Abstract
Objective:
To assess the in vitro antibacterial, antifungal and cytotoxic activities of amblyone, a triterpenoid isolated from Amorphophallus campanulatus (Roxb).
Methods:
Disc diffusion technique was used for in vitro antibacterial and antifungal screening. Cytotoxicity was determined against brine shrimp nauplii. In addition, minimum inhibitory concentration (MIC) was determined using serial dilution technique to determine the antibacterial potency.
Results:
Large zones of inhibition were observed in disc diffusion antibacterial screening against four Gram-positive bacteria (Bacillus subtilis, Bacillus megaterium, Staphylococcus aureus and Streptococcus pyogenes) and six Gram-negative bacteria (Escherichia coli, Shigella dysenteriae, Shigella sonnei, Shigella flexneri, Pseudomonas aeruginosa and Salmonella typhi). The MIC values against these bacteria ranged from 8 to 64 μg/ml. In antifungal screening, the compound showed small zones of inhibition against Aspergillus flavus, Aspergillus niger and Rhizopus aryzae. Candida albicans was resistant against the compound. In the cytotoxicity determination, LC50 of the compound against brine shrimp nauplii was 13.25 μg/ml.
Conclusions:
These results suggest that the compound has good antibacterial activity against the tested bacteria, moderate cytotoxicity against brine shrimp nauplii and insignificant antifungal activity against the tested fungi.
Keywords: Gram-negative, Gram-positive, MIC, triterpenoid
Introduction
The frequency of life-threatening infections caused by pathogenic microorganisms has increased worldwide and is becoming an important cause of morbidity and mortality in immunocompromised patients in developing countries.[1] Although a large number of antimicrobial agents have been discovered, pathogenic microorganisms are constantly developing resistance to these agents.[1] In recent years, attempts have been made to investigate the indigenous drugs against infectious diseases.[2] This may help to develop safer antimicrobial drugs.[2]
Amorphophallus campanulatus (Roxb.) Bl. (family: Araceae), locally known as Ol Kachu, is a perennial herb with rounded tuberous root stock (corm). The plant is widely distributed in Bangladesh, India and Africa.[3–5] The tuberous roots of the plant have been used traditionally for the treatment of piles, abdominal pain, tumours, enlargement of spleen, asthma and rheumatism.[3–5] The tuberous roots of the plant have also been reported to possess tonic, stomachic and appetizer properties.[4,5] Previously, we have reported the possible antibacterial, antifungal and cytotoxic activities of tuberous roots of Amorphophallus campanulatus.[24] The present study was conducted to determine the antibacterial, antifungal and cytotoxic activities of amblyone, a triterpenoid isolated from tuberous roots of the plant (Fig 1).
Figure 1.
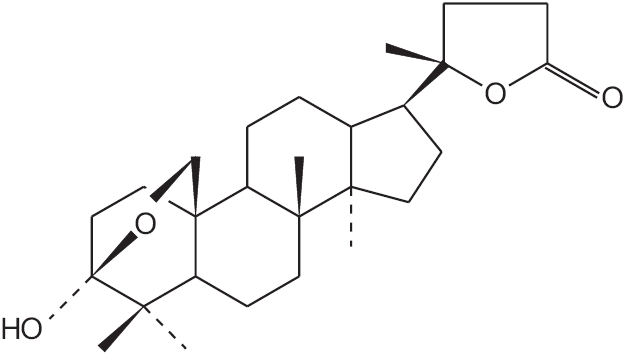
Structure of amblyone
Materials and Methods
Plant material
The tuberous roots of Amorphophallus campanulatus was collected during January 2004 from Katakhali area of Rajshahi district of Bangladesh and identified by Prof. A.T.M. Naderuzzaman, Department of Botany, University of Rajshahi, where its voucher specimen (No. AC9642) was deposited. The tuberous roots were cut, air-dried and ground into powder.
Plant material extraction and fractionation
Powdered dried roots (600 g) of the plant were extracted (cold) with ethanol (4 l) in flat bottom glass containers, through occasional shaking and stirring for 10 days.[6] The whole extract was filtered and the solvent was evaporated to dryness in vacuo with rotary evaporator at 40-50°C to afford a blackish green mass (34 g), which was further extracted with petroleum ether (3 × 50 ml), chloroform (3 × 50 ml) and methanol (3 × 50 ml) and to afford petroleum ether (17 g), chloroform (8 g) and methanol (7 g) fractions, respectively.[7] The preliminary phytochemical screening of different fractions was carried out by chemical tests and thin layer chromatographic methods.[8]
Isolation of compound
The petroleum ether soluble fraction (3 g) was subjected to column chromatography using n-hexane, chloroform and methanol of increasing polarity. Column chromatography fractions eluting with 80% chloroform in n-hexane to 100% chloroform were subjected to preparative TLC (Silica gel PF254) with solvent system ethyl acetate:cyclohexane (3:1) to yield compound 1 (27.6 mg). Its structure was confirmed on the basis of spectroscopic methods [IR, liquid chromatography/electrospray-mass spectroscopy (LC/ES-MS), 1H and 13C NMR including JMOD, COSY, NOESY, HMBC, HSQC]. The LC/ES-MS in the positive ion mode of 1 showed molecular [M + H]+ peak at m/z 431.5 corresponding to a molecular formula of C27H42O4. 1H and 13C NMR data of compound 1 were in good agreement with 1H and 13C NMR data of amblyone published in literature.[9]
Antibacterial activity and minimum inhibitory concentration (MIC) were determined against four Gram-positive bacteria (Bacillus subtilis, Bacillus megaterium, Staphylococcus aureus and Streptococcus pyogenes) and six Gram-negative bacteria (Escherichia coli, Shigella dysenteriae, Shigella sonnei, Shigella flexneri, Pseudomonas aeruginosa and Salmonella typhi). Antifungal screening was carried out against four fungi (Aspergillus flavus, Aspergillus niger, Candida albicans and Rhizopus aryzae). All these tested organisms were available in the Microbiology Research Laboratory of Pharmacy Department, Rajshahi University, Bangladesh. Cytotoxicity was determined against brine shrimp nauplii that were obtained by hatching brine shrimp eggs (Carolina Biological Supply Company, Burlington, NC, USA) in artificial seawater (3.8% NaCl solution) for 48 h.
Media
Nutrient agar media (Difco laboratories) pH 7.2, nutrient broth media (Difco laboratories) pH 6.8, Sabouraud dextrose agar media (Biolife Vole Monza) pH 5.6 and artificial seawater (3.8% NaCl solution) pH 8.4 were used for antibacterial screening, MIC determination, antifungal screening and cytotoxicity determination, respectively.
Antibacterial screening
In vitro antibacterial screening was carried out by disc diffusion method,[10,11] which is a qualitative to semi-quantitative test. Briefly, 20 ml quantities of nutrient agar were plated in Petri dish with 0.1 ml of a 10−2 dilution of each bacterial culture (18 h old). Filter paper discs (6 mm in diameter) impregnated with various concentration of amblyone were placed on test organism-seeded plates. Methanol was used to dissolve the compound and was completely evaporated before application on test organism-seeded plates. Blank disc impregnated with solvent methanol followed by drying off was used as negative control. The activity was determined after 18 h of incubation at 37°C. The diameters of zone of inhibition produced by the amblyone were then compared with the standard antibiotic kanamycin 30 μg/disc. Each sample was used in triplicate for the determination of antibacterial activity.
Minimum inhibitory concentration determination
Serial tube dilution technique[12,13] was used to determine MIC of the compound against these bacteria. Amblyone (1.024 mg) was dissolved in 2-ml nutrient broth media (three drops of Tween 80 added to facilitate dissolution) to obtain a stock solution having concentration of 512 μg/ml. The serial dilution technique was used to obtain 256-2 μg/ml dilutions. One drop (0.02 ml) of prepared suspension of organism (107 organism/ml) was added to each broth dilution. These dilutions were incubated at 37°C for 18 h and examined for the growth. The MIC of amblyone was taken as the lowest concentration that showed no growth. Growth was observed in those tubes where the concentration of the amblyone was below the inhibitory level and the broth medium was turbid. The nutrient broth media with three drops of Tween 80 and kanamycin were used as negative and positive control, respectively.
Antifungal screening
This was also carried out by disc diffusion method.[10,11] In this method, 20 ml of Sabouraud dextrose was plated in Petri dish with 0.2 ml of a 10−2 dilution of each fungal culture (10 h old). The activity was determined after 72 h of incubation at 30°C. The diameter of zone of inhibition produced by the amblyone was then compared with the standard antibiotic nystatin 30 μg/disc. Each sample was used in triplicate for the determination of antifungal activity.
Cytotoxicity assay
The cytotoxicity assay was performed on brine shrimp nauplii using Mayer method.[14,15] Dissolution of compound was performed in artificial seawater using DMSO. Each 5 ml solution of different concentrations (0.5, 1, 2, 5, 10, 20 and 40 μg/ml) of the compound was taken in different vials where brine shrimp nauplii were placed and observed for mortality for 24 h. The resulting data were transformed to probit analysis[16] for the determination of LC50 values of the compound. Artificial seawater medium containing DMSO was used as control. Gallic acid and vincristine sulphate were used as standards in this assay.
Results
The results of antibacterial activity of amblyone against the test bacteria are presented in Table 1. In comparison to reference standard kanamycin (30 μg/disc), amblyone exhibited significant antibacterial activity at 160 μg/disc. Amblyone showed highest activity against Bacillus megaterium and lowest against Pseudomonas aeruginosa. The MIC values against Gram-positive and Gram-negative bacteria ranged from 8 to 32 and 16 to 64 μg/ml, respectively [Table 2].
Table 1.
In vitro antibacterial activity of amblyone and kanamycin
| Test organism | Strain No. | Zone of inhibition (diameter in mm) | ||
|---|---|---|---|---|
| Amblyone (80 μg/disc) | Amblyone (160 μg/disc) | Kanamycin (30 μg/disc) | ||
| Gram-positive | ||||
| Bacillus subtilis | QL 40 | 21 ± 1.5 | 24 ± 1.3 | 30 ± 2.1 |
| Bacillus megaterium | QL 38 | 22 ± 1.3 | 26 ± 2.2 | 28 ± 0.9 |
| Staphylococcus aureus | ATCC 259233 | 19.5 ± 0.8 | 23 ± 1.5 | 31 ± 1.4 |
| Streptococcus pyogenes | CRL | 20.5 ± 1.4 | 25 ± 2.1 | 25 ± 1.7 |
| Gram-negative | ||||
| Escherichia coli | FPFC 1407 | 15 ± 1.0 | 20.5 ± 1.1 | 24 ± 1.4 |
| Shigella dysenteriae | AL 35587 | 18.5 ± 0.8 | 21.5 ± 0.9 | 30 ± 2.4 |
| Shigella sonnei | AJ 8992 | 15 ± 1.1 | 20 ± 1.0 | 32 ± 1.7 |
| Shigella flexneri | AL 30372 | 16 ± 0.8 | 21 ± 1.1 | 28 ± 1.2 |
| Pseudomonas aeruginosa | CRL | 12 ± 0.7 | 16 ± 0.9 | 31 ± 1.7 |
| Salmonella typhi | B 56 | 17 ± 1.1 | 21 ± 1.2 | 28 ± 2.1 |
The control disc used for solvent had no zone of inhibition; hence, this data has not been shown. Data shown in mean ± SEM (n = 3)
Table 2.
Minimum inhibitory concentration of amblyone and kanamycin
| Bacteria | MIC values of amblyone (μg/ml) | MIC values of kanamycin (μg/ml) |
|---|---|---|
| Bacillus subtilis | 32 | 2 |
| Bacillus megaterium | 8 | 4 |
| Staphylococcus aureus | 16 | 8 |
| Streptococcus pyogenes | 16 | 8 |
| Escherichia coli | 32 | 8 |
| Shigella dysenteriae | 16 | 2 |
| Shigella sonnei | 32 | 4 |
| Shigella flexneri | 16 | 16 |
| Pseudomonas aeruginosa | 64 | 16 |
| Salmonella typhi | 32 | 4 |
The negative control containing solvent had no MIC value; hence, this data has not been shown
Amblyone showed weak antifungal activity against a number of tested fungi [Table 3]. It was observed that amblyone is inactive against Candida albicans. In cytotoxicity assay, the LC50 value of amblyone was 13.25 μg/ml. The cytotoxicity of amblyone was compared with that of standard gallic acid and vincristine sulphate, whose LC50 values were 4.53 and 0.76 μg/ml, respectively [Table 4]. No mortality was found in the control group. An approximate linear correlation was observed between logarithm of concentration and percentage of mortality.
Table 3.
In vitro antifungal activity of amblyone and nystatin
| Test organism | Zone of inhibition (diameter in mm) | ||
|---|---|---|---|
| Amblyone (80 μg/disc) | Amblyone (160 μg/disc) | Nystatin (30 μg/disc) | |
| Aspergillus flavus | 7 ± 0.7 | 9 ± 0.8 | 17 ± 1.3 |
| Aspergillus niger | 9 ± 0.6 | 10 ± 1.1 | 16 ± 1.4 |
| Candida albicans | 0 | 0 | 18 ± 0.9 |
| Rhizopus aryzae | 8 ± 0.8 | 10 ± 1.0 | 15 ± 1.1 |
The control disc used for solvent had no zone of inhibition; hence, this data has not been shown. Data shown in mean ± SEM (n = 3)
Table 4.
Cytotoxicity of amblyone, gallic acid and vincristine sulphate
| Sample | LC50 (μg/ml) | 95% confidence limit (μg/ml) | Regression equation | X2 value |
|---|---|---|---|---|
| Amblyone | 13.25 | 9.39-18.69 | Y = 2.01 + 2.65X | 1.71 |
| Gallic acid | 4.53 | 3.33-6.15 | Y = 3.93 + 1.62X | 1.25 |
| Vincristine sulphate | 0.76 | 0.57-0.82 | Y = 3.16 + 2.98X | 0.62 |
LC50 values, confidence limits, regression equations and X2 values were calculated by probit analysis
Discussion
Several plants have been used for the treatment of piles, abdominal pain, tumours, enlarged spleen, asthma and rheumatism.[3–5] Analgesic activity of Amorphophallus campanulatus tuber[22] and inhibition of amylase, trypsin and chymotrypsin by Amorphophallus campanulatus tuber[23] have also been determined. The antibacterial, antifungal and cytotoxic activities of tuberous roots of Amorphophallus campanulatus were also reported.[24] Amblyone was previously isolated from aerial part of Cleome amblyocarpa (Capparidaceae)[9] and Salvia aspera (Labiatae).[17] Isolation of amblyone from Amorphophallus campanulatus and antibacterial, antifungal and cytotoxic studies of amblyone are being for the first time reported how.
Although amblyone showed activity against all tested bacteria, it was better against Gram-positive bacteria than Gram-negative bacteria. Highest activity against Bacillus megaterium and lowest activity against Pseudomonas aeruginosa was observed and it was supported by serial tube dilution technique. The antifungal activity seems to be clinically insignificant. Cytotoxicity of amblyone against brine shrimp nauplii and its comparison with standard gallic acid and vincristine sulphate indicated a moderate cytotoxicity of amblyone.
Previous reports of antibacterial, antifungal and cytotoxic activities[24] and traditional uses (against tumours and enlarged spleen[3–5]) of tuberous root of the plant support the findings of present studies. Moderate cytotoxicity of amblyone indicate that it can be selected for further cell line assay, because many scientists have shown a correlation between cytotoxicity and activity against the brine shrimp nauplii using extracts or isolated compounds from terrestrial plants.[18–21] However, more studies are needed to elucidate the structure activity relationship of amblyone, toxicological evaluation and to identify other active constituents of the plant Amorphophallus campanulatus if any.
References
- 1.Al-Bari MA, Sayeed MA, Rahman MS, Mossadik MA. Characterization and antimicrobial activities of a phenolic acid derivative produced by Streptomyces bangladeshiensis a novel specis collected in Bangladesh. Respir J Med Sci. 2006;1:77–81. [Google Scholar]
- 2.Rahman MM, Wahed MII, Biswas MH, Sadik GM, Haque ME. In vitro antibacterial activity of the compounds of Trapa bispinosa Roxb. Science. 2001;1:214–6. [Google Scholar]
- 3.Bhattacharya S. Chironjib banoushadi. 1st ed. Vol. 2. Calcutta: Anand Publishing Ltd; India; 1990. pp. 63–9. [Google Scholar]
- 4.Ghani A. Medicinal Plants of Bangladesh. Dhaka, Bangladesh: Asiatic society of Bangladesh; 1998. pp. 77–8. [Google Scholar]
- 5.Kirtikar KR, Basu BD. Indian Medicinal Plants. 2nd ed. Vol. 4. India: Dehra Dun publisher Ltd; 1994. pp. 2609–10. [Google Scholar]
- 6.Trease EG, Evans WC. Textbook of Pharmacognosy. 14th ed. UK: WB Saunders Co; 1997. p. 119. [Google Scholar]
- 7.Jeffery GH, Bassett J, Mendham J, Denney RC. Vogel's Textbook of Quantitative Chemical Analysis. 5th ed. England: Longman Group UK Ltd; 2000. p. 161. [Google Scholar]
- 8.Harbone JB. Phytochemical methods: A guide to modern technique of plant analysis. London: Chapman and Hall Ltd; 1998. p. 52. [Google Scholar]
- 9.Harraz FM, Ulubelen A, Ökasüz S, Tan N. Dammarane triterpenes from Cleome amblyocarpa. Phytochemistry. 1995;39:175–8. [Google Scholar]
- 10.Carson CF, Hammer KA, Riley TV. Broth microdilution method for determination susceptibitity of Escherichia coli and Staphylococcus aureus to the essential oil of Melaleuca altenifolia (tea tree oil) Microbios. 1995;82:181–5. [PubMed] [Google Scholar]
- 11.Dash S, Nath LK, Bhise S, Bhuyan N. Antioxidant and antimicrobials activities of Heracleum nepalense D Don root. Trop J Pharm Res. 2005;4:341–7. [Google Scholar]
- 12.Rahman MM, Mosaddik MA, Wahed MI, Haque ME. Antimicrobial activity and cytotoxicity of Trapa bispinosa. Fitoterapia. 2000;71:704–6. doi: 10.1016/s0367-326x(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 13.Mosaddik MA, Haque ME. Cytotoxicity and antimicrobial activity of goniothalamin isolated from Bryonopsis laciniosa. Phytother Res. 2003;17:1155–7. doi: 10.1002/ptr.1303. [DOI] [PubMed] [Google Scholar]
- 14.Hossain MS, Hossain MA, Islam R, Alam AH, Kudrat-e-Zahan, Sarkar S, Farooque MA. Antimicrobial and cytotoxic activities of 2-aminobenzoic acid and 2-aminophenol and their coordination complexes with Magnesium (Mg-II) Pak J Biol Sci. 2004;7:25–7. [Google Scholar]
- 15.Islam MA, Sayeed MA, Islam MA, Khan GR, Mosaddik MA, Bhuyan MS. Terpenes from bark of Zanthoxylum budrunga and their cytotoxic activities. Rev Latinoamer Quím. 2002;30:24–8. [Google Scholar]
- 16.Finney DJ. Probit Analysis. 3rd ed. Cambridge, UK: University Press; 1971. pp. 18pp. 37–77. [Google Scholar]
- 17.Esquivel B, Guerrero F, Toscano RA. Tri-nordammarane triterpenoids and neoclerodane diterpenoids from Salvia aspera (Labiatae) Nat Prod Lett. 2002;16:129–35. doi: 10.1080/10575630290020046. [DOI] [PubMed] [Google Scholar]
- 18.Gurkan E, Tuzun OT, Hirlak F. Cytotoxicity assay of some papaver alkaloids using Artemia salina (Brine shrimp) Fitoterapia. 1995;66:544–5. [Google Scholar]
- 19.Martin-Cordero G, Saenz MT, Ayuso MJ. Cytotoxic activity of Retama spaerocarpa. Fitoterapia. 1995;16:495–8. [Google Scholar]
- 20.Mongelli E, Desmarchelier C, Giulietti A. Bioactivity of certain medicinal latexes used by the Eséejas. J Ethnopharmacol. 1995;47:159–63. doi: 10.1016/0378-8741(95)01262-c. [DOI] [PubMed] [Google Scholar]
- 21.Desmarchelier C, Mongelli E, Coussio J, Ciccia G. Studies on the cytotoxicity, antimicrobials and DNA-binding activities of plants used by the Eséejas. J Ethnopharmacol. 1996;50:91–6. doi: 10.1016/0378-8741(95)01334-2. [DOI] [PubMed] [Google Scholar]
- 22.Shilpi JA, Ray PK, Sarder MM, Uddin SJ. Analgesic activity of Amorphophallus campanulatus tuber. Fitoterapia. 2005;76:367–9. doi: 10.1016/j.fitote.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Prathibha S, Nambisan B, Leelamma S. Enzyme inhibitors in tuber crops and their thermal stability. Plant Foods Hum Nutr. 1995;48:247–57. doi: 10.1007/BF01088446. [DOI] [PubMed] [Google Scholar]
- 24.Khan A, Rahman M, Islam S. Antibacterial, antifungal and cytotoxic activities of tuberous roots of Amorphophallus campanulatus. Turk J Biol. 2007;31:167–72. [Google Scholar]


