Abstract
Anticipation is a central component of anxiety and the anterior insula appears to be an important neural substrate in which this process is mediated. The anterior insula is also thought to underlie the interoceptive representation of one’s affective state. However, the degree to which individual differences in anticipation-related insula reactivity are associated with variability in the subjective experience of anxious anticipation is untested. To assess this possibility, functional magnetic resonance images were acquired while participants completed an auditory anticipation task with trial-by-trial self-report ratings of anxious anticipation. We hypothesized that the anterior insula would be positively associated with an individual’s subjective experience of anticipatory anxiety. The results provide evidence for an amygdalo-insular system involved in anxious auditory anticipation. Reactivity in the right anterior insula was predictive of individuals’ subjective experience of anxious anticipation for both aversive and neutral stimuli, whereas the amygdala was predictive of anticipatory anxiety for aversive stimuli. In addition, anxious anticipatory activation in the left insula and left amygdala covaried with participants’ level of trait anxiety, particularly when the anticipated event was proximal.
Keywords: amygdala, insula, anxiety, anticipation, auditory
INTRODUCTION
The anticipation of undesirable events is an essential characteristic of anxiety. Although moderate levels of anxious anticipation may be adaptive and allow individuals to prepare for negative events, excessive anticipation can lead to debilitating disruptions in daily life (Nitschke et al., 2006). Indeed, dysfunctional levels of anticipation appear to manifest in a number of anxiety disorders including specific phobia, generalized anxiety disorder, social anxiety disorder and panic disorder. Knowledge about the neural substrates of anxious anticipation in healthy individuals may facilitate our understanding of this process in clinical populations. Neuroimaging research has consistently implicated the insula as an important substrate of anxious anticipation (Phelps et al., 2001; Simmons et al., 2004; Dalton et al., 2005; Nitschke et al., 2006; Onoda et al., 2008; Waugh et al., 2008), especially in highly anxious samples (Lorberbaum et al., 2004; Simmons et al., 2006; Straube et al. 2007). Although differences in anxiety are associated with anticipatory insula activity (Lorberbaum et al., 2004; Simmons et al., 2006; Straube et al., 2007), it is unclear how the temporal proximity of the anticipated event influences this association. Additionally, it is unknown whether this anticipatory insula reactivity is associated with variability in the subjective experience of anxious anticipation.
In addition to the insula, neuroimaging research in healthy populations has revealed that aversive anticipation involves the amygdala (Phelps et al., 2001; Nitschke et al., 2006; Onoda et al., 2008) and areas (including medial, dorsolateral, orbitofrontal, ventrolateral and anterior cingulate cortex) of the prefrontal cortex (Simpson et al., 2001; Simmons et al., 2004, 2006; Nitschke et al., 2006; Straube et al., 2007; Onoda et al., 2008). It is unclear to what extent the neural system(s) involved in the anticipation of aversive events across sensory domains recruit common and/or unique neural structures. It would be expected that structures such as the amygdala, which are involved in detecting and appraising potential threats across sensory modalities (LeDoux, 1996; Zald, 2003) would be common in most forms of threat detection and aversive anticipation. However, the structures representing the actual preparatory response may depend upon the specific anticipated event. In particular, the insula is generally involved in the processing of disgust (Phillips et al., 1997; Wright et al., 2004) and interoceptive body states including pain (Craig, 2003), but the insula is also thought to underlie the conscious perception of affective feelings (Critchley et al., 2004). While the insula has previously been implicated in the negative anticipation of painful (Phelps et al., 2001) and visual (Simmons et al., 2004; Dalton et al., 2005; Nitschke et al., 2006; Onoda et al., 2008; Waugh et al., 2008) stimuli, many negative visual stimuli (e.g. mutilations and insects) have an inherent disgust quality and it is therefore unclear whether this insula activity reflects a (i) specific preparatory pain or disgust response or (ii) general feeling state of anxious anticipation. Given that loud bursts (100 dB) of white noise are aversive, but are neither painful nor disgusting, they may serve as a relatively clean stimulus-probe to assess the role of the insula (and other neural structures) in anticipatory anxiety.
Here, participants completed a functional magnetic resonance imaging (fMRI) task with a cue plus countdown paradigm, which signaled a future aversive or neutral auditory event. Participants rated their level of anxious anticipation during each trial. The primary aim of this study was to assess whether individuals’ feelings of anxious anticipation were associated with their anticipatory insula response. As secondary aims we examined whether the insula and amygdala were involved in anticipation of aversive auditory stimuli and the extent to which anticipatory reactivity in these structures is predicted by trait anxiety. We hypothesized that self-reports of anxious anticipation would be positively associated with individuals’ insula reactivity during anxious anticipation. In addition, based on previous research discussed above, we hypothesized that both the insula and the amygdala would be activated during aversive auditory anticipation and that activity in these areas would be positively associated with individual differences in trait anxiety.
METHODS
Participants
Thirty-five (19 male and 16 female) healthy adults between the ages of 18 and 48 (M = 23.91, s.d. = 6.64) participated in the study. Thirty-one reported being right-handed and four reported being left-handed. Potential participants were screened for prescription and recreational drug usage, neurological and psychological histories, and for presence of metal implants. Advertisements were used for recruitment and monetary compensation was provided to participants. This study was approved by the Institutional Review Board of Stony Brook University; all participants provided informed consent. Twenty-three participants completed the auditory anticipation task with participant feedback described below and 12 completed an alternate version1 that did not contain participant feedback.
Assessment of trait anxiety
Prior to scanning, participants completed the trait scale of the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1970). Trait anxiety scores in the current sample were between 20 and 53 (M = 33.69, s.d. = 7.34), which is similar to the published normal range for working adults (Spielberger et al., 1970). Male (M = 33.79) and female (M = 33.56) participants had equivalent levels of trait anxiety (P > 0.1). Participant age was not associated with anxiety (P > 0.1).
Stimuli and equipment
The auditory anticipation task included both auditory events and visual cues. All visual stimuli were presented using a mirror attached to the head coil, which reflected onto a screen positioned behind participants while they lay in the scanner. Visual stimuli were presented on the screen using an MRI-compatible 60 Hz projector with a 1024 × 768 resolution. Auditory stimuli were presented through SereneSound (Resonance Technology Inc., Northridge, CA, USA) 30 dB external noise attenuating MRI-compatible headphones. Auditory and visual stimuli were presented and the task was programmed with E-Prime 1.2 (Psychology Software Tools, Pittsburg, PA, USA). Initiation of the experiment was triggered by the first radiofrequency pulse of the echo planar imaging (EPI) sequence.
Experimental procedure
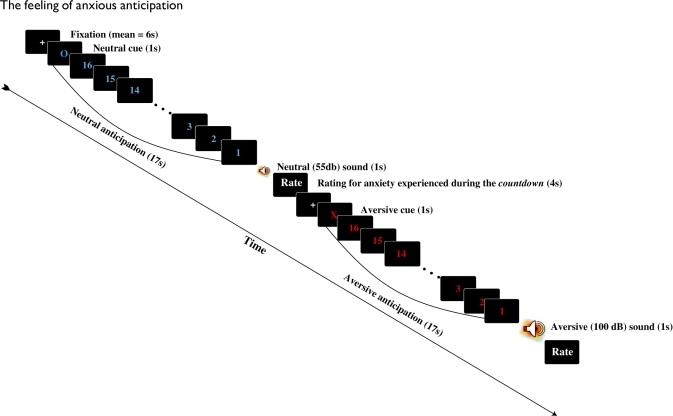
The auditory anticipation task consisted of 20 trials/blocks of anticipation: 10 aversive and 10 neutral. As displayed in Figure 1, each trial began with a white fixation cue presented in the center of a black screen (jittered 4000–8000 ms). The fixation cue was immediately followed by a red ‘X’ or a blue ‘O’ for 1000 ms. Participants were informed that the red ‘X’ indicated that they would hear a loud (100 dB) burst of white noise (aversive event), while the blue ‘O’ indicated that they would hear a soft (55 dB) presentation of the same white noise (neutral event). Preceding the presentation of aversive and neutral events was a 16 s period (block) of anticipation. During this block of anticipation, a countdown from 16 to 1 (16 s; red text for aversive and blue for neutral) was numerically presented in the center of the screen. Aversive and neutral auditory events immediately followed this period of anticipation and were 1000 ms in duration. After the presentation of the aversive or neutral event, a screen appeared which asked participants to rate their level of anxiety during the countdown on a 4-point scale (from 1 = ‘not anxious’ to 4 = ‘very anxious’).
Fig. 1.
Each trial of the anticipation task began with a fixation cue. Fixation was followed by a red ‘X’ or a blue ‘O,’ which respectively, indicated that a loud or soft burst of white noise would be presented after a 16 s countdown. The 17 s period of cue + countdown was considered a block of either anxious (X cue) or neutral (O cue) anticipation. After the auditory event participants reported the level of anxiety they experienced during the countdown.
Image acquisition
A 3 Tesla Siemens Trio whole body scanner equipped with an eight-channel SENSE head coil was used to acquire T2*-weighted scans with an EPI sequence sensitive to BOLD signal using the following parameters: TR = 2500 ms, TE = 22 ms, flip angle = 83°, matrix dimensions = 96 × 96, FOV = 224 × 224 mm, slices = 36, slice thickness = 3.5 mm, gap = 0. We additionally acquired anatomical T1-weighted structural scans. A total of 232 volumes were collected during a single functional run.
Image analysis
Standard preprocessing procedures were performed in SPM5, including image realignment corrections for head movements, slice timing corrections for order of slice acquisition, normalization to standard 2 × 2 × 2 mm Montreal Neurological Institute space, and spatial smoothing with a Gaussian full-width-at-half-maximum 6 mm filter. Using the general linear model in SPM5, first-level single subject statistical parameter maps were created from a model, which specified both blocks of anticipation (aversive and neutral; cue + countdown: 17 s) and auditory events (aversive and neutral; 0 s). Bilateral amygdala and insula masks were created using the Masks for Regions of Interest Analysis software (Walter et al., 2003). Region of interest (ROI) analyses were preformed using a false discovery rate search volume corrected α = 0.05 with an extent threshold = 10 continuous voxels.
RESULTS
Analyses of participants’ self-reported anticipatory anxiety
These analyses only included the 23 individuals who completed the version of the task with self-reported feedback of anxious anticipation.
Behavioral data
A paired samples t-test of participants self-reported anxiety ratings during the countdown revealed that overall participants felt more anxious in anticipation of the aversive (M = 2.50) relative to the neutral (M = 1.17) sound, t(22) = 8.35, P < 0.001. This result serves as a manipulation check verifying that on average participants felt more anxious in anticipation of loud relative to soft sounds.
Neural correlations with self-report anxious anticipation states
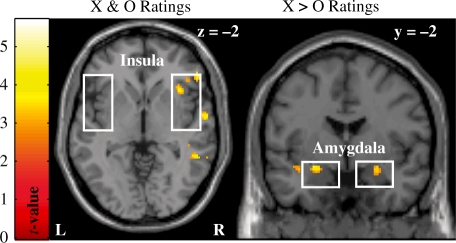
Participants’ trial-by-trial self-reported anxiety ratings was included as regressors in the first-level SPMs. Contrast files for these regressors were then used at the second-level to assess whether anticipatory reactivity in our ROIs was associated with individuals’ subjective experience of anxious anticipation. As displayed in Figure 2, the right anterior insula was positively associated with participants’ self-reported anxiety during both aversive and neutral trials. On the other hand, the amygdala bilaterally covaried to a greater extent with participants’ self-report anxiety during aversive compared to neutral anticipation (see Table 1). Thus, the right anterior insula was associated the subjective experience of anxious anticipation during both high- and low-anxiety inducing trials, whereas the amygdala was selectively associated with feelings of anxiety during highly aversive trials.
Fig. 2.
Displayed on the left is activity in the right anterior insula that was predictive of intertrial differences in participants’ self-reported level of anxious anticipation during both aversive and neutral anticipation trials. The amygdala (right panel) was bilaterally positively associated with participants’ ratings of anxious anticipation during aversive compared to neutral trials. Activation displayed at Puncorrected < 0.005 for illustrative purposes.
Table 1.
Aversive auditory anticiption-related activity
| MNI Coordinates |
Maximally activated voxel |
||||||
|---|---|---|---|---|---|---|---|
| Analysis and region | Hemisphere | x | y | z | Voxels | t-value | P-value |
| Aversive > Neutral anticipation (ROI) | |||||||
| Amygdala | R | 18 | 2 | −18 | 19 | 4.30 | <0.05SVC |
| Insula | L | −44 | 14 | −4 | 62 | 5.57 | <0.05SVC |
| R | 40 | 18 | −8 | 98 | 5.13 | <0.05SVC | |
| Aversive > neutral anticipation (whole brain) | |||||||
| Brainstem/inferior colliculus | L | −10 | −32 | −12 | 24 | 5.69 | <0.05FDR |
| R | 14 | −28 | −12 | 51 | 5.24 | ||
| Insula | L | −44 | 20 | −6 | 163 | 5.65 | <0.05FDR |
| R | 40 | 18 | −8 | 42 | 5.13 | <0.05FDR | |
| Amygdala | R | 16 | −4 | −20 | 23 | 5.13 | <0.05FDR |
| Superior frontal gyrus | L–R | 4 | 8 | 56 | 51 | 5.31 | <0.05FDR |
| Dorsolateral prefrontal cortex | L | −30 | 46 | 28 | 20 | 4.52 | <0.05FDR |
| Cerebellum | L | −22 | −66 | −18 | 50 | 4.92 | <0.05FDR |
| Trait anxiety (ROI) | |||||||
| Amygdala | L | −30 | −2 | −26 | 31 | 4.27 | <0.05SVC |
| Insula | L | −42 | −16 | 0 | 26 | 3.70 | <0.005uncor |
| X and O ratings (ROI) | |||||||
| Insula | R | 38 | 12 | −16 | 66 | 4.28 | <0.05SVC |
| X > O ratings (ROI) | |||||||
| Amygdala | L | −24 | −2 | −14 | 23 | 4.18 | <0.05SVC |
| R | 26 | 0 | −14 | 28 | 4.21 | <0.05SVC | |
Analyses of aversive auditory anticipation
These analyses include all 35 individuals who completed either version of the auditory anticipation task.
Aversive auditory anticipation-related activity
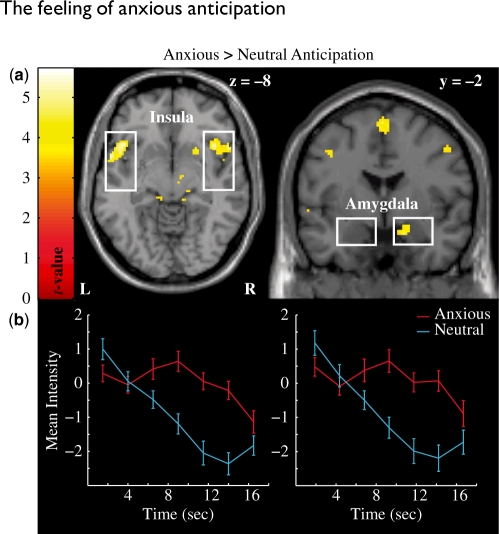
An aversive vs neutral anticipation second-level t-test contrast was created to assess the neural correlates of aversive auditory anticipation. These ROI analyses revealed that the right amygdala and bilateral insula were more active during periods of aversive compared to neutral anticipation (Figure 3a). Time-series data were extracted for each condition from the active voxels within right amygdala and insula. The data were linearly interpolated, detrended and then averaged across voxels and trials for each participant. As can be seen in Figure 3b, the amygdala and insula responded in a similar fashion where activation was increased in aversive relative to neutral trials ∼6 s and sustained throughout the period of anxious anticipation. An additional whole brain analysis of aversive vs neutral anticipation with a false discovery rate corrected α = 0.05 and an extent threshold = 20 continuous voxels was used for further exploration. In addition to the insula and amygdala, a number of other structures including the left dorsolateral prefrontal cortex (DLPFC), bilateral brainstem/inferior colliculus, medial superior frontal gyrus, and left cerebellum all displayed increased activation during aversive compared to neutral auditory anticipation (see Table 1 for details).
Fig. 3.
(a) The bilateral insula (upper left panel) and right amygdala (upper right panel) were activated across participants during the anxious anticipation of a loud sound. Activation displayed at Puncorrected < 0.001 for illustrative purposes. (b) The time-series data indicate an elevated response within both the right insula (lower left panel) and amygdala (lower right panel) during anxious relative to neutral anticipation that begins ∼6 s post cue and sustains throughout the countdown.
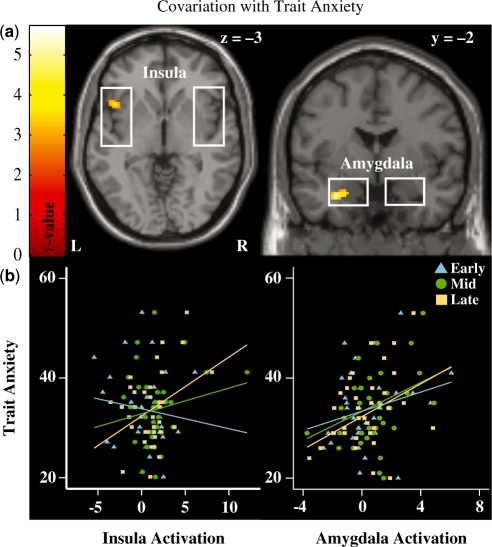
Correlations with trait anxiety
Trait anxiety was included as a covariate in the ROI analyses to assess the extent to which this variable predicted activity in the amygdala and insula during the anticipation of aversive sounds. As shown in Figure 4a, trait anxiety was positively associated with anxious anticipatory activation in the left amygdala. At a reduced threshold of Puncorrected < 0.005, the left insula was positively associated with trait anxiety. Extracted activity from these clusters was linearly interpolated, detrended and then averaged across voxels and trials for each participant. The first 2 s of these interpolated time-series were discarded, while the remaining 15 s was split into early (3–7 s), middle (8–12 s) and late (13–17 s) phases of anticipation. Trait anxiety was positively correlated with left amygdala activity during the middle (r = 0.39, P < 0.05) and late (r = 0.35, P < 0.05) phases of anticipation. The left insula positively correlated with trait anxiety during the late (r = 0.36, P < 0.05), but not middle (r = 0.17, P > 0.1), phase of anticipation (see Figure 4b). Neither structure was associated with trait anxiety for the early phase of anticipation (amygdala: r = 0.24, P > 0.1, insula: r = −0.13, P > 0.1).
Fig. 4.
(a) Anxious anticipation-related activity within the left anterior insula (upper left panel) and amygdala (upper right panel) were sensitive to individual differences in trait anxiety. Individuals with higher levels of anxiety also had higher levels of activation in the left anterior insula and amygdala during aversive relative to neutral anticipation trials. Activation displayed at Puncorrected < 0.005 for illustrative purposes. (b) Trait anxiety was significantly correlated with insula (lower left panel) reactivity during the late (r = 0.36, P < 0.05), but not early (r = −0.13, P > 0.1) or middle (r = 0.18, P > 0.1), phases of aversive anticipation. Amygdala (lower right panel) activity correlated with trait anxiety during the middle (r = 0.39, P < 0.05) and late (r = 0.35, P < 0.05), but not early (r = 0.24, P > 0.1) phases of anticipation.
DISCUSSION
Participants’ feelings of anxiety were positively associated with activation in the right anterior insula during anticipation of both aversive and neutral sounds. Conversely, activation in the amygdala positively covaried with anxious feelings, but only during anxious anticipation (Figure 2). In addition, the right amygdala and bilateral insula were generally activated during periods of anxious auditory anticipation (Figure 3). Anxious anticipation-related activation of the left amygdala and insula were positively associated with individual differences in trait anxiety (Figure 4). These findings suggest that across individuals there is an amygdalo-insular network for the anticipation of negative events and that activity within this network is relatively elevated in highly anxious individuals during aversive auditory anticipation. Furthermore, activation in the amygdala and insula is predictive of an individual’s feeling state of anxious anticipation.
Role of the amygdala and insula in feeling anticipatory anxiety
The right anterior insula was sensitive to variability in individuals’ anxious feelings during anticipation which is consistent with its proposed role in conscious awareness of one’s own feeling states (Craig, 2003; Critchley et al., 2004). Unlike the insula, amygdala activity was only associated with variability in individual’s feeling states during anxious (relative to neutral) anticipation. That is, the degree to which an individual’s amygdala response was predictive of their self-reported level of anxious anticipation was restricted to the aversive/loud sound trials. Given that the amygdala is known to elicit and modulate physiological responses to threat (Davis and Whalen, 2001; LeDoux, 1996) and that physiological responses contribute to one’s feeling state (Craig, 2003), the amygdala may play an indirect role in modulating individuals’ feeling states in highly arousing circumstances. Indeed, models of amygdala functionality (Davis and Whalen, 2001; LeDoux, 1996) do not view this structure as the neural substrate underlying the subjective experience of emotion (or even specifically fear). On the other hand, the anterior insula was predictive of participants’ feeling states in both low and high arousal conditions, which suggests that activation in the insula is more generally predictive of one’s internal feelings. Taken together the findings in this study and previous research (Craig, 2003; Critchley et al., 2004) suggest a general role of the insula in the processing and representation of one’s internal feeling state, while the amygdala may play a more specific role in contributing to anxious worry states by modulating physiological responding under conditions of high arousal.
Neural correlates of aversive auditory anticipation
To the best of our knowledge, the current findings provide the first evidence that the right amygdala and bilateral insula are activated during periods of anxious auditory anticipation. The finding of anticipatory insula reactivity to an aversive auditory stimulus, which was neither disgusting nor painful, suggests that the role of the insula in aversive anticipation is not that of a specific preparatory response to disgust or pain. Rather, our findings suggest that the role of the insula in aversive anticipation appears to be more consistent with its previously implicated role in mediating interoceptive representations (Craig, 2003) and conscious affective feeling states (Critchley et al., 2004). On the other hand, the amygdala is known to be sensitive to a variety of threatening stimuli including social (Morris et al., 1996) and fear conditioned (Knight et al., 2005) cues. The amygdala response to potential threat appears to rely upon limited sensory information (Morris et al., 1998; Whalen et al., 1998; Pasley et al., 2004; Williams et al., 2004) and results in a rapid modulation of physiology (Morris et al., 1998) and attention-related behavior including modulations of sensory cortex (Carlson et al., 2009). Given the characteristics of the amygdala and insula as well as the similar time course of the anxious anticipation-related reactivity in these structures (see Figure 3b), we speculate that these two affective processes may occur in parallel. That is, during periods of anxious anticipation the amygdala may detect threat cues and initiate the fear response including modulations in physiology and attention. At the same time (at least at the temporal resolution of fMRI), visceral changes associated with this response are represented within the insula and contribute to an anxious feeling state of aversive anticipation, which may in turn modulate processing in the amygdala.
Surprisingly, we did not find activation in the anterior cingulate cortex (ACC), a region which has previously been shown to be activated during aversive anticipation (Nitschke et al., 2006; Straube et al., 2007; Onoda et al., 2008). This may be due to the methodology we employed to assess anxious auditory anticipation. In particular, the countdown in our study may have reduced or eliminated the temporal uncertainty associated with the anticipated undesirable event. Indeed, another fMRI study of aversive anticipation utilizing the countdown methodology also did not report activations in the anterior cingulate (Phelps et al., 2001). Previous research indicates that the ACC is involved in conflict monitoring and the processing of expectations (Botvinick, 2007; Sallet et al., 2007). In anticipation paradigms the ACC may play a similar role where the ACC ‘monitors’ ones expectations about the temporal proximity of the anticipated aversive event. However, future research that directly manipulates temporal certainty is needed to test this speculative role of the ACC.
In addition to the amygdala and insula, the left DLPFC, bilateral brainstem/inferior colliculus, medial superior frontal gyrus and left cerebellum were all activated during anxious auditory anticipation. The extent to which each component of this anticipatory system is involved in different anticipatory situations is unknown. Although it is unclear whether the BOLD signal reflects local inhibitory or excitatory neuronal activity, it may be expected that in anticipation of a loud sound a general dampening or gating of auditory processing would be adaptive. Consistent with this notion, we found that activity in the inferior colliculus was modulated by aversive auditory anticipation, which may represent subcortical gating of auditory processing. The DLPFC activation is consistent with previous findings of anticipatory PFC activations (Simpson et al., 2001; Simmons et al., 2004, 2006; Nitschke et al., 2006; Straube et al., 2007; Onoda et al., 2008). Activation in this region is also implicated in cognitive reappraisal (Ochsner et al., 2002) and may indicate an unsolicited attempt of participants to regulate their anticipatory response. Thus, similar to other sensory modalities, aversive auditory anticipation recruits a distributed network of brain structures including the amygdala, insula, and DLPFC among others, but also recruits auditory specific structures such as the inferior colliculus.
Association with trait anxiety
Anxious anticipation-related activation of the left amygdala and insula were positively associated with individual differences in trait anxiety. Although anxiety-amygdala associations have been observed in paradigms designed to elicit automatic responses (Rauch et al., 2000; Etkin et al., 2004; Armony et al., 2005; Bryant et al., 2008), these associations have also been reported in anticipation paradigms (Lorberbaum et al., 2004). Previous research has also indicated that the insula is elevated in high anxiety individuals during aversive anticipation (Lorberbaum et al., 2004; Simmons et al., 2006; Straube et al., 2007). Thus, the observed anxiety associations in the amygdala and insula during aversive anticipation are consistent with previous findings and indicate an elevated anticipatory response in anxiety. Here, we add to this literature with our finding of an elevated amygdala response in highly trait anxious individuals that starts in the middle and is maintained into the late phase of aversive anticipation. In contrast, insula reactivity was only associated with trait anxiety during the late phase of anticipation. These results indicate that in highly anxious individuals there is a relatively early sustained amygdala sensitivity to anticipated threat that is accompanied by a later elevated insula response as the anticipated aversive event becomes more proximal.
Given that the STAI assesses several facets of anxiety and that scores on this scale correlate with measures of depression, future studies should clarify whether insula and amygdala reactivity are associated with a general disposition for negative affect or more specific symptoms of anxiety such as worry or somatic arousal. In addition to using scales that focus on distinct features of anxiety (e.g. the Penn State Worry Questionnaire; Meyer et al., 1990) future efforts would benefit from inclusion of autonomic measures.
CONCLUSION
Consistent with previous findings in other sensory modalities (Phelps et al., 2001; Simmons et al., 2004; Dalton et al., 2005; Nitschke et al., 2006; Onoda et al., 2008; Waugh et al., 2008), we found that the amygdala and insula were activated in anticipation of an aversive auditory stimulus. Activity in the amygdala and insula were found to positively covary with trait anxiety during anxious anticipation. More specifically, the amygdala was associated with trait anxiety during the middle and late phases of aversive anticipation whereas the insula was associated with trait anxiety only during the late phase of aversive anticipation. Activity in the right anterior insula was predictive of individuals’ subjective experience of anxious anticipation throughout the task, while the amygdala was selectively associated with feelings of anxiety during aversive trials. These findings provide evidence for an amygdalo-insular system involved in the anticipation of aversive auditory events. Activation in this system appears to be sensitive to individual differences in trait anxiety and is reflective of an individual’s internal feeling state of anxious anticipation.
Acknowledgments
This research was supported by the Office of Naval Research #N0014-04-1-005 (LRMP) and the National Institutes of Health #5M01-RR-10710 (General Clinical Research Center).
Footnotes
1 Twelve participants completed an alternate version of the auditory anticipation task containing 212 volumes acquired on a separate scanner (Philips 3T) using the same acquisition parameters described in the text. This version of the task did not include the participant feedback screen. In place of the feedback screen was an additional period of fixation (set at 2000 ms) that followed the auditory event and preceded the beginning of the next trial.
REFERENCES
- Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. American Journal of Psychiatry. 2005;162(10):1961–3. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective, and Behavioural Neuroscience. 2007;7(4):356–66. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Human Brain Mapping. 2008;29(5):517–23. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Reinke KS, Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47(5):1386–9. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion on Neurobiology. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005;17(6):969–80. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6):1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26(4):1193–200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain the Mysterious Mnderpinnings of Emotional Life. London: Weidenfeld and Nicholson; 1996. [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15(18):2701–5. [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–95. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–70. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29(1):106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Toki S, Ueda K, Shishida K, Kinoshita A, et al. Anterior cingulate cortex modulates preparatory activation during certain anticipation of negative picture. Neuropsychologia. 2008;46(1):102–10. doi: 10.1016/j.neuropsychologia.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Mayes LC, Schultz RT. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42(1):163–72. doi: 10.1016/s0896-6273(04)00155-2. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–41. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Sallet J, Quilodran R, Rothe M, Vezoli J, Joseph JP, Procyk E. Expectations, gains, and losses in the anterior cingulate cortex. Cognitive, Affective, and Behavioural Neuroscience. 2007;7(4):327–36. doi: 10.3758/cabn.7.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15(14):2261–5. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60(4):402–9. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of National Academy of Sciences USA. 2001;98(2):688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionaire. Palo Alto, CA: Consulting Psychology Press; 1970. [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37(4):1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, et al. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses. Paper presented at the Ninth International Conference on Functional Mapping of the Human Brain: NY, New York; 2003. [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Social Cognitive and Affective Neuroscience. 2008;3(4):322–32. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. Journal of Neuroscience. 2004;24(12):2898–904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15(15):2347–51. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]