Abstract
Objective:
To find out the hypoglycemic and hypolipidemic activity of an ethanolic extract of the aerial part of Salvadora oleoides Decne in euglycemic and alloxan-induced diabetic albino rats.
Materials and Methods:
Diabetes was induced in albino rats by administration of alloxan monohydrate (120 mg/kg, i.p.). Normal as well as diabetic albino rats were divided into groups (n = 6) receiving different treatments: vehicle (control), ethanolic extract (1 g and 2 g/kg b.w), and standard antidiabetic drug tolbutamide (0.5 g/kg b.w.). Blood samples were collected by cardiac puncture and were analyzed for blood glucose and lipid profile on days 0, 7, 14, and 21.
Results:
The ethanolic extract of S oleoides produced significant reduction (P < 0.001) in blood glucose and also had beneficial effects (P < 0.001) on the lipid profile in euglycemic as well as alloxan-induced diabetic rats at the end of the treatment period (21st day). However, the reduction in the blood glucose and improvement in lipid profile was less than that achieved with the standard drug tolbutamide.
Conclusion:
We concluded that an ethanolic extract of S oleoides is effective in controlling blood glucose levels and improves lipid profile in euglycemic as well as diabetic rats.
Keywords: Antidiabetic, arteriosclerosis, diabetes mellitus, hypercholesterolemia, sulphonylureas
Diabetes mellitus is heterogeneous primary disorder of carbohydrate metabolism with multiple etiological factors; it generally involves absolute or relative insulin deficiency, or insulin resistance, or both. Whatever the cause, diabetes ultimately leads to hyperglycemia, which is the landmark of this disease syndrome.[1] NIDDM has also been associated with an increased risk for premature arteriosclerosis due to increase in triglycerides and low density lipoprotein levels. About 70-80% of deaths in diabetic patients are due to vascular disease. An ideal treatment for diabetes would be a drug that not only controls the glycemic level but also prevents the development of arteriosclerosis and other complications of diabetes.[2]
Long before the use of insulin became common, indigenous remedies were used for the treatment of diabetes mellitus and hyperlipidemia. There has been an increasing demand from patients for the use of natural products with antidiabetic and antihyperlipidemic activity. This is largely because insulin cannot be used orally and insulin injections are associated with the risk of hypoglycemia and impairment of hepatic and other body functions. The undesirable side effects and contraindications of synthetic drugs, and the fact that they are not suitable for use during pregnancy, have made scientists look towards hypoglycemic agents of plant origin.[3] Many herbs and plant products have been shown to have antihyperglycemic and antihyperlipidemic action.[4–6]
Salvadora oleoides Decne (Salvadoraceae family), commonly known in India as meetha jal is an oil-yielding medicinal and multipurpose tree. It can grow in arid and alkaline conditions.[7] The leaves of S. oleoides are used to relieve cough and for treatment of enlarged spleen and fever. The leaves of S. oleoides are said to possess anti-inflammatory, analgesic, and antiulcer activity.[8] The objective of the present study was to evaluate the hypoglycemic and hypolipidemic activity of an ethanolic extract of the aerial parts (stem and leaves) of S. oleoides in normal and alloxan-induced diabetic rats.
Materials and Methods
Animals
Adult albino rats of 16-19 weeks age and of either sex, weighing 180-200 g, were procured from the Disease-Free Small-Animal House, Haryana Agricultural University, Hissar (India). The animals were kept in clean and dry plastic cages, with 12 h: 12 h light-dark cycle at 25 ± 2°C temperature and 45-55% relative humidity. The animals were fed with standard pellet diet (Hafed, Rohtak) and water was given ad libitum. For experimental purpose the animals were kept fasting overnight but allowed free access to water. The Institutional Animal Ethics Committee of the Department of Biosciences, M. D. University, Rohtak approved the study.
Preparation of the extract
Aerial parts (stem and leaves) of S. oleoides Decne (Salvadoraceae) were collected in April-June from Matanhail, Jhajjar District, Haryana (India). The plant material was authenticated by the FRI, Dehradun. A voucher specimen (No. 14/153533) was deposited at FRI, Dehradun.
The plant material was dried under shade and powdered in a grinder. The powdered material (100 g) was extracted with 70% ethanol by hot continuous percolation method in a Soxhlet apparatus. The extract was evaporated to dryness under vacuum and dried in a vacuum desiccator to obtain a residue of 13.65 g.
Drug administration
The quantities of the individual drugs to be administered were calculated and suspended in vehicle (1% w/v suspension of carboxymethylcellulose (CMC) in water 10 ml/kg b.w). The drug was administered continuously for 21 days orally using an infant feeding tube. The results were compared with that of the standard drug tolbutamide which was also given continuously for 21 days.
Determination of LD50 of S. oleoides
LD50 was calculated by the probit analysis method.[9]
Induction of experimental diabetes
A single dose (120 mg/kg b.w, i.p.) of alloxan monohydrate (1%) dissolved in sterile normal saline was used for induction of diabetes mellitus in the rats. Diabetes was confirmed 1 week after alloxan injection by determining the blood glucose concentration; only animals with blood glucose of 200-300 mg/dl (mild diabetes) were used for the experiment. The diabetic animals were allowed free access to tap water and pellet diet and were maintained at room temperature in plastic cages.
Collection of blood and experimental setup
The rats were anesthetized with diethyl ether and blood samples were drawn from the heart of the animals. The rats were divided into two groups as follows:
Euglycemic rats: Euglycemic rats were divided into four groups, each group having six animals. Group I served as control and received 1% w/v suspension of CMC in water at a dose of 10 ml/kg b.w. Group II and III received ethanolic extract of S. oleoides in 1% CMC at a dose of 1 g and 2 g/kg b.w, respectively. Group IV received the standard drug tolbutamide at a dose of 0.5 g/kg b.w. Blood glucose and lipid profile were estimated before starting the treatment and weekly (7, 14, and 21 days) thereafter up to the end of treatment period.
Diabetic rats: Diabetic rats were also divided in to four groups as described above. Blood glucose and lipid profile were determined at day 0, 7, 14, and 21.
Estimation of blood glucose and lipid profile
Fasting blood glucose was determined using the ortho-toluidine method.[10] Total cholesterol estimation was done using the Erba diagnostic kit.[11] Serum triglycerides were estimated using Enzokit (Ranbaxy).[12] HDL-cholesterol was determined using the Erba diagnostic kit.[13] VLDL (very low density lipoproteins)-cholesterol was calculated as: triglycerides/5; LDL (low density lipoproteins) cholesterol was calculated by the equation:
LDL-cholesterol = total cholesterol − (HDL + VLDL).
All estimations were done using the Erba Transasia auto-analyzer.
Statistical analysis
The blood glucose and lipid levels before and after the administration of extract were compared using Student's ‘t’ test. The data on blood glucose level was also analyzed by one-way ANOVA. The minimum level of significance was fixed at P < 0.05.
Probit analysis method
Ten rats were given S. oleoides ethanolic extract in doses of 0.5, 1, 2, 3, 5, 6, and 10 g/kg b.w. The rats were kept under observation for 21 days to monitor mortality. The percentage of mortality was calculated and values were transformed to probit scale [Table 1]. Calculating the value from table, the LD50 was found to be 29.51.
Table 1.
Log concentration/probit mortality regression equation for rats exposed to different doses of S oleoides
| Doses of S oleoides (g/kg b.w.) | No. of rats exposed | % mortality | Log conc. (X) | Probit | X2 mortality (Y) | Y2 | XY |
|---|---|---|---|---|---|---|---|
| 0.5 | 10 | 0 | −0.3010 | 0 | 0.0906 | 0 | 0 |
| 1 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 10 | 0 | 0.3010 | 0 | 0.0906 | 0 | 0 |
| 3 | 10 | 0 | 0.4771 | 0 | 0.2276 | 0 | 0 |
| 5 | 10 | 0 | 0.6990 | 0 | 0.4886 | 0 | 0 |
| 6 | 10 | 30 | 0.7781 | 4.48 | 0.6055 | 20.07 | 3.48 |
| 10 | 10 | 40 | 1 | 4.75 | 1 | 22.56 | 4.75 |
| ΣX = 2.95 | ΣY = 9.23 | ΣX2 = 2.50 | ΣY2 = 42.63 | ΣXY = 8.27 |
X = 0.422 Y = 1.318
Regression equation Y = −0.15 + 3.484X
Correlation factor for SSX = ΣX2 − (ΣX)2 / N = 1.26
Correlation factor for SSY = ΣY2 − (ΣY2) / N = 41.74
Correlated sum of products of XY (C) = ΣXY − (ΣX. ΣY)/N = 4.39
In the integration equation equation Y = a + bX is the slop value; where ‘b’ = C /SSX = 3.484; and ‘a’ = Y − b.X = − 0.15; Y = a + b X: If ‘Y’ = 5; 5 = −0.15 + 3.484 X; X = 1.47;
LD50 was calculated by formula; LD50 = X log; = log 1.47 (antilog 29.51); thus, the LD50 was 29.51
Results
Effect of ethanolic extract of S oleoides on euglycemic rats
A significant reduction (P < 0.001) in blood glucose levels was observed at the end of the second week (14th day) of treatment with ethanolic extract (1 g/kg and 2 g/kg b.w.) of S. oleoides in the euglycemic rats; this was further lowered after 21 days of treatment. The maximum reduction in blood glucose level was seen at a dose of 2 g/kg b.w, the fall being 10.98, 19.99, and 26.92%, respectively, after 7 days, 14 days, and 21 days of S. oleoides extract administration. However the effect of S. oleoides ethanolic extract was less than that of tolbutamide, which showed 36.05% reduction in blood glucose levels after 21 days of treatment [Figure 1].
Figure 1.
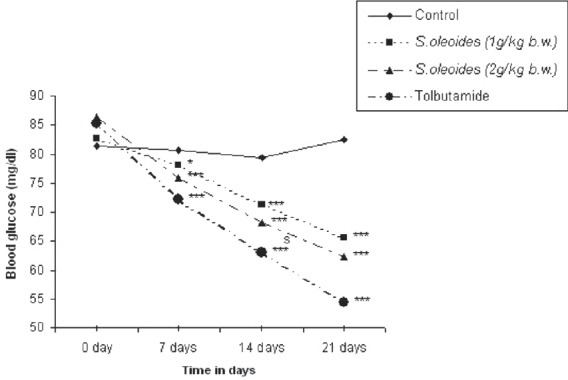
Effect of S oleoides on blood glucose level in euglycemic rats. Significantly different from control (n = 6), *P < 0.05;**P < 0.01;***P < 0.001
As the blood glucose-lowering effect of 2 g/kg b.w. of the ethanolic extract was more, only the effect of this dose on the lipid profile of normal as well diabetic albino rats is shown in Table 2. Administration of the ethanolic extract led to a significant fall (P < 0.001) in the level of triglycerides, total cholesterol, LDL, and VLDL, and improved the HDL levels, in normal rats. Tolbutamide also showed reduction in the levels of triglycerides, total cholesterol, LDL, and VLDL, and improved the HDL, after 21 days [Table 2].
Table 2.
Effect of ethanolic extract (2 g/kg b.w.) of S oleoides on lipid profile in euglycemic rats
| Lipid profile | Period | Control | Ethanolic extract | Tolbutamide |
|---|---|---|---|---|
| TG | 0 day | 84.46 ± 1.50 | 82.28 ± 1.65 | 85.32 ± 1.78 |
| 7 days | 80.32 ± 2.08 | 77.25 ± 1.82*** | 76.21 ± 1.63*** | |
| 14 days | 81.04 ± 1.70 | 71.02 ± 1.46*** | 69.35 ± 1.14*** | |
| 21 days | 80.61 ± 1.94 | 64.70 ± 2.01*** | 62.03 ± 1.46*** | |
| TCH | 0 day | 78.23 ± 1.75 | 79.06 ± 1.89 | 76.14 ± 1.23 |
| 7 days | 79.13 ± 0.71 | 73.72 ± 1.06*** | 70.09 ± 1.37*** | |
| 14 days | 77.65 ± 1.30 | 69.08 ± 1.57*** | 65.43 ± 1.11*** | |
| 21 days | 76.45 ± 0.90 | 62.56 ± 1.78*** | 58.22 ± 1.93 | |
| HDL | 0 day | 22.18 ± 1.01 | 18.21 ± 1.19 | 21.65 ± 1.08 |
| 7 days | 21.06 ± 1.82 | 19.42 ± 1.42NS | 22.78 ± 0.98NS | |
| 14 days | 22.47 ± 1.13 | 20.22 ± 1.53* | 24.61 ± 1.02*** | |
| 21 days | 39.16 ± 1.32 | 21.52 ± 1.23*** | 26.52 ± 1.36*** | |
| LDL | 0 day | 42.01 ± 1.04 | 44.40 ± 2.16 | 42.67 ± 1.72 |
| 7 days | 38.98 ± 1.77 | 38.85 ± 1.89*** | 34.25 ± 1.53*** | |
| 14 days | 39.41 ± 1.53 | 34.66 ± 2.31*** | 29.72 ± 1.19*** | |
| 21 days | 16.98 ± 1.50 | 28.70 ± 1.87*** | 25.92 ± 1.02*** | |
| VLDL | 0 day | 16.89 ± 1.50 | 16.45 ± 1.42 | 17.81 ± 1.24 |
| 7 days | 16.06 ± 1.81 | 15.45 ± 0.92NS | 15.87 ± 1.17NS | |
| 14 days | 16.20 ± 1.62 | 14.20 ± 1.40* | 13.93 ± 1.34* | |
| 21 days | 16.12 ± 1.60 | 12.94 ± 0.74*** | 12.36 ± 1.35*** |
Values are mean ± SD, n = 6 in each group. NS = nonsignificant
P < 0.05
**P < 0.01
P < 0.001 when compared with the vehicle-treated group
Effect of S oleoides ethanolic extract on alloxan-induced diabetic rats
On repeated administration of ethanolic extract at doses of 1g and 2 g/kg b.w.for 21 days, a significant (P < 0.001) dose-dependent decrease in blood glucose of the diabetic rats was seen as compared to the vehicle-treated group. Tolbutamide showed a 29.37% decrease as compared to the control group [Figure 2].
Figure 2.
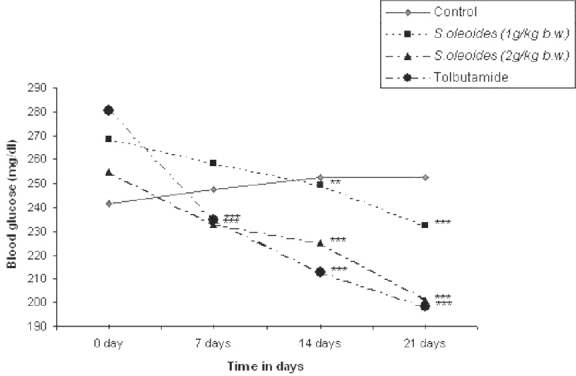
Effect of S oleoides on blood glucose level in alloxan induced diabetic rats. Significantly different from control (n = 6), *P < 0.05; **P < 0.01; ***P < 0.001
Administration of vehicle to alloxan-induced diabetic rats resulted in an increase in the level of triglycerides, total cholesterol, LDL, and VLDL, and decreased HDL, after 21 days. Continuous administration of the ethanolic extract (2 g/kg b.w.) of S. oleoides led to significant decrease (P < 0.001) in the level of triglycerides, total cholesterol, LDL, and VLDL in the diabetic rats, while it increased (P < 0.01) the level of HDL [Table 3].
Table 3.
Effect of ethanolic extract (2 g/kg b.w.) of S oleoides on lipid profile in alloxan-induced diabetic rats
| Lipid profile | Period | Control | Ethanolic extract | Tolbutamide |
|---|---|---|---|---|
| TG | 0 day | 130.06 ± 3.42 | 122.05 ± 3.31 | 132.26 ± 3.04 |
| 7 days | 132.27 ± 3.76 | 119.46 ± 3.62*** | 127.34 ± 3.41*** | |
| 14 days | 136.28 ± 3.33 | 108.23 ± 2.43*** | 116.69 ± 3.56*** | |
| 21 days | 138.94 ± 2.42 | 99.12 ± 2.78*** | 101.63 ± 3.52*** | |
| TCH | 0 day | 125.452 ± 3.93 | 112.62 ± 2.76 | 120.11 ± 2.71 |
| 7 days | 126.34 ± 2.21 | 106.24 ± 2.89** | 110.07 ± 2.93*** | |
| 14 days | 128.60 ± 3.12 | 98.92 ± 1.86*** | 101.27 ± 2.42*** | |
| 21 days | 131.45 ± 2.90 | 90.46 ± 1.80*** | 93.08 ± 2.32*** | |
| HDL | 0 day | 17.94 ± 1.44 | 15.62 ± 1.89 | 16.43 ± 1.87 |
| 7 days | 17.02 ± 1.63 | 16.95 ± 1.62NS | 18.23 ± 1.56NS | |
| 14 days | 16.17 ± 1.02 | 17.81 ± 1.06* | 19.86 ± 1.72** | |
| 21 days | 15.36 ± 1.57 | 18.76 ± 0.81*** | 21.16 ± 1.22*** | |
| LDL | 0 day | 81.50 ± 2.24 | 72.59 ± 2.15 | 82.51 ± 1.96 |
| 7 days | 82.85 ± 2.77 | 65.40 ± 2.16*** | 74.21 ± 2.72*** | |
| 14 days | 85.18 ± 2.01 | 59.40 ± 2.49*** | 65.15 ± 2.36*** | |
| 21 days | 88.04 ± 2.81 | 51.88 ± 2.23*** | 56.48 ± 2.17*** | |
| VLDL | 0 day | 26.01 ± 2.81 | 24.41 ± 1.76 | 27.19 ± 1.76 |
| 7 days | 26.47 ± 2.88 | 23.89 ± 0.72NS | 25.98 ± 1.10NS | |
| 14 days | 27.25 ± 1.82 | 21.64 ± 1.12** | 23.18 ± 1.16*** | |
| 21 days | 27.79 ± 2.41 | 19.82 ± 0.71*** | 20.32 ± 1.28*** |
Values are mean ± SD, n = 6 in each group. NS = nonsignificant
P < 0.05
P < 0.01
P < 0.001 when compared with the vehicle treated group
Discussion
Diabetes mellitus is a chronic disorder caused by partial or complete insulin deficiency, which produces inadequate glucose control and leads to acute and chronic complications. Premature and extensive arteriosclerosis involving renal, peripheral, and cardiovascular vessels remain the major complication of diabetes mellitus. Alteration in the serum lipid profile is known to occur in diabetes and this is likely to increase the risk for coronary heart disease. A reduction in serum lipids, particularly of the LDL and VLDL fraction and triglycerides, should be considered as being beneficial for the long-term prognosis of these patients.[14] Lowering of blood glucose and plasma lipid levels through dietary modification and drug therapy seems to be associated with a decrease in the risk of vascular disease.
In the present study, treatment with S. oleoides ethanolic extract (2 g/kg b.w.) in euglycemic rats produced significant decrease in blood glucose level. The hypoglycemic effect may be due to increased secretion of insulin from the b-cells of the pancreas, i.e., pancreatotrophic action.[15] S. oleoides contain several organic sulfur compounds and it is well known that sulfur derivatives show hypoglycemic effects. In fact, many plants containing sulfur are used traditionally as antidiabetics.[16–17] These compounds produce an increase in insulin, probably by inhibition of some substances competing with insulin for their SH-group. The results were comparable with that of tolbutamide, which acts by stimulation of insulin release,[18] thus further confirming that the extract lowers the blood glucose by a pancreatotrophic action.
Moreover, S. oleoides produced significant beneficial effects in the lipid profile in euglycemic rats, reducing triglycerides, total cholesterol, LDL, and VLDL, and increasing HDL, significantly. The ethanolic extract increased secretion of insulin from b-cells of pancreas; this increased secretion of insulin stimulates fatty acid biosynthesis and also the incorporation of fatty acids into triglycerides in the liver and adipose tissue.[19]
Alloxan, a beta cytotoxin, induces ‘chemical diabetes’ in a wide variety of animal species by damaging the insulin-secreting cells of the pancreas.[19] Literature sources indicate that alloxan rats are hyperglycemic.[20] The use of lower doses of alloxan (120 mg/kg b.w.) produced a partial destruction of pancreatic b-cells even though the animals became permanently diabetic.[21] Thus, these animals have surviving b-cells and regeneration is possible.[22] It is well known that the sulfonylureas (tolbutamide) act by directly stimulating the b-cells of the islets of Langerhans to release more insulin and these compounds are active in mild alloxan-induced diabetes where as they.[23] Since our results show that tolbutamide reduced the blood glucose levels in the diabetic animals, the state of diabetes is not severe.
Prolonged administration of an ethanolic extract of S. oleoides leads to significant reduction in blood glucose level, which is in agreement with other studies.[24,25] The hypoglycemic activity of the drug was due to the regeneration of pancreatic cells that were partially destroyed by alloxan, and potentiation of insulin secretion from surviving b-cells of the islets of Langerhans.[26]
Diabetic rats were observed to have increased plasma lipids, which are responsible for several cardiovascular disorders.[27] The higher lipid levels seen in diabetic rats was due to increased mobilization of free fatty acids from peripheral depots and also due to lipolysis caused by hormones.[28,29] The ethanolic extract leads to regeneration of the b-cells of the pancreas and potentiation of insulin secretion from surviving b-cells; the increase in insulin secretion and the consequent decrease in blood glucose level may lead to inhibition of lipid peroxidation and control of lipolytic hormones. In this context, a number of other plants have also been reported to have antihyperglycemic, antihyperlipidemic, and insulin stimulatory effects.[30–32]
It is well known that LDL plays an important role in arteriosclerosis and that hypercholesterolemia is associated with a defect relating to the lack of LDL receptors. The decrease of cholesterol and LDL levels achieved by administration of ethanolic extract, demonstrates a possible protection against hypercholesterolemia and the harm this condition brings about. Further studies are needed to identify the chemical constituents of the ethanolic extract of S. oleoides that may be responsible for the hypoglycemic and hypolipidemic activity.
Acknowledgments
Sushila Saini would like to thank the UGC for the award of a JRF/SRF.
References
- 1.Olefasky JM. In: Text Book of Medicine. Smith LH, Wyngarden JB, Bennett JC, editors. Philadalphia: WB Saunders; 1992. [Google Scholar]
- 2.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 2nd ed. Oxford: Clarendon Press; 1985. [DOI] [PubMed] [Google Scholar]
- 3.Berger W. Incidence of severe side effects during therapy with sulphonylureas and biguanides. Hormones Metabolic Res. 1985;17:111–5. [PubMed] [Google Scholar]
- 4.Elder C. Ayurveda for diabeties mellitus: A review of the biomedical literature. Altern Ther Health Med. 2004;10:44–50. [PubMed] [Google Scholar]
- 5.Srinvasan K. Plant foods in the management of diabetes mellitus: Spices as beneficial antidiabetic food adjuncts. Int J Food Sci Nutr. 2005;56:399–414. doi: 10.1080/09637480500512872. [DOI] [PubMed] [Google Scholar]
- 6.Badole S, Patel N, Bodhankar S, Jain B, Bhardwaj S. Antihyperglycemic activity of aqueous extract of leaves of Cocculus hirsutus (L.). Diels in alloxan-induced diabetic mice. Indian J Pharmacol. 2006;38:49–53. [Google Scholar]
- 7.Singh U, Wadhwani AM, Johri MB. Dictionary of Economic plants. New Delhi: Indian Council of Agricultural Research; 1996. [Google Scholar]
- 8.Anonymous . The Wealth of India-Raw Materials. IX, PID, New Delhi: CSIR; 1972. [Google Scholar]
- 9.Finney DJ. Probit analysis: A statistical treatment of sigmoid response curve. London: Cambridge University Press; 1971. [Google Scholar]
- 10.Dubowski KM. Blood sugar estimation by o-toluidine method. Clin Chem. 1962;8:215–35. [PubMed] [Google Scholar]
- 11.Roeschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974;12:226. [PubMed] [Google Scholar]
- 12.Buccolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 13.Burstein M, Scholnick HR, Morfin R, Scholnick HR, Morfin R. Rapid method for the isolation of lipoprotein from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–95. [PubMed] [Google Scholar]
- 14.Chattopadhay RF, Bandyopadhyay M. Effects of Azadirachta indica leaf extract on serum lipid profile changes in normal and streptozotocin induced diabetic rats. Afr J Biomed Res. 2005;8:101–4. [Google Scholar]
- 15.Trivedi NA, Mazumdar B, Bhatt JD, Hemavathi KG. Effect of Shilajit on blood glucose and lipid profile in alloxan induced diabetic rats. Indian J Pharmacol. 2004;36:373–6. [Google Scholar]
- 16.Kupiecki FP, Ogzewalla CD, Schell FM. Isolation and characterization of a hypoglycemic agent from Xanthium strumarium. J Pharm Sci. 1974;63:1166–7. doi: 10.1002/jps.2600630736. [DOI] [PubMed] [Google Scholar]
- 17.Oliver-Bever B. Medicinal plants in Tropical West Africa. London: Cambridge University Press; 1986. [Google Scholar]
- 18.Hardy KJ, McNutty SJ. Oral hypoglycemic agents. Med Digest. 1997;23:5–9. [Google Scholar]
- 19.Best CH, Taylor NB. Biological effects of insulin. In: Williams W, Wilkins, editors. Physiological basis of medical practice. London: Cambridge University Press; 1989. [Google Scholar]
- 20.Bopanna KN. Anti diabetic and antihyperlipidemic alloxan diabetic rabbits. Indian J Pharmacol. 1997;29:162–7. [Google Scholar]
- 21.Prince SM, Menon VP. Hypoglycemic and other related actions of Tinospora cardifolia roots in alloxan induced diabetic rats. J Ethanopharmacol. 2000;70:9–15. doi: 10.1016/s0378-8741(99)00136-1. [DOI] [PubMed] [Google Scholar]
- 22.Ayber MJ, Riera AN, Grau A, Sanchez SS. Hypoglycemic effect of the water extract of Smallanthus soncifolius (Yacon) leaves in normal and diabetic rats. J Ethanopharmacol. 2001;74:125–32. doi: 10.1016/s0378-8741(00)00351-2. [DOI] [PubMed] [Google Scholar]
- 23.Gomes A, Vedasiromoni JR, Das M, Sharma RM, Ganguly DK. Antihyperglycemic effect of black tea (Cemellia sinensis) in rat. J Ethnopharmacol. 1995;27:243–75. doi: 10.1016/0378-8741(95)01223-z. [DOI] [PubMed] [Google Scholar]
- 24.Badole S, Patel N, Bodhankar S, Jain B, Bhardwaj S. Antihyperglycemic activity of aqueous extract of leaves of Cocculus hirsutus (L.). Diels in alloxan-induced diabetic mice. Indian J Pharmacol. 2006;38:49–53. [Google Scholar]
- 25.Latha M, Pari L. Antihyperglycaemic effect of Cassia auriculata in experimental diabetes and its effects on key metabolic enzymes involved in carbohydrate metabolism. Clin Exp Pharmacol Physio. 2003;30:38–43. doi: 10.1046/j.1440-1681.2003.03785.x. [DOI] [PubMed] [Google Scholar]
- 26.Suba V, Murugesan T, Bhaskara RR, Ghosh L, Pal M, Mandal SC, Saha BP. Antidiabetic potential of Barleria lupulina extract in rats. Fitoterapia. 2004;75:1–4. doi: 10.1016/s0367-326x(03)00163-1. [DOI] [PubMed] [Google Scholar]
- 27.Alarcon-Aguilar FJ, Campos-Sepulveda AE, Xolalopa-Molina S, Hernandez-Galicia E, Roman-Ramos R. Hypoglycemic activity of Ibervillea sonorae root in healthy and diabetic mice and rats. Pharma Biol. 2002;40:570–5. [Google Scholar]
- 28.Ei-Soud NH, Khalil MY, Oraby FS, Farrag AR. Antidiabetic effects of Fenugreek alkaloid extract in streptozotocin induced hypoglycemic rats. J App Sci. 2007;3:1073–83. [Google Scholar]
- 29.Nikkhila EA, Kekki M. Plasma triglyceride transport kinetics in diabetes mellitus. Metabolism. 1973;22:1–22. doi: 10.1016/0026-0495(73)90024-3. [DOI] [PubMed] [Google Scholar]
- 30.Odetola AA, Akinloye O, Egunjobi C, Adekunle WA, Ayoola AO. Possible antidiabetic and antihyperlipidaemic effect of fermented Parkia biglobosa (JACQ) extract in alloxan-induced diabetic rats. Clin Exp Pharmacol Physiol. 2006;33:808–12. doi: 10.1111/j.1440-1681.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes NP, Lagishetty CV, Panda VS, Naik SR. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Compl Altern Med. 2007;7:29–37. doi: 10.1186/1472-6882-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramalingam S, Pari L. Antihyperlipidemic and antiperoxidative effect of Diasulin, a polyherbal formulation in alloxan induced hyperglycemic rats. BMC Complement Altern Med. 2005;5:14–23. doi: 10.1186/1472-6882-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


