Abstract
Prefrontal cortex (PFC) has been implicated in the experience and regulation of emotional states. Emotional experience is a complex construct, encompassing a range of more specific processes. This exploratory study aimed to delineate which (if any) aspects of emotional experience rely critically on either the ventromedial frontal (VMF) or lateral frontal (LF) lobes. The affective experience of individuals with damage to these regions was surveyed in detail using several measures and compared with that of control participants. Dependent measures included subjective and observer ratings of both dispositional affect and transient responses to laboratory mood inductions. VMF damage was associated with greater negative dispositional affect relative to controls and to individuals with LF damage; however, transient responses to emotional stimuli were largely normal. In contrast, LF damage was associated with an exaggerated subjective reactivity to sad emotional stimuli relative to control participants, but normal dispositional affect. Interestingly, neither form of PFC damage affected spontaneous emotion recovery following the mood inductions. These findings suggest a role for VMF in modulating dispositional negative affect; in contrast, LF areas appear to be critical in regulating transient emotional responses while emotional stimuli are present. This study also illustrates the dissociability of different aspects of emotional experience in patients with focal brain injury.
Keywords: mood, emotion, emotion regulation, lesion, prefrontal cortex
INTRODUCTION
Prefrontal cortex (PFC) has been implicated in affect generation and regulation (e.g. Ochsner and Gross, 2005). However, empirical support for this claim is heterogeneous at both conceptual and methodological levels. Affective experience encompasses a complex set of processes that vary both in time course (transient vs dispositional) and in content (e.g. fear, happiness). Furthermore, individual affective episodes include subjective sensations, objective behaviors and autonomic responses (Russell, 2003). Studies of the neural basis of affect have variously operationalized affect as a mood-like trait (e.g. Zald et al., 2002), as a relatively transient emotional state (e.g. Schaefer et al., 2002), or as a disorder (e.g. Mayberg et al., 1999), and each of these constructs has been measured in a variety of ways. Perhaps unsurprisingly, the result is a patchwork of findings that resists easy interpretation.
Notwithstanding this conceptual and experimental heterogeneity, many studies have suggested that PFC is somehow involved in affective experience. Some of the most compelling data in this area come from clinical observations of the effects of PFC damage, which typically have captured affective experience at a fairly general level, whether in early descriptions of emotional changes after PFC damage (as in the case of Phineas Gage; Damasio, 1994) or of the effects of frontal lobotomy on mood and mood disorders (Valenstein, 1986), or in more recent questionnaire-based observational studies of patients with PFC damage (e.g. Barrash et al., 2000). While such work supports a role for PFC in emotion, the specific emotional processes and their relation to specific subregions within PFC remain ill-defined.
More recently, functional imaging has been applied to explore the neural basis of many facets of affective experience. Such experiments in the burgeoning field of affective neuroscience have led to increasingly detailed, component-level models of how emotion is generated and regulated in the healthy brain, and in mood disorders. This work suggests that particular regions within PFC, including ventromedial [VMF, encompassing orbitofrontal and adjacent ventromedial prefrontal cortex (vmPFC)] and dorsal and ventral lateral frontal (LF) cortex, may be playing distinct roles (for reviews see Barrett et al., 2007; Kober et al., 2008; Lee and Siegle, in press).
VMF areas have been implicated in the experience of transient affect (Damasio et al., 2000) and in longer-term affective states (Zald et al., 2002). VMF also has been implicated in depression (e.g. Davidson et al., 2002), with ‘over-activity’ within subgenual anterior cingulate (a subregion of VMF) proposed to be a critical pathophysiological mechanism (e.g. Mayberg et al., 2005). LF regions also have been implicated in the experience of transient affect (e.g. Goldin et al., 2005), as well as in dispositional affect, particularly during depressed states (e.g. Mayberg et al., 1999). However, these findings are not simple to interpret in a component process account of affective experience, given that low mood is not an essential criterion for depression.
It has been proposed that LF activations—particularly those seen in transient emotional perturbations—may reflect the regulation of emotional experience. Transient emotional states represent a balance between emotional perturbation and regulation, with top–down regulation of emotion plausibly present even during the onset of an emotional state. Several researchers have reported an inverse association between LF activity and in brain regions thought to be important in negative affect (e.g. Ochsner et al., 2004), suggesting that LF regions act to dampen transient emotional changes mediated by limbic and subcortical structures. However, similar patterns have also been reported for VMF regions (e.g. Hariri et al., 2003).
Although this functional imaging work provides a more detailed view of the role of PFC in emotional experience, models that derive from neuroimaging data require converging support from other methods. Given the complex processes that comprise emotions (e.g. facial expression, subjective experience; see Russell, 2003), it is difficult to know which of these components is reflected in the various activations observed in neuroimaging studies. Furthermore, as discussed above, observed activations may represent reactivity, regulation, or both.
Studies of patients with focal brain injury can help to answer two questions: (i) does a particular brain region play a necessary role in a given process and (ii) are putative component processes of affective experience in fact dissociable? There are remarkably few loss-of-function studies that address the roles of PFC in emotional experience at the component process level. Transient emotion, in particular, has been little studied. Rule et al. (2002) and Roberts et al. (2004) found a diminished ability among individuals with damage to orbitofrontal cortex (OFC; a region within VMF) to ‘filter’ responses to aversive stimuli such as shock and loud noises. OFC damage also has been reported to impair more complex transient emotional experiences, such as regret (Camille et al., 2004), pride and embarrassment (Beer et al., 2003). Even these few studies are difficult to reconcile, in that they report enhancement of one transient emotional response and blunting of others.
Questionnaire-based studies of patients with focal VMF injury have identified general changes in emotion (e.g. ‘blunted emotional experience’) by informant report (Barrash et al., 2000) and self-reported changes in emotional experience for certain cardinal emotions (Hornak et al., 2003). The latter study reported that damage to medial frontal and OFC areas was associated with changes in emotional experience, but the specific pattern of change (affected emotions, direction of change) varied widely across individuals. Finally, despite the plausibility of LF involvement in affective experience based on neuroimaging studies, there have been few lesion studies examining LF contributions to affective processes.
In sum, the existing loss-of-function literature provides insufficient evidence to conclude that the PFC plays a necessary role in either transient emotional experience and regulation or longer-term mood, much less to specify what this role might be. Here we report a study of the effects of PFC damage affecting either VMF or LF lobes on three aspects of affective experience: short-term induced emotion, spontaneous recovery from emotional states and long-term ‘dispositional’ affect. This exploratory work aimed to begin to disentangle the component processes of affective experience supported by these two regions of PFC. We focused on sad and happy emotional states because of the relevance of these emotions to understanding the neural substrates of depression.
A WORD ABOUT TERMINOLOGY
The current work will treat ‘affect’ as an umbrella term for emotions and moods, following the lead of Davidson (2000) and Gross (1998). It is customary to distinguish ‘moods’ from ‘emotions’ based on various features, including duration and whether there is a readily identifiable object of the valenced state (e.g. Gross, 1998; Russell, 2003). Moods are generally associated with longer duration and lack of a specific object, whereas emotions are of shorter duration and have an identifiable object. For these reasons, the relatively transient and stimulus-driven affective manipulations in the laboratory in the present study are referred to as ‘emotion’. Nevertheless, the conventional term ‘mood induction’ is used to refer to the experimental manipulations.
METHODS
Participants
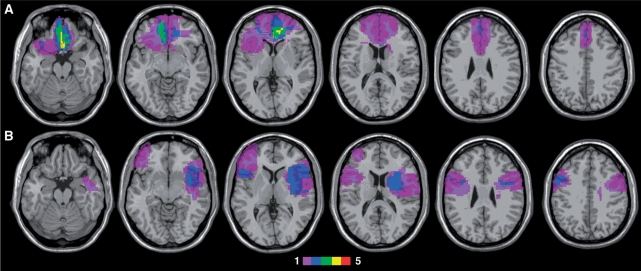
Participants included 7 individuals with VMF damage, 8 with LF damage and 15 healthy controls (CTRL) whose age and education closely matched those of the two patient groups (see Table 1). Individuals with damage to the PFC were recruited through the Center for Cognitive Neuroscience patient database at the University of Pennsylvania. All individuals with focal damage principally involving cortex anterior to the precentral sulcus were eligible to participate. Lesion subgroups were defined a priori, following the boundaries laid out in Stuss and Levine (2002): those with damage involving primarily medial orbitofrontal and/or ventral medial prefrontal cortex (VMF group), and those with damage affecting the frontal convexity and sparing VMF (LF group). As can be seen in Figure 1, the LF group primarily comprised participants with damage to the inferior and/or middle frontal gyrus. In two LF cases, damage extended into the adjacent anterior insula.
Table 1.
Group means (s.d.) for demographic variables
| Group | Age (years) | Female (%) | Education (years) | IQ* | BDI* | Lesion volume (cc) | IADL** |
|---|---|---|---|---|---|---|---|
| VMF (n = 7) | 53.4 (11.0) | 57.1 | 13.0 (2.1) | 110.6 (6.2) | 15.7 (9.7) | 22.1 (24.8) | 15.4 (3.7) |
| LF (n = 8) | 59.0 (10.0) | 62.5 | 14.4 (2.3) | 117.9 (10.7) | 11.5 (8.3) | 42.0 (38.2) | 20.0 (1.6) |
| CTRL (n = 15) | 56.8 (7.3) | 73.3 | 14.9 (2.2) | 125.1 (7.8) | 6.2 (4.2) | n/a | n/a |
Note: cc = cubic centimeters. n/a = not applicable. IADL = Instrumental Activities of Daily Living scale. BDI = Beck Depression Inventory. *ANOVA, P < 0.05. **t-test, P < 0.01.
Fig. 1.
Location and overlap of brain lesions. (A) The lesions of the seven subjects with VMF damage, (B) shows those of the eight subjects with LF damage, projected on axial slices of the MNI brain. Different colors indicate the number of subjects (from one to five) who had damage to a particular area, according to the color key. VMF damage was due to rupture of anterior communicating artery aneurysm in six cases and bilateral anterior cerebral artery stroke in one case. LF damage followed ischemic or hemorrhagic stroke in seven participants and resection of a low-grade glioma in one.
Individuals with damage to PFC were recruited on the basis of lesion location alone, without regard to the presence of mood symptoms to avoid introducing selection bias (i.e. systematically excluding the very individuals whose lesions directly affected brain regions involved in emotion processing). Demographic information for all participants is summarized in Table 1. The Beck Depression Inventory (BDI; Beck et al., 1996) was used as a screening measure of depressive symptoms. BDI scores were higher in the VMF group (P = 0.004) and marginally higher in the LF group (P = 0.07) relative to the CTRL group, but the VMF and LF groups did not differ significantly (Table 1). A total of two individuals in the VMF group and three in the LF group had any history of clinically significant mood disturbance: one person in each group had a past history of depression that preceded brain injury and two additional individuals in the LF group and one in the VMF group had developed mild mood lability or mild depressive symptoms subsequent to their brain injury. Three individuals in the LF group were being treated with selective serotonin reuptake inhibitors (SSRIs) at the time of testing. Rottenberg et al. (2002) reported no significant effects of psychoactive medications on subjective, observer-rated or autonomic measures of emotion in a mood induction paradigm in a large sample of depressed patients, making brain injury a more likely determinant of any observed effects in the present study. One person in the VMF group was taking methylphenidate and donepezil. As a group, VMF subjects were more likely than those with LF damage to be dependent for instrumental activities of daily living (banking, shopping; Table 1), but had comparable lesion volumes and estimated pre-morbid IQ. The two groups performed similarly on neuropsychological screening tests of memory and executive function (Table 2).
Table 2.
Group means (s.d.) from neuropsychological screening
| Group | Digit span forward | Animal fluency | F fluency | Trails B errors* | Reversal learning errors | Verbal recall (5-min delay) |
|---|---|---|---|---|---|---|
| VMF | 5.0 (0) | 14.3 (5.3) | 9.5 (3.3) | 4.2 (3.6) | 9.2 (3.5) | 3.6 (1.5) |
| LF | 4.9 (0.4) | 16.8 (3.5) | 10.5 (3.0) | 0.6 (0.5) | 6.9 (2.2) | 3.9 (0.9) |
Note: Not all subjects completed all tests. *t-test, P < 0.05.
Healthy control participants had no history of major neurological or psychiatric disorders, were not taking psychoactive medications, and scored above 27 on the Folstein Mini-mental status examination and below 15 on the BDI. All participants provided written informed consent in accordance with the Declaration of Helsinki and were paid a nominal fee for their time. The Institutional Review Board of the University of Pennsylvania approved the study protocol. The same participants also completed an emotion recognition task; results are reported in Heberlein et al. (2008).
Dispositional affect
Measurement of dispositional affect
Dispositional affect was measured with the Positive and Negative Affect Scale (PANAS; Watson et al., 1988). This widely-used scale consists of 10 positive affect (PA) and 10 negative affect (NA) adjectives. Participants rated the extent (from one, ‘very slightly or not at all’ to five, ‘extremely’) to which they generally experience these emotions; individuals with lesions were instructed to base their ratings on their emotional experience subsequent to their brain injury. Each participant selected an informant who independently completed the informant version of the PANAS. Informant ratings were collected due to concern about the validity of the self reports among patient with frontal lobe damage—i.e. that the brain regions responsible for affective experience might be the same ones required for accurate reporting about affective experiences. Informants were family members or friends of the participants.
Transient affect
Mood induction procedure
The experiment lasted ∼90 min. A multimodal mood induction was used to maximize the likelihood of producing the target emotions. All participants underwent a negative, ‘neutral’ and positive multimodal mood induction, in that order; each mood induction was followed by an uninstructed recovery period. The negative mood induction was done first in order to give participants maximal recovery time from the negative mood induction prior to leaving the testing session; the neutral induction was done second to separate the negative and positive inductions in order to minimize any carryover effects from the negative induction. This fixed-order design was chosen for these advantages, and because of the practical impossibility of counterbalancing order in a way that would disambiguate order effects from lesion effects given sample size constraints. The mood inductions each comprised a movie clip lasting ∼3 min followed by recall of an autobiographical memory (happy or sad experience) while listening to a 3-min musical excerpt (see Table 3); in the neutral condition participants were asked to recall a mundane errand (e.g. a recent trip to the grocery store).
Table 3.
Basic structure and stimuli used in the three mood induction conditions
| Condition | Movie clip | Music/recall | Recovery |
|---|---|---|---|
| Negative | ‘The Champ’ | Barber’s ‘Adagio for Strings’ | Uninstructed time alone |
| BREAK | |||
| Neutral | ‘Winged Migration’ | Hammersmith’s Opus 22 | Uninstructed time alone |
| BREAK | |||
| Positive | Bill Cosby ‘Chocolate Cake’ sketch | Bach’s Brandenburg Concerto No. 2, first movement | Uninstructed time alone |
Note: Each segment lasted ∼3 min. Recovery lasted as long as necessary for mood ratings (gathered every 3 min) to return to within 10 mm of baseline levels; typically recovery occurred within 6 min.
Upon completing each induction, participants were told that ‘this portion of the experiment is over, but some time must elapse before continuing with the next part.’ This instruction was intended to allow spontaneous recovery from the mood induction (rather than explicit, instructed mood regulation). The recovery phase continued until subjective ratings of the targeted emotion state were within 10 mm of pre-induction ratings, typically achieved within 6 min of the end of the induction. The next induction began following a short break. At the completion of the experiment, participants were asked about the content of their autobiographical recall and debriefed. A qualitative assessment revealed no apparent group differences in the content of memories; the vast majority of participants (77%) across groups recalled the sickness and/or death of a loved one in the sad condition. Responses in the happy condition were more variable and included themes related to children (e.g. ‘first grandchild’), celebrations (e.g. ‘Dad’s 90th birthday’), vacation and leisure (e.g. ‘going to the mountains with my husband’) and successful experiences (e.g. ‘winning the lottery’). Analyses revealed no significant group differences in the participants’ ratings of the sadness, F(2,23) = 1.84, P = 0.18, or happiness, F(2,24) = 0.17, P = 0.85, of their recalled memories.
The experimenter entered the room every 3 min to start or stop stimulus presentation and to obtain subjective emotion ratings. Videotape of participants’ facial expressions was continuously and unobtrusively recorded throughout. Two of seven VMF and five of eight LF individuals were unable to travel to the testing site and were tested in their homes. In such cases, every attempt was made to replicate the quiet and isolated conditions of the laboratory. There were no significant differences in the participants’ subjective mood ratings or emotion behavior as a function of location of testing for either happiness (Ps = 0.15–0.54) or sadness (Ps = 0.35–0.87).
Mood induction measures
Subjective ratings of emotion
Subjective emotion was assessed at 3-min intervals throughout the experiment, using two 100 mm visual analog scales (VAS). Anchors were ‘not at all happy (sad)’ and ‘extremely happy (sad)’.
Ratings of emotion behavior
Emotional facial expression (‘emotion behavior’) was determined through analysis of the videotapes. Eight minutes of video, comprising 2 min from the movie, music/recall and each of the two recovery blocks, was extracted for each mood condition and each participant; video was not available for two participants. Video segments were divided into 15-s clips and presented in random order to three raters who were blind to experimental condition and lesion group. These raters scored the degree of expressed happiness and sadness on a scale from zero (‘not at all’) to four (‘extremely’). Inter-rater reliability for ratings of happiness was excellent with an intraclass correlation (ICC) of 0.91 and for ratings of sadness was acceptable (ICC = 0.61). The greater agreement for happiness replicated previous reports (e.g. Calder et al., 2003) that sadness is more difficult to recognize reliably than is happiness.
Validation of VAS use
In order to familiarize participants with the use of VAS as a means of rating continuous variables, participants completed three rating tasks using scales identical in appearance to those used for subjective mood ratings. The first task required rating the degree of pressure detected in response to standard somatosensory stimulation of the fingertip with Semmes-Weinstein monofilaments. Similarly, a subset of the participants rated photographs of three individuals for apparent age and weight. All participants correctly ordered the somatosensory stimuli and apparent age. Weight judgments were more variable but correct ratings did not differ significantly by group (Ps > 0.56).
Statistical analysis
The current study used alpha = 0.05 (two-tailed) for all tests of statistical significance. Covarying for BDI did not substantively change any of the reported mood induction effects; thus the analyses reported omit this covariate. BDI was not included as a covariate in the PANAS analyses because the two instruments measure overlapping constructs (Watson et al., 1988); covarying for BDI scores therefore could obscure differences in the outcome measure of interest.
PANAS ratings
In order to reduce the number of statistical comparisons, and in the absence of hypotheses concerning specific affective dimensions (e.g. distressed, active) measured by the PANAS, item scores were summed to yield total scores for PA and NA. Overall (across groups) tests of PA vs NA were based on paired samples t-tests. One-way ANOVA was used to test for effects of lesion group; significant effects were examined using follow-up independent t-tests. Finally, the PA and NA scale scores were entered into 3 × 2 repeated measures ANOVAs separately for self- and informant reported PANAS scores, with lesion group (LF, VMF and CTRL) and affect (PA and NA) entered as between- and within-subject factors, respectively; this analysis provided a test for interactions between lesion group and valence of dispositional affect. For hypothesis generating purposes, we also report the effects of PFC damage on the individual affective dimensions measured with this scale (Table 4).
Table 4.
Mean (s.d.) ratings of dispositional affect by lesion group
| Group | |||
|---|---|---|---|
| CTRL | LF | VMF | |
| Positive affect | |||
| Interested | 4.1a (0.5) | 3.6 (0.7) | 3.3b (1.1) |
| Excited | 2.6 (0.9) | 3.0 (0.9) | 3.3 (0.8) |
| Strong | 3.5 (0.8) | 3.3 (0.9) | 3.0 (1.2) |
| Enthusiastic | 3.6a (0.6) | 3.4 (0.5) | 2.7b (1.1) |
| Proud | 3.6 (0.9) | 3.9 (1.1) | 3.1 (1.3) |
| Alert | 3.9a (0.7) | 3.4 (0.7) | 2.6b (1.3) |
| Inspired | 3.3 (0.9) | 3.5 (0.5) | 2.4 (1.6) |
| Determined | 3.6 (1.1) | 4.1 (0.8) | 3.3 (1.3) |
| Attentive | 3.9a (0.5) | 3.9 (0.6) | 2.3b (1.4) |
| Active | 3.5a (0.6) | 3.4 (0.7) | 2.7b (1.1) |
| Negative affect | |||
| Distressed | 1.4a (0.6) | 2.0 (1.1) | 2.4b (1.4) |
| Upset | 1.4a (0.5) | 2.5b (1.4) | 2.0 (1.2) |
| Guilty | 1.4a (0.6) | 2.3b (1.4) | 1.7 (0.8) |
| Scared | 1.3 (0.6) | 1.6 (0.7) | 2.1 (1.7) |
| Hostile | 1.1a (0.3) | 1.6 (1.2) | 2.3b (1.6) |
| Irritable | 1.6 (0.5) | 2.1 (1.4) | 2.4 (1.3) |
| Ashamed | 1.3a (0.4) | 1.8 (1.4) | 2.6b (1.1) |
| Nervous | 1.4a (0.5) | 2.4 (1.7) | 2.4b (1.1) |
| Jittery | 1.1a (0.3) | 2.4 (1.4) | 2.6b (1.1) |
| Afraid | 1.3a (0.6) | 1.4a (0.7) | 2.9b (1.2) |
Note: Values in a row with different superscripts differ significantly at P < 0.05, uncorrected.
Mood inductions
Overall (across groups) tests of the effects of the various mood inductions were based on paired-samples t-tests. Group comparisons were performed first with one-way ANCOVAs (controlling for baseline measures of reported sadness or happiness) to test for any significant differences between groups; significant findings were probed further with t-tests that compared groups directly.
RESULTS
Effects of VMF and LF damage on dispositional affect
Participant reports
Positive affect
Across groups, participants reported experiencing more PA than NA, t(29) = 8.48, P < 0.0001. There was a significant effect of group on total reported PA, F(2,27) = 3.35, P = 0.05, with significantly less PA among the VMF group vs the CTRL group, t(20) = 2.28, P = 0.04, and marginally less PA compared to the LF group, t(13) = 2.06, P =0.06. There was no significant difference between the LF and CTRL groups, t(21) = 0.17, P = 0.87.
Negative affect
Similarly a significant effect of group was detected for NA, F(2,27) = 6.21, P = 0.006; individuals in the VMF group reported significantly more NA than did the CTRL group, t(20) = 4.83, P = 0.0001. The LF group also reported significantly greater NA than did the CTRL group, t(21) = 2.34, P = 0.03, but the two PFC lesion groups did not differ significantly on this measure, t(13) = 0.71, P = 0.49. There was a significant interaction between group and valence (PA or NA) on reported affect, F(2,27) = 9.34, P = 0.0008. As can be seen in Figure 2, the PA vs NA difference was greatest in the CTRL group, smaller (P = 0.05) in the LF group, and smallest in the VMF group (P = 0.03 and P = 0.0003 vs the LF and CTRL groups, respectively). The specific affective dimensions driving these effects are shown in Table 4.
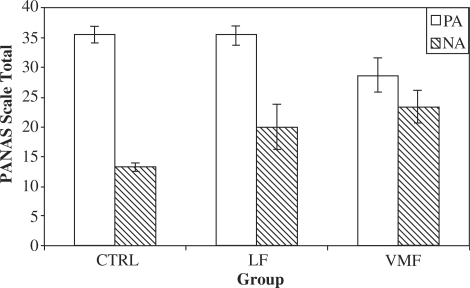
Fig. 2.
Lesion group by affect valence interaction on self ratings of dispositional affect. PA and NA refer to positive and negative affect, respectively.
Informant reports
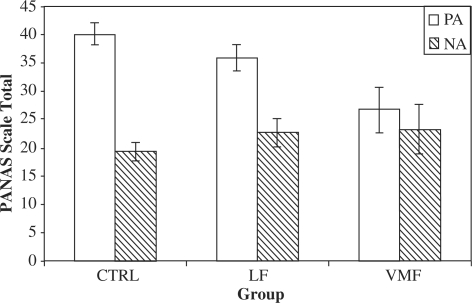
Across groups, informants reported more PA than NA, t(29) = 6.07, P < 0.0001 (Figure 3). There was a significant effect of group on total reported PA, F(2,27) = 6.82, P = 0.004; informants reported significantly less PA among the VMF group vs the CTRL group, t(20) = 3.51, P = 0.002, and marginally less PA compared to the LF group, t(13) = 2.04, P = 0.06. The CTRL and LF groups did not differ significantly, t(21) = 1.39, P = 0.18. Contrary to the self-report NA findings there was no significant effect of lesion group on informant reports of NA, F(2,27) = 0.80, P = 0.46, nor any significant interactions between specific affective dimension and group for either PA (P = 0.55) or NA (P = 0.17). Finally, there was a significant interaction between group and valence on reported affect, F(2,27) = 5.39, P = 0.01. The informant-reported PA vs NA difference was greatest in the CTRL group, non-significantly smaller (P = 0.15) in the LF group, and smallest in the VMF group (P = 0.12 and P = 0.003 vs the LF and CTRL groups, respectively; see Figure 3).
Fig. 3.
Lesion group by affect valence interaction on informant ratings of dispositional affect. PA and NA refer to positive and negative affect, respectively.
Effects of LF and VMF damage on transient affective experience
We now present the results from the transient mood inductions. We begin with emotion reactivity findings for sadness (subjective reports, observer ratings); happiness reactivity data are presented next in the same order. These analyses provide a test of whether emotional reactivity differs as a function of lesion group. Emotion recovery findings are presented next, in the same order as for reactivity.
Emotion reactivity
Sadness
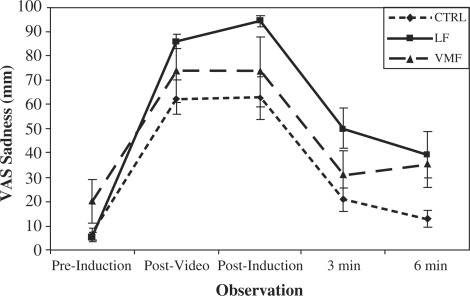
Subjective reports
Peak mood induction sadness was calculated as the mean of the mood ratings given after each mood induction segment (sad movie and autobiographical memory/music). There was a significant increase in sadness from pre- to post-induction, t(29) = 12.84, P < 0.0001, indicating that sad mood inductions were highly effective. There was a significant effect of group, F(3,26) = 3.71, P = 0.03; the LF group reported greater subjective reactivity to the sad mood induction (see Figure 4). Pairwise comparisons confirmed a significant difference in sadness reactivity between the LF and CTRL groups, t(20) = 3.02, P = 0.007, and between the LF and VMF groups, t(12) = 2.29, P = 0.04. Sadness reactivity in LF patients taking an SSRI did not differ significantly from LF patients not taking this class of medication, nor was SSRI status a significant predictor of sad mood reactivity across groups (Ps = 0.14–0.55).
Fig. 4.
Sadness ratings before and after the sad mood induction.
Emotion behavior
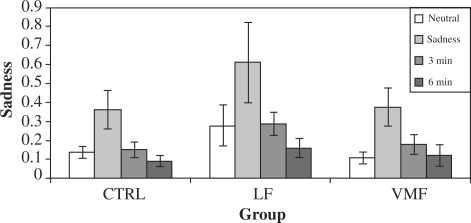
Reactivity based on observer ratings was calculated in a similar fashion as was subjectively reported reactivity, by taking the mean of the sadness ratings following the two phases of the sad mood induction. We controlled for potential group differences in ratings of sadness across the board (e.g. one group might look sad in all conditions) by using sadness ratings in the neutral condition as a baseline against which to compare sadness ratings in the sad condition (Figure 5). Raters observed significantly greater levels of sadness in the sad condition vs the neutral condition, t(27) = 3.25, P = 0.003. The direction of group differences was consistent with the pattern observed in subjective ratings, with greatest sadness observed among the LF group (see Figure 5); however, these differences were not statistically significant.
Fig. 5.
Observer ratings of sad emotion behavior. Rating of sadness in the neutral condition is used as a baseline. ‘3 min’ and ‘6 min’ refer respectively to the first and second 3-min blocks of recovery time following the mood inductions.
Happiness
Subjective reports
Peak happiness was calculated in the same way as peak sadness. There was a significant increase in happiness from pre- to post-induction, t(29) = 5.00, P < 0.0001, but no significant effect of group, F(2,26) = 1.38, P = 0.27.
Emotion behavior
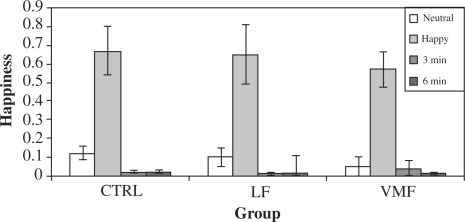
Happiness reactivity was calculated in a similar way as for sadness (see above); results indicated a significant effect of the happy mood induction across all participants, t(27) = 7.04, P < 0.0001 (see Figure 6). There was no effect of group status on happiness reactivity, F(3,24) = 0.62, P = 0.61.
Fig. 6.
Observer ratings of happy emotion behavior. Rating of happiness in the neutral condition is used as a baseline. ‘3 min’ and ‘6 min refer respectively to the first and second 3-min blocks of recovery time following the mood inductions.
Emotion recovery
We next assessed the extent to which participants recovered from the induced sad and happy emotions. These analyses tested whether individuals with PFC damage would show deficits in their ability to recover from negative moods.
Sadness
Subjective reports
Degree of mood recovery was calculated as the difference between peak sadness ratings and mood ratings at 3 min post-mood induction and between ratings at peak and at 6 min post-mood induction. There was a significant effect of time at both post-induction assessments (Ps < 0.0001), showing that across groups, reported sadness dropped significantly after the sad mood inductions. However, there was no significant effect of group on recovery from sadness for either recovery period (Ps > 0.63). Indeed, inspection of Figure 4 shows that the slopes of the lines during the period when most mood recovery occurred (from peak to 3 min post-induction) are nearly parallel.
Emotion behavior
Observer-rated sadness recovery was calculated as the difference between ratings of sadness during the sad mood inductions and sadness ratings during the 3- and 6-min recovery periods. There was a significant effect of time at the 3- and 6-min recovery periods (Ps < 0.008). Group status did not predict observer-rated sadness recovery at any time point, controlling for sadness ratings during the neutral condition (all Ps > 0.51; see Figure 5).
Happiness
Subjective reports
Individuals’ happiness ratings decreased following the happy mood induction (from peak to 3 min post-induction), t(29) = 3.31, P = 0.003. There were no significant differences between groups in their recovery at either the 3- or the 6-min time periods (Ps > 0.27).
Emotion behavior
Participants displayed significantly less happiness during the recovery phase vs the induction phase, t(27) = 7.75, P < 0.0001 (see Figure 6). Analyses of observer ratings of recovery from the happy mood induction yielded no significant effect of group on recovery (Ps > 0.93).
DISCUSSION
This study explored the roles of VMF and LF regions in two aspects of affective experience: dispositional affect in everyday life, and transient changes in emotional state in the context of a laboratory mood induction. VMF damage was associated with a more negative profile of dispositional affect according to both self- and informant ratings, while transient responses to sad and happy emotional stimuli in the laboratory were nearly identical to the responses of control participants. In contrast, LF damage was linked to a significantly greater emotional response to sad stimuli during the mood induction, but did not have a detectable impact on dispositional affect in everyday life. Findings from the two lesion groups are discussed in turn.
VMF
This study confirms findings in the literature suggesting emotional changes in everyday life after VMF damage, but provides more specific information about the nature of those changes. Both self- and informant-reports indicated significantly less PA, as well as self-reported greater NA, compared to the control group. It is worth noting that these dispositional changes do not reflect emotional ‘blunting’, which would reduce both positive and negative affect, but rather indicate a consistent bias toward negative (and away from positive) dispositional emotion.
In contrast, the VMF group was consistently similar to healthy controls in the mood induction experiment. Their subjective reactivity and recovery from happiness and sadness were virtually indistinguishable from those of healthy controls, arguing against a necessary role for VMF regions in emotion experience and recovery occurring over minutes. The current happiness data are in accord with findings by Beer et al. (2003), who reported that participants with damage to OFC showed similar amusement as controls following a ‘teasing interaction’ in a laboratory setting. However, these results seem at odds with neuroimaging reports (e.g. Ohira et al., 2006) showing the activation of VMF (along with lateral PFC regions) during emotion suppression. One possibility is that the intentional regulation of negative emotion may rely more on VMF regions, while passive negative emotion recovery—as featured in the current study as well as in the paradigm used in Hariri et al. (2003)—may depend to a greater extent on lateral PFC regions (see Mauss et al., 2007, for an extended discussion of automatic emotion regulation). The current study cannot address this issue directly because it did not include a condition in which participants were asked to exert conscious control over their emotional responses.
We next turn our attention to the findings for the LF group, in which we found a very different pattern of results.
LF
The LF group showed an exaggerated sadness response during the mood induction, but mood recovery that was indistinguishable from that reported by the CTRL and the VMF groups. It appears that damage to lateral PFC regions leads to an enhanced emotional impact of actively attended sad stimuli; once those stimuli are no longer present, LF damage did not affect the ability to recover from this mood perturbation.
Studies using neuroimaging paradigms have demonstrated a role for lateral PFC regions in the regulation of negative affect (e.g. Mayberg et al., 1999; Ochsner et al., 2004; Ohira et al., 2006). Findings by Hariri and colleagues (2003) suggest that lateral PFC attenuates the fear-related amygdala response in the presence of negative images; the authors concluded that this region may be involved in ‘a system by which humans can control and direct their emotional responses’ (p. 500).
Several authors have reviewed data that suggest a distinction between dorsolateral and ventrolateral PFC in the regulation of affective states. For example, Ochsner and Gross (2005) in a review of their work and others concluded that ventral PFC plays a primary role in matching affective responses to the evaluations of stimuli; dorsal PFC on the other hand has a greater role in higher-order cognitive regulation of emotional stimuli, including reappraisal (see also Gross and Ochsner, 2008). Phillips et al. (2003) also propose a ventrolateral and dorsolateral distinction in emotion regulation; in their model, ventrolateral regions are responsible for assigning affective valence to stimuli and producing the appropriate affective response, while dorsal regions are involved to a greater degree in the regulation of these affective states. Given that the LF group in the present sample included patients with both dorsolateral and ventrolateral PFC damage, we are unable to draw finer-grained conclusions about the critical contributions of these subregions. However, the hyper-responsiveness of this group of patients to the sad mood induction is in keeping with disruption of emotion regulation, at least in the presence of emotional stimuli.
The present data argue for a distinction between modulation of emotional experience while exposed to emotional stimuli (either externally presented or internally generated), which the present results suggest requires intact lateral PFC, and recovery of emotion in the absence of ongoing emotional input, which does not appear to rely on the frontal lobes. This finding raises the interesting possibility that mood homeostasis is fractionated, with active ‘executive control’ (see, e.g. Ochsner and Gross, 2005) involved only in modulating on-going emotional input. In the absence of such input, recovery may primarily reflect spontaneous ‘decay’ of the activity initially triggered by these inputs, a process which here occurred over a few minutes.
Despite the greater transient sadness reactivity reported by the LF group, these individuals by and large reported dispositional affect that was not significantly different from that of the CTRL group. This result raises intriguing questions about the determinants of dispositional affect, arguing against its being simply an integration or average of transient affective experience. The dissociability of dispositional and transient affect suggested by the present study will be an interesting direction for future work.
This study set out to clarify the role of different PFC regions in affective experience using a component process framework. Taken together, the findings support the usefulness of this component process approach. By breaking down affective experience according to timeframe (dispositional vs. transient) and phase (reactivity vs recovery), and by looking at specific dimensions of affective experience (e.g. positive vs negative, happy vs sad, subjective experience vs objective behavior), it is possible to move from a general clinical impression that PFC damage leads to emotional changes to a more specific description of the affective processes in which particular PFC regions play a critical role. These results suggest that dispositional and transient affect are distinct processes that rely on at least partly distinct neural substrates. Furthermore, the findings suggest that the ability to modulate emotional reactions in the presence of a negative stimulus is separable from the process of regulating a negative emotional state once the stimulus has been removed.
Strengths and limitations
Several important experimental controls make it unlikely that either low-level difficulties in using the self-report scales (VAS) or sensitivity to demand characteristics contributed substantially to the current results. As discussed earlier, all groups used VAS appropriately to rate perceptual stimuli. Also, the greater sadness response for the LF group was specific to the sad condition and therefore unlikely to reflect a general tendency among this group to give extreme ratings. Group differences in the observer ratings of transient emotion behavior were in the same direction as the subjective reports (although the former were not detectable at the 0.05 significance level), suggesting that the greater sadness reactivity among participants with damage to lateral PFC regions was not an artifact of these individuals’ agreeableness with the experimenters’ expectations or of other extraneous factors. Similarly, the observer ratings of emotion behavior suggest that the subjectively reported VMF emotional experiences are not merely demand effects of the mood induction. Likewise, the consistent finding of a smaller PA:NA ratio among individuals with damage to VMF according to both self- and informant reports increases the confidence that can be placed in this result.
This exploratory study involved a very detailed examination of affective experience, but in a relatively small sample. As with all lesion studies, replication of the current findings in a different group of individuals with damage to PFC will be important. Further work in a larger sample will also be required to more clearly specify the regions within lateral PFC that are important for sadness over-reactivity. For example, larger studies may be able to clarify whether patients with LF lesions that include damage to the insula differ in any systematic way from patients with intact insulae, as well as clarifying the specific contributions of dorsolateral and ventrolateral subregions. In addition, larger studies may be able to determine whether there are differential effects of specific aspects of the mood induction (e.g. video vs sad memory recall, order of mood induction). Finally, it will be important to determine whether the effects observed here are specific to passive emotional recovery vs more intentional emotion regulation; paradigms that include instructions for the regulation of induced emotional states may address this question.
CONCLUSIONS
These findings suggest a critical role of lateral PFC in emotion modulation in the presence of sadness-evoking emotional stimuli; additionally, they provide evidence that individuals with VMF damage can experience positive and negative emotional states, and recover normally from these transient emotions. The consistent disruptions in dispositional affect seen among the VMF group demonstrate the importance of this region in modulating the ‘tonic’ aspect of emotional experience; furthermore, they show the dissociability of dispositional affect and transient emotional responses.
Conflict of Interest
None declared.
Acknowledgments
We thank Hilary Gerstein for providing technical assistance and Marianna Stark for assistance recruiting and scheduling patients. This work was supported by National Institutes of Health (NIH) R21-DA01586, Canadian Institutes of Health Research (CIHR) MOP-77583 and a CIHR Clinician-Scientist award to L.K.F. S.J.G. was funded by NIH F31-MH073363. A.S.H. was funded by NIH T32-NS07413. M.J.F. was funded by NIH R01- HD043078, R01-DA18913, R01-DA14129 and R21-DA01586.
REFERENCES
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18:355–81. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annual Review of Psychology. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd. 1996. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manly T, et al. Facial expression across the adult life span. Neuropsychologia. 2003;41:195–202. doi: 10.1016/s0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamerl J.-R, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–70. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ Error: Emotion, Reason and the Human Brain. New York: 1994. Grosset/Putnam. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. American Psychologist. 2000;55:1196–214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, et al. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Hutcherson C.AC, Ochsner KN, Glover GH, Gabrieli J.DE, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. NeuroImage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998;2:271–99. [Google Scholar]
- Gross JJ, Ochsner KN. Cognitive emotion regulation: insights from social cognitive and affective science. Current Directions in Psychological Science. 2008;17:153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. Journal of Cognitive Neuroscience. 2008;20:721–33. doi: 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ. Social Cognitive and Affective Neuroscience. 2009. Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. doi:10.1093/scan/nsp001 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Bunge SA, Gross JJ. Automatic emotion regulation. Social and Personality Psychology Compass. 2007;1:146–67. [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, et al. Association of neural and physiological responses during emotion suppression. NeuroImage. 2006;29:721–33. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Roberts NA, Beer JS, Werner KH, et al. The impact of orbital prefrontal cortex damage on emotional activation to unanticipated and anticipated acoustic startle stimuli. Cognitive, Affective, and Behavioral Neuroscience. 2004;4:307–16. doi: 10.3758/cabn.4.3.307. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–46. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:264–70. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110:145–72. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience. 2002;14:913–21. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53:401–33. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Valenstein ES. Great and Desperate Cures: The Rise and Decline of Psychosurgery and Other Radical Treatments for Mental Illness. New York: Basic Books; 1986. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences. 2002;99:2450–4. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]