with greater aggression indicating its normal function is to exert inhibitory control over aggressive impulses. Concurrent FPC activation likely reflects foresight of harmful consequences that result from aggressive acts. The correlation of age-dependent activation changes and cortical thinning demonstrates ongoing maturation of the FPC during adolescence towards a refinement of social and cognitive information processing that can potentially facilitate mature social behavior in aggressive contexts.
Keywords: ventromedial prefrontal cortex, frontopolar cortex, fMRI, cortical thickness, neurodevelopment, trait anger
INTRODUCTION
Aggressive behavior is thought to reflect a failure in the integrity of brain structures promoting emotion regulation (Davidson et al., 2000), moral decision making (Miczek et al., 2007) and utilization of knowledge about social conventions (Grafman et al., 1996). The prefrontal cortex (PFC) plays a key role in the regulation of aggressive behavior. Findings in adult patients with brain damage (Damasio et al., 1994, Grafman et al., 1996, Anderson et al., 1999, Blair and Cipolotti, 2000), aggressive populations (Siever et al., 1999, Soloff et al., 2000) and healthy adults (Pietrini et al., 2000) indicate that the ventromedial prefrontal cortex (vmPFC) is critically involved in the control of aggressive behavior. During adolescence, aggressive behavior and challenging adult-derived social rules is a common consequence of adolescents’ efforts to resist parental dependence (Kelley et al., 2004). Although developmental neuroimaging studies show that adolescence is a critical time for changes in the neural processes underling social behavior (Blakemore, 2008b), the neural correlates of aggressive behavior in adolescents are obscure.
The frontal cortex undergoes significant developmental changes at least until the late 20s (Giedd, 2008). Frontal grey matter reaches peak thickness in pre-adolescent boys at age 10.5 years and then begins to gradually decrease, currently thought to be related to a combination of synaptic elimination and increasing white matter volume from ongoing myelination (Giedd, 2004). It has been argued that the relatively late onset of synaptic elimination in the adolescent PFC and asynchronized neural development with other brain regions limits the efficiency of social information processing and may increase the vulnerability for adolescent-onset psychiatric problems such as antisocial and risky behavior (Blakemore, 2008b). Specifically, the frontopolar cortex (FPC) has been shown to undergo continued cortical thinning during adolescence (O’Donnell et al., 2005), reaching peak thickness at about age 10 years (Shaw et al., 2008), and activation changes in this region were found in studies investigating the neural underpinnings of retaliation (Lotze et al., 2007) and imagined physical aggression (Pietrini et al., 2000) in adults.
Here, we used functional and structural magnetic resonance imaging (MRI) to investigate brain activation changes during imagined physical aggression and cortical thickness in healthy male adolescents 14–17 years of age. In a block-designed functional magnetic resonance imaging (fMRI) experiment, participants imagined their own aggressive or non-aggressive (neutral and pleasant) behavior during interactions that were initiated by a fictitious male adolescent that they incidentally met in a parking garage. Trials consisted of screen-prompted instructions, a period of imagination of the scene with closed eyes that started and ended with a beep, followed by emotional ratings using a self-assessment manikin (SAM) (Lang et al., 1993). Cortical thickness values were derived from structural MRI images to investigate whether structural changes as adolescents aged were associated with activation differences between aggressive and non-aggressive conditions in the FPC. Prior to scanning, we assessed individuals’ trait aggression scores (aggression questionnaire, AQ) (Buss and Warren, 2000), judgments of severity of aggressive behavior outcomes and their ability to visualize scenes (vividness of visual imagination questionnaire, VVIQ) (Marks, 1972).
We hypothesized reduced activations in the vmPFC during imagined aggressive behavior compared with imagined non-aggressive acts based on findings in adults (Pietrini et al., 2000). We further predicted reduced cortical thickness in the FPC with increasing age (O’Donnell et al., 2005, Shaw et al., 2008). Finally, we predicted that these age-related differences in brain structure are likely reflected in age-dependent changes in activation levels in the FPC during imagined aggression.
METHODS
Participants
Twenty healthy male adolescents (mean ± s.d.; years of age: 15.7 ± 1.1, range 14–17; years of education: 9.8 ± 1.3, range 8–12) who had no history of psychiatric or neurological illness, including conduct disorder, antisocial traits and attention deficit hyperactivity disorder, participated in the fMRI experiment. All participants, except for one participant who was ambidextrous, were right-handed (Edinburgh inventory; laterality quotient: 87.5 ± 14.3, range 47.4–100.0) (Oldfield, 1971), native English speakers, had normal or corrected-to-normal vision, and normal intellectual abilities (Wechsler Abbreviated Scales of Intelligence, WASI; intelligence quotient: 113 ± 14, range 87–135) (Wechsler, 1999). After complete description of the study, parents gave written informed consent and adolescents gave their written assent for the procedures that were approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board at the National Institutes of Health. Participants received financial compensations for their participation.
Procedure
Participants underwent first a screening (pre-scanning phase), then the fMRI scan (scanning phase) and finally completed questionnaires (post-scanning phase). They came in twice for testing, and all parts of the study were completed within three to five weeks.
Pre-scanning phase
During visit 1, participants were screened for child psychiatric conditions and trauma symptoms using the Schedule for Affective Disorders and Schizophrenia for School Aged Children – Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1996) and the Trauma Symptom Checklist for Children (TSCC) (Briere, 1996). Participants were also tested for intelligence (WASI) (Wechsler, 1999) and handedness (Edinburgh Inventory) (Oldfield, 1971). The VVIQ (Marks, 1972) was used to assess participants’ ability to form mental pictures in a standardized manner. Furthermore, we administrated the AQ (Buss and Warren, 2000) to measure trait aggression and a severity rating of 13 scenes describing aggressive behavior (see Methods, Procedure, Questionnaires in Supplementary data). The psychiatric screening was performed by a board-certified child psychiatrist. In addition, participants underwent a neurological examination by a board-certified neurologist to rule out neurological disorders.
Scanning phase
In the beginning of visit 2, participants completed a paper and pencil version of the SAM (Hodes et al., 1985, Lang et al., 1993) to control for variation in baseline emotional status before performing the aggression task in the scanner. The SAM is a three-dimensional, non-verbal, visual measure of the emotional content of stimuli and events that requires participants to choose one of five manikins with visual expressions to indicate their degree of happiness (valence), excitement (arousal) and control (dominance). Faces of manikins in the valance dimension vary from smiling (happy) to frowning (unhappy). In the arousal dimension, manikins show different degrees of excitement through a sleepy face with eyes closed (calm) to a jumping manikin with his eyes wide open (excited). Finally, dominance is depicted by manikins that differ in size with the smallest manikin indicating the feeling of being controlled (submissiveness) and the largest manikin depicting being in control (dominance). In this phase of the experiment, participants marked their emotional state below the appropriate manikin once for each dimension. Correlational data with similar instruments such as the semantic differential scale and findings from numerous studies using different populations and designs indicate that the SAM’s three dimensions capture a primary organizing system of affective experience (Lang et al., 1993). Studies using the International Affective Picture System to assess emotional experience towards a wide range of picture stimuli revealed that the SAM has high internal consistency and re-test reliability (rs = 0.94–0.99) (Lang et al., 2001). Furthermore, it has been shown that adolescent boys judge picture stimuli from the International Affective Picture System similar to adults using the SAM confirming reliability of the SAM for our studies’ age group (McManis et al., 2001).
Then, participants were familiarized with the experimental task. They were introduced to a general scenario that they were asked to imagine during all scenes in the experiment. After viewing a picture of a parking garage, participants were asked to close their eyes and imagine that a tall, mean-looking teenager was walking toward them and stopping right in front of them in the otherwise empty garage. Participants were asked to imagine the scenario in great detail and as vividly as possible. They were told that they would imagine interactions between themselves and the other teenager in this general scene while in the scanner (see Methods, Procedure, Task instructions in supplementary data).
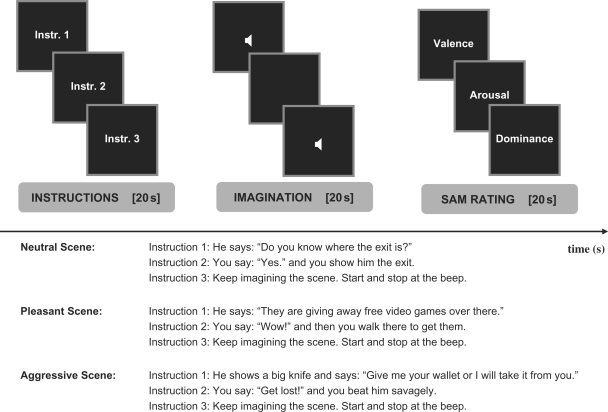
During scanning, we employed a blocked fMRI design that required participants to imagine three types of interactions with the tall, mean-looking teenager (two control and one experimental condition): neutral interactions (e.g. a request by the other teenager for directions to the exit), pleasant interactions (e.g. the other teenager informing our participant that video games are given away for free at a near location) and aggressive interactions (e.g. a physical fight during which the participant beats up the other teenager in response to a threat to take away his belongings) (see example instructions in Figure 1). Although blocked fMRI designs have been criticized for their neuropsychological drawbacks and numerous assumptions involved, they are known to elicit robust MRI signals (Amaro and Barker, 2006). We have chosen this design for our study to enable the investigation of brain responses to aggressive and non-aggressive interactions. Our main goal was to elicit imagery of complex social behavior that extends over a longer period of time and involves a course of actions rather then a brief, single act that might better be presented in an event-related design. Previous studies including PET and fMRI studies of motor imagery, social planning, emotional correlates of past events and imagery of obsessive compulsive associations to visual stimuli have used blocked designs to investigate brain responses over relatively longer periods of time (Partiot et al., 1995, Ghaem et al., 1997, Kimbrell et al., 1999, Damasio et al., 2000, Pietrini et al., 2000, Mataix-Cols et al., 2004, Solodkin et al., 2004, Stanton et al., 2007). Imagining of aggressive interactions had been successfully shown by some of us to reduce brain glucose metabolism in adults (Pietrini et al., 2000)—a finding that we attempted to replicate with the current study in an adolescent sample. Participants completed a practice version of the task prior to the scan. Neutral and pleasant control conditions were used to compare aggressive with non-aggressive interactions.
Fig. 1.
Experimental design. A trial consisted of three phases: instructions (20 s), imagining of the scene (20 s) and SAM ratings of emotions (20 s). During the instruction phase (insets 1−3), participants viewed three screens including a verbal description of the scene. During the imagination phase, a beep at the beginning and end indicated when participants had to start and stop imagining the scene. During the SAM rating phase, participants rated their emotional valence, arousal and dominance on a 5-point Likert scale. The experiment included one experimental and two control conditions. For each condition, an example of the word material used in an individual trial is shown.
At the beginning of each run, participants viewed the picture of the parking garage and then saw a statement reminding them of the presence of the tall, mean-looking teenager. At the beginning of each trial, instructions including a description of the present scene were shown for 20 s on three consecutive screens (Figure 1, Instr. 1–3) followed by a period of another 20 s where participants imagined the scene with their eyes closed (blank screen). Headphones were given to allow for auditory input and beeps indicated when participants had to start and stop imagining the scene and close and open their eyes, respectively. Finally, by pressing buttons on a response box, participants rated their emotional state along the dimensions valence, arousal and dominance using a 5-point rating scale version of the SAM. Ratings lasted for a total of 20 s and were cued by the corresponding manikins that were presented on three consecutive screens starting with the manikins that represent variations in valance, followed by the arousal, and dominance manikins. Over the experiment, four runs were employed and each run included two trials of each of the control conditions and four trials of the experimental condition, for a total of eight trials per run. Across the four runs, trials were repeated once so that participants imagined each scene twice throughout the experiment. All trials were presented randomly within runs and between participants (see Methods, Procedure, Scanning set-up in supplementary data).
Post-scanning phase
Immediately after scanning, the state anger sub-scale of the state trait anger expression inventory (STAXI) (Spielberger, 1999) and the state anxiety subscale of the State Trait Anxiety Inventory (14 years of age: STAI-C; 15–17 years of age: STAI-Y) (Spielberger, 1973, 1983) were given to assess participants’ emotional state. In addition, they rated their emotional state using the SAM rating again to ensure their emotional well-being after performing the aggression task in the scanner. Participants also rated the vividness of their imagination during the fMRI task using eight statements on a 5-point-rating scale ranging from 0–4 that were based on a questionnaire developed by Pietrini and colleagues (visualization scale) (Pietrini et al., 2000).
We performed follow-up emotional evaluations to ensure participants’ well-being 1 day and 2 weeks after participation in the experiment (see Methods, Procedure, Follow-up assessment in supplementary data).
Data acquisition
Imaging data were collected using a 3 Tesla General Electric (GE Medical Systems, Waukesha, WI) whole-body MRI scanner equipped with a standard head coil. Anatomical scans were performed using a T1-weighted 3D MP-RAGE (magnetization-prepared rapid acquisition gradient echo) sequence (TR 9 ms, TE 4 ms, flip angle 12°, field of view 256 mm, matrix size 256 × 256, 1.2 mm thick, in-plane resolution, 0.8594 × 0.8594 mm2). Functional images were acquired using a T2*-weighted 2D gradient EPI (echo-planar image) sequence, optimized for the blood oxygenation level-dependent (BOLD) response (TR 2 s, TE 23 ms, flip angle 90°, 30 slices, 3-mm thick, in-plane resolution 3.75 × 3.75 mm2, field of view 240 mm). In each run, 278 volume images were taken parallel to the anterior commissure-posterior commissure line. The first five volumes were discarded to allow for T1 equilibration effects.
Data analyses
FMRI data
FMRI and cortical thickness data analyses were performed using BrainVoyager QX (Brain Innovation, Maastricht, The Netherlands, http://www.BrainVoyager.com). Functional data were preprocessed as follows: slice scan time correction was performed using sinc interpolation; small serial head movements were corrected by spatially aligning all volumes to the first volume using rigid body transformation; following a linear trend removal, low-frequency non-linear drifts of three or fewer cycles for the time series were removed by temporal high-pass filtering. Spatial smoothing of the functional images with a Gaussian filter of 4-mm full-width at half-maximum was performed. Preprocessing of the anatomical data included reassembling into 1-mm resolution and normalizing into Talairach space using a piece-wise linear transformation. Functional data were co-registered with the individual’s 3D anatomical images and then reassembled into 3 × 3 × 3 mm3 isotropic voxels. Brodmann areas were determined by using the Talairach Daemon Client software (Research Imaging Center, San Antonio, TX, USA, http://ric.uthscsa.edu/).
A general linear model (GLM) corrected for first-order serial correlation was applied (Friston et al., 1999). Random-effects analyses were performed on the multi-participant level (n = 20) to explore brain regions that were associated with imagining aggressive vs non-aggressive scenes. The GLM model consisted of a set of eight regressors: instruction phase (n = 1), imagined scenes phase (n = 3, aggressive interactions, neutral interactions and pleasant interactions), rating phase (n = 1) and parametric effects of emotion ratings (n = 3, valence, arousal and dominance). Regressors for emotion ratings were orthogonalized and scaled between 0 and 1. Including these emotional ratings as predictors of no interest into our GLM allowed us to control brain activation changes for emotional differences between conditions. Note that these emotional ratings assessed with the SAM were separate from those SAM ratings acquired prior to engaging in the fMRI task and after completion of the experiment prior to dismissal from the study. The latter were merely used as a clinical assessment of the post-task emotional well-being of participants. Regressor time courses were adjusted for the hemodynamic response delay by convolution with a double-gamma hemodynamic response function (Buchel et al., 1998). Multiple regression analyses were performed independently for the time course of each individual voxel. After computing the coefficients (parameter estimates) for all regressors, t tests were performed between coefficients resulting from contrasting experimental vs control conditions. Statistical models at the multi-participant level were fit for the linear contrast of aggression vs non-aggression conditions.
The cluster-level statistical threshold approach (Forman et al., 1995) as implemented in BrainVoyager QX was used to correct for multiple comparisons by calculating the minimum cluster size to achieve a false activation probability α = 0.05. First, the voxel-level threshold was set at t = 3.22 (P < 0.005, uncorrected) for the aggression vs non-aggression contrast. This thresholded map was then submitted to a whole-brain correction criterion based on the estimate of the map’s spatial smoothness and on an iterative procedure (Monte Carlo simulation) for estimating cluster level false-positive rates. After 1000 iterations, the minimum cluster size of 123 mm3 was applied to the statistical map. Clusters that exceeded this threshold were used to create functional ROIs. At the second level, bivariate correlations (Pearson’s correlation) were computed between participants’ trait aggression scores and participants’ activation based upon contrasts of parameter estimates derived from the peak voxels for aggressive vs non-aggressive conditions.
Cortical thickness
Cortical thickness maps for each participant were calculated based on the Laplace method (Jones et al., 2000) (see Data analyses, Cortical thickness in supplementary data). Whole-brain correlations were performed across cortical thickness maps between age and cortical thickness. A priori ROIs to explore developmental differences across age (based on previous neuroimaging studies on aggression) included the vmPFC (Pietrini et al., 2000) and FPC (Pietrini et al., 2000, Lotze et al., 2007). Brain regions were identified that showed a significant correlation between participant’s age and aggression-related activation based upon contrasts of parameter estimates derived from the aggressive vs non-aggressive conditions. After the functional correlation map was overlaid onto the group cortical thickness map, participants’ cortical thickness values were extracted for each region from the group cortical thickness map at the co-location between the peak correlation of the functional and the cortical thickness map. Pearson’s correlations were computed among participants’ activation (parameter estimates), age, cortical thickness and behavioral aggression. In addition, cortical thickness was correlated with participants’ IQ to address recent structural neuroimaging findings indicating that cortical thickness in the PFC is positively correlated with general intelligence in adolescents (Shaw et al., 2006) (see Data analyses, Behavioral data in supplementary data).
RESULTS
Behavioral results
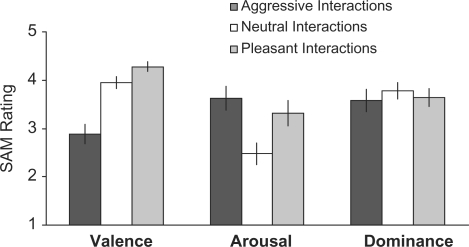
In our behavioral analysis, we found that the adolescent group had normal trait aggression scores (mean ± s.d., 43.4 ± 4.3) on the AQ and an average ability to visualize scenes (2.2 ± 0.8) on the VVIQ (note that participants, on average, rated their performance during imagining the scenes in our experiment using the visualization scale as clear and vivid above average). Emotional SAM ratings that were completed following each imagined scene varied across experimental conditions for valence (unhappy–happy scale) (F2,38 = 45.47, P < 0.001) and arousal (calm–excited scale) (F2,38 = 14.22, P < 0.001), but not for dominance (controlled–control scale) (F2,38 = 0.38, P = 0.615, Figure 2). To control for these emotional differences, we included the ratings as predictors of no interest in the GLM analysis of our fMRI data.
Fig. 2.
SAM Ratings. Ratings for valence (1 = unhappy, 5 = happy), arousal (1 = calm, 5 = excited) and dominance (1 = submissive, 5 = dominant) for the neutral, pleasant and aggressive conditions are shown. The valence ratings differed significantly across conditions (F2,38 = 45.47, P < 0.001). The valence ratings in the pleasant and neutral conditions were significantly higher (more happiness) than those in the aggression condition (Ps < 0.01), and pleasant and neutral conditions differed significantly from one another with higher levels of happiness in the pleasant condition (P < 0.01). The arousal ratings differed across conditions (F2,38 = 14.22, P < 0.001). They were significantly lower (less excitement) in the neutral condition compared with the aggression and pleasant conditions (Ps < 0.01). The aggression and pleasant conditions did not differ significantly from one another (P = 0.650). The dominance ratings (participants felt in control) did not differ significantly across the three conditions (F2,38 = 0.38, P = 0.615).
Participants’ age correlated with physical aggression (r = 0.50, P < 0.025) and hostility (r = 0.45, P < 0.044) assessed with the AQ, indicating that aggression increased with age in our study group. Participants’ age did not correlate with severity ratings of aggressive acts (r = −0.10, P = 0.667).
FMRI results
In our fMRI analysis, we were interested in adolescents’ brain activation changes associated with aggressive behavior. Using a GLM approach, we found reduced activation in the left vmPFC (Brodmann area, BA10; Talairach coordinates, x, y, z; −3, 44, −8; peak activation, t = −4.67, P < 0.05, corrected) during the aggressive compared with the non-aggressive conditions (Figure 3a). In addition, we found increased activation in the left primary visual cortex (BA17; −21, −91, −8; t = 4.00, P < 0.05, corrected) in the same contrast. Furthermore, participants’ AQ trait anger values correlated negatively with individuals’ activation in the left vmPFC (BA10; −3, 47, −8; r = −0.54, P < 0.016) based upon the contrast of parameter estimates (peak voxels) derived from the aggressive vs non-aggressive conditions, indicating that the higher the trait anger the lower the activation during imagining aggressive behavior relative to the activation during imagining non-aggressive behavior (Figure 3b).
Fig. 3.
vmPFC activation during imagined aggression and its relationship with trait anger. (a) Comparing aggressive vs non-aggressive conditions revealed reduced activation in the left vmPFC (BA10; peak activation, −3, 44, −8; t = −4.67, P < 0.05, corrected). (b) Participants’ trait anger correlated negatively with activation in the left vmPFC (BA10; −3, 47, −8; r = −0.54, P < 0.016) based upon the contrast of parameter estimates derived from the aggressive vs non-aggressive conditions, indicating that the higher the trait anger the lower the activation in this region. Statistical images were overlaid onto Brain Voyager’s single participant canonical T1 image in Talairach space.
Cortical thickness
In our cortical thickness analysis, we were interested in the association between structural changes in grey matter with increasing age and with brain activation changes during aggression. We first identified those regions of aggression-related activation that correlated with adolescents’ age. We found positive correlations between age and brain activation in the left FPC (BA10, −9, 56, 13; peak correlation, r = 0.47, P < 0.038) (Figure 4a), left rostral anterior cingulate cortex (rACC, BA24; −9, 26, 1; r = 0.66, P < 0.002), and right lateral orbitofrontal cortex (lOFC, BA10; 27, 50, 10; r = 0.56, P < 0.013) based upon the contrast of parameter estimates derived from the aggressive vs non-aggressive conditions, indicating that activation in these regions while imagining aggression ‘increased’ with age.
Fig. 4.
Co-localization of age-correlated activation and cortical thickness in the FPC. (a), Participants’ age correlated positively with activation in the left FPC (BA10; −9, 56, 13; r = 0.47, P < 0.038) based upon the contrast of parameter estimates derived from the aggressive vs non-aggressive conditions, indicating that the older the adolescents the higher the activation in this region. (b) Participants’ age correlated negatively with cortical thickness in the left FPC (r = −0.54, P < 0.014), indicating that the older the participants the thinner the cortex in this region.
Next, we tested whether cortical thickness in these regions correlated with age in our sample. We found a negative correlation only between FPC thickness and age (r = −0.54, P < 0.042, Bonferroni corrected) (Figure 4b), but not with lOFC and rACC (lOFC: r = −0.29; rACC: r = 0.01, Ps > 0.05), indicating that cortical thickness in the left FPC decreased with age. Finally, activation in the FPC correlated negatively with cortical thickness in the same region (r = −0.48, P < 0.034), indicating that activation increased while cortical thickness decreased. In addition, we were interested in what cognitive process may underlie the activation changes in the FPC during imagined aggressive behavior. We found a negative correlation between FPC activation and adolescents’ ratings of the severity of physical harm inflicted by hypothetical aggressive acts based upon the contrast of parameter estimates derived from the aggressive vs non-aggressive conditions, indicating that as FPC activation increased, judged consequences of aggressive acts diminished (r = −0.46, P < 0.041). Cortical thickness in the FPC did not correlate with participants’ IQ (r = −0.25, P = 0.287).
DISCUSSION
In the present study, we investigated brain activation changes during imagined physical aggression and cortical thickness in normally developing male adolescents 14–17 years of age. First, we demonstrated that imagined aggressive behavior is associated with reduced activation in the vmPFC and increased activation in the visual cortex, the latter indicating that participants may have been more engaged in imagining aggressive compared with non-aggressive behavior (Ganis et al., 2004). Our finding of reduced vmPFC activation during imagined aggression is consistent with data from a previous PET study that found reduced vmPFC activation during imagined aggression in adults (Pietrini et al., 2000).
Previous studies using anger induction designs indicate that the ventral PFC is more ‘activated’ when healthy adults imagine autobiographical scenes of experienced anger, compared with lower activation during neutral control scenes, or during viewing angry compared with sad facial expressions (Blair et al., 1999, Dougherty et al., 1999, Kimbrell et al., 1999). Furthermore, it has been shown that ‘activation’ in the left vmPFC is associated with social norm violations, modulation of aggressive behavior tendencies, and the prevention of inappropriate, aggressive behavior (Berthoz et al., 2002). These findings are in contrast with studies evoking imagined aggressive actions (rather than thought) but are consistent with the idea that the orbitofrontal cortex (OFC) and the vmPFC are involved in the inhibition of acting aggressively on anger impulses (Blair, 2004).
But what social-cognitive mechanisms can explain the effect of aggressive situational cues on ‘reduced’ vmPFC activation? It has been suggested that the vmPFC is involved in the reenactment of social event knowledge on the basis of simulation mechanisms (Damasio, 1989, Barsalou, 1999, Moll et al., 2005, Krueger et al., 2007, 2009). Since the vmPFC has reciprocal connections with brain regions that are associated with emotional processing (amygdala), memory (hippocampus), reward processing (basal ganglia including the striatum and the nucleus accumbens) and higher-order sensory processing (temporal visual association areas) (Ongur et al., 2003), it is well suited to capture the affective and reward value accompanying experiences in social situations. For example, when aggressive situations are experienced in real life (e.g. observed at school) or through media, the vmPFC captures diverse exemplars of those situations, establishing a summary representation that encompasses a multi-modal representation distributed throughout the brain’s association and modality-specific areas. Those dynamic summary representations provide the underlying cognitive structure for the development of traits that organize and guide one’s potential behavior in future social interactions (Krueger et al., 2009). Typically, those representations in the vmPFC modulate aggressive behavior by exerting inhibitory control over aggressive impulses, a process that can be inferred based on findings from numerous studies in patients with lesions in this region (Damasio et al., 1994, Grafman et al., 1996, Anderson et al., 1999, Blair and Cipolotti, 2000), anger induction studies (Blair et al., 1999, Dougherty et al., 1999, Kimbrell et al., 1999) and investigations on representations of social norms (Berthoz et al., 2002, King et al., 2006). However, in the case of imagined aggressive behavior in our study, this inhibitory control is loosened, presumably via bottom-up signaling, so one can engage in aggressive behavior. This is associated with a dampening of vmPFC activation, disengaging the control function of the PFC in situations of aggressive engagement.
While our findings of reduced vmPFC activation during aggression may be indicative of a diminished control function in the vmPFC associated with aggressive behavior, we did not detect activation increases in the amygdala or changes in other regions of the emotional brain corresponding to aggressive behavior. The amygdala has been shown to be inhibited by PFC structures such as the OFC and vmPFC in studies of patients with intermittent explosive disorder and major depressive disorder with anger attacks (Dougherty et al., 2004, Coccaro et al., 2007). It may be, however, that the interaction between the vmPFC and the amygdala is not uniformly inhibitory suggesting that diminished processing in the vmPFC would not necessarily lead to changes in amygdala activity (Koenigs et al., 2008).
Our results from the correlational analysis of vmPFC activation and anger indicate that the higher adolescents’ score on self-reported trait anger, the lower the vmPFC activation during imagined aggressive behavior relative to imagined non-aggressive behavior. This indicates that higher levels of trait anger that our adolescent subjects experienced in real life led to greater dampening of the vmPFC, although some previous research has described increased vmPFC activation during anger experience (Blair et al., 1999, Dougherty et al., 1999, Kimbrell et al., 1999). This inconsistency might be explained by differential effects of trait vs state anger on the neural mechanisms of aggression, indicating that trait anger as assessed in our study can impose a risk for reduced control over aggressive impulses exerted by the PFC.
Numerous other findings from structural and functional imaging studies in healthy controls and patients with elevated levels of aggression provide evidence for the aggression- and anger-related engagement of medial as well as lateral ventral PFC structures. Lieberman (2007) proposed that neural activity associated with social behavior can be distinguished as externally vs internally focused core processes engaging medial vs lateral PFC structures, respectively. In our study, we found reduced vmPFC activation while participants mentally engaged in aggressive behavior and emotions connecting the imagined interactions with autobiographical scripts. Other studies exposing participants to displays of aggressive acts in the media uniformly found lateral OFC activation (Mathiak and Weber, 2006, Kelly et al., 2007) supporting the idea that activation in this structure reflects an external, physical focus enabled through the actual visual experience of aggressive behavior.
Second, in our developmental analysis, we found increased activation and cortical thinning in the FPC as adolescents aged as well as an increase in activation with decreased cortical thickness in this region. In addition, participants’ age correlated with physical aggression and hostile thoughts experienced in life. Finally, our results revealed that the FPC is involved in adolescents’ judgments of the severity of potential physical harm to a person who was the victim of the aggressive act.
In agreement with our results, activation in the FPC was found to be increased during retaliation in adults (Lotze et al., 2007) indicating that FPC activity is modulated by aggressive behavior. Furthermore, the FPC is the most complex cytoarchitectonic structure within Broadman area 10 with the most prominent and well developed cortical layer III (Ongur et al., 2003) making it suitable for higher order cognitive functions such as multi-tasking and reasoning (Christoff et al., 2001, Gilbert et al., 2006) and moral judgments that require sophisticated social-cognitive thought processes (Moll et al., 2001). We suggest that the FPC, in guiding social behavior, is recruited for predicting the consequences of harmful aggressive behavior in situations that present aggressive cues.
Our findings also demonstrate that developmental processes are associated with FPC function. The FPC was increasingly activated during imagined aggressive behavior as adolescents aged and physical aggression and hostility also increased with age. Predicting outcomes of aggressive behavior may become more relevant during adolescence because adolescents become increasingly experienced with aggressive behavior through real life and media exposure, which may have caused the changes in FPC activation across age. Furthermore, reduced cortical thickness in this region with increasing age in the current study confirmed results from previous structural neuroimaging studies demonstrating FPC thinning in typically developing adolescents (O’Donnell et al., 2005, Shaw et al., 2008). With this co-localization between functional and structural conditions in the FPC, we show a correlation between aggression-related activation changes that vary across participants’ age and changes in grey matter structure in the same group of adolescents. These structural changes indicate synaptic reorganization in adolescents’ FPC and are thought to eventually promote more specialized functional processes in mature regions (Blakemore, 2008b).
However, excess synapses during ongoing pruning can temporarily decrease the efficiency of neural processes in the PFC and affect behavioral performance on tasks associated with PFC function (Blakemore and Choudhury, 2006). For example, studies investigating executive function (Anderson et al., 2001) and face recognition (Carey et al., 1980) during adolescence have shown that behavioral performance changes in cognitive and social tasks do not always follow a linear trend towards improvement, but may also reflect stagnation and decline. Similarly, functional imaging studies have demonstrated that increased or decreased brain activation patterns can be associated with changes in age-related social task performance, that is also dependent upon other factors such as emotional content and attention demands of the task, strategies used and gender (Blakemore, 2008a). We propose that the age-dependent increase in FPC activation that was associated with lower severity ratings of consequences of aggressive behavior may result from a combination of perturbations of neural processes and experience-driven changes in social judgment during this developmental period. Alternatively, it could be expected that older adolescents, by virtue of a more mature FPC and more experience with aggressive behavior, imagined the aggressive scenes to a more or a less severe extent. Although, our analysis did not indicate a relationship between participants’ age and ratings of severity, a positive association between age and aggressive behavior and hostile thought was found indicating that more experience with aggression may have caused the negative correlation between FPC activation and the severity ratings for the consequences of aggressive behavior.
Our study combines the investigation of adolescent behavior in the context of brain development and function to address developmental effects of aggression. Although this approach allowed us to identify associations between aggressive behavior and structural and functional cortical development, the normative changes in cortical thickness represent a potential caveat for brain activation patterns associated with aggression. Our results are therefore preliminary and remain to be confirmed in future analyses of adolescent brain development and aggressive behavior. Furthermore, previous research has shown that changes in cortical thickness across childhood and adolescence are associated with intellectual ability, indicating that adolescents’ PFC thickness is positively correlated with IQ (Shaw et al., 2006). We addressed this potential confound and found no association between cortical thickness and IQ in our study group. Finally, our findings of reduced vmPFC activation and concurrent developmental effects in the FPC may be gender-specific given that our study group included only males. Findings from a recent structural imaging study, that compared female and male adolescents’ grey matter density correlations with observed aggressive parent–adolescent interactions, imply that the OFC might be involved in gender specific regulations of aggressive behavior (Whittle et al., 2008). Our findings in male adolescents may reflect gender-specific vmPFC responses to aggressive behavior and the effect of female adolescents’ aggression on PFC activation patterns including vmPFC and FPC remains to be explored in future studies.
In conclusion, we demonstrated the importance of the vmPFC and—in the context of development—FPC in modulating physical aggression in 14–17-year old healthy male adolescents. Although both regions fall within the boundaries of BA10, the cytoarchitectonic complexity within area 10 increases along the medial axis of the PFC with the most prominent and well-developed cortical layer III located in the FPC (Ongur et al., 2003). This difference in architechtonic complexity may be associated with two distinct neural processes related to physical aggression in adolescents. First, reduced vmPFC activation is associated with diminished access to social knowledge and the extent to which adolescent males experience anger in everyday life. Second, changes in FPC activation are associated with the anticipation of the consequences of adolescents’ current aggressive behavior based on their previous experience with physical altercations. Activation in the FPC corresponded to age-related differences in cortical thickness in the FPC demonstrating the conjoint development of higher-order social functions and structural refinement in healthy adolescents without a history of abnormal aggressive behavior. This co-localization of age-dependent activation changes and cortical thinning indicates an ongoing maturation of the FPC during adolescence towards a refinement of social information processing that can potentially facilitate mature social behavior in situations that present aggressive cues.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was funded by the intramural research program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke. We thank Dr Eric Wassermann, Dr Dimitrios Kapogiannis and Dr Edward Huey for performing the neurological examinations in our participants.
REFERENCES
- Amaro E, Jr, Barker GJ. Study design in fMRI: basic principles. Brain and Cognition. 2006;60:220–32. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Anderson AV, Anderson P, Northam E, Jacobs R, Catroppa C. Development of executive functions through late childhood and adolescence in an Australian sample. Developemental Neuropsychology. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Natural Neuroscience. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behavioural Brain Science. 1999;22:577–609. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123(Pt 6):1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett D., I., Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(Pt 5):883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Development of the social brain during adolescence. Quarterly Journal of Experimental Psychology. 2008a;61:40–9. doi: 10.1080/17470210701508715. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Natural Review of Neuroscience. 2008b;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Briere J. Trauma Symptom Checklist for Children: Professional Manual. Lutz, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–8. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Buss AH, Warren WL. The Aggression Questionnaire. Los Angeles, CA: Western Psychological Services; 2000. [Google Scholar]
- Carey S, Diamond R, Woods B. The develoment of face recognition - a maturational component. Developmental Psychology. 1980;16:257–69. [Google Scholar]
- Christoff K, Prabhakaran, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–49. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62(2):168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Computation. 1989;1:123–32. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Natural Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Rauch SL, Deckersbach T, Marci C, Loh R, Shin LM, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Archives of General Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, et al. Anger in healthy men: a PET study using script-driven imagery. Biological Psychiatry. 1999;46:466–72. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Research Cognitive Brain Research. 2004;20:226–41. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, et al. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 1997;8:739–44. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. Journal of Adolescence Health. 2008;42:335–43. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognition and Neuroscience. 2006;18:932–48. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–8. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Hodes RL, Cook EW, 3rd, Lang PJ. Individual differences in autonomic response: conditioned association or conditioned fear? Psychophysiology. 1985;22:545–60. doi: 10.1111/j.1469-8986.1985.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Jones SE, Buchbinder BR, Aharon Three-dimensional mapping of cortical thickness using Laplace's equation. Human Brain Mapping. 2000;11:12–32. doi: 10.1002/1097-0193(200009)11:1<12::AID-HBM20>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Diagnostic Interview. Kiddie-Sads-Present and Lifetime Version. (1st) 1996;2006 Vol. 2006. [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Annals of the New York Academy of Sciences. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Kelly CR, Grinband J, Hirsch J. Repeated exposure to media violence is associated with diminished response in an inhibitory frontolimbic network. PLoS ONE. 2007;2:e1268. doi: 10.1371/journal.pone.0001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell TA, George MS, Parekh P., I., Ketter TA, Podell DM, Danielson AL, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biological Psychiatry. 1999;46:454–65. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- King JA, Blair RJ, Mitchell DG, Dolan RJ, Burgess N. Doing the right thing: a common neural circuit for appropriate violent or compassionate behavior. Neuroimage. 2006;30:1069–76. doi: 10.1016/j.neuroimage.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont, Cheon B, Solomon J, Wassermann EM, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Natural Neuroscience. 2008;11:232–7. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends in Cognigitive Science. 2009;13:103–9. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Krueger F, Moll J, Zahn R, Heinecke A, Grafman J. Event frequency modulates the processing of daily life activities in human medial prefrontal cortex. Cerebral Cortex. 2007;17:2346–53. doi: 10.1093/cercor/bhl143. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): instruction manual and affective ratings) Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2001. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lotze M, Veit R, Anders S, Birbaumer N. Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: an interactive fMRI study. Neuroimage. 2007;34:470–8. doi: 10.1016/j.neuroimage.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Marks DF. Individual differences in the vividness of visual imagery and their effects. In: Sheehan PW, editor. The function and nature of imagery. New York: Academic Press; 1972. pp. 83–108. [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Weber R. Toward brain correlates of natural behavior: fMRI during violent video games. Human Brain Mapping. 2006;27:948–56. doi: 10.1002/hbm.20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38:222–31. [PubMed] [Google Scholar]
- Miczek KA, de Almeida RM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. Journal of Neuroscience. 2007;27:11803–6. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Eslinger PJ, Oliveira-Souza R. Frontopolar and anterior temporal cortex activation in a moral judgment task: preliminary functional MRI results in normal subjects. Arquivos de Neuro-Psiquiatria. 2001;59:657–64. doi: 10.1590/s0004-282x2001000500001. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Natural Review of Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24:948–54. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of computational nurology. 2003;460:425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Partiot A, Grafman J, Sadato N, Wachs J, Hallett M. Brain activation during the generation of non-emotional and emotional plans. Neuroreport. 1995;6:1397–400. doi: 10.1097/00001756-199507100-00009. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. American Journal of Psychiatry. 2000;157:1772–81. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, et al. d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–23. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cerebral Cortex. 2004;14:1246–55. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biological Psychiatry. 2000;47:540–7. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory for Children: Professional Manual. 1973. Mind Garden, Redwood City, CA. [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory for Adults: Manual. 1983. Mind Garden, Redwood City, CA. [Google Scholar]
- Spielberger CD. State Trait Anger Expression Inventory. Lutz, FL: Psychological Assessment Resources, Inc; 1999. [Google Scholar]
- Stanton BR, Williams C, V., Leigh PN, Williams SC, Blain CR, Giampietro PV, Simmons A. Cortical activation during motor imagery is reduced in Amyotrophic Lateral Sclerosis. Brain Research. 2007;1172:145–51. doi: 10.1016/j.brainres.2007.07.044. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Whittle S, Yap MB, Yucel M, Fornito A, Simmons JG, Barrett A, et al. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent-adolescent interactions. Proceedings of the National Academy of Science. USA. 2008;105:3652–7. doi: 10.1073/pnas.0709815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.