Abstract
The Beauty-is-Good stereotype refers to the assumption that attractive people possess sociably desirable personalities and higher moral standards. The existence of this bias suggests that the neural mechanisms for judging facial attractiveness and moral goodness overlap. To investigate this idea, we scanned participants with functional magnetic resonance imaging while they made attractiveness judgments about faces and goodness judgments about hypothetical actions. Activity in the medial orbitofrontal cortex increased as a function of both attractiveness and goodness ratings, whereas activity in the insular cortex decreased with both attractiveness and goodness ratings. Within each of these regions, the activations elicited by attractiveness and goodness judgments were strongly correlated with each other, supporting the idea of similar contributions of each region to both judgments. Moreover, activations in orbitofrontal and insular cortices were negatively correlated with each other, suggesting an opposing relationship between these regions during attractiveness and goodness judgments. These findings have implications for understanding the neural mechanisms of the Beauty-is-Good stereotype.
Keywords: fMRI, face, moral, orbitotofrontal cortex, insula
INTRODUCTION
‘Physical beauty is the sign of an interior beauty, a spiritual and moral beauty’ [Schiller, 1882, cited by Dion et al. (1972)]. This quote illustrates the Beauty-is-Good stereotype, which is pervasive in human societies, and has been the focus of social psychological research for over three decades (Dion et al., 1972). Compared to unattractive people, attractive individuals are assumed to have better personalities and be morally good (Dion et al., 1972; Eagly et al., 1991; Langlois et al., 2000). For example, one study found that facial attractiveness was positively linked to socially desirable personality traits, such as kindness, honesty, friendliness, trustworthiness, etc. (Dion et al., 1972). The Beauty-is-Good stereotype has been demonstrated in a variety of everyday domains, such as undergraduates' teaching evaluations of instructors (Hamermesh and Parker, 2005) and voters' preferences for political candidates (Efran and Patterson, 1974). Attractive people are more likely to get hired (Marlowe et al., 1996) and earn on average 12% more than unattractive people (Hamermesh and Biddle, 1994). Unlike the case of race, gender, ethnicity, disability and age, there is no legislation against attractiveness-related discrimination. However, the most somber social impact of the Beauty-is-Good stereotype is within the justice system, as studies of mock trials have shown that defendants who are less attractive are more likely to be found guilty (Efran, 1974; Piehl, 1977; Kulka and Kessler, 1978; Burke et al., 1990) and receive longer sentences(Friend and Vinson, 1974; Seligman et al., 1977; Weiten, 1980; Burke et al., 1990; Castellow et al., 1990; Wuensch et al., 1993).
Although the Beauty-is-Good stereotype has been the focus of many studies in social psychology, very little is known regarding the neural mechanisms involved. By definition, the Beauty-is-Good stereotype reflects the influence of aesthetic evaluation on moral evaluation. One possibility is that brain regions involved in the aesthetic processing influence brain regions involved in the moral judgments. A second possibility is that interaction between aesthetic and moral processing occurs because a set of brain regions mediates both types of judgments. The present functional magnetic resonance imaging (fMRI) study investigated the second alternative by identifying overlaps between activations elicited by aesthetic judgments and by moral judgments. Although several regions have been associated with ‘one’ of these domains, such as the amygdala with facial attractiveness (Kranz and Ishai, 2006), and medial prefrontal cortex with moral evaluation (Greene et al., 2001, 2004; Moll et al., 2002, 2007; Heekeren et al., 2005; Schaich Borg et al., 2008), we focused on two brain regions that have been associated with ‘both’ aesthetic and moral domains: the medial orbitofrontal cortex and the insular cortex.
The medial orbitofrontal cortex (OFC) has been associated with processing ‘positive stimuli’. Within the aesthetic domain, functional neuroimaging studies have shown that the medial OFC shows greater activity when people view attractive faces rather than unattractive faces (O'Doherty et al., 2003b; Kranz and Ishai, 2006; Bray and O'Doherty, 2007; Ishai, 2007), as well as beautiful pictures rather than ugly pictures (Kawabata and Zeki, 2004). Within the moral domain, medial OFC activations have been reported during the processing of morally positive stimuli (Moll et al., 2006; Zahn et al., 2008). Beyond functional neuroimaging, patients with OFC lesions show poor practical judgments (Damasio et al., 1994) and impaired moral behavior (Anderson et al., 1999), and people with smaller OFC gray matter volume display higher psychopathy scores (de Oliveira-Souza et al., 2008). Although the medial OFC has been independently linked to aesthetic and moral judgments, it is unclear if exactly the same OFC regions mediate both types of judgments and show similar activation patterns within participants.
In contrast with the medial OFC, the insular cortex has been associated with processing ‘negative stimuli’. Within the aesthetic domain, there is evidence that insular activity is greater for viewing unattractive than attractive faces (O'Doherty et al., 2003b; Krendl et al., 2006).Within the moral domain, insular activations have been reported during the processing of morally negative stimuli (Krendl et al., 2006; Hsu et al., 2008; Zahn et al., 2008). Moreover, the insular cortex has been linked to the feeling of being hurt emotionally during a social interaction, or ‘social pain’ (Eisenberger et al., 2003; Sanfey et al., 2003), and socially negative signals from faces (Phillips et al., 1997; Winston et al., 2002). As in the case of the medial OFC, although insular activations have been found in both aesthetic and moral judgments, it is uncertain whether or not the regions are involved in and the activation patterns are same for both types of judgments.
To investigate whether the same medial OFC and insular regions show similar activation patterns in response to aesthetic and moral judgments, we scanned participants while rating the attractiveness of faces and the goodness of hypothetical actions, and then used these ratings as parametric regressors to indentify brain regions where activity increased or decreased as a function of both types of ratings. On the basis of separate studies in aesthetic and moral domains, we predicted that (i) medial OFC activity would ‘increase’ as a function of both attractiveness and goodness ratings, whereas (ii) insular activity would ‘decrease’ as a function of both types of ratings. Finally, assuming an opposing relationship between these two regions, we predicted (iii) a negative correlation between OFC and insula activities during both attractiveness and goodness ratings.
MATERIALS AND METHODS
Subjects
Twenty-two right-handed, college-aged female Caucasian participants were recruited from the Duke University community and paid for their participation. All subjects were English native speakers. The data from two subjects were excluded from analyses because of equipment malfunction. Thus, our analyses included data from 20 subjects with an average age of 23.4 years (s.d. = 3.1). All participants gave informed consent to a protocol approved by the Duke University Institutional Review Board.
Stimuli
The fMRI study included three tasks: (i) face attractiveness rating task, (ii) action goodness rating task and (iii) brightness rating task. For the ‘face attractiveness rating task’, we selected photos of 270 different Caucasian male faces from several face databases, including the NimStim Face Stimulus Set (Tottenham et al., 2009), the AR Face Database (Martinez and Benavente, 1998), the CVL Face Database (http://www.lrv.fri.uni-lj.si/facedb.html), the PICS database (pics.psych.stir.ac.uk/), FERET Database (Phillips et al., 1998, 2000) and the Frontal Face Dataset (http://www.vision.caltech.edu/archive.html). To have enough faces in the highly attractive range, we also included photos from male fashion models found in online catalogs. Given that most of our participants are Caucasian, we decided to limit the study to Caucasian participants and Caucasian faces to avoid potential differences in perceiving faces across races [‘other race effect’ (Rhodes et al., 2005)]. The reason for using only male faces and female participants is that our pilot studies showed that attractiveness ratings were most consistent across participants when female participants rated male faces. All stimuli were converted into grayscale images with dimensions of 256 × 256 pixels on a white background. For the ‘action goodness rating task’, we created 270 short sentences describing hypothetical past actions performed by men that varied from very negative (e.g. ‘He raped a little girl.’) to very positive (e.g. ‘He saved his sister from drowning.’). These sentences were 3–11 words in length and used only familiar words. A pilot study with female participants confirmed that attractiveness ratings for faces (very unattractive to very attractive) and goodness ratings for actions (very bad to very good) were widely distributed across an 8-point scale. For the ‘brightness rating task’ as a control for stimulus perception and motor response, we used eight grayscale swatches (256 × 256 pixels) with different levels of brightness evenly spread from black to white. Examples of experimental stimuli are illustrated by Figure 1.
Fig. 1.
Behavioral paradigm. (A) An example in the ‘face attractiveness judgment task’. Female participants were presented with faces of Caucasian young males for 2.5 s each, and judged each face in an attractiveness 8-point scale from very unattractive to very attractive. (B) An example of the ‘action goodness judgment task’. Participants were presented with sentences describing hypothetical actions for 4 s each, and judged each action in a goodness 8-point scale from very bad to very good. (C) An example of the ‘brightness judgment task’, which was used as control task. Subjects were presented with swatches of varying brightness for 2.5 s each and judged each swatch in a brightness 8-point scale from very dark to very bright. In all tasks, trials were separated by a jittered fixation interval (0.5–5 s).
Experimental tasks
Stimuli were presented for 2.5 s (brightness and attractiveness rating tasks) or 4 s (goodness rating task) with a jittered 0.5–5 s intertrial interval, and participants rated them in an 8-point scale. Trial duration was decided on the basis of the results of a behavioral pilot study, which showed that participants needed more time to read sentences in the goodness rating task than to see faces in the attractiveness task or to see swatches in the brightness task. In the ‘brightness rating task’ (scan 1), 90 grayscale swatches were presented, and participants rated them from very dark (level 1) to very bright (level 8). In the ‘face attractiveness rating task’ (scans 2–4), 270 faces were presented, and participants rated them from very unattractive (level 1) to very attractive (level 8). Each face was rated only once. In the ‘action goodness rating task’ (scans 5–7), 270 sentences of hypothetical actions were presented, and participants rated them from morally very bad (level 1) to very good (level 8). The presentation order of experimental stimuli was randomized between subjects in all tasks. Trials in which no response was made were not included in fMRI analyses.
The sensory properties of attractiveness and goodness rating tasks were very different (i.e. faces vs sentences), but this is an advantage for our goals because overlaps in activation are more likely to reflect similarities in processes rather than similarities in stimuli. To ensure that overlaps were not due to similarities in simple decision or motor processes, the brightness rating task was employed as a control task.
Image acquisition and data analysis
All MRI data acquisition was conducted using a 4-T GE scanner. Stimuli were presented using liquid crystal display goggles, and behavioral responses were recorded using an 8-button fiber optic response box. Scanner noise was reduced with earplugs, and head motion was minimized using foam pads and a headband. Anatomical scans began by first acquiring a T1 weighted sagittal localizer series. Second, high-resolution T1-weighted structural images (256 × 256 matrix, TR = 12 ms, TE = 5 ms, FOV = 24 cm, 68 slices, 1.9 mm slice thickness) were collected. Coplanar functional images were subsequently acquired utilizing an inverse spiral sequence (64 × 64 matrix, TR = 1500 ms, TE = 31 ms, Flip angle = 60°, FOV = 24 cm, 34 slices, 3.8 mm slice thickness).
The preprocessing and statistical analyses for all images were performed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK). In the preprocessing analysis, after discarding the first four volumes, images were corrected for slice-timing and motion, then spatially normalized into the Montreal Neurological Institute (MNI) template and spatially smoothed using a Gaussian kernel of 8 mm FWHM. For each subject trial-related activity was modeled by convolving a vector of trial onsets with a canonical hemodynamic response function (HRF) within the context of the General Linear Model (GLM). Confounding factors (head motion, magnetic field drift) were also included in the model.
To identify regions where activity increased or decreased simply as a function of attractiveness, goodness and brightness ratings at the subject level, the ratings of each participant were entered as first-order parametric modulators in SPM5 in their original (i.e. 1, 2, 3, 4, 5, 6, 7, 8) and reverse formats. Additionally, given evidence that the amygdala activity may show a quadratic function in response to facial attractiveness and trustworthiness (Winston et al., 2007; Said et al., 2009), we conducted exploratory analyses with a U-shaped regressor (i.e. 8, 4, 2, 1, 1, 2, 4, 8). For sake of completeness and because of the relationship to reaction times (see below), we also investigated an inverted-U regressor (i.e. 1, 2, 4, 8, 8, 4, 2, 1). Following subject-level analyses, regions shared by attractiveness and goodness ratings were identified at the group level using random effects analyses. To be considered significant, an activation had to increase (or decrease) parametrically (i) for attractiveness and goodness judgments taken together (P < 0.05, uncorrected); (ii) for attractiveness judgments to a greater extent than for brightness judgments (P < 0.05, uncorrected) and (iii) for goodness judgments to a greater extent than for brightness judgments (P < 0.05, uncorrected). In the statistical analysis, the first contrast was masked inclusively by the second and third contrasts. By using this procedure, we isolated activations reflecting processes shared by the attractiveness and goodness judgments but not by the brightness judgment. Similar procedures were used to examine U and inverted-U responses in the amygdala. Given that the last two contrasts are independent, the joint probability of the conjunction can be estimated at P < 0.0025. Additionally, because a minimum spatial extent of 15 contiguous voxels was required, Monte Carlo simulations of spatially correlated data yield a probability estimate of P < 0.0001 (Forman et al., 1995). All coordinates of activations were converted from MNI to Talairach space (Talairach and Tournoux, 1988) using the MNI2TAL tool (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
To further examine if the regions identified by parametric analyses play a similar role in face attractiveness and action goodness ratings, we measured correlations between the two tasks across participants. We extracted for each participant the peak activity level (effect size) in the contrasts of attractiveness vs brightness and of goodness vs brightness (all levels collapsed), and then, we computed Pearson correlations between the two effect-size vectors across participants. These analyses answered the question of whether regions identified in the parametric modulation analyses showed a same pattern across participants for attractiveness and goodness tasks. Additionally, to examine functional connectivity between the regions identified in the parametric modulation analysis, we calculated Pearson correlations between activations in these regions.
To examine if regions showing simply increased or decreased activations make different directions of contributions to rating performance, we conducted a three-step correlation analysis based on activity extracted from individual trials (Rissman et al., 2004). As a first step, we created GLM, in which each individual trial was modeled by a separate covariate, yielding different parameter estimates for each individual trial from all subjects. Second, mean activity (effect sizes of difference between trial and fixation baseline) was extracted from regions-of-interest (ROIs) in the medial OFC and insular cortex, with each ROI defined as a sphere with 5 mm radius centered at the peak of the parametric activation. Finally, the Spearman’s rank correlation between activations (effects sizes) extracted from individual trials and rating scores were computed for each region and for each judgment task, and then were analyzed to evaluate their significance at a threshold of P < 0.05.
RESULTS
Behavioral data
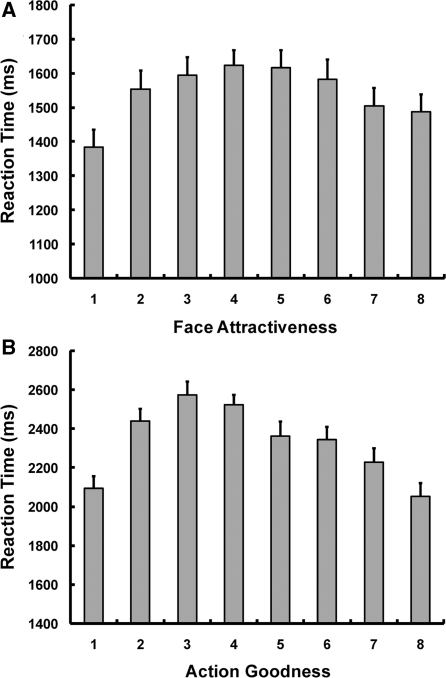
Consistent with the way in which stimuli were selected, attractiveness and goodness ratings were widely distributed, yielding a substantial number of trials in each of the eight levels of both attractiveness and goodness scales (Table 1). Mean numbers of ‘No Response’ trials were very small in both attractiveness (M : 8.7, s.d. : 15.3) and goodness (M : 5.7, s.d. : 11.6) tasks. Whereas the distribution of ratings reflects the selection of the stimuli, response times (RTs) are more interesting because they provide an indirect measure of judgment at different points of each scale. An observation of Figure 2A and B suggests that RTs were faster in the attractiveness than in the goodness task (compare y-axes) and that both tasks showed a similar inverted-U pattern with slower RTs for intermediate ratings and faster RTs for extreme ratings. To investigate these ideas, we conducted a 2 (task) × 8 (levels) ANOVA, which yielded significant main effects of task [F(1,19) = 522.6, P < 0.001] and levels [F(7,133) = 29.9, P < 0.001] and a significant task × level interaction [F(7,133) = 13.4, P < 0.001]. To confirm the inverted-U pattern, we conducted post-hoc tests comparing intermediate ratings (mean of levels 4 and 5) to extreme ratings (mean of levels 1 and 8) in each task. These tests yielded significant differences for both attractiveness ratings [intermediate: 1619 ms, s.d. : 205; extreme: 1425 ms, s.d. : 229; t(19) = 5.02, P < 0.01] and goodness ratings [intermediate: 2406 ms, s.d. : 279; extreme: 2072 ms, s.d. : 288; t(19) = 7.07, P < 0.01]. These RT effects suggest that the judgments were more demanding for intermediate than extreme judgments, possibly because stimuli in the middle of the scale were ambiguous regarding attractiveness and goodness. This finding is important because it indicates that fMRI analyses focused on linear increases or decreases in activation are not confounded with differences in task difficulty (which shows an inverted-U rather than a linear increase or decrease). It is worth noting that this RT pattern differs from the pattern found in a few studies where RTs were longer for highly attractive than for medium and low attractive faces (Aharon et al., 2001; Kranz and Ishai, 2006). This discrepancy can be easily explained by procedural differences. For example, in the study by Aharon et al. (2001), RTs were measured during a task, in which participants increased or decreased viewing time by themselves, whereas in our task, viewing time was fixed. In the study by Kranz and Ishai (2006), the task was similar to ours but the rating scale had only 3 points, which probably made the task easier than ours.
Table 1.
Mean number of trials for each level in attractiveness and goodness rating tasks
| Task | L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 |
|---|---|---|---|---|---|---|---|---|
| Face attractiveness | 19.5 | 42.0 | 50.5 | 46.8 | 38.6 | 31.5 | 22.2 | 10.4 |
| Action goodness | 29.8 | 29.9 | 23.9 | 27.0 | 44.6 | 41.6 | 37.8 | 30.0 |
L, Scaling Levels
Fig. 2.
Results of reaction time data. (A) Participants rated the attractiveness of faces from very unattractive (level 1) to very attractive (level 8). Reaction times in this task showed an inverted-U function indicating greater difficulty for intermediate judgments. (B) Participants rated sentences of hypothetical actions from morally very bad (level 1) to very good (level 8). Reaction times in this task also showed an inverted- U function. All error bars represent standard errors.
fMRI data
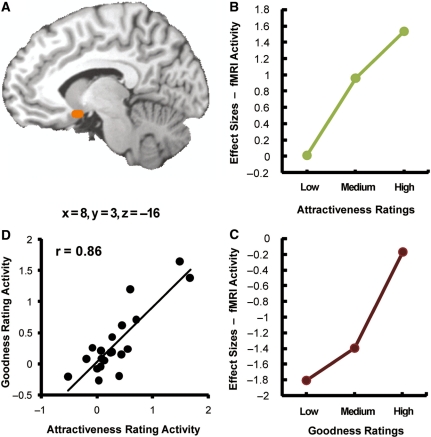
Consistent with our first prediction, OFC activity increased as a function of linearly increased format in both types of ratings (Table 2). Activity within the right medial OFC region (Figure 3A) increased as a function of ratings in the facial attractiveness task (Figure 3B) and in the action goodness task (Figure 3C). These changes in activity cannot be attributed to decision difficulty because the most difficult judgments, as indicated by slower RTs, were those in the middle of the scale, not in the extremes. To confirm a similar role of the medial OFC in attractiveness and goodness judgments, we calculated the correlation between the OFC activities during the two tasks across participants. As illustrated by Figure 3D, we found a highly significant positive correlation (r = 0.86, P < 0.01), indicating that participants with stronger medial OFC activation for face attractiveness ratings also showed stronger medial OFC activations for action goodness ratings. To confirm that the correlation was not driven by two potential outliers (Figure 3D), we redid the analysis without these points and the correlation remained significant (r = 0.65, P < 0.01). Also, the correlations remained significant when performed on unsubtracted data. Another region where activity increased as function of both type of judgments was the posterior cingulate cortex (Table 1). This region also showed a highly positive correlation between activations during the attractiveness and goodness judgment tasks (r = 0.62, P < 0.01).
Table 2.
Regions showing similar activation patterns in response to judgments of face attractiveness and action goodness
| Regions | L/R | BA | Coordinates |
Z-value | Voxel size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Linear increases for both attractiveness and goodness ratings | |||||||
| Medial OFC | R | 25 | 8 | 3 | −16 | 4.26 | 19 |
| Posterior cingulate cortex | L | 30 | −8 | −51 | 13 | 3.89 | 91 |
| Linear decreases for both attractiveness and goodness ratings | |||||||
| Insular cortex | R | 34 | −15 | −6 | 4.80 | 25 | |
| Inferior frontal gyrus | L | 44 | −53 | 19 | 10 | 4.41 | 32 |
| Supramarginal gyrus | L | 40 | −60 | −45 | −4 | 5.12 | 111 |
| Supramarginal gyrus | R | 40 | 53 | −50 | 24 | 3.07 | 64 |
| U-shaped responses for both attractiveness and goodness ratings | |||||||
| Anterior cingulate cortex | L | 32 | −4 | 44 | 1 | 5.27 | 96 |
| Lingual gyrus | R | 18 | 15 | −71 | −9 | 3.70 | 35 |
| Cerebellum | L | −4 | −64 | −29 | 3.55 | 21 | |
| Inverted-U responses for both attractiveness and goodness ratings | |||||||
| No significant activation | |||||||
R, Right; L, Left; BA, Brodmann area.
Fig. 3.
(A) Right medial OFC region where activity increased simply as a function of both attractiveness and goodness ratings. (B) Increase in OFC activity as a function of attractiveness ratings. ‘Low’ corresponds to ratings 1–3, ‘Medium’ to ratings 4–5 and ‘High’ to ratings 6–8. (C) Increase in OFC activity as a function of goodness ratings. ‘Low’, ‘Medium’ and ‘High’ correspond to ratings 1–3, 4–5 and 6–8, respectively. (D) Changes in OFC activity as a function of attractiveness and goodness ratings were highly correlated (r = 0.86, P < 0.01).
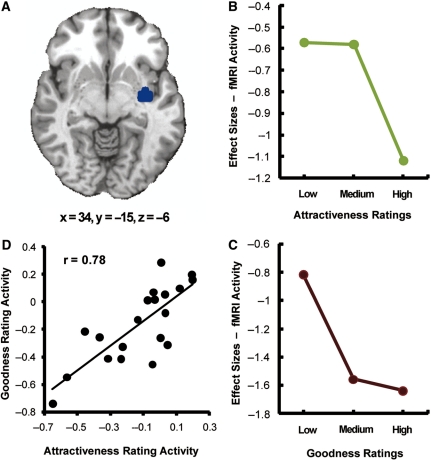
Consistent with our second prediction, insular activity decreased as a function of both attractiveness and goodness (Table 2). Activity within the right insular region (Figure 4A) decreased as a function of ratings in the face attractiveness task (Figure 4B) and in the action goodness task (Figure 4C). As in the case of the medial OFC, these changes cannot be attributed to difficulty, which was greater in the center of the scale. Confirming a similar role of the insular cortex in the two types of judgments, we found a highly significant positive correlation in insular activations during attractiveness and goodness ratings across participants (Figure 4D, r = 0.78, P < 0.01). To confirm that the correlation was not driven by two possible outliers (see Figure 4D), we recalculated the correlation without these points and it remained significant (r = 0.63, P < 0.01). As in the case of medial OFC correlations, insula correlations remained significant when performed on unsubtracted data. Other regions where activity decreased as function of both types of judgments were the left inferior frontal gyrus and the supramarginal gyrus in both hemispheres (Table 2). These regions also showed highly positive correlations between activities during the attractiveness and goodness judgment tasks (left inferior frontal gyrus: r = 0.63, P < 0.01; left supramarginal gyrus: r = 0.61, P < 0.01; right supramarginal gyrus: r = 0.83, P < 0.01).
Fig. 4.
(A) Right insular cortex region where activity decreased as a function of both attractiveness and goodness ratings. (B) Decrease in insular activity as a function of attractiveness ratings. For ratings groupings, see caption of Figure 3. (C) Decrease in insular activity as a function of goodness ratings. For ratings groupings, see caption of Figure 3. (D) Changes in insular activity as a function of attractiveness and goodness ratings were highly correlated (r = 0.78, P < 0.01).
In addition to the main linear analyses, we conducted analyses to identify brain regions showing U-shaped or inverted-U activation patterns as a function of both attractiveness and goodness ratings. As indicated by Table 2, U-shaped responses were found in the anterior cingulate gyrus, lingual gyrus and cerebellum. The lack of U-shaped responses in the amygdala is interesting because U-shaped amygdala responses have been previously found for facial attractiveness and trustworthiness (Winston et al., 2007; Said et al., 2009). Inverted-U responses were not found in any region.
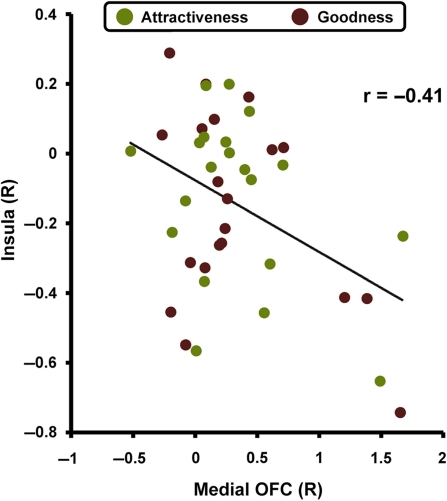
Finally, given medial OFC and insular regions showed increased or decreased responses to the attractiveness and goodness, we tested whether the OFC and insular regions should interact during both judgments of attractiveness and goodness. Confirming this prediction, in the correlation analysis between right medial OFC and right insular activations, they were negatively correlated across participants (Figure 5, r = –0.41, P < 0.01). This correlation is consistent with the existence of direct anatomical connections between the OFC and insular cortex (Van Eden et al., 1992). Moreover, when we extracted activity from individual trials within participants and calculated Spearman’s rank correlation, we found that the medial OFC activations were positively correlated with both beauty and goodness ratings, whereas the insular activations were negatively correlated with both types of ratings (Table 3). Thus, these two regions have opposing effects on attractiveness and goodness judgments.
Fig. 5.
Significantly negative correlation (r = –0.41, P < 0.01) between right medial OFC activity and right insular cortex activity during attractiveness and goodness ratings tasks. Activations during the attractiveness rating task are plotted in green and activations during the goodness rating task are plotted in brown.
Table 3.
Results of the Spearman's rank correlation between activity in the medial OFC and insular cortex and rating score during the face attractiveness and action goodness ratings
| Regions | Face attractiveness |
Action goodness |
||
|---|---|---|---|---|
| Z-value | P-value | Z-value | P-value | |
| OFC | 2.27 | 0.02 | 2.64 | <0.01 |
| Insular cortex | −3.54 | <0.001 | −3.62 | <0.001 |
DISCUSSION
Three main findings emerged from the present study. First, medial OFC activity increased as a function of perceived attractiveness and goodness, and the activity was significantly correlated between the two judgment tasks. Second, insular activity decreased as a function of perceived attractiveness and goodness, and the activity showed a significant correlation between the two judgment tasks. Third, medial OFC and insular cortex displayed an opposing relationship during attractiveness and goodness judgments. These findings are discussed in separate sections below.
Medial OFC: attractiveness and goodness
Activity in the medial OFC, which has been known as a region associated with processing positive emotions and reward, increased as a function of both attractiveness and goodness ratings, and was correlated across participants between these ratings (Figure 3). The findings in Figure 3 are in harmony with evidence from separate fMRI studies that OFC activity increases as a function of facial attractiveness (O'Doherty et al., 2003b; Kranz and Ishai, 2006; Winston et al., 2007; Ishai, 2007; Bray et al., 2008; Cloutier et al., 2008;) and as a function of moral goodness (Moll et al., 2006; Zahn et al., 2008). Also, patients with OFC lesions show poor practical judgments (Damasio et al., 1994) and impaired moral behavior (Eslinger and Damasio, 1985; Anderson et al., 1999; Ciaramelli et al., 2007), and people with smaller OFC gray matter volume display higher psychopathy scores (de Oliveira-Souza et al., 2008). However, our study is the first one to clearly show that medial OFC activations for positive aesthetic and moral judgments overlap within the same participants and are significantly correlated with each other.
Given the strong link between medial OFC and reward processing (Rolls, 2000; Martin-Soelch et al., 2001; McClure et al., 2004; O'Doherty, 2004), one possible explanation of increased activity for both facial attractiveness and action goodness is that both are rewarding. This idea is consistent with several lines of evidence, including neuroimaging (Aharon et al., 2001; Cloutier et al., 2008) and developmental research. For example, infants who are presented with pairs of faces spend more time looking at the most attractive face in each pair (Langlois et al., 1987, 1991). Evolutionary accounts suggest that physical beauty may be associated with better genetic fitness and reproductive capacities (Fink and Penton-Voak, 2002; Rhodes, 2006). Sexual preference may also play a role. For instance, an fMRI study found that medial OFC activity was greater for male than female faces when the participants were heterosexual females and homosexual males, but showed the opposite pattern when the participants were heterosexual males and homosexual females (Kranz and Ishai, 2006). The link between positive moral judgments and reward is also supported by several neuroimaging studies (Bartels and Zeki, 2004; Singer et al., 2004a; Izuma et al., 2008) that suggest positive social impressions about people are processed within reward-related circuits, including OFC and the striatum. In addition, neuropsychological studies have reported that patients with OFC damages are impaired in the judgment of moral behaviors (Eslinger and Damasio, 1985; Ciaramelli et al., 2007). In the present study, increasing activity as a function of both facial attractiveness and action goodness was identified in the medial OFC region, which is one of areas associated with approaching social rewards (Davey et al., in press; Lebreton et al., 2009). However, it is worth noting that a few studies have associated medial OFC with the processing of morally-negative actions (e.g. Moll et al., 2007). The reasons for the inconsistency with the present study and evidence linking medial OFC to processing moral goodness (Moll et al., 2006; Zahn et al., 2008) and rewarding stimuli (Rolls, 2000; Martin-Soelch et al., 2001; McClure et al., 2004; O'Doherty, 2004) are uncertain and deserve further research.
Insular cortex: unattractiveness and badness
Activity in the insular cortex, which has been known as a region associated with processing negative emotions and pain, increased as a function of both negative attractiveness and goodness ratings, and was correlated across participants between these ratings (Figure 4). The findings in Figure 4 are consistent with evidence from separate fMRI studies that insular activity is greater for unattractive than attractive faces (O'Doherty et al., 2003b; Krendl et al., 2006) and for negative than positive moral stimuli (Krendl et al., 2006; Hsu et al., 2008; Zahn et al., 2008). However, this study is the first one to demonstrate that insular activations for negative aesthetic and moral judgment overlap within the same participants and are significantly correlated across participants.
One possible explanation of the increasing insular activity for both unattractiveness and badness mediated is in terms of the role of this region in the processing of punishment (O'Doherty et al., 2003a). Functional neuroimaging studies have linked insular activations to emotions of disgust and fear (Phan et al., 2002), as well to a variety of negative social situations, including social exclusion (Eisenberger et al., 2003), unfairness (Sanfey et al., 2003), socially negative signals from faces (Phillips et al., 1997; Winston et al., 2002) and unreciprocated cooperation (Rilling et al., 2008). The insula has also been linked to the processing of pain (Critchley et al., 2000), and aversive conditioning (Seymour et al., 2004). The posterior insular region identified in the present study could be one of areas associated with both disgust and pain (Benuzzi et al., 2008), and with self-experienced pain but not with empathy for the pain experienced by others, which has been associated with the anterior insula (Singer et al., 2004b). The present findings suggest that the posterior insular cortex, which showed responses to unattractive faces and bad actions, could mediate avoidance of socially negative stimuli.
Opposing relationship between medial OFC and insular activities
The third finding of the study was a contrasting relationship between medial OFC and insular cortex during aesthetic and moral judgments. Activations in these regions were negatively correlated with each other (Figure 5) and had opposite-sign correlations between attractiveness and goodness ratings (Table 2). This finding has implications for theoretical accounts of the Beauty-is-Good stereotype, i.e. the assumption that attractive individuals have better personalities and higher moral standards (Dion et al., 1972; Eagly et al., 1991; Langlois et al., 2000). This stereotype could reflect (i) a positive bias toward attractiveness, (ii) a negative bias against unattractiveness or (iii) a combination of both mechanisms (Griffin and Langlois, 2006). Although this issue has been examined by various lines of behavioral studies (Wilson and Daly, 2004; Olson and Marshuetz, 2005; Hayden et al., 2007), fMRI data can be more informative. The ‘positive bias hypothesis’ suggests that the mechanisms of aesthetic and moral judgments are likely to overlap in brain regions associated with processing positive stimuli, whereas the ‘negative bias hypothesis’ suggests that the overlap should occur in regions associated with processing negative stimuli. The ‘dual process hypothesis’ predicts that both types of regions should be involved, possibly in opposition to each other. Thus, the current results are consistent with the dual process account of the Beauty-is-Good stereotype.
One way of explaining the opposition between medial OFC and insular cortex is in terms of approach and avoidance. The Beauty-is-Good stereotype could reflect an approach response toward beauty and goodness, mediated by medial OFC, combined with an avoidance response away from unattractiveness and badness, mediated by the insular cortex. The link between medial OFC and approach responses is supported by evidence that this region shows greater activity when people approach friends rather with other people (Guroglu et al., 2008), and when hungry participants perceive food-related rather than food-unrelated stimuli under hunger conditions (Fuhrer et al., 2008). The link between insular cortex and avoidance responses is consistent with findings that insular activity is associated with the anticipation of threat (Seymour et al., 2007), the avoidance of risky options in decision-making tests (Kuhnen and Knutson, 2005) and the individual differences in avoidance learning (Samanez-Larkin et al., 2008).
It is worth noting, however, that the implications of the current findings for the Beauty-is-Good stereotype are indirect. Although we identified overlaps in the neural mechanisms of attractiveness and goodness judgments, we did not observe an interaction between these two types of judgments within the same task. Thus, further research is required to corroborate the direct involvement of OFC and insular cortex in the Beauty-is-Good stereotype.
CONCLUSION
In sum, the goal of the present study was to identify shared neural mechanisms of aesthetic and moral judgments. Activity in region previously associated with reward, medial OFC, increased as a function of both attractiveness and goodness ratings, whereas activity in a region previously associated with disgust, pain and punishment, the insula, decreased as a function of both attractiveness and goodness ratings. In both regions, activations for attractiveness and goodness judgments were positively correlated across participants. This second finding is independent from the first one and it strengthens the link between aesthetic and moral processing by showing that individual differences in the reactivity of these regions are similar for attractiveness and goodness judgments. Finally, we found an opposing relationship between medial OFC and insular activity during aesthetic and moral judgments. These findings are consistent with the idea that the Beauty-is-Good stereotype involves a positive bias toward attractiveness and goodness coupled with a negative bias against unattractiveness and badness. The notion of opposing mechanism fits with the roles of medial OFC activity in approaching rewards and the role of insular cortex in avoiding punishment.
Acknowledgments
This research was supported by National Institutes of Health grants NS41328 and AG23770 to R.C. T.T. was supported by MEXT/ KAKENHI (21119503) and Cosmetology Research Foundation program.
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–51. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Benuzzi F, Lui F, Duzzi D, Nichelli PF, Porro CA. Does it look painful or disgusting? Ask your parietal and cingulate cortex. The Journal of Neuroscience. 2008;28:923–31. doi: 10.1523/JNEUROSCI.4012-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, O’Doherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. Journal of Neurophysiology. 2007;97:3036–45. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Bray S, Rangel A, Shimojo S, Balleine B, O’Doherty JP. The neural mechanisms underlying the influence of pavlovian cues on human decision making. The Journal of Neuroscience. 2008;28:5861–6. doi: 10.1523/JNEUROSCI.0897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Ames MA, Etherington R, Pietsch J. Effects of victim's and defendant's physical attractivenss on the perception of responsibility in an ambiguous domestic violence case. Journal of Family Violence. 1990;5:199–207. [Google Scholar]
- Castellow WA, Wuensch KL, Moore CH. Effects of physical attractiveness of the plaintiff and defendant in sexual harassment judgments. Journal of Social Behaviour and Personality. 1990;5:547–62. [Google Scholar]
- Ciaramelli E, Muccioli M, Ladavas E, di Pellegrino G. Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Social Cognitive and Affective Neuroscience. 2007;2:84–92. doi: 10.1093/scan/nsm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J, Heatherton TF, Whalen PJ, Kelley WM. Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience. 2008;20:941–51. doi: 10.1162/jocn.2008.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan R.J. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yucel M. Being liked activates primary reward and midline self-related brain regions. Human Brain Mapping. in press doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–13. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Dion K, Berscheid E, Walster E. What is beautiful is good. Journal of Personality and Social Psychology. 1972;24:285–90. doi: 10.1037/h0033731. [DOI] [PubMed] [Google Scholar]
- Eagly AH, Ashmore RD, Makhijani MG, Longo LC. What is beautiful is good, but..: A meta-analytic review of research on the physical attractiveness stereotype. Psychological Bulletin. 1991;110:109–28. [Google Scholar]
- Efran MG. The effect of physical appearance on the judgment of guilt, interpersonal attraction, and severity of recommended punishment in a simulated jury task. Journal of Research in Personality. 1974;8:45–54. [Google Scholar]
- Efran MG, Patterson E. Voters vote beautiful: the effect of physical appearance on a national debate. Canadian Journal Behavioural Science. 1974;6:352–6. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fink B, Penton-Voak I. Evolutionary psychology of facial attractiveness. Current Directions in Psychological Science. 2002;11:154–8. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friend RM, Vinson M. Learning over backwards: Junors responses to defendants' attractiveness. Journal of Communication. 1974;24:124–9. [Google Scholar]
- Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Griffin AM, Langlois JH. Stereotype directionality and attractiveness stereotyping: is beauty good or is ugly bad? Social Cognition. 2006;24:187–206. doi: 10.1521/soco.2006.24.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroglu B, Haselager GJ, van Lieshout CF, Takashima A, Rijpkema M, Fernandez G. Why are friends special? implementing a social interaction simulation task to probe the neural correlates of friendship. Neuroimage. 2008;39:903–10. doi: 10.1016/j.neuroimage.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Hamermesh DS, Biddle JE. Beauty and the labor market. The American Economic Revenue. 1994;84:1174–94. [Google Scholar]
- Hamermesh DS, Parker A. Beauty in the classroom: professorial pulchritude and putative pedagogical productivity. Economics of Education Review. 2005;24:369–76. [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proceedings. Biological Sciences. 2007;274:1751–6. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, Villringer A. Influence of bodily harm on neural correlates of semantic and moral decision-making. Neuroimage. 2005;24:887–97. doi: 10.1016/j.neuroimage.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Hsu M, Anen C, Quartz SR. The right and the good: distributive justice and neural encoding of equity and efficiency. Science. 2008;320:1092–5. doi: 10.1126/science.1153651. [DOI] [PubMed] [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. International Journal Psychophysiology. 2007;63:181–5. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Zeki S. Neural correlates of beauty. Journal of Neurophysiology. 2004;91:1699–705. doi: 10.1152/jn.00696.2003. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–8. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Krendl AC, Macrae CN, Kelley WM, Fugelsang JA, Heatherton TF. The good, the bad, and the ugly: an fMRI investigation of the functional anatomic correlates of stigma. Social Neuroscience. 2006;1:5–15. doi: 10.1080/17470910600670579. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kulka RA, Kessler JB. Is justice really blind?-The influence of litigant physical attractiveness on juridical judgment. Journal of Applied Social Psychology. 1978;8:366–81. [Google Scholar]
- Langlois JH, Kalakanis L, Rubenstein AJ, Larson A, Hallam M, Smoot M. Maxims or myths of beauty? A meta-analytic and theoretical review. Psychological Bulletin. 2000;126:390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Ritter JM, Roggman LA, Vaughn LS. Facial diversity and infant preferences for attractive faces. Developmental Psychology. 1991;27:79–84. [Google Scholar]
- Langlois JH, Roggman LA, Casey RJ, Ritter JM. Infant preferences for attractive faces: Rudiments of a stereotype? Developmental Psychology. 1987;23:363–9. [Google Scholar]
- Lebreton M, Barnes A, Miettunen J, et al. The brain structural disposition to social interaction. The European Journal of Neuroscience. 2009;29:2247–52. doi: 10.1111/j.1460-9568.2009.06782.x. [DOI] [PubMed] [Google Scholar]
- Marlowe CM, Schneider SL, Nelson CE. Gender and attractiveness biases in hiring decisions: are more experienced managers less biased? Journal of Applied Psychology. 1996;81:11–21. [Google Scholar]
- Martin-Soelch C, Leenders KL, Chevalley AF, et al. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Research. Brain Research Reviews. 2001;36:139–49. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Benavente R. The AR Face Database. CVC Technical Report. 1998;24 [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–8. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, et al. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002;22:2730–6. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Garrido GJ, et al. The self as a moral agent: linking the neural bases of social agency and moral sensitivity. Social Neuroscience. 2007;2:336–52. doi: 10.1080/17470910701392024. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15623–8. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003a;23:7931–9. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003b;41:147–55. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Olson IR, Marshuetz C. Facial attractiveness is appraised in a glance. Emotion. 2005;5:498–502. doi: 10.1037/1528-3542.5.4.498. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Moon H, Rizvi SA, Rauss PJ. The FERET evaluation methodology for face recognition algorithms. IEEE Transaction on. Pattern Analysis and Machine Intelligence. 2000;22:1090–104. [Google Scholar]
- Phillips PJ, Wechsler H, Huang J, Rauss PJ. The FERET database and evaluation procedure for face recognition algorithms. Image and Vision Computing Journal. 1998;16:295–306. [Google Scholar]
- Piehl J. Integration of information in the "courts:" Influence of physical attractiveness on amount of punishment for a traffic offender. Psychological Reports. 1977;41:551–6. [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annual Review of Psychology. 2006;57:199–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Lee K, Palermo R, et al. Attractiveness of own-race, other-race, and mixed-race faces. Perception. 2005;34:319–40. doi: 10.1068/p5191. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Goldsmith DR, Glenn AL, et al. The neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia. 2008;46:1256–66. doi: 10.1016/j.neuropsychologia.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–63. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereberal Cortex. 2000;10:284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Said CP, Baron SG, Todorov A. Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience. 2009;21:519–28. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychological Science. 2008;19:320–3. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–58. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schaich Borg J, Lieberman D, Kiehl KA. Infection, incest, and iniquity: investigating the neural correlates of disgust and morality. Journal of Cognitive Neuroscience. 2008;20:1529–46. doi: 10.1162/jocn.2008.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman C, Brickman J, Koulack D. Rape and physical attractiveness: assigning responsibility to victims. Journal of Personality. 1977;45:554–63. doi: 10.1111/j.1467-6494.1977.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–7. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nature Reviews Neuroscience. 2007;8:300–11. doi: 10.1038/nrn2119. [DOI] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004a;41:653–62. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004b;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Proportional System: An Approach to Cerebral Imaging. Stuttgart: Georg Thieme; 1988. Co-Planar Stereotactic Atlas of the Human Brain: 3-dimensional. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden CG, Lamme VA, Uylings HB. Heterotopic cortical afferents to the medial prefrontal cortex in the rat. A Combined retrograde and anterograde tracer study. European Journal of Neuroscience. 1992;4:77–97. doi: 10.1111/j.1460-9568.1992.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Weiten W. The attraction-leniency effect in jury research: An examination of external validity. Journal of Applied Social . 1980;10:340–347. [Google Scholar]
- Wilson M, Daly M. Do pretty women inspire men to discount the future? Proceedings. Biological Sciences. 2004;271(Suppl 4):S177–9. doi: 10.1098/rsbl.2003.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wuensch KL, Chia RC, Castellow WA, Chuang CJ, Cheng BS. Effects of physical attractiveness, sex, and type of crime on mock juror decisions: a replication with Chinese students. Journal of Cross-Cultural Psychology. 1993;24:414–27. [Google Scholar]
- Zahn R, Moll J, Paiva M, et al. The Neural basis of human social values: evidence from functional MRI. Cerebral Cortex. 2008;19:276–83. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]