Abstract
In an attempt to understand the nature of selectivity in Ti-mediated reductive cross-coupling between homoallylic alcohols and imines, we investigated whether thermodynamic equilibration of the presumed organometallic intermediate plays a role in selectivity. No evidence could be found for olefin exchange in preformed azatitanacyclopentanes – an observation that is consistent with a model based on kinetically controlled selective carbometalation.
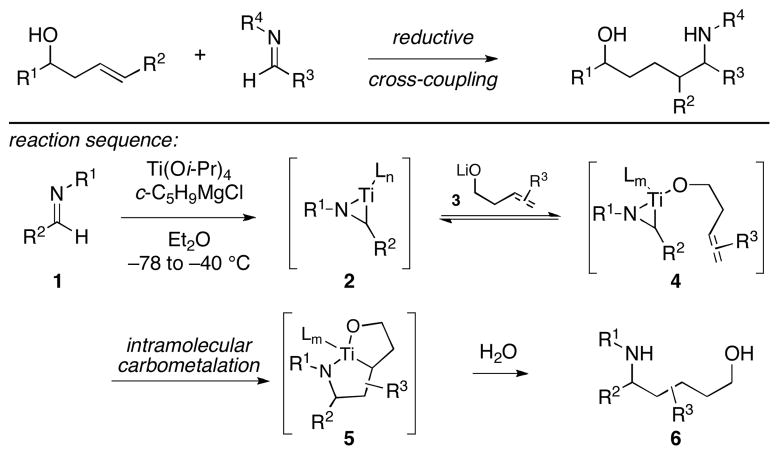
Reductive cross-coupling is emerging as a powerful strategy for bimolecular C–C bond formation.1 This mode of reactivity has historically been limited to a relatively small substrate scope as a result of firmly established barriers associated with the control of reactivity and selectivity in the bimolecular C–C bond forming event.2 In a program aimed at advancing a suite of methods for reductive cross-coupling that overcome these limitations, we recently described highly regio- and stereoselective coupling reactions of substituted homoallylic alcohols with aromatic imines (Figure 1).3 This process, proceeding with stoichiometric use of an inexpensive and non-toxic metallic species (Ti(Oi-Pr)4), was accomplished by: 1) initial conversion of an aromatic imine to an azatitanacyclopropane, 2) introduction of a homoallylic alkoxide, and 3) protonation of the presumed bicyclic azametallacyclopentane intermediate. A mechanistic proposal to account for the enhanced reactivity and high selectivity observed was put forth based on: 1) rapid and reversible ligand exchange at titanium, and 2) site- and stereoselective intramolecular carbometalation under kinetic control.
Figure 1.
Reductive cross-coupling of homoallylic alcohols with imines.
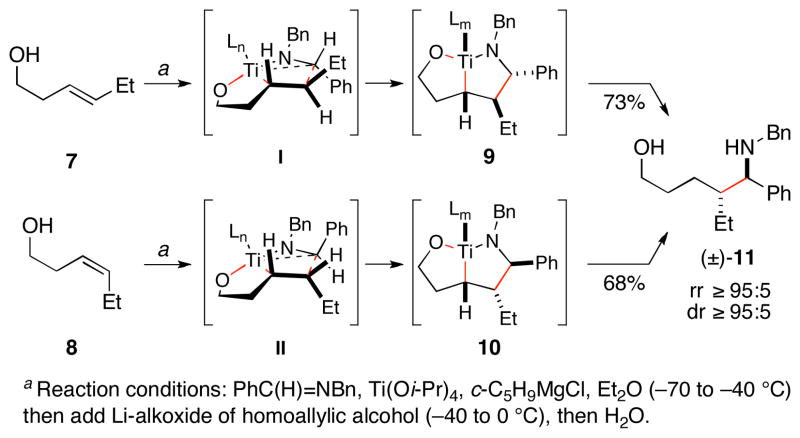
During the course of these studies, we observed a stereoconvergence in reductive cross-coupling between disubstituted alkenes and aromatic imines. As illustrated in Figure 2, coupling of the homoallylic alcohol 7 or 8 with a simple aromatic imine provided the anti-1,5-amino alcohol 11 in 68 and 73% yield with very high levels of stereoselection (dr ≥ 95:5).3 This observation was consistent with a mechanistic proposal whereby each alkene underwent selective carbometalation by way of distinct geometries (I and II). While the number of ligands on Ti in the transition state for these coupling reactions remains unclear, we expected that the development of a cis-fused bicyclo-[3.3.0] system would be favoured.4 Further, minimization of eclipsing 1,2-interactions about the developing C–C bond was thought to be important for the control of stereochemistry en route to intermediates 9 and 10. Subsequent protonation would then furnish the 1,5-amino alcohol 11. Overall, the high anti-selectivity and stereoconvergence was consistent with this mechanistic proposal based on kinetic control.
Figure 2.
Stereoconvergence in alkene–imine coupling.
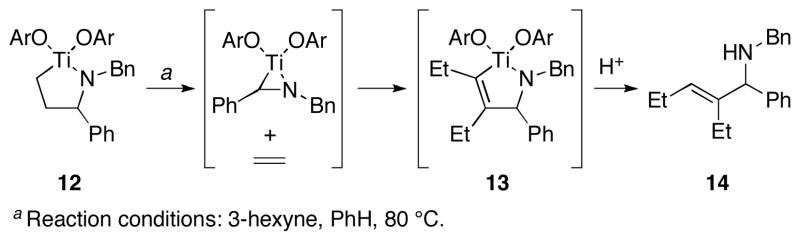
Previous reports in other laboratories have demonstrated that a variety of metallacyclopentane intermediates can readily undergo fragmentation with loss of an alkene ligand (Figure 3).5,6 These observations led us to speculate whether the selectivity of our alkene–imine coupling was a result of thermodynamic, rather than kinetic, control.
Figure 3.
C–C bond cleavage from a preformed azatitanacyclopentane.5
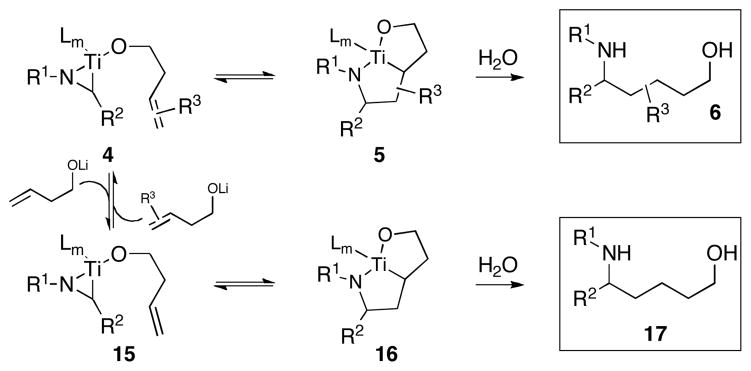
Our studies aimed at addressing this question began with an analysis of the basic reaction process – we accepted that: 1) Ligand exchange at Ti was readily reversible and fast with respect to C–C bond formation,7 and 2) azatitanacyclopentane formation followed from syn-carbometalation across the alkene. As depicted in Figure 4, if C–C bond-formation is readily reversible under the reaction conditions (4 ⇌ 5), then rapid and reversible ligand exchange at Ti should allow for alkene exchange (4 ⇌ 15). Subsequent carbometalation and protonation would then deliver 1,5-amino alcohol 17 from the preformed azametallacyclopentane 5.
Figure 4.
Potential equilibria for alkene exchange.
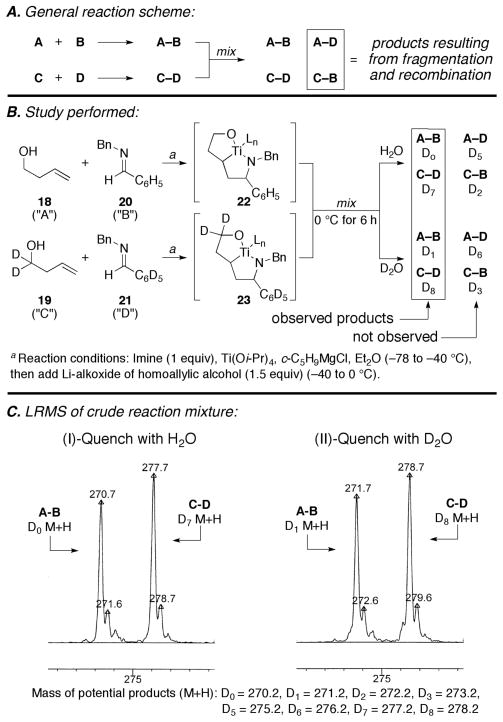
To determine whether such an alkene exchange could occur under the reaction conditions employed in our reductive cross-coupling, we explored the general reaction scheme depicted in Figure 5A. Here, metallacyclopentane intermediates with similar steric and electronic properties were sought to avoid biasing any potential equilibria. With this goal in mind, homoallylic alcohol 18 was coupled to imine 20 alongside the deuterated analogs 19 and 21.8, 9 After reductive coupling was judged complete, the reaction mixtures were combined and stirred for an additional six hours at 0 °C to allow for equilibration. Subsequent hydrolysis of the presumed organometallic intermediates (H2O) provided two of the four possible products (Figure 5B and 5C-I). Lack of evidence for D5 (M +H = 275.2) or D2 (M+H = 272.2)-containing products is consistent with the proposition that the equilibrium discussed in Figure 4 does not play a role in product distribution.
Figure 5.
Cross-over experiments.
To confirm that the experimental procedure was sufficient for the conclusion reached, we needed to validate that quenching of the organometallic intermediates did not occurr during the mixing process. As such, a related experiment was performed where the organometallic intermediates were quenched with D2O. In this experiment, only D1 and D8-containing products were identified (no evidence was found for the D6 or D3-containing cross-over products; Figure 5B and 5C-II).
In conclusion, we provide evidence that alkene exchange in titanium alkoxide-mediated reductive cross-coupling of imines with homoallylic alcohols does not occur under the reaction conditions described. Because rapid and reversible ligand exchange at Ti would allow for alkene exchange from mixed titanate esters (i.e. 4 and 15), we conclude that carbometalation under these reaction conditions occurs in an irreversible manner. While more substituted alkenes may behave differently in this coupling process, the current study describes conclusive evidence in support of kinetic selectivity for the reductive cross-coupling of simple homoallylic alcohols with aromatic imines.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support of this work by the National Institutes of Health - NIGMS (GM080266 and GM080266-04S1).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and spectral data are included for all new compounds. See http://dx.doi.org/10.1039/b000000x/
Notes and references
- 1.For recent reviews, see: Reichard HA, McLaughlin M, Chen MZ, Micalizio GC. Eur J Org Chem. 2009 doi: 10.1002/ejoc.200901094.Ng S-S, Ho C-Y, Schleicher KD, Jamison TF. Pure Appl Chem. 2008:929. doi: 10.1351/pac200880050929.Montgomery J, Sormunen GJ. Top Curr Chem. 2007;279:1.Skucas E, Ngai MY, Komanduri V, Krische MJ. Acc Chem Res. 2007;40:1394. doi: 10.1021/ar7001123.
- 2.The majority of reductive methods for cross-coupling are limited to a relatively small subset of coupling partners. See ref. 1 for reviews.
- 3.Takahashi M, Micalizio GC. J Am Chem Soc. 2007;129:7514. doi: 10.1021/ja071974v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For the strain energy of cis- and trans-fused bicyclo-[3.3.0]-octane, see: Chang S, Boyd RH, McNally D, Sharyteh S, Hickey MJ. J Am Chem Soc. 1970;92:3109.For an example where a transfused bicyclo-[3.3.0] Zr-containing system is preferred, see: Nugent WA, Taber DF. J Am Chem Soc. 1989;111:6435.
- 5.Hill JE, Fanwick PE, Rothwell IP. Organometallics. 1992;11:1775. [Google Scholar]
- 6.For related examples of C–C bond cleavage in metallacyclopentanes, see: McDermott JX, Wilson ME, Whitesides GM. J Am Chem Soc. 1976;98:6529.Grubbs RH, Miyashita A. J Am Chem Soc. 1978;100:1300.Dorf U, Engel K, Erker G. Angew Chem Int Ed Engl. 1982;21:914.Cohen SA, Auburn PR, Bercaw JE. J Am Chem Soc. 1983;105:1136.Cohen SA, Bercaw JE. Organometallics. 1985;4:1006.Erker G, Czisch P, Kruger C, Wallis JM. Organometallics. 1985;4:2059.Erker G, Dorf U, Rheingold AL. Organometallics. 1988;7:138.Takahashi T, Tamura M, Saburi M, Uchida Y, Negishi E. J Chem Soc Chem Commun. 1989:852.Takahashi T, Fujimori T, Seki T, Saburi M, Uchida Y, Rousset CJ, Negishi E. J Chem Soc, Chem Commun. 1990:182.Ito H, Taguchi T, Hanzawa Y. Tetrahedron Lett. 1992;33:4469.Taber DF, Louey JP, Lim JA. Tetrahedron Lett. 1993;34:2243.Taber DF, Louey JP, Wang Y, Nugent WA, Dixon DA, Harlow RL. J Am Chem Soc. 1994;116:9457.Harris MCJ, Whitby RJ, Blagg J. Tetrahedron Lett. 1995;36:4287.
- 7.For a discussion of ligand exchange with titanium alkoxides, see: Woodard SS, Finn MG, Sharpless KB. J Am Chem Soc. 1991;113:106.
- 8.In each case, 1.5 eq of homoallylic alcohol were used to ensure that, along with free isopropoxide (generated during the formation of the Ti-imine complex), the bicyclic metallacyclopentane intermediates have the potential to undergo ligand exchange with excess alkoxide.
- 9.For the preparation of 19, see: Negishi E, Boardman LD, Sawada H, Bagheri V, Stoll AT, Tour JM, Rand CL. J Am Chem Soc. 1988;110:5383.For the preparation of 20, see: Simion A, Simion C, Kanda T, Nagashima S, Mitoma Y, Yamda T, Mimura K, Tashiro MJ. Chem Soc, Perkin Trans. 2001;1:2071.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.