Abstract
Background/Aim:
Resistance to metronidazole is one of the most common reasons for Helicobacter pylori treatment failure with the classic triple therapy. The clarithromycin-based regimen is not cost-effective for use in developing countries. Though furazolidone is a great substitute it has many side effects. Decreasing the duration of treatment with furazolidone to 1 week may help decrease the drug's side effects.
Aim:
To study the efficacy and side effects of furazolidone when given for 1 week in combination with bismuth subcitrate, amoxicillin, and omeprazole.
Patients and Methods:
One hundred and seventy-seven patients with duodenal ulcer were randomly divided into two groups. Group I received omeprazole 2 Χ 20 mg + amoxicillin 2 Χ 1 g + bismuth subcitrate 4 Χ 120 mg for 2 weeks, with furazolidone 2 Χ 200 mg in the first week only. Group II received the same regimen, except that 1 week of furazolidone was followed by 1 week of metronidazole in the second week. Control endoscopy was performed after 6 weeks. Three biopsies from the antrum and three from the corpus were taken for urease testing and histology. Eradication was concluded if all tests were negative for H pylori.
Results:
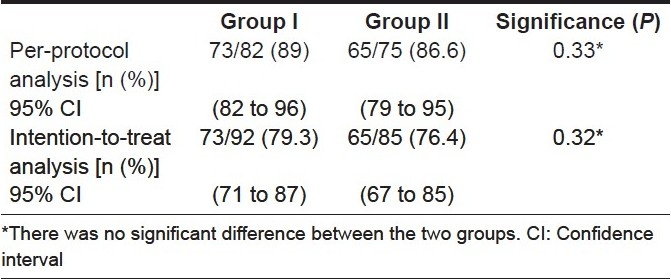
One hundred and fifty-seven patients completed the study. Two subjects from group I and three from group II did not tolerate the regimen and were excluded from the analysis. No serious complication was detected in any patient. The eradication rates by per-protocol (PP) analysis and intention-to-treat (ITT) analysis were 89% and 79.3% in group I and 86.6% and 74.4% in group II, respectively.
Conclusion:
One week of furazolidone in combination with 2 weeks of amoxicillin, omeprazole, and bismuth subcitrate is a safe and cost-effective regimen for the eradication of H pylori. Adding metronidazole to the above regimen does not increase the eradication rate.
Keywords: Eradication, furazolidone, Helicobacter pylori
Helicobacter pylori infection plays an important role in the pathogenesis of peptic ulcer disease. Its eradication leads to cure of the disease and prevents relapse. [1–5]The optimal regimen should have high efficacy, tolerable side effects, simple dosage and should be economical. Intensive efforts are being made to identify such an optimal regimen, but there are many obstacles hindering the achievement of this goal. The H pylori strains have high diversity, with resistance to antibiotics varying in the different regions in the world.[6]The number of strains of H pylori that are resistant to metronidazole and clarithromycin has been increasing during the past few years. Clinical experience shows that regimens recommended for H pylori eradication in developed countries do not show the same efficacy in developing countries.[6–9] In Iran, many strains of H pylori are resistant to metronidazole and clarithromycin but not to furazolidone.[10–11]Furazolidone is an effective antibiotic against H pylori infection but its serious (though rare) adverse effects at the therapeutic dose (400 mg/day) limits its widespread use.[12]The sensitivity of H pylori to metronidazole and clarithromycin can increase the efficacy of furazolidone.[13]
In our previous study, we observed that there is significant increase in the efficacy of furazolidone in H pylori eradication when the dose is increased from 100 mg to 400 mg a day; however, the side effects also increase.[14]Although previous studies in Iran have shown that metronidazole could be replaced with furazolidone,[6,12,15] a 2-week course of furazolidone at 400 mg/day is associated with significant side effects; unfortunately, eradication cannot generally be achieved with a dose under 400 mg/day.[14] In addition, reports from Iran have also shown that a period of treatment of less than 14 days reduces the chances of cure.[10–11] So, the aim of present study was to assess the cure rate and side effects of a 1-week course of furazolidone (400 mg/day) given in combination with 2 weeks of bismuth subcitrate, amoxicillin, and omeprazole. Additionally, in one group of patients, metronidazole was added in the second week of the treatment period to find out how this affected the rate of H pylori eradication.
PATIENTS AND METHODS
A total of 177 patients with H pylori-associated duodenal ulcer, proven by endoscopic findings, were enrolled in this study. The exclusion criteria were as follows: Age < 15 years; gravidity or lactation; glucose-6-phosphate dehydrogenase deficiency (which is common in this region); history of gastric surgery; history of upper gastrointestinal bleeding in the last 4 weeks; intake of antibiotics or any acid suppressant or nonsteroidal anti-inflammatory drugs in the preceding 4 weeks; history of drug allergy; presence of chronic hepatic, renal, or pulmonary disease; contraindication to any of the drugs used in the study; and history of having undergone eradication therapy in the past.
The aim and nature of the trial was fully explained to the patients and their consent obtained. The enrolled patients were randomly allotted into two groups to receive the following medications:
Group I: Omeprazole (capsules, 20 mg) b.i.d (before breakfast and dinner), amoxicillin (capsules, 1 g) b.i.d (with breakfast and dinner), bismuth subcitrate tablets,120 mg q.i.d for 2 weeks, and furazolidone tablets, 200 mg b.i.d (with breakfast and dinner) for 1 week.
Group II: Received the same regimen as group I, except that in the second week metronidazole tablets, 500mg b.i.d. was given instead of furazolidine (i.e., 1 week furazolidone and 1 week metronidazole).
Subjects were questioned 2 weeks post treatment regarding any adverse effects and drug compliance. Use of more than 80% of the recommended drugs was considered as good compliance. Control endoscopy was done 8 weeks after completion of treatment, and three specimens were taken from the antrum and three from the corpus for rapid urease test and histologic examination.
Hematoxylin-eosin, Alcian blue, and Giemsa stains were used for morphologic examination of helicobacter-like organisms (HLO) and the updated Sydney system was used for grading. Histopathology was performed by a specialized pathologist. Eradication was assumed if all six specimens were negative for H pylori.
Statistical analysis
Statistical calculations were performed with SPSS, version 10.0 (SPSS Inc., Chicago, IL., USA). Demographic characteristics, H pylori cure rates, and side effects were compared using the two-tailed Pearson chi-square test. All patients were evaluated in an ITT analysis, in which patients without final H pylori determination or with protocol violation were considered treatment failures. The PP analysis included all subjects who took at least 80% of each study medication as prescribed and who completed the final H pylori status assessment. Statistical significance was set at P < 0.05.
RESULTS
Of 177 patients who were enrolled, 157 patients completed the study; 55.4% were males and 44.6% were females. The mean age was 38.84 ± 10.42 years (range: 14-80 years). There were no significant differences between the two groups with regard to age, gender, smoking habits, and endoscopic findings [Table 1].
Table 1.
Patient demographics and clinical characteristics
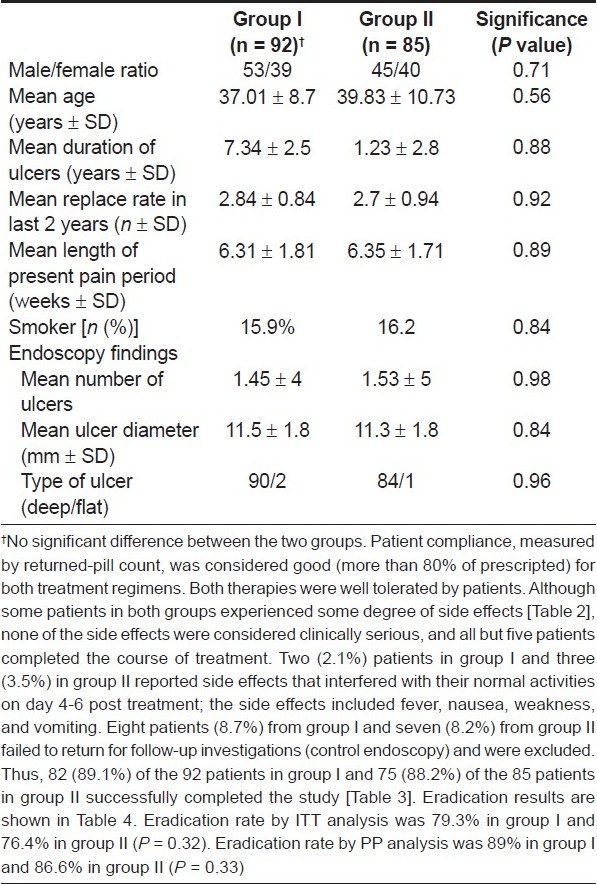
Only two patients from group I and three from group II did not tolerate the regimen; these patients were excluded from the study [Table 2]. There were no serious complications. Thus, decreasing the duration of furazolidone treatment to 1 week with a dose of 400 mg/ day was not associated with severe adverse effects.
Table 2.
Adverse treatment effects
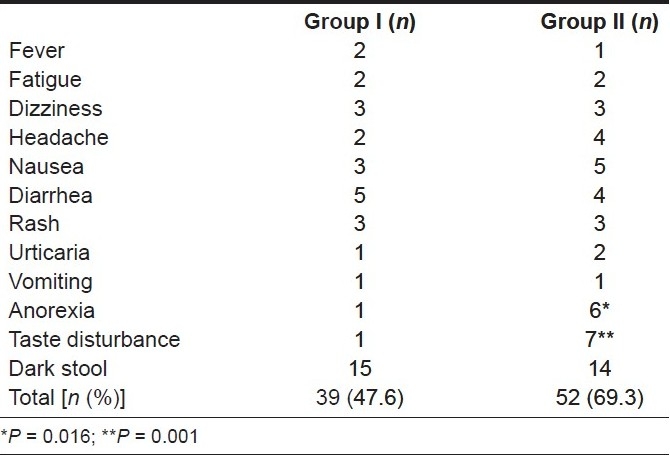
There was no significant difference between the eradication rates in the two groups both by PP and ITT analysis. The addition of metronidazole in the second week of treatment did not result in an increase in the eradication rate, but it was associated with more side effects [Tables 3 and 4].
Table 3.
Overview of treatment results
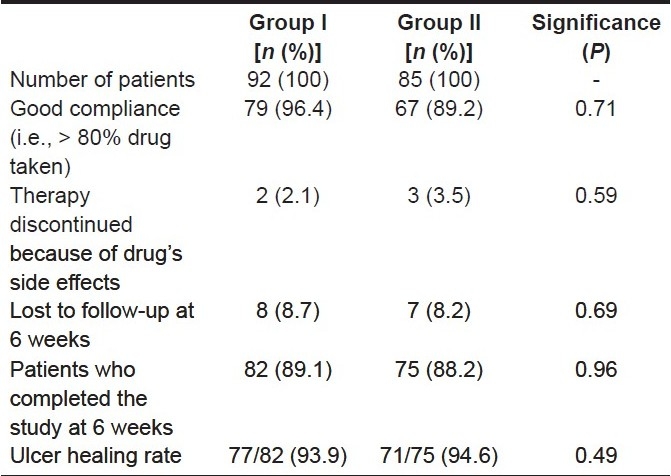
Table 4.
Comparison of eradication between the two groups
DISCUSSION
The eradication rate of H pylori infection in peptic ulcer patients in developing countries is far from optimal with the recommended regimens from developed countries. [16] About 40-70% of H pylori strains are resistant to metronidazole, which is believed to be the main cause of treatment failure.[4,13,17–20] The increase in the prevalence of resistant strains observed in developing countries, as well as in European countries and North America, is the reason for the poor efficacy of metronidazole-based regimens.[2,3,21–27]
Clarithromycin, a macrolide antibiotic, has been used and recommended in the European and North American consensus conferences as an effective drug to replace metronidazole.[28,29] However, this drug is not a feasible option in developing countries because of its high cost. Reports about the development of strains resistant to this drug are also increasing.[9,20,22,30–33] O'Morain et al. reported an increase in the prevalence of resistant strains from 1% to 13%.[27] Graham et al. has described the problems faced when treating clarithromycin-resistant strains of H pylori. [7] The results obtained with various regimens in Iran reveal the presence of special and more toxic strains that do not respond to the optimal regimens recommended in developed countries. This is a challenging situation for developing countries, necessitating the use of a new drug in place of clarithromycin.
Furazolidone is an antibiotic that was introduced in the late 1950s. It is effective against both gram-negative and gram-positive bacteria.[34,35] It has good enteral absorption as well as tissue distribution.[35] It has an ability to promote peptic ulcer healing, which is now known to be via its action on H pylori.[21,36]
In the past few years, there have been many reports about the efficacy of furazolidone when used in combination regimens. [15,35–41] and many authors have considered this drug as a good replacement for clarithromycin.[21,23] The sensitivity of H pylori to furazolidone is reported to be very high in South Korea.[18] In various reports, fewer than 4% of strains were found to be resistant to furazolidone. [18,42,43] In Iran, where the H pylori strains have been shown to be resistant to metronidazole in 37-40% of cases and against clarithromycin in 15% of cases, none of the H pylori isolates were resistant to furazolidone.[43] Its severe but rare side effects, such as episodes of hypotension, sudden fever, headache, and rash, limit the widespread use of this drug. It seems that its monoaminoxidase-inhibitor effect causes these side effects, which can probably be prevented by avoiding tyramine-containing foods such as old cheese.[42] Our previous study revealed that most of the serious side effects with a doze of 400 mg/day occur in the second half of the therapy period, 10 days after the beginning of drug therapy.[14]
Microbiological investigations have also revealed that low concentrations of furazolidone in medium (3 mg/cc) can suppress the growth of H pylori, without development of drug resistance. Thus, dose adjustment and reduction in the duration of treatment appear to be reasonable approaches.[43]
In our previous study, we observed a significant decrease in both side effects and efficacy of furazolidone (in the eradication of H pylori) when the dose was lowered from 400 mg to 100 mg.[14] Fakheri et al. has reported similar results.[43] Thus, a shorter period of treatment with furazolidone and the addition of another drug (such as bismuth), in combination with amoxicillin and omeprazole, seems to be reasonable.[44]
Studies from developing countries such as Iran have shown that regimens shorter than 2 weeks were not as effective as they were reported to be in the developed countries.[10] Our previous study found that furazolidone at a dose of 400 mg daily for more than 1 week led to severe adverse effects.[14] In the present study we advised furazolidone for only 1 week in order to decrease the drug′s side effects. We found that this improved compliance.
Xiao et al. treated patients with furazolidone in combination with amoxicillin and omeprazole for 1 week and found a high eradication rate of 86% and 87% by ITT and PP analysis, respectively.[38] In another study, furazolidone was given (in combination with amoxicillin and metronidazole) only for 5 days but at a high dose of 600 mg daily, and the authors reported that serious complications were not seen. The eradication rate did not differ when the duration of therapy was prolonged to 10 days.[34]
Another aspect of therapy is the improvement in efficacy seen when bismuth is given in combination with furazolidone plus amoxicillin and omeprazole for H pylori eradication.[44] In the present study we decided not only to reduce the duration of treatment with furazolidone but also to add bismuth subcitrate to the regimen.
De Boer et al. has reported that metronidazole administered at a dose of over 750 mg per day could overcome bacterial resistance.[44] Therefore, in the present study, after 1 week of furazolidone therapy we administered 1000 mg/day of metronidazole in group II.
CONCLUSION
This study showed that, as compared with clarithromycin-containing regimens, 400mg/day of furazolidone for 1 week given in combination with 2 weeks of amoxicillin, omeprazole, and bismuth subcitrate is a good, safe, and cost-effective regimen for the eradication of H pylori in Iran. There are few side effects with this regimen and therefore patient compliance is very good. The addition of metronidazole in the second week of the treatment period does not appear to increase the eradication rate.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 1995;9:59–69. [PubMed] [Google Scholar]
- 2.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;6(272):65–9. [PubMed] [Google Scholar]
- 3.Abu-Mahfouz MZ, Prasad VM, Santogade P, Cutler AF. Helicobacter pylori recurrence after successful eradication: 5-year follow-up in the United States. Am J Gastroenterol. 1997;92:2025–8. [PubMed] [Google Scholar]
- 4.Tytgat GN. Review article: Treatments that impact favourably upon the eradication of Helicobacter pylori and ulcer recurrence. Aliment Pharmacol Ther. 1994;8:359–68. doi: 10.1111/j.1365-2036.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 5.De Boer WA, Tytgat GN. Regular review: Treatment of Helicobacter pylori infection. BMJ. 2000;320:31–4. doi: 10.1136/bmj.320.7226.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakheri H, Malekzadeh R, Merat S, Khatibian M, Fazel A, Alizadeh BZ, et al. Clarithromycin vs.furazolidone in quadruple therapy regimens for the treatment of Helicobacter pylori in a population with a high metronidazole resistance rate. Aliment Pharmacol Ther. 2001;15:411–6. doi: 10.1046/j.1365-2036.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 7.Graham DY, Qureshi WA. Antibiotic-resistant H pylori infection and its treatment. Curr Pharm Des. 2000;6:1537–44. doi: 10.2174/1381612003399077. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe H, Watari J, Shibata N, Satoh T, Yokota K, Kohgo Y. Usefulness of new triple therapy containing PPI. Nippon Rinsho. 2001;59:314–8. [PubMed] [Google Scholar]
- 9.van der Hulst RW, Keller JJ, Rauws EA, Tytgat GN. Treatment of Helicobacter pylori infection: A review of the world literature. Helicobacter. 1996;1:6–19. doi: 10.1111/j.1523-5378.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 10.Malekzadeh R, Merat S, Derakhshan MH, Siavoshi F, Yazdanbod A, Mikaeli J, et al. Low Helicobacter pylori radication rates with 4- and 7-day regimens in an Iranian population. J Gastroenterol Hepatol. 2003;18:13–7. doi: 10.1046/j.1440-1746.2003.02897.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaviani MJ, Malekzadeh R, Vahedi H, Sotoudeh M, Kamalian N, Amini M, et al. Various durations of a standard regimen (amoxycillin, metronidazole, colloidal bismuth sub-citrate for 2 weeks or with additional ranitidine for 1 or 2 weeks) on eradication of Helicobacter pylori in Iranian peptic ulcer patients.A randomized controlled trial. Eur J Gastroenterol Hepatol. 2001;13:915–9. doi: 10.1097/00042737-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, Osato MS, Hoffman J, Opekun AR, Anderson SY, El-Zimaity HM. Furazolidone combination therapies for Helicobacter pylori infection in the United States. Aliment Pharmacol Ther. 2000;14:211–5. doi: 10.1046/j.1365-2036.2000.00640.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994;89:S116–28. [PubMed] [Google Scholar]
- 14.Roghani HS, Massarrat S, Shirekhoda M, Butorab Z. Effect of different doses of furazolidone with amoxicillin and omeprazole on eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2003;18:778–82. doi: 10.1046/j.1440-1746.2003.03058.x. [DOI] [PubMed] [Google Scholar]
- 15.Malekzadeh R, Ansari R, Vahedi H, Siavoshi F, Alizadeh BZ, Eshraghian MR, et al. Furazolidone versus metronidazole in quadruple therapy for eradication of Helicobacter pyloriin duodenal ulcer disease. Aliment Pharmacol Ther. 2000;14:299–303. doi: 10.1046/j.1365-2036.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- 16.Saberi-Firoozi M, Massarrat S, Zare S, Fattahi M, Javan A, Etaati H, et al. Effect of triple therapy or amoxycillin plus omeprazole or amoxycillin plus tinidazole plus omeprazole on duodenal ulcer healing, eradication of Helicobacter pylori, and prevention of ulcer relapse over a 1-year follow-up period: A prospective, randomized, controlled study. Am J Gastroenterol. 1995;90:1419–23. [PubMed] [Google Scholar]
- 17.Roghani HS, Massarrat S, Pahlewanzadeh MR, Dashti M. Effect of two different doses of metronidazole and tetracycline in bismuth triple therapy on eradication of Helicobacter pyloriand its resistant strains. Eur J Gastroenterol Hepatol. 1999;11:709–12. doi: 10.1097/00042737-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pyloriisolates from Korea. J Antimicrob Chemother. 2001;47:459–61. doi: 10.1093/jac/47.4.459. [DOI] [PubMed] [Google Scholar]
- 19.Drouin E. Helicobacter pylori: Novel therapies. Can J Gastroenterol. 1999;13:581–3. doi: 10.1155/1999/485237. Review. [DOI] [PubMed] [Google Scholar]
- 20.Thijs JC, Van Zwet AA, Moolenaar W, Oom JA, De Korte H, Runhaar EA. Short report: Clarithromycin, an alternative to metronidazole in the triple therapy of Helicobacter pyloriinfection. Aliment Pharmacol Ther. 1994;8:131–4. doi: 10.1111/j.1365-2036.1994.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 21.Dani R, Queiroz DM, Dias MG, Franco JM, Magalhães LC, Mendes GS, et al. Omeprazole, clarithromycin and furazolidone for the eradication of Helicobacter pyloriin patients with duodenal ulcer. Aliment Pharmacol Ther. 1999;13:1647–52. doi: 10.1046/j.1365-2036.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- 22.Fennerty MB, Lieberman DA, Vakil N, Magaret N, Faigel DO, Helfand M. Effectiveness of Helicobacter pyloritherapies in a clinical practice setting. Arch Intern Med. 1999;159:1562–6. doi: 10.1001/archinte.159.14.1562. [DOI] [PubMed] [Google Scholar]
- 23.Mégraud F. Resistance of Helicobacter pylorito antibiotics. Aliment Pharmacol Ther. 1997;11:43–53. doi: 10.1046/j.1365-2036.11.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 24.Osato MS, Reddy R, Graham DY. Metronidazole and clarithromycin resistance amongst Helicobacter pyloriisolates from a large metropolitan hospital in the United States. Int J Antimicrob Agents. 1999;12:341–7. doi: 10.1016/s0924-8579(99)00079-5. [DOI] [PubMed] [Google Scholar]
- 25.Reddy R, Osato M, Gutierrez 0. Metronidazole resistance is high in Korea and Colombia and appears to be rapidly increasing in the US (Abstract) Gastroenterology. 1996;110:A236. [Google Scholar]
- 26.O'Morain C, Montague S. Challenges to therapy in the future. Helicobacter. 2000;5:S23–6. doi: 10.1046/j.1523-5378.2000.0050s1023.x. discussion S27-31. [DOI] [PubMed] [Google Scholar]
- 27.New antidepressant drug opens up important treatment and safety options. Ir Med J. 1996;89:214. [PubMed] [Google Scholar]
- 28.McNamara D, O'Morain C. Consensus guidelines: Agreement and debate surrounding the optimal management of Helicobacter pyloriinfection. Can J Gastroenterol. 2000;14:511–7. doi: 10.1155/2000/604563. [DOI] [PubMed] [Google Scholar]
- 29.Tytgat GNJ. Aspects of Anti-Helicobacter pylori, Basic Mechanism to Clinical Cure 1996. Dordrecht: Kluwer Academic. 1996:304–47. [Google Scholar]
- 30.al-Assi MT, Ramirez FC, Lew GM, Genta RM, Graham DY. Clarithromycin, tetracycline, and bismuth: A new non-metronidazole therapy for Helicobacter pyloriinfection. Am J Gastroenterol. 1994;89:1203–5. [PubMed] [Google Scholar]
- 31.Jawetz E. Basic and Clinical Phar-macology. In: K-atzung BG, editor. Drugs with specialized indication and urinary antiseptic. 6th ed. California: Appleton and Lange; 1995. p. 738. [Google Scholar]
- 32.Smith JN. Goth's Medical Pharmacology, 13 th ed. In: dark WG, Brater DC, Johnson AR, editors. Drugs used to treat amebiasis and other intestinal protozoal infections. St Louis: New York Mosby; 1992. p. 689. [Google Scholar]
- 33.Howden A, Boswell P, Tovey F. In-vitro sensitivity of Campylobacter pyloridis to furazolidone. Lancet. 1986;2:1035. doi: 10.1016/s0140-6736(86)92639-5. [DOI] [PubMed] [Google Scholar]
- 34.Van Zwet AA, Thijs JC, van der Wouden EJ, Kooy A. Low cure rate of Helicobacter pyloriinfection with omeprazole and furazolidone dual therapy for one week. Aliment Pharmacol Ther. 1997;11:533–5. doi: 10.1046/j.1365-2036.1997.00166.x. [DOI] [PubMed] [Google Scholar]
- 35.Xiao SD, Liu WZ, Xia DH, Jiang SJ, Wang RN, Zhang ZH, et al. The efficacy of furazolidone and metronidazole in the treatment of chronic gastritis associated with Helicobacter (Campylobacter) pylori-a randomized double-blind placebo-controlled clinical trial. Hepatogastroenterology. 1990;37:503–6. [PubMed] [Google Scholar]
- 36.Liu WZ, Xiao SD, Hu PJ, Lu H, Cui Y, Tytgat GN. A new quadruple therapy for Helicobacter pyloriusing tripotassium dicitrato bismuthate, furazolidone, josamycin and famotidine. Aliment Pharmacol Ther. 2000;14:1519–22. doi: 10.1046/j.1365-2036.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu WZ, Xiao SD, Shi Y, Wu SM, Zhang DZ, Xu WW, et al. Furazolidone-containing short-term triple therapies are effective in the treatment of Helicobacter pyloriinfection. Aliment Pharmacol Ther. 1999;13:317–22. doi: 10.1046/j.1365-2036.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 38.Xiao SD, Liu WZ, Hu PJ, Ouyang Q, Wang JL, Zhou LY, et al. A multicentre study on eradication of Helicobacter pyloriusing four 1-week triple therapies in China. Aliment Pharmacol Ther. 2001;15:81–6. doi: 10.1046/j.1365-2036.2001.00895.x. [DOI] [PubMed] [Google Scholar]
- 39.Kwon DH, Lee M, Kim JJ, Kim JG, El-Zaatari FA, Osato MS, et al. Furazolidone- and nitrofurantoin-resistant Helicobacter pylori: Prevalence and role of genes involved in metronidazole resistance. Antimicrob Agents Chemother. 2001;45:306–8. doi: 10.1128/AAC.45.1.306-308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendonça S, Ecclissato C, Sartori MS, Godoy AP, Guerzoni RA, Degger M, et al. Prevalence of Helicobacter pyloriresistance to metronidazole, clarithromycin, amoxicillin, tetracycline, and furazolidone in Brazil. Helicobacter. 2000;5:79–83. doi: 10.1046/j.1523-5378.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 41.Malekzadeh R, Pourkhajeh A, Siavoshi F. Antibiotic resistance of Helicobacter pylorito antibiotics in Iran. Gastroenterology. 1999;116:244. [Google Scholar]
- 42.Gilman AG, Rail TW, Niles AS, Taylor P. 8 ed. NewYork: Pergamon Press; 1991. Goodman and Gillman′s Pharmacological basis of therapeutics. [Google Scholar]
- 43.Fakheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylorieradication. Aliment Pharmacol Ther. 2004;19:89–93. doi: 10.1046/j.1365-2036.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 44.de Boer WA. Bismuth triple therapy: Still a very important drug regimen for curing Helicobacter pyloriinfection. Eur J Gastroenterol Hepatol. 1999;11:697–700. [PubMed] [Google Scholar]


