Abstract
In multicellular organisms, cell-cell interactions are mediated in part by cell junctions, which underlie tissue architecture. Throughout spermatogenesis, for instance, preleptotene leptotene spermatocytes residing in the basal compartment of the seminiferous epithelium must traverse the blood-testis barrier to enter the adluminal compartment for continued development. At the same time, germ cells must also remain attached to Sertoli cells, and numerous studies have reported extensive restructuring at the Sertoli-Sertoli and Sertoli-germ cell interface during germ cell movement across the seminiferous epithelium. Furthermore, the proteins and signaling cascades that regulate adhesion between testicular cells have been largely delineated. These findings have unveiled a number of potential “druggable” targets that can be used to induce premature release of germ cells from the seminiferous epithelium, resulting in transient infertility. Herein, we discuss a novel approach with the aim of developing a nonhormonal male contraceptive for future human use, one that involves perturbing adhesion between Sertoli and germ cells in the testis.
I. Introduction
In mammals, cells are adhered together, forming either an epithelium or an endothelium, by anchoring junctions near the apical portion of two adjacent cells, just behind the tight junctions. Desmosomes, in turn, are located behind the anchoring junctions; collectively, they are referred to as junctional complexes (Alberts et al., 1994). However, junctions at the cell-cell interface, in particular anchoring junctions (including cell-cell actin-based adherens junctions and cell-matrix intermediate filament-based desmosomes), undergo extensive restructuring during development (e.g., embryogenesis and postnatal tissue and/or organ maturation), maintenance of normal tissue function (e.g., inflammatory responses in tissues, replacement of epidermis in skin), and under pathological conditions (e.g., tumorigenesis). During spermatogenesis in adult mammalian testes, such as the rat, type A spermatogonia in the basal compartment of the seminiferous epithelium undergo mitotic division to give rise to type B spermatogonia, which subsequently undergo meiosis and differentiate into zygotene, preleptotene, and leptotene spermatocytes. Leptotene spermatocytes are the germ cells that traverse the blood-testis barrier to enter the adluminal compartment for further development (Hess, 1990; Russell, 1993b; Vogl et al., 1993, 2000). Once these cells enter the adluminal compartment, they differentiate into haploid round spermatids and undergo spermiogenesis, typified by the condensation of nuclear material in the head, formation of the acrosome, and elongation of the tail (de Kretser and Kerr, 1998). These spermatids then traverse the seminiferous epithelium while developing into mature elongated spermatids (e.g., spermatozoa) that are eventually released into the tubule lumen at spermiation. Studies spanning the past decade or so have demonstrated that these cellular events involve disassembly and reassembly of Sertoligerm cell junctions (Cheng and Mruk, 2002; Mruk and Cheng, 2004b). As such, germ cell movement during spermatogenesis occurs via cycles of anchoring junction restructuring. However, the mechanism(s) that regulates this event at the Sertoli-Sertoli and Sertoli-germ cell interface during spermatogenesis has remained largely unknown until recent years. In this review, we focus our discussion on recent advances in the field regarding the biology and regulation of anchoring junctions between testicular cells in the seminiferous epithelium of the testis. These findings have also provided some insights into developing novel contraceptives for men.
Major advances in developing hormonal male contraceptives have been made in recent years (Anderson and Baird, 2002; Lopez et al., 2005; Amory et al., 2006), and a hormonal contraceptive is anticipated to reach the consumer market sometime in the foreseeable future because phase III clinical trials are now under way in different countries, including the United States (Amory et al., 2006). Much research effort has also been placed on nonhormonal contraceptive approaches for men, which would provide an attractive alternative to hormone-based contraceptives for several reasons. First, it can take several weeks for a hormonal male contraceptive, such as those based on testosterone or a testosterone-progestin combination, to induce male infertility. This is because it takes this long to suppress systemic and testicular endogenous androgen levels, thereby interrupting spermatogenesis and leading to azoospermia (Amory et al., 2006). Second, the use of hormones to suppress spermatogenesis may elicit systemic side effects because the testis is not the only target organ of androgens. Because nonhormonal contraceptives would not interfere (that is, if they could exert their effects locally inside the seminiferous epithelium) with the hypothalamic-pituitary-testicular axis, serum follicle-stimulating hormone (FSH),1 luteinizing hormone (LH), and testosterone levels would not be affected. Therefore, the functions of androgen-dependent organs and structures such as prostate, muscle, skin, hair follicles, and bone would not be likely to be compromised after administration. Examples of this would be contraceptives that exerted their effects in the seminiferous epithelium, such as those perturbing the adhesion of germ cells to Sertoli cells. Finally, long-term use of hormone-based male contraceptives might have other adverse effects similar to those reported previously for female oral contraceptives, including elevated risks of cancer and cardiovascular disease (Lech and Ostrowaska, 2006; Birrell et al., 2007; Lurie et al., 2007; Princemail et al., 2007).
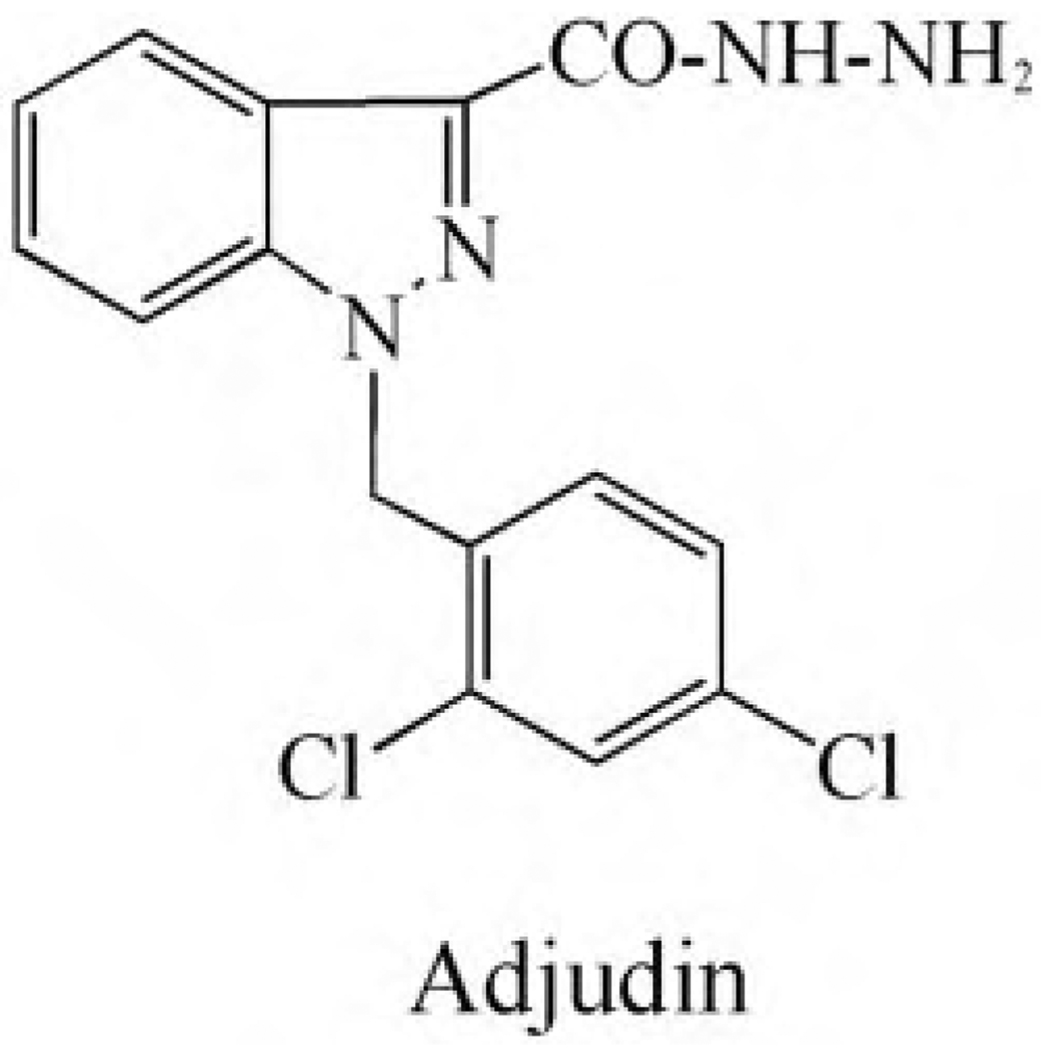
It is noteworthy that several potential nonhormonal contraceptives, such as CDB-4022 and adjudin (formerly called AF-2364), have been shown to exert their effects, at least in part, on Sertoli-germ cell anchoring junctions, leading to depletion of germ cells from the seminiferous epithelium and reversible infertility (Cheng et al., 2001; Grima et al., 2001; Hild et al., 2007, 2001). This approach of male contraception is associated with minimal side effects, because its effects are exerted at the site of Sertoligerm cell contact. Equally important, if adhesion between Sertoli cells and spermatogonia remains largely unaffected after treatment, fertility is regained, as is the case with adjudin (Cheng et al., 2001, 2005a; Grima et al., 2001). Thus, it is critical that we understand the molecular architecture and the regulatory mechanisms underlying Sertoligerm cell adhesion, given its importance in spermatogenesis and in the development of nonhormonal male contraceptives. In this review, we discuss recent advances in the field of cell adhesion with the hope that this information will help us better understand the mechanisms of action of contraceptive drugs that affect Sertoli-germ cell adhesion. As such, our goals are to provide a critical overview of current advances in the field and to discuss new insights in translation of findings from the laboratory bench to product development, and perhaps the consumer market.
II. General Review of Anchoring Junctions
In broad terms, anchoring junctions are categorized as follows: those that connect two cells (e.g., adherens junction and desmosomes) or those that connect cells to the substratum or extracellular matrix (e.g., focal contacts and hemidesmosomes). Another obvious distinction between junction types is that adherens junctions and focal contacts link indirectly to actin filaments in the cytoplasm, whereas the other two junction types attach to intermediate filaments. In this review, we limit our discussion to anchoring junctions, as well as to ectoplasmic specializations, because recent studies have reported anchoring junction proteins to be targets of selected compounds for male contraception. The ultimate goal is to understand the biology of anchoring junctions in the testis because this may lead to the development of safe, effective, and reversible nonhormonal male contraceptives.
A. Cell-Cell Actin-Based Adherens Junctions
1. Cadherin-Catenin Multiprotein Complex
a. Classic cadherins
The cadherin superfamily of cell adhesion molecules, which includes classic cadherins, protocadherins, and atypical cadherins, is composed of more than 100 members involved in many aspects of tissue morphogenesis (Fig. 1). In this section, we briefly highlight recent findings relating to classic cadherins, particularly E- and N-cadherins, with emphasis on the testis. For additional background information, we refer readers to several excellent reviews in the field (Gumbiner, 1988; Kemler, 1993; Herrenknecht, 1996; Yap et al., 1997; Steinberg and McNutt, 1999; Vleminckx and Kemler, 1999; Xu et al., 2001; Wheelock and Johnson, 2003; Halbleib and Nelson, 2006; Weis and Nelson, 2006; Nejsum and Nelson, 2007; Pokutta and Weis, 2007; Takeichi, 2007).
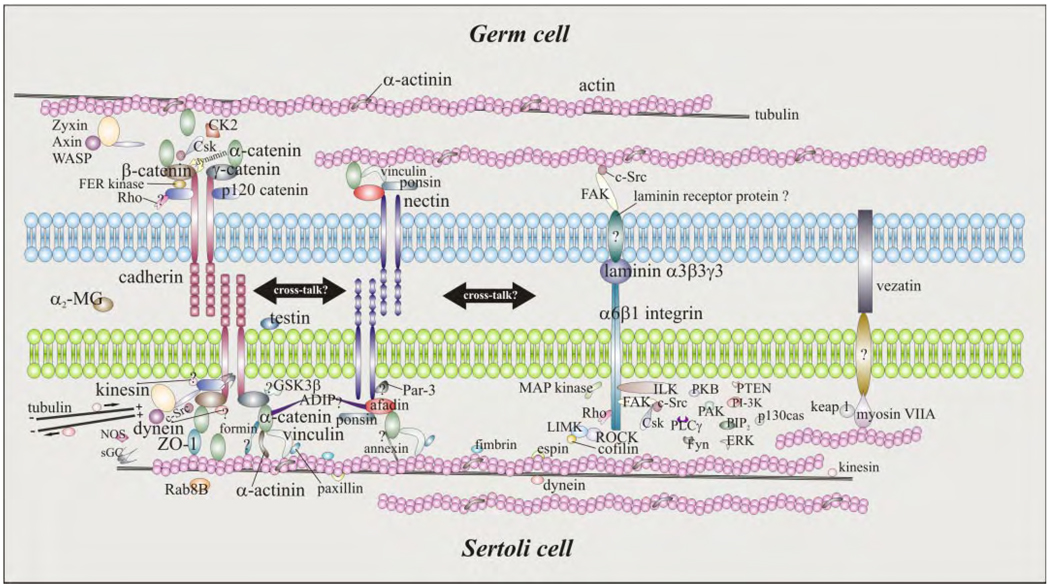
FIG. 1.
Schematic drawing illustrating the molecular architecture of the apical ectoplasmic specialization in the adult rat testis. Ectoplasmic specialization function is constituted by at least four multiprotein complexes: 1) cadherin-catenin, 2) nectin-afadin, 3) integrin-laminin, and 4) vezatin-myosin, and these link indirectly to the cytoskeleton. As discussed in this review, germ cell adhesion to Sertoli cells is regulated by many molecules, including phosphatases and kinases, cytokines, and GTPases. Recent studies have also suggested that there is cross-talk between different multiprotein complexes at the anchoring junction, which is likely to contribute significantly to the regulation of Sertoli-germ cell adhesion in the testis. Thus, many of these proteins can become “druggable” targets for nonhormonal male contraceptive development. This figure was prepared based on several recently published original research and review articles in the field, and these are cited throughout the text. Molecules that are seemingly important in cell adhesion but that have yet to be studied in detail in the testis are denoted with a question mark.
There exists presently an enormous amount of research on the regulation of E-cadherin function in different epithelia. So far, it is known that E-cadherin can be regulated by 1) changes in protein-protein interactions, particularly those between E-cadherin and catenin/p120 catenin, as well as those between α-catenin and the actin cytoskeleton; 2) tyrosine phosphorylation; 3) small GTPases such as Rac, Rho, and Cdc42; 4) proteolytic cleavage; and 5) endocytosis (Halbleib and Nelson, 2006). Loss of E-cadherin-mediated cell adhesion can also occur through down-regulation of E-cadherin expression via promoter hypermethylation and transcriptional repression (Halbleib and Nelson, 2006). For instance, the transcription factors Snail, Slug, ZEB, basic helix-loop-helix, and Twist are all known repressors of E-cadherin expression (Bolós et al., 2003; Conacci-Sorrell et al., 2003; Peinado et al., 2004, 2007; Yang et al., 2004; Vesuna et al., 2008). In addition, important functional and regulatory differences between E- and N-cadherin have been noted. First, N-cadherin-mediated cell adhesion has been shown to be weaker than E-cadherin-mediated adhesion (Chu et al., 2004; Panorchan et al., 2006). Second, E- and N-cadherins seem to bind different isoforms of p120 catenin, an Armadillo-related protein known to facilitate cell adhesion. For instance, N-cadherin binds a larger phosphorylated isoform of p120 catenin, whereas E-cadherin binds a smaller nonphosphorylated isoform (Seidel et al., 2004), suggesting that each cadherin may have other unique binding partners. This may also contribute directly to their difference in mediating adhesive strength.
In the testis, Sertoli and germ cells have been shown to express both E- and N-cadherin (Wu et al., 1993; Lee et al., 2003), but the expression of E-cadherin by germ cells was considerably higher than that of N-cadherin (Lee et al., 2003, 2004). In addition, E-cadherin expression in undifferentiated type A spermatogonia was recently demonstrated in the mouse testis (Tokuda et al., 2007), which clearly illustrates that spermatogonia use this cell adhesion protein predominantly to adhere to Sertoli cells. On the other hand, N-cadherin localization was restricted to the basal ectoplasmic specialization present between adjacent Sertoli cells in all stages of the epithelial cycle (Lee et al., 2003, 2004; Xia and Cheng, 2005; Xia et al., 2005b) but in stages V to VI, it was also found to localize to the apical ectoplasmic specialization by immunofluorescent microscopy (Johnson and Boekelheide, 2002; Lee et al., 2004). The extent of cross-talk between these two pools of N-cadherin in the testis—one at the basal, and the other at the apical ectoplasmic specialization—is not yet known, but it certainly does pose an intriguing question as to how they communicate with each other. It is noteworthy that N-cadherin and occludin (a structural tight junction protein) colocalization was evident at the blood-testis barrier, and these two proteins were shown to associate with each other via the peripheral membrane adaptors catenin and zonula occludens-1 [ZO-1, a tight junction-associated protein] (Yan and Cheng, 2005) (Fig. 1). Given the uniqueness of the blood-testis barrier, which is composed largely of tight junctions and ectoplasmic specializations, additional testis-unique linker proteins that would connect these two important multiprotein complexes are very likely to exist. In addition, cross-talk between nectin-afadin and occludin-ZO-1 complexes has not yet been reported, clearly revealing that additional research is required.
Data has emerged to indicate that the plus-ends of microtubules interact with proteins of the adherens junction, as well as regulate its assembly and disassembly (Bacallao et al., 1989; Ivanov et al., 2006; Stehbens et al., 2006; Ligon and Holzbaur, 2007). This seemingly suggests that the plus-ends of microtubules are rich in biochemical interactions. For example, kinesin [a microtubule plus-end directed motor protein that was recently found in the testis at the apical ectoplasmic specialization (Vaid et al., 2007)] has been shown to associate with p120 catenin (Chen et al., 2003a), dynein (a microtubule minus-end directed motor protein) with β-catenin (Ligon et al., 2001) and KIF3 kinesin with N-cadherin (Teng et al., 2005) (Fig. 1). Ligon and Holzbaur (2007) illustrated the importance of dynein in junction assembly in an elegant study showing that disruption of dynein function inhibited the formation of junctions. In addition, initiation of cell-cell contact reportedly stabilized microtubule plus-ends (Waterman-Storer et al., 2000), illustrating that cadherins regulate microtubule dynamics. The reverse also seems to be true. Important functional studies have shown that adversely affecting microtubules, which resulted in their depolymerization, perturbed the ability of cells to accumulate E-cadherin at cell contacts (Stehbens et al., 2006). Likewise, treatment with nocodazole, an agent that affects microtubule plusend dynamics, adversely affected the localization of catenin at sites of cell-cell contact in lung epithelial cells (Waterman-Storer et al., 2000). Equally important, the trafficking of N-cadherin to the cell surface in fibroblasts required not only the microtubule network but also the active participation of kinesin (Mary et al., 2002). Testicular E- and N-cadherin were also shown to interact indirectly with intermediate filaments (Johnson and Boekelheide, 2002; Lee et al., 2003, 2004) (Fig. 1). Taken collectively, these studies reveal that microtubules are important regulators of cell adhesion. Although complementary functional studies in the testis are clearly lacking, it is important that future studies be expanded to include the role of microtubules in the regulation of other types of junctions such as the ectoplasmic specialization and desmosome. However, note that microtubules in the Sertoli cell are not organized as they would be in a typical epithelial cell, with plus-ends projecting toward the cell periphery, and that the microtubule-organizing center has not yet been identified (Vogl et al., 1993).
b. Other cadherin family members
Protocadherins form the largest subfamily within the cadherin superfamily, consisting of more than 80 members, including α-, β- and γ-protocadherins (Gumbiner, 2005; Redies et al., 2005; Halbleib and Nelson, 2006; Morishita and Yagi, 2007; Takeichi, 2007), yet little is known regarding their function and regulation. They differ from classic cadherins in two important aspects. First, protocadherins have six or more conserved cadherin repeats in their extracellular domains, the role of which is to mediate adhesion between adjacent cells. Protocadherin 15, for example, possesses 11 conserved cadherin repeats (Ahmed et al., 2003; El-Amraoui and Petit, 2005). Second, although many protocadherins exhibit weak homophilic adhesion in cell aggregation assays (Chen and Gumbiner, 2006), and selected members have been shown to localize to cell contacts in vitro, it is not entirely clear at present whether they have a direct role in cell adhesion. Moreover, protocadherins do not seem to interact directly with cytoskeletal proteins (Angst et al., 2001; Halbleib and Nelson, 2006; Takeichi, 2007), and little is known regarding their cytoplasmic binding partners, which would provide critical information on their possible functions. Even though relatively little protocadherin-related research has been performed in the testis, protocadherins are known to be expressed abundantly in this organ (Johnson et al., 2000, 2004). For example, protocadherin α3 was shown to localize to the spermatid acrosome, intercellular bridge, and flagellum but not to the classic cadherin-based adhesion junction nor the ectoplasmic specialization (Johnson et al., 2004). These observations illustrate that protocadherins are unique in their functions and are likely to have other physiological properties in addition to (or instead of) cell adhesion.
Atypical cadherins comprise a subfamily of proteins, namely Dachsous, Flamingo, and Fat, primarily involved in planar cell polarity (Fanto and McNeill, 2004; Jones and Chen, 2007). Atypical cadherins also seem to have roles in cell adhesion, but these are presently not well defined. For instance, Flamingo was shown to mediate cell adhesion in vitro when it was expressed in Drosophila melanogaster S2 cells (Usui et al., 1999). In addition, Fat1, a mammalian homolog of D. melanogaster Fat, has been reported to bind and recruit Ena/VASP proteins (a family of proteins known to regulate actin dynamics), as well as to localize to the leading edge of lamellipodia and filopodia (Moeller et al., 2004). Fat1 also localized to intercellular junctions in epithelial NRK-52E and neuronal HN33 cells (Moeller et al., 2004), but its distribution was different from that of E-cadherin (Moeller et al., 2004), suggesting that Fat function is independent of the cadherin-catenin complex. Besides mammalian Fat1, other Fat proteins (e.g., Fat2, Fat3, and Fat-J) have also been reported to exist (Tanoue and Takeichi, 2004). On the other hand, Celsr is the mammalian homolog of D. melanogaster Flamingo. Three Celsr proteins have been identified (Celsr 1–3), all of which are present in the testis (Beall et al., 2005). Specifically, Celsr1 and Celsr 2 expression was detected in Sertoli cells, whereas Celsr3 was a germ cell (elongating spermatid) product (Beall et al., 2005). However, Celsr2 and Celsr3 failed to colocalize with classic cadherins and catenins in the testis (Beall et al., 2005), similar to findings previously reported for Fat1 in NRK-52E and HN33 cells. In addition, Celsr2 and Celsr3 immunoreactivity was detected at neither the ectoplasmic specialization nor desmosome-like junction in the testis (Beall et al., 2005). As is the case for the protocadherin subfamily, these results are suggestive of distinct roles for Celsr proteins outside of cell adhesion. For instance, Celsr proteins such as Celsr3 may contribute to maintaining germ cell polarity and orientation during spermiogenesis, similar to that described previously for its homolog Flamingo. Orientation of elongating/elongated spermatids within the epithelium is critical to the maintenance of spermatogenesis, and the Sertoli cell apical ectoplasmic specialization and germ cell acrosome have been postulated to contribute collectively to spermatid polarity (Russell, 1980, 1983; Russell and Peterson, 1985; Mruk and Cheng, 2004b). As such, additional research effort is needed to determine the precise role of Celsr proteins in the testis because these findings are likely to provide new insights on important cellular events that underlie spermatogenesis and fertility.
c. Classic catenins
Catenins comprise a family of well studied proteins that support adherens junction function in different epithelia (Perez-Moreno and Fuchs, 2006; Scott and Yap, 2006) including the testis (Chen et al., 2003b; Lee et al., 2003; Lee et al., 2004; Lee and Cheng, 2005). Catenins have long been regarded as proteins that link cadherins to the actin cytoskeleton either directly or indirectly (Fig. 1), but they can also participate in signal transduction events, as is the case for β-catenin (Nelson and Nusse, 2004; Bienz, 2005; Harris and Peifer, 2005) and γ-catenin (Simcha et al., 1998; Miravet et al., 2002; Maeda et al., 2004). Generally speaking, α-catenin mediates cell adhesion and tissue organization by existing in a multiprotein complex consisting of cadherin, β-catenin, p120 catenin, and actin. However, this concept was recently revised by findings demonstrating that α-catenin cannot bind β-catenin and actin simultaneously (Drees et al., 2005; Yamada et al., 2005b) (Fig. 1), suggesting that α-catenin is in a dynamic relationship with cadherin–β-catenin–p120 catenin instead of a stable one. This observation raised new and important questions, the most important one being how α-catenin interacts with the actin cytoskeleton to regulate adherens junction dynamics. Interestingly, α-catenin associates with an array of proteins known to regulate actin dynamics. For instance, α-catenin can interact with formin-1 to regulate actin polymerization (Kobielak et al., 2004), as well as bind other actin-associated proteins, such as vinculin (Watabe-Uchida et al., 1998; Weiss et al., 1998), ZO-1 (Imamura et al., 1999), and afadin (Mandai et al., 1997) (Fig. 1), which in turn may regulate actin dynamics. Moreover, three distinct α-catenins are known to exist (i.e., αE-, αN-, and αT-catenin) (Herrenknecht et al., 1991; Hirano et al., 1992; Janssens et al., 2001). Of these, αT-catenin is expressed highly in the testis, where it localized to elongating spermatids at stages II to VIII of the seminiferous epithelial cycle (Goossens et al., 2007b). On the other hand, β- and/or γ-catenin were shown to interact with a unique list of proteins in the testis, including soluble guanylate cyclase (Sarkar et al., 2006), nitric-oxide synthase (Lee et al., 2005), c-Src (normal cellular Rous sarcoma virus, which is the counterpart of viral sarcoma, v-Src), and casein kinase 2 (Lee and Cheng, 2005) (Fig. 1), demonstrating that catenins recruit a diverse array of proteins to regulate cadherin-based cell adhesion. This is likely to contribute to the unique nature of cell junctions in the testis.
d. p120 catenin family
Several p120 catenin subfamily members are known to exist in mammals: 1) p120 catenin, 2) Armadillo repeat gene deleted in velo-cardio-facial syndrome (ARVCF), 3) p0071, 4) plakophilins 1, 2, 3, and 5) δ-catenin, all of which bind classic cadherins (Anastasiadis, 2007; McCrea and Park, 2007; Reynolds, 2007). In this section, we select two of these proteins, namely p120 catenin and ARCVF, for further discussion. Discussions on p0071 and the plakophilins are also included in this review (see B., 2. Armadillo Proteins, below), but δ-catenin (also known as neural plakophilin-related arm protein, NPRAP) is not because it is expressed specifically by neurons.
p120 Catenin is a multifunctional protein that is structurally related to β-catenin. It has a structural as well as a signaling role, and this is reflected in its cytoplasmic and nuclear localization (Daniel, 2007; Reynolds, 2007; Xiao et al., 2007). In terms of its cytoplasmic function, p120 catenin promotes the lateral clustering of cadherins, which form the adhesive bond (Yap et al., 1998; Thoreson et al., 2000), and is required for turnover and stabilization of cadherin-mediated cell contacts (Ireton et al., 2002; Davis et al., 2003; Xiao et al., 2003). In the absence of p120 catenin, cadherin is internalized and degraded (Ireton et al., 2002; Davis et al., 2003), indicating that p120 catenin is critical to adherens junction function. However, the reverse does not seem to be true, because the stability of p120 catenin was unaffected in the absence of cadherin (Van Hengel et al., 1999). In addition, p120 regulates cadherin-actin interactions by 1) binding and inhibiting RhoA (Anastasiadis and Reynolds, 2001; Castaño et al., 2007) or 2) activating Rac1 and Cdc42 via Vav2 (Noren et al., 2000). It is noteworthy that only cadherins can target p120 catenin to the plasma membrane (Thoreson et al., 2000), and membrane localization of p120 catenin is sufficient to induce its phosphorylation (Xia et al., 2006b). Deletion of p120 catenin in Caenorhabditis elegans resulted in germline cytokinesis defects and sterility (Pettitt et al., 2003; Skop et al., 2004), whereas a loss-of-function study in D. melanogaster, which only contain p120 catenin and not other members of this protein family, showed that p120 catenin is not an essential component of the adherens junction (Myster et al., 2003). As discussed above, this is in sharp contrast to studies in mammals, which suggest that p120 is indispensable for adhesive function. Several interesting and potentially important p120 catenin protein interactions have been demonstrated in addition to those previously reported for cadherin (Reynolds et al., 1994) and Rho (Magie et al., 2002). These include kinases [e.g., Fer, Fyn, and Yes are all members of the nonreceptor tyrosine kinase Src family (Kim and Wong, 1995; Piedra et al., 2003; Xu et al., 2004)] and phosphatases [e.g., PTPµ, DEP1 and SHP-1 (Zondag and Moolenaar, 1997; Keilhack et al., 2000; Mariner et al., 2001; Holsinger et al., 2002)], as well as associations with Kaiso [a zinc finger transcriptional repressor (Daniel and Reynolds, 1999; Prokhortchouk et al., 2001; Daniel, 2007)], Gli-similar 2 [Glis2, a Kruppel-like transcriptional repressor (Hosking et al., 2007)], Functional regulator of Dishevelled in ontogenesis [Frodo, a regulator of the Wnt pathway (Park et al., 2006a)], Nanos1 [a zinc finger protein that functions in germ cell development (Strumane et al., 2006)], and cortactin (Boguslavsky et al., 2007). In the testis, p120 catenin was shown to structurally interact with the adaptor proteins axin, zyxin, and Wiskott-Aldrich syndrome protein [WASP (Lee et al., 2004)], as well as with soluble guanylate cyclase (Sarkar et al., 2006) (Fig. 1), suggesting that p120 catenin sequesters different molecules to regulate cadherin-based cell adhesion during spermatogenesis. For example, WASP, a known regulator of actin dynamics, recruits actin-related protein 2/3 and profilin to induce actin reorganization (Caron, 2002; Takenawa and Suetsugu, 2007). In this way, p120 catenin may function as a signaling platform by recruiting other molecules to regulate actin dynamics and Sertoligerm cell adhesion. As discussed above, p120 catenin also binds to kinesin (Fig. 1) to facilitate the movement of cadherin along microtubules and to regulate microtubule dynamics (Chen et al., 2003a; Franz and Ridley, 2004; Yanagisawa et al., 2004; Ichii and Takeichi, 2007). An inflammatory role for p120 catenin in the epidermis was described previously (Perez-Moreno et al., 2006). Taken collectively, these results illustrate the importance of p120 catenin in adherens junction function.
ARVCF is homologous to p120 catenin (Sirotkin et al., 1997), and it can bind to cadherin (Kaufmann et al., 2000; Mariner et al., 2000; Waibler et al., 2001), but the function of ARVCF in cell adhesion seems to be different from that of p120 catenin. For instance, ARVCF has been shown to interact with ZO-1 and ZO-2 (Kausalya et al., 2004), as well as with erbin [a PDZ domain-containing protein and tumor suppressor (Laura et al., 2002)], but similar protein associations were not reported for p120 catenin. Moreover, ARVCF can localize to the nucleus (Mariner et al., 2000), and ZO-2 has been proposed by Kausalya et al. (2004) to play a key role in AFVCF’s nuclear localization, but the relevance of these findings is presently unknown. The consensus is that ARVCF does not have a structural role in cell junctions because its level is so low compared with that of p120 catenin (Mariner et al., 2000). ARVCF and p120 catenin are known to compete for binding to E-cadherin (Mariner et al., 2000), and localization of ARVCF to the plasma membrane is required for stable cell adhesion (Kausalya et al., 2004). Although ARVCF expression seems to be ubiquitous (Mariner et al., 2000), its presence in the testis remains to be reported. In light of these significant findings, the function of ARVCF in the testis should be carefully examined, in particular its signaling role in cadherin-mediated Sertoli-germ cell adhesion.
2. Nectin-Afadin Multiprotein Complex
Nectins comprise a relatively small family of Ca2+-independent immunoglobulin-like adhesion molecules involved in homophilic and heterophilic interactions (Takai and Nakanishi, 2003; Sakisaka et al., 2007; Rikitake and Takai, 2008) (Fig. 1). By comparison, nectin heterophilic trans-interactions are stronger than homophilic trans-interactions [nectin-3 + nectin-1 versus nectin-3 + nectin-3, respectively (Satoh-Horikawa et al., 2000; Irie et al., 2004; Martinez-Rico et al., 2005; Ogita and Takai, 2006)]. Moreover, nectin trans-interactions seem to be more stable and robust than cadherin trans-interactions (Satoh-Horikawa et al., 2000). Nevertheless, the role of nectin in cell adhesion is to initiate the formation of adherens junctions, and this is followed by the recruitment of cadherins (Takai and Nakanishi, 2003). Recruitment of cadherins has been shown to be mediated by nectin-induced activation of Rac, Cdc42 and Rap1 through c-Src, leading to reorganization of the actin cytoskeleton (Sakisaka and Takai, 2004; Ogita and Takai, 2006). After sequestration of cadherins at the adherens junction, nectins continue to regulate cadherin function by (i) inhibiting the endocytosis of cadherin and (ii) affecting the conformation of cadherin extracellular domains to facilitate trans-interactions (Hoshino et al., 2005; Sato et al., 2006). Nectins also regulate cytoskeletal dynamics via F-actin binding proteins that interact directly with nectin [e.g., afadin, α-catenin, α-actinin, vinculin, annexin II and IQGAP (Ogita and Takai, 2006; Miyoshi and Takai, 2007)] (Fig. 1), and establishment of nectin-based adherens junctions required annexin II and IQGAP in MDCK cells (Katata et al., 2003; Yamada et al., 2005a, 2006a). Furthermore, nectin-afadin can also recruit tight junction proteins such as claudin, occludin, JAM-A, and ZO-1 to the junctional complex (Yokoyama et al., 2001; Fukuhara et al., 2002; Takai and Nakanishi, 2003; Sato et al., 2006), suggesting the existence of cross talk between different junction types. In addition, nectin-1 and nectin-3 have been shown to bind directly to Par-3 (Takekuni et al., 2003), a cell polarity protein, but Par-3 was not required for nectin-based cell adhesion (Ooshio et al., 2007). Instead, Par-3 was needed for colocalization of nectin with afadin (Ooshio et al., 2007).
Afadin is a well studied nectin-binding protein that connects nectin to the actin cytoskeleton (Fig. 1). It has two splice variants, l-afadin and s-afadin, but only the larger splice variant (l-afadin) can bind to actin (Mandai et al., 1997). s-Afadin (also known as AF-6), on the other hand, can localize to both the plasma membrane and nucleus (Buchert et al., 2007) and has also been reported to form a complex with and serve as a substrate for Fam [a deubiquitinating enzyme (Taya et al., 1998)]. This implies that proteolytic degradation of AF-6 occurs via the ubiquitin-proteasome pathway.
Of special interest are recent studies showing cross-talk between nectin-afadin and cadherin-catenin multiprotein complexes. Initial studies demonstrated that cross-talk between these two multiprotein complexes was mediated by interactions between: 1) l-afadin and α-catenin (Pokutta et al., 2002), 2) ponsin [binds to afadin and vinculin (Mandai et al., 1999)] and vinculin [binds to F-actin and α-catenin (Jockusch and Isenberg, 1981; Menkel et al., 1994; Weiss et al., 1998)], and/or 3) afadin dilute domain-interacting protein [ADIP (Asada et al., 2003)] and α-actinin [binds to α-catenin (Knudsen et al., 1995)]. Note that ADIP localization was not only restricted to the adherens junction, where it was proposed to function in the organization of the actin cytoskeleton (Asada et al., 2003). ADIP was also found to localize to the perinuclear region, specifically the Golgi complex, and bind to β′-COP in vitro and in vivo (Asada et al., 2004), a subunit protein of the coatomer complex involved in protein trafficking events (Schekman and Orci, 1996). The fourth and most recently described type of cross-talk involves LIM domain only 7, an l-afadin and α-actinin binding protein that connects the two multiprotein complexes via α-actinin (Ooshio et al., 2004). Specifically, LIM domain only 7 associated with both nectin-afadin and cadherin-catenin adhesion complexes by immunoprecipitation (Ooshio et al., 2004) and failed to localize to the desmosome and tight junction by immunofluorescence and immunoelectron microscopy (Ooshio et al., 2004).
Nectin is critical for spermatogenesis. Mice lacking either nectin-2 [expressed by Sertoli and germ cells (Bouchard et al., 2000; Ozaki-Kuroda et al., 2002; Takai and Nakanishi, 2003)] or nection-3 [expressed exclusively by spermatids (Ozaki-Kuroda et al., 2002; Takai and Nakanishi, 2003)] were infertile, resulting from malformations in the head and midpiece of elongated spermatids (Mueller et al., 2003; Inagaki et al., 2006). In addition, nectin-like molecule-2 [TSLC1, also known as IGSF4 (Wakayama et al., 2003)], a member of the nectin family of cell adhesion proteins and a germ cell product, was shown to be equally important for spermatogenesis and fertility (Wakayama et al., 2003; Fujita et al., 2006; Surace et al.,2006; Yamada et al., 2006b). It is noteworthy that TSLC1 did not bind afadin or recruit E-cadherin to the adherens junction. Instead, it associated with other proteins such as Pals2 (Shingai et al., 2003), a tight junction-associated adaptor protein involved in cell polarity, suggestive of cross-talk between nectin-based adherens and tight junctions.
B. Cell-Cell Intermediate Filament-Based Desmosome Junctions
Desmosomes are anchoring junctions present between adjacent cells that mediate strong adhesion (Kowalczyk et al., 1999; Green and Gaudry, 2000; Jamora and Fuchs, 2002; Holthöfer et al., 2007). From an ultrastructural perspective, they appear as highly symmetrical, electron-dense plasma membrane domains associating with intermediate filaments (Fig. 2). Desmosomes are generally found in tissues that are subjected to mechanical stress (e.g., skin and heart), but their presence in other organs, such as the testis, has also been reported (Russell, 1977a, 1993b; Ren and Russell, 1992) (Fig. 2). The physiological importance of desmosomes has been exemplified best by studies examining the clinical manifestations of human diseases in which desmosomal proteins were affected by mutations or the presence of autoimmune antibodies (Cheng et al., 2005b; Kottke et al., 2006; Uitto et al., 2007). It is noteworthy that the ability of cells to assemble stable desmosomes is dependent on the presence of functional adherens junctions (Huber, 2003; Yin and Green, 2004). Similar to adherens junctions, desmosomes are composed of proteins from three major gene families: 1) desmosomal cadherins, 2) armadillo proteins, and 3) plakins. These are discussed below.
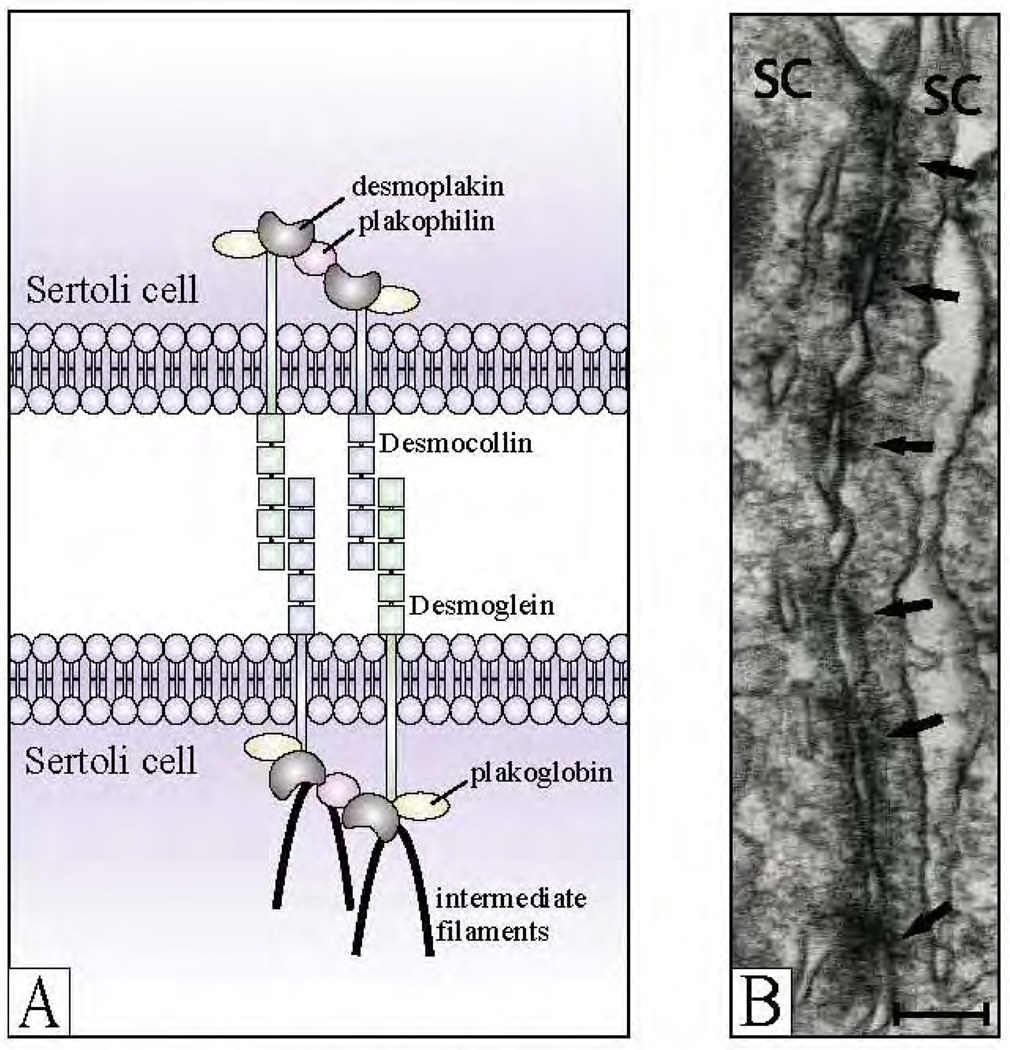
FIG. 2.
The desmosome-like junction in the adult rat testis. A, schematic illustration showing constituent proteins of the desmosome junctions. The desmosome junction uses intermediate filaments for cytoplasmic attachment. B, electron micrograph showing typical ultrastructural features of the desmosome-like junction in the testis. This junction type is characterized by the presence of electron dense material (see arrows) between two apposing Sertoli cell (SC) plasma membranes. They were called desmosome-like junctions because they shared properties of both desmosomes and gap junctions (Russell, 1977a). Bar in B, 0.25 µm.
1. Desmosomal Cadherins
Desmosomal cadherins, transmembrane proteins that mediate Ca2+-dependent cell adhesion, are composed of two protein subfamilies: 1) desmogleins (desmogleins 1–4) and 2) desmocollins (desmocollins 1–3) (Fig. 2). Both desmogleins and desmocollins show significant sequence homology to classical cadherins and a similar organization of their ectodomains (Dusek et al., 2007). Among the desmosomal cadherins, desmoglein 2 and desmocollin 2 are the only proteins that are expressed ubiquitously in all desmosome-containing cells and tissues (Schäfer et al., 1994; Nuber et al., 1995), whereas the others have a unique tissue distribution. For example, desmoglein 4 expression was highest in the testis (Whittock and Bower, 2003), but little is known about its functional significance in spermatogenesis and fertility. It is noteworthy that both desmoglein- and desmocollin-specific peptides were needed to perturb desmosomal adhesion (Tselepis et al., 1998), illustrating that desmosomal cadherins favor heterotypic interactions to facilitate cell adhesion.
2. Armadillo Proteins
Plakoglobin, plakophilins, and p0071 are all examples of armadillo proteins (Fig. 2). The function of plakoglobin (also known as γ-catenin), a close relative of β-catenin (Butz et al., 1992), is to facilitate the lateral clustering of desmosomal cadherins (Bornslaeger et al., 2001; Koeser et al., 2003), thereby mediating strong adhesion. Plakoglobin is an important protein for many other reasons as well. First, plakoglobin is present at both desmosomes and adherens junctions (Cowin et al., 1986), although its binding affinity for desmosomal cadherins was reported to be five times stronger that that for classic cadherins (Chitaev et al., 1996). Second, plakoglobin was shown to be required for stable cell adhesion in l-fibroblasts expressing desmoglein 1 and desmocollin 2 (Marcozzi et al., 1998), and its loss resulted in the mixing of desmosomes and adherens junctions (Ruiz et al., 1996). This seems to suggest that plakoglobin functions in the segregation of desmosomal and adherens junction proteins (Koeser et al., 2003) and also in their cross-talk, because plakoglobin was found to localize to both cell structures. Third, desmosome assembly required that plakoglobin associate with E-cadherin (Lewis et al., 1997) because free plakoglobin (non–junction-associated) interacted with the ubiquitin ligase β-TrCP and was subsequently degraded by the proteosome (Sadot et al., 2000). Binding of plakoglobin to desmoglein also prevented plakoglobin from interacting with α-catenin (Sacco et al., 1995; Witcher et al., 1996). In the testis, studies have confirmed the association of plakoglobin with cadherin- (Lee et al., 2003) and desmosome-based (D.D.M. and C.Y.C., unpublished observations) cell adhesion complexes. Taken collectively, these results illustrate the importance of plakoglobin in desmosome and adherens junction dynamics.
In addition to its role in cell junction dynamics, plakoglobin was also found to localize to the nucleus (Zhurinsky et al., 2000). Similar to β-catenin, plakoglobin can activate the Wnt signaling pathway, transduce signals to the nucleus, and activate lymphoid enhancer binding factor/T cell factor-dependent transcription (Simcha et al., 1998; Miravet et al., 2002; Maeda et al., 2004; Shimizu et al., 2008). It is noteworthy that plakoglobin, as well as E-cadherin, increased after Wnt-1 expression in PC-12 cells, and this strengthened cell-cell contacts (Bradley et al., 1993). Plakoglobin also forms a complex with adenomatous polyposis coli (APC) and glycogen synthase kinase 3β (Rubinfeld et al., 1993, 1995, 1996; Hülsken et al., 1994), which are other members of the Wnt signaling cascade.
Three plakophilins (plakophilins 1–3) have been identified, and all three have been shown to localize to desmosomes (Fig. 2) as well as to the nucleus of different cell types (Hatzfeld, 2007). Of these, plakophilin 2 displays the broadest expression pattern (Hatzfeld, 2007), and plakophilins in general have been reported to interact with many proteins including desmogleins, desmocollins, desmoplakins, plakoglobin, β-catenin, keratin, tubulin, and actin (Hatzfeld et al., 2000; Hatzfeld, 2007). Plakophilins function as scaffolding proteins by recruiting and stabilizing proteins at the desmosome (Hatzfeld et al., 2000; South et al., 2003), thereby regulating the number of desmosomes present, as well as their size. For example, lack of plakophilin 1 reduced desmosome stability and increased the migratory activity of keratinocytes (South et al., 2003). In intercalated discs of cardiomyocytes, plakophilin 2 and desmoplakin (a plakin; see 3. Plakins, below) colocalization was evident in both desmosomes and adherens junctions (Franke et al., 2006). In this same in vivo system, plakophilin 2 interacted specifically with αT-catenin (Goossens et al., 2007a), seemingly suggesting that desmosomes and adherens junctions are mixed in cardiac intercalated discs. Surprisingly, plakoglobin, which supposedly functions in the segregation of desmosomes and adherens junctions, was present in the heart (Cowin et al., 1986) and shown to be essential for its function (Ruiz et al., 1996).
p0071, the final member of the armadillo superfamily, is referred to occasionally as plakophilin 4 because of its striking resemblance to the three bona fide plakophilins. Similar to plakoglobin and plakophilin 2, p0071 interacts with both classic and desmosomal cadherins (Hatzfeld and Nachtsheim, 1996; Hatzfeld et al., 2003), revealing that it has dual functions—one at the adherens junction, and the other at the desmosome—depending on the cell type studied. In addition, Hatzfeld et al. (2003) reported that the C-terminal head domain of p0071 localized to the nucleus when it was ectopically expressed in MCF-7 cells. In this context, it is of interest to note that p0071 is essential for cell division (Wolf et al., 2006; Keil et al., 2007) because both knockdown and overexpression of p0071 adversely affected cytokinesis, resulting in the formation of multinucleated cells and the induction of apoptosis (Wolf et al., 2006). In terms of protein-protein interactions, p0071 has been shown to associate with desmocollin 3a (Hatzfeld et al., 2003), desmoplakin (Calkins et al., 2003), plakoglobin (Hatzfeld et al., 2003), and cadherin (Calkins et al., 2003; Hatzfeld et al., 2003). p0071 also interacts with erbin (Izawa et al., 2002; Jaulin-Bastard et al., 2002) and plakophilin-related armadillo repeat protein-interacting PDZ protein [papin (Deguchi et al., 2000; Ohno et al., 2002)], two PDZ-domain containing proteins that play roles in cell junction dynamics.
3. Plakins
Two isoforms of desmoplakin exist, desmoplakin I and desmoplakin II, and both are abundant in all types of epithelia (Ruhrberg and Watt, 1997; Jefferson et al., 2004; Sonnenberg and Liem, 2007). Desmoplakin’s function is to link intermediate filaments to the plasma membrane (Bornslaeger et al., 1996) (Fig. 2), but it also has additional roles in desmosome assembly and the maintenance of desmosome stability (Gallicano et al., 1998). Similar to plakoglobin, desmoplakin seems to function in the segregation of desmosome and adherens junction proteins (Bornslaeger et al., 1996). This is because expression of dominant-negative desmoplakin resulted in the formation of adhesive contacts that contained components of both cell junction types (Bornslaeger et al., 1996). Desmoplakin has also been implicated in the dynamics of the microtubule cytoskeleton during epidermal differentiation (Lechler and Fuchs, 2007), suggesting that it may have yet another function that is not directly related to cell adhesion.
Plectin, another member of the plakin family, has a critical function in mediating cytoskeletal interactions and is known to associate with actin, microtubules, and intermediate filaments (Foisner et al., 1988; Seifert et al., 1992; Nikolic et al., 1996; Svitkina et al., 1996). It is present in both desmosomes and hemidesmosomes (Wiche et al., 1983; Hieda et al., 1992; Okumura et al., 1999), and its presence in Sertoli cells has been previously reported (Guttman et al., 1999). In particular, plectin acts as a linker to stabilize cells and tissues as was exemplified in plectin-deficient mice when the connection between intermediate filaments and hemidesmosomal proteins was severed (Andrä et al., 1998). Plectin also serves as a scaffolding platform for signaling molecules. For instance, plectin was shown to bind and sequester receptor for activated C kinase, a protein kinase C binding partner (Ron et al., 1994), to the cytoskeleton, thereby affecting the protein kinase C signaling pathway (Osmanagic-Myers and Wiche, 2004). Plectin also interacts with other proteins such as desmoplakin (Eger et al., 1997), fodrin (Herrmann and Wiche, 1987; Eger et al., 1997), β4 integrin (Rezniczek et al., 1998), spectrin (Herrmann and Wiche, 1987), Fer kinase (Lunter and Wiche, 2002), AMP-activated protein kinase (Gregor et al., 2006), lamin B (Foisner et al., 1991), nesprin-3 [an outer nuclear envelope protein (Wilhelmsen et al., 2005; Ketema et al., 2007)], and seven in absentia homolog [Siah, an ubiquitin E3 ligase (House et al., 2003)]. The interaction between plectin and Siah may suggest that plectin facilitates the degradation of cytoplasmic proteins such as β-catenin (Liu et al., 2001; Matsuzawa and Reed, 2001; Park et al., 2006b).
In this section, we have summarized recent findings in the field regarding the physiological significance of desmosomes, as well as several desmosome proteins in epithelia. The goal in the near future is not to simply repeat these findings in the testis. Instead, functional studies should be performed to determine the role of desmosome-like junctions in the seminiferous epithelium during spermatogenesis and whether there exists a testis-specific desmosomal protein that may become a “druggable” target for male contraception. For instance, would ablation of a desmosomal protein impair the function of Sertoli cell tight junctions because these junctions are known to coexist with desmosome-like junctions at the blood-testis barrier (Fig. 3)? Would the same be true for ectoplasmic specializations, which also associate intimately with desmosome-like junctions at the blood-testis barrier (Fig. 3)? Morphological studies in the testis have illustrated that the desmosome is the major type of anchoring junction in that it confers Sertoli-Sertoli and Sertoli-germ cell adhesion before the formation of the apical ectoplasmic specialization, which occurs in step 8 elongating spermatids. Thus, future studies are likely to provide new and interesting findings on desmosome dynamics in the testis.
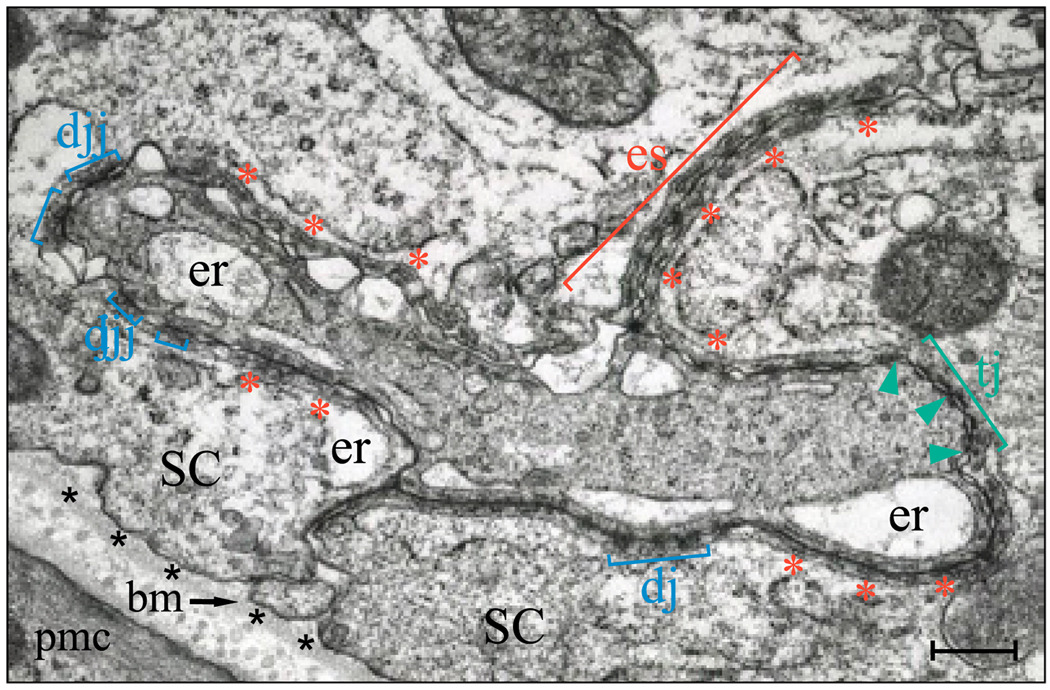
FIG. 3.
Blood-testis barrier in the adult rat testis. Electron micrograph of the blood-testis barrier present between adjacent Sertoli cells (SC) near the basement membrane (bm, see arrow and asterisks), which is a modified form of the ECM that appears as an amorphous substance. Underneath this lies a layer of type I collagen, and cross-sectioned collagen bundles are clearly visible. This is followed by peritubular myoid cells (pmc). The blood-testis barrier is characterized largely by the coexistence of tight junctions (tj; see green bracket), basal ectoplasmic specializations (es; see red bracket), and desmosome-like junctions (dj; see blue brackets). The basal ectoplasmic specialization is typified by the presence of actin filament bundles (see red asterisks) sandwiched between the endoplasmic reticulum (er) and the Sertoli cell plasma membrane. Desmosomes are typified by electron dense material present between two adjacent Sertoli cells, whereas tight junctions are characterized by “kisses,” regions of close contact between two apposing Sertoli cell plasma membranes (see green arrowheads). Bar, 0.75 µm. [Reproduced from Sarkar O, Mathur PP, Cheng CY, and Mruk DD (2008) Interleukin-1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod 78:445–454. Copyright © 2008 Society for the Study of Reproduction. Used with permission.]
C. Cell-Matrix Intermediate Filament-Based Hemidesmosomes
Hemidesmosomes are cell junctions that morphologically resemble desmosomes, but they are biochemically and functionally distinct. Their function is to connect intermediate filaments of cells to the underlying basement membrane (Borradori and Sonnenberg, 1999; Litjens et al., 2006). Thus far, hemidesmosome function has been shown to be conferred by at least five distinct proteins: α6β4 integrin, bullous pemphigoid antigen 180 (BP180), BP230, tetraspanin CD151, and plectin (Jones et al., 1998; Borradori and Sonnenberg, 1999). Of these, α6β4 integrin seems to be the most important in that it has both structural and signaling roles (Giancotti, 1996; Borradori and Sonnenberg, 1999), and its interaction with plectin was required for hemidesmosome integrity (Geerts et al., 1999; Koster et al., 2001). Moreover, this is the only integrin family member known to attach to intermediate filaments; other integrins have been shown to connect to actin and function in cell migration at focal contacts, a type of cell-matrix actin-based anchoring junction (Mercurio et al., 2001; Delon and Brown, 2007).
In the testis, the existence of hemidesmosomes on the basal surface of Sertoli cells was first reported by Connell (1977). Unlike those present in other organs, such as the skin, hemidesmosomes do not seem to be well developed structurally, but they are still readily distinguishable as such by electron microscopy (Mruk and Cheng, 2004b). Nevertheless, it is interesting to note that there are no reported cases of hemidesmosome disassembly in the testis, suggestive of stable and robust adhesion. Moreover, α6β1 integrin does not seem to localize to the intermediate filament-based hemidesmosome in the testis but apparently to the actin-based basal ectoplasmic specialization (Chapin et al., 2001; Mulholland et al., 2001). In addition, except for a single report published nearly 2 decades ago that described the presence of an uncharacterized 120-kDa protein in the testis referred to as 1–2B7B (Zhang et al., 1991), there has been no advancement in the biology of the hemidesmosome in this organ. However, it is especially important that future studies address the possibility of cross-talk between hemidesmosomes and the blood-testis barrier, given their physical proximity. For instance, identification of a testis-specific hemidesmosomal protein would be a welcomed break-through in the field and would probably provide additional insight on the regulation of the blood-testis barrier. Finally, hemidesmosomes have also been demonstrated to exist between type A spermatogonia and the basement membrane (Russell, 1993b).
D. Cell-Matrix Actin-Based Focal Contacts
Focal contacts are cell adhesions that attach cells to their substrate. This connection is facilitated by integrins, different adaptor proteins, the actin cytoskeleton within cells, and collagens and laminins in the extracellular matrix (Burridge and Fath, 1989; Lo, 2006). Generally speaking, numerous studies have linked focal contact function to cell spreading and migration. Although the existence of focal contacts in the testis has never been described, several studies from different laboratories have reported the presence of putative focal contact proteins in this organ, including α6β1 integrin (Salanova et al., 1995, 1998; Mulholland et al., 2001), integrin-linked kinase [ILK (Mulholland et al., 2001)], focal adhesion kinase [FAK (Siu et al., 2003)], c-Src (Lee and Cheng, 2005; Wong et al., 2005; Zhang et al., 2005), vinculin (Grove et al., 1990; Pfeiffer and Vogl, 1991), profilin III (Braun et al., 2002), talin (Santoro et al., 2000), paxillin (Wine and Chapin, 1999), zyxin (Lee et al., 2004), fimbrin (Grove and Vogl, 1989), and laminin (Koch et al., 1999; Siu and Cheng, 2004a; Yan and Cheng, 2006). It is noteworthy that many of these focal contact proteins were shown to localize to the ectoplasmic specialization rather than at the Sertoli cell-basement membrane interface, as expected. The ectoplasmic specialization is a specialized type of anchoring junction that is found in the testis at two distinct sites: 1) either basally between Sertoli cells at the blood-testis barrier or 2) apically between Sertoli cells and elongating/elongated spermatids [see III., C. The Ectoplasmic Specialization, a Testis-Specific Anchoring Junction, below (Russell, 1993b; Vogl et al., 1993)]. The fact that focal contact proteins function in ectoplasmic specialization dynamics is interesting, revealing that the ectoplasmic specialization has a hybrid-like character, which is needed for the regulation of germ cell movement during spermatogenesis. In other words, the ectoplasmic specialization is a mixed type of junction representing a unique category of its own because it adopts features from one of the most effective cell adhesive structures known to facilitate cell movement: the focal contact. On a final note, focal contact proteins (e.g., FAK and paxillin) have also been shown to regulate cadherin-based cell adhesion in HeLa cells (Yano et al., 2004).
III. Review of Anchoring Junctions in the Testis and Their Regulation
A. Cellular Organization of the Seminiferous Epithelium in the Testis
The organization of cells in the seminiferous epithelium of the adult mammalian testis is extremely complex (Fig. 4). All cells (Sertoli and germ cells) in the epithelium sit on top of the tunica propria, which is composed of the following two zones: 1) an acellular zone (basement membrane and a layer of type I collagen) and 2) a cellular zone [peritubular myoid cells and the lymphatic endothelium (Dym, 1994; Mruk and Cheng, 2004b)]. However, direct contact with the basement membrane is made only with Sertoli cells and different types of spermatogonia (Fig. 4). In rodents, spermatogonia can be distinguished microscopically by determining whether heterochromatin is present in the nucleus and by examining its distribution. The different types of spermatogonia are 1) undifferentiated type A spermatogonia [Asingle (also known as spermatogonial stem cells), Apaired, and Aaligned], 2) differentiated type A spermatogonia (A1, A2, A3 and A4), 3) intermediate spermatogonia, and 4) type B spermatogonia, which subsequently give rise to preleptotene spermatocytes (de Rooij and Russell, 2000; Hess, 1990). Besides spermatogonia, preleptotene spermatocytes are the only other class of germ cells that reside in the basal compartment outside the blood-testis barrier. Preleptotene spermatocytes differentiate into leptotene and then zygotene spermatocytes, and these are the germ cells that traverse the blood-testis barrier at stages VIII to XI of the seminiferous epithelial cycle during spermatogenesis in adult rat testes (Russell, 1977b, 1980). Behind the blood-testis barrier, primary and secondary spermatocytes constitute the next layers of developing germ cells, followed by spermatids and spermatozoa. In brief, spermatocytes give rise to spermatids via two meiotic divisions at stage XIV of the epithelial cycle in adult rats, and round spermatids undergo spermiogenesis and differentiate from step 1 to 19 elongating/elongated spermatids. Spermatozoa are the germ cells that are released into the tubule lumen at spermiation, and they collect in the rete testis in preparation for transit to the epididymis for further maturation. In light of the intimate association between cells in the seminiferous epithelium and those in the tunica propria, cross-talk between these two compartments is likely to exist. For instance, peritubular myoid cells are likely to regulate the adhesion of Sertoli cells and spermatogonia to the basement membrane, but studies addressing this area of research have yet to be performed.
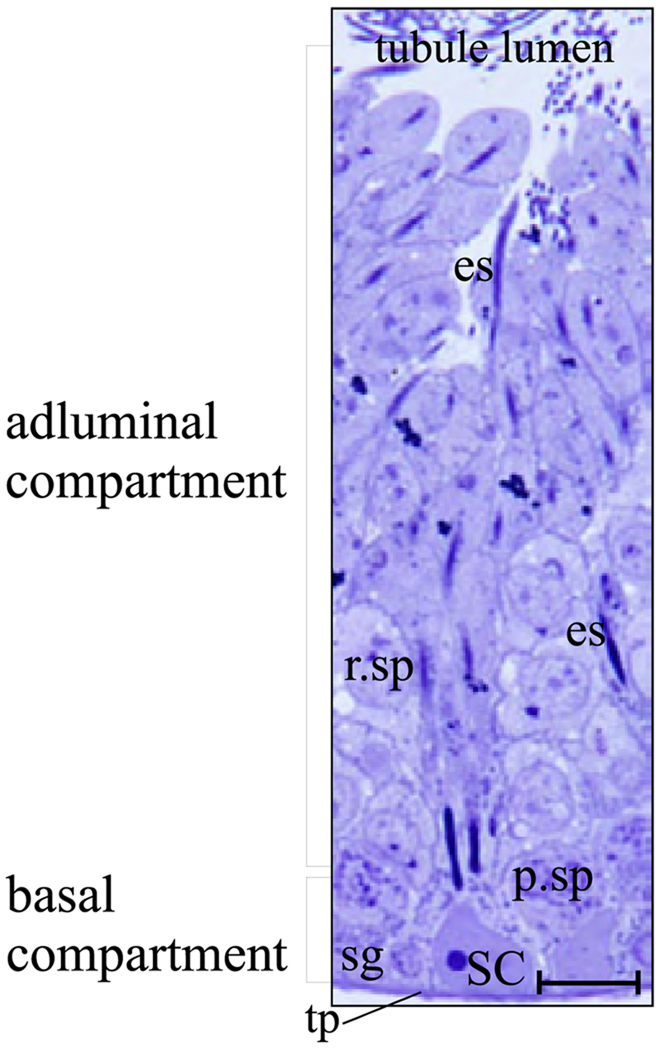
FIG. 4.
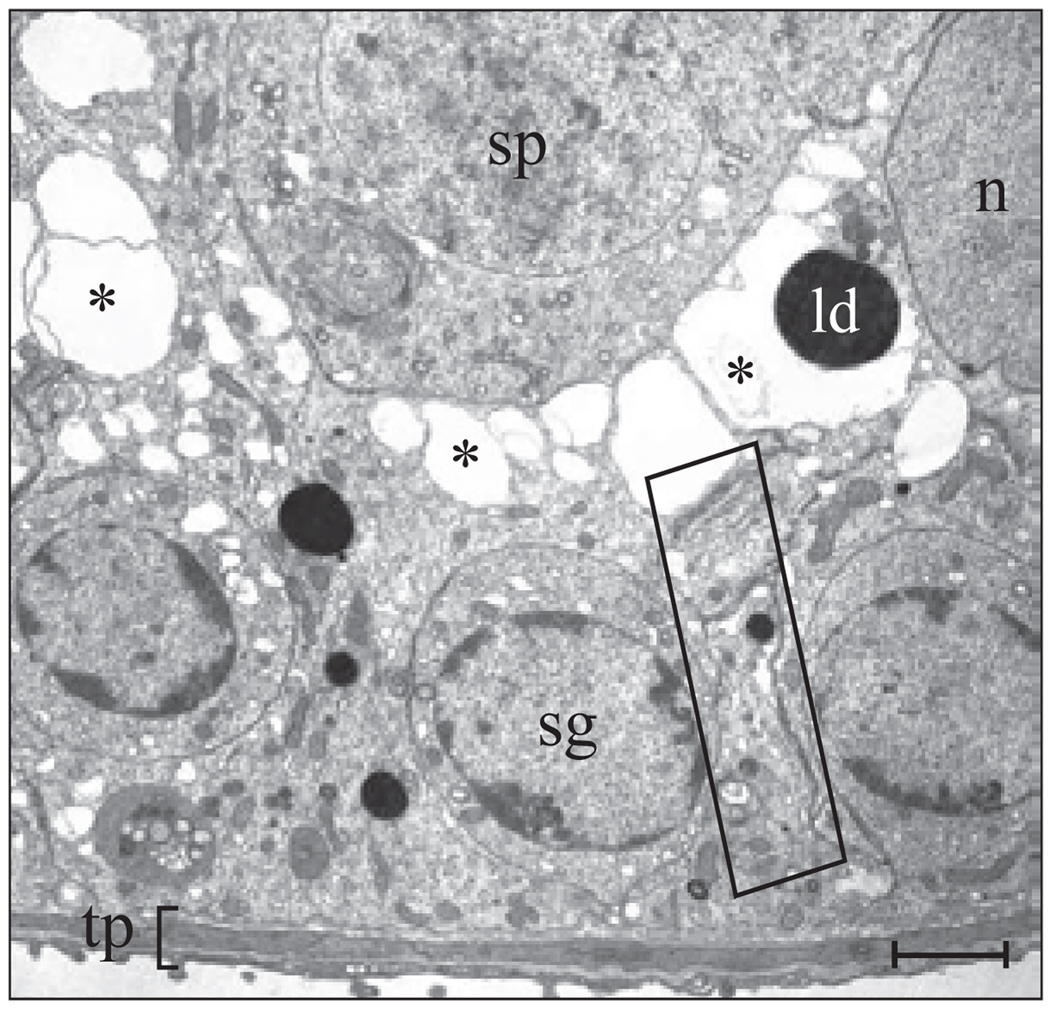
Cross-section of a seminiferous tubule illustrating the intimate relationship between Sertoli and germ cells in the seminiferous epithelium of an adult rat testis. All cells in the seminiferous epithelium sit on top of the tunica propria (tp). A Sertoli cell nucleus (SC) is visualized in this micrograph and located basally within the seminiferous epithelium. It is noteworthy that each Sertoli cell has the ability to support ~30 to 40 germ cells at various stages of development, including spermatogonia (sg), pachytene spermatocytes (p.sp), round (r.sp), and elongating (es) spermatids. During spermiation, elongated spermatids release into the tubule lumen and then travel to the epididymis for further development. The blood-testis barrier (see Fig. 3), which is formed by adjacent Sertoli cells, physically divides the seminiferous epithelium into basal and adluminal compartments. Bar, 12 µm.
Early morphological investigations using cross-sections of seminiferous tubules from adult rats and mice stained with periodic acid-Schiff reaction have shown that germ cells in a particular phase of development associate with one another and with Sertoli cells in a unique pattern (LeBlond and Clermont, 1952; de Kretser and Kerr, 1988). These cell associations, known as stages of the seminiferous epithelial cycle (Fig. 5), are defined most accurately by the morphology of developing spermatid heads and acrosomes, as well as by the relative position of spermatids and primary spermatocytes within the seminiferous epithelium during spermiogenesis (LeBlond and Clermont, 1952; Parvinen, 1982; Hess et al., 1990). Fourteen stages have been assigned in the rat testis with one complete cycle lasting approximately 12.9 days (Fig. 5), whereas there are only 6 and 12 stages in the human and mouse, respectively. However, germ cells are not the only cells known to contribute to the seminiferous epithelial cycle and spermatogenesis. Studies have illustrated that Sertoli cells, the somatic constituents of the seminiferous epithelium, also contribute to the epithelial cycle. For instance, Sertoli cells have been shown to assume various shapes throughout different stages of the epithelial cycle (Russell, 1993c; Vogl et al., 1993; Hess and Franca, 2005). In fact, it has been reported that as much as ~40% of the Sertoli cell surface is in contact with elongated spermatids alone (Russell et al., 1986; de França et al., 1993), illustrating that the interaction between the Sertoli cell and this germ cell type is enormous. Indeed, a single Sertoli cell is known to support ~30–40 germ cells at different stages of development (Weber et al., 1983; Wong and Russell, 1983).
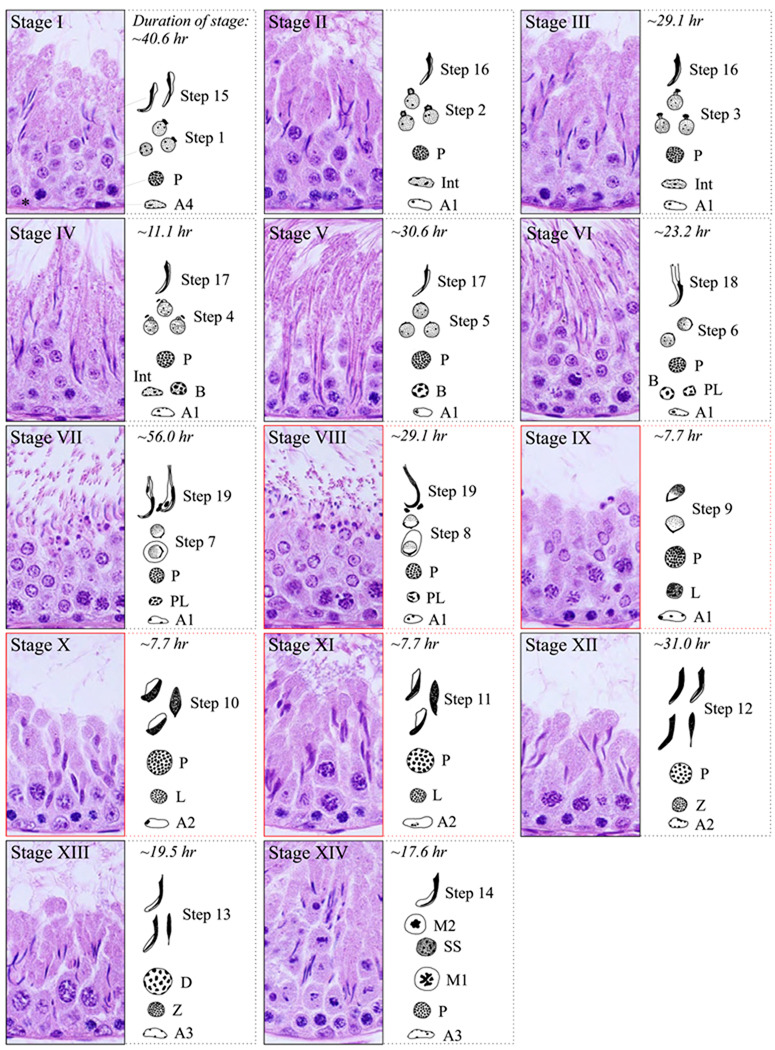
FIG. 5.
Stages of the seminiferous epithelial cycle in the adult rat testis. Each of the 14 stages shown consists of three parts: 1) a cross-section of the seminiferous epithelium (paraffin-embedded testes) stained with hematoxylin and eosin, 2) an illustration of the different types of germ cells associating with that particular stage, and 3) the estimated length of each stage (in hours). Stages II and III are the most difficult to distinguish correctly. Thus, the duration of these two stages has been combined into a single time point. One complete seminiferous epithelial cycle in the rat lasts for 12.9 days. Spermatogonial development is subdivided into type A1–4, intermediate (Int), and type B (B). Spermiogenesis is subdivided into steps 1 to 19 to more accurately define the morphological changes that occur in spermatids during development. In the figure, stages VIII to XI have been enclosed in red boxes to define the approximate time when leptotene spermatocytes traverse the blood-testis barrier, entering into the adluminal compartment for further development. P, pachytene spermatocyte; PL, preleptotene spermatocyte; L, leptotene spermatocyte; Z, zygotene spermatocyte; D, diplotene spermatocyte; SS, secondary spermatocyte; M1, meiosis I; M2, meiosis II. [Prepared based on earlier reports in Stages 2.2, a graphical program designed by Drs. Rex Hess and David Scott (University of Illinois, Urbana-Champaign, IL), and Russell et al., 1990.]
Spermatogenesis is the process by which germ cells develop into mature spermatids under the influence of FSH, testosterone, LH, and estrogen (Steinberger, 1971; de Kretser and Kerr, 1988; Hess et al., 2001; Hess, 2003; Carreau et al., 2008). Throughout spermatogenesis, germ cells traverse the seminiferous epithelium, and this process associates with extensive restructuring of Sertoli-Sertoli and Sertoli-germ cell junctions. In the testis, three types of cell junctions have been identified (Russell, 1993b; Vogl et al., 1993; Cheng and Mruk, 2002; Mruk and Cheng, 2004b), and they are similar functionally to those found in other epithelia (Shin et al., 2006; Holthöfer et al., 2007; Meçse et al., 2007; Pokutta and Weis, 2007). These are tight, anchoring, and gap junctions. Two additional types of testis-specific junctions are also present: ectoplasmic specializations and tubulobulbar complexes. Both are believed to have specialized functions related to spermatogenesis.
B. Concept of the Blood-Testis Barrier
The blood-testis barrier, a blood-tissue barrier present between adjacent Sertoli cells in the testis (Fig. 3), is one of the tightest blood-tissue barriers known to exist because it was shown to restrict the diffusion of small molecules (e.g., dyes) from blood vessels present in the interstitium into the seminiferous epithelium (Goldman, 1909; Fawcett et al., 1970; Fawcett, 1975). Its functions are to 1) create a specialized environment for postmeiotic germ cell development, 2) regulate the passage of molecules into and out of the seminiferous epithelium, 3) serve as an immunological barrier, and 4) confer cell polarity (Dym and Fawcett, 1970; Setchell and Waites, 1975). Herein, we discuss briefly the biology of the blood-testis barrier as it relates to spermatogenesis and fertility. This information, if adequately expanded in future studies, should shed insight on how compounds such as adjudin and CDB-4022 traverse the blood-testis barrier to affect spermatogenesis because this tissue barrier poses an obstacle in the delivery of contraceptives that target cells in the seminiferous epithelium.
The blood-testis barrier is different from blood-tissue barriers present in other organs such as the brain. First, the blood-testis barrier physically separates the seminiferous epithelium into two compartments: 1) the basal compartment in which spermatogonia and early primary spermatocytes reside and 2) the adluminal compartment in which late primary and secondary spermatocytes and spermatids are sequestered from the systemic circulation and allowed to complete meiosis and differentiation (Dym and Fawcett, 1970; Setchell, 1980; Pelletier and Byers, 1992) (Fig. 4). This is a unique characteristic of the blood-testis barrier because in no other organ does a blood-tissue barrier create two functionally distinct yet interdependent compartments within an epithelium. Second, the blood-testis barrier must open (or restructure) transiently to allow leptotene spermatocytes to enter the adluminal compartment without significantly affecting the homeostasis of the epithelium. The opening/restructuring of the blood-testis barrier has been shown to roughly span stages VIII to XI of the epithelial cycle, revealing that it would be opened for approximately one tenth of the 12.9-day epithelial cycle in the rat (Fig. 5). Third, blood-tissue barriers are known to be constituted largely by tight junctions, but in some organs, such as the intestine, adherens junctions are known to be spatially intermixed and to cofunction with tight junctions. In the testis, the tight junction is a primary component of the blood-testis barrier, but the classic adherens junction seems to be replaced by the ectoplasmic specialization (Russell and Peterson, 1985). Desmosome junctions, as well as gap junctions and tubulobulbar complexes (the tubulobulbar complex is a testis-specific type of cell junction known to internalize intact cell junctions after restructuring of the blood-testis barrier, as well as during sperm release), also contribute to blood-testis barrier function (Fig. 3), making this barrier unique from other blood-tissue barriers. Finally, the relative location of the tight junction in Sertoli cells of the testis is different; it is situated approximately one-two germ cells away from the basal lamina, whereas in other epithelial cells it occupies the apical portion of cells.
The regulation of tight junctions in the testis is understood poorly compared with other epithelia. Although this seems to be due to the lack of suitable models that can be used to study tight junction assembly and disassembly, in reality several excellent experimental models are available. In essence, these include 1) culturing Sertoli cells at high density in the presence of calcium to initiate junction assembly, 2) calcium and ATP depletion and repletion experiments, 3) culturing cells in the presence of various factors such as growth factors and cytokines, and 4) detachment of cells from their substrate/extracellular matrix (Denker and Nigam, 1998; Grima et al., 1998; Ben-Shaul and Ophir, 2001; Siu and Cheng, 2004b; Xia et al., 2005a). However, only a few of these in vitro systems have been used successfully to study tight junction dynamics in the testis (Byers et al., 1986; Janecki et al., 1991; Grima et al., 1992). For instance, cultures of Sertoli cells have proved to be a useful system to study tight junction dynamics in the testis because a very good picture has evolved regarding the biochemical architecture of this cellular structure. We know that the proteins that constitute the tight junction in the testis are not different from those found in other organs such as the brain, kidney, or small intestine. However, this in vitro system is not ideal. For example, tight junctions between Sertoli cells (isolated from 20-day-old rat testes) were considerably less tight than those between keratinocytes and MDCK cells when assessed by TER [<100 Ω · cm2 versus >1000 Ω · cm2, respectively] (Gumbiner and Simons, 1986; Janecki et al., 1991; Grima et al., 1992). Although Sertoli cell tight junctions seem to be “leaky” in vitro, this is not an accurate representation of the situation in vivo, because when dye was injected into the vasculature, most tissues were labeled except the brain, testis, and placenta (Goldman, 1909). At this point, it is not entirely clear whether some crucial factor(s) are missing from Sertoli cell cultures in vitro. For example, germ cells, especially preleptotene and leptotene spermatocytes, which associate intimately with the blood-testis barrier, are absent from this in vitro system. Yet, we speculate that they play an important role in the restructuring of the blood-testis barrier at stages VIII to XI of the epithelial cycle. Moreover, peritubular myoid cells, which are known to contribute to the making of the extracellular matrix (Fig. 3), have been reported to serve as a selective barrier in the rat in vivo similar to the one present between adjacent Sertoli cells (Setchell, 1978; Plöen and Setchell, 1992). Because peritubular myoid cells encircle seminiferous tubules, this barrier would provide the first line of protection by prohibiting the entry of harmful substances into the seminiferous epithelium. Yet peritubular myoid cells do not seem to possess tight junctions because these cells were not immunoreactive for tight junction proteins such as occludin and ZO-1 (Mruk and Cheng, 2004b). It is also not known why this barrier-like property of peritubular myoid cells is restricted to rodents only. Nevertheless, it should be noted that no in vitro or in vivo studies in the literature address the role of germ or peritubular myoid cells in the assembly and/or disassembly of Sertoli cell tight junctions. Thus, although Sertoli cell cultures have proved to be important for assessing many physiological and toxicological parameters, results obtained from the use of this in vitro model should be interpreted with some degree of caution.
In this section, we have described several in vitro models that can be used to successfully study blood-testis barrier dynamics. Through the use of these models in the past, much information has been acquired relating to the regulation of Sertoli cell junctions, because the blood-testis barrier is constituted exclusively by Sertoli cells (Fig. 3). After decades of morphological and biochemical studies, we know that Sertoli cells isolated from rat testes become polarized and establish functional junctions when they are plated on a reconstituted basement membrane such as Matrigel at high cell density (~0.5–1.0 × 106 cells/cm2) in a two-dimensional environment (Chung et al., 1999; Grima and Cheng, 2000; Wong et al., 2000; Chung and Cheng, 2001; Lee et al., 2003). We also know that Sertoli cells cultured under these conditions are functionally similar to those found in the testis in vivo, because they secrete transferrin, androgen binding protein (ABP), testin, and α2-macroglobulin in a polarized fashion (Grima et al., 1992). Furthermore, the ultrastructural features of basal ectoplasmic specializations and tight junctions found in these two-dimensional cultures are indistinguishable from those observed in vivo by electron microscopy (Siu et al., 2005). The three-dimensional culture system has recently sparked added interest among investigators. In this model, cells are grown embedded within a three-dimensional extracellular matrix (ECM), and they form tubule-like structures characterized by a lumen (Fischbach et al., 2007; Pampaloni et al., 2007; Yamada and Cukierman, 2007). The primary advantage of using the three-dimensional culture model over the two-dimensional system lies in the fact that cell junctions seem to be more extensively developed in the former in vitro system, and this is especially important in processes such as wound healing, tumor migration, and metastasis, which depend largely on the directed movement of cells. While studies from our laboratory and others have not yet reported findings using Sertoli cells cultured in a three-dimensional environment, preliminary findings have shown that Sertoli cells formed tubule-like structures when cultured under these conditions (D.R.M. and C.Y.C., unpublished observations).
C. The Ectoplasmic Specialization, a Testis-Specific Anchoring Junction
In the past several decades, the Sertoli cell has received considerable attention, largely because a unique arrangement of actin filaments was found to exist in two distinct areas of this cell known as the apical and basal ectoplasmic specialization, a testis-specific type of anchoring junction (Russell, 1977c; Vogl et al., 1993, 2000) (Fig. 6). At the ectoplasmic specialization, actin filaments are not arranged as they would be in a typical epithelial cell but in hexagonal bundles with unipolar orientation (Russell, 1977c, 1993b; Grove and Vogl, 1989; Mruk and Cheng, 2004a). Another notable feature of actin filaments at the ectoplasmic specialization is that they are noncontractile in nature (Vogl and Soucy, 1985), suggesting that this structure is not likely to facilitate the movement of germ cells. Despite this observation, actin filaments at the ectoplasmic specialization are in a continuous state of disassembly and reassembly (e.g., depolymerization and polymerization), especially during the different stages of the epithelial cycle (Russell, 1993a,c). It should also be noted that actin filaments are scattered diffusely throughout the Sertoli cell, but at the ectoplasmic specialization, they are most well organized and their density is the greatest (Russell, 1977c, 1993b; Vogl et al., 1993) (Fig. 6).
FIG. 6.
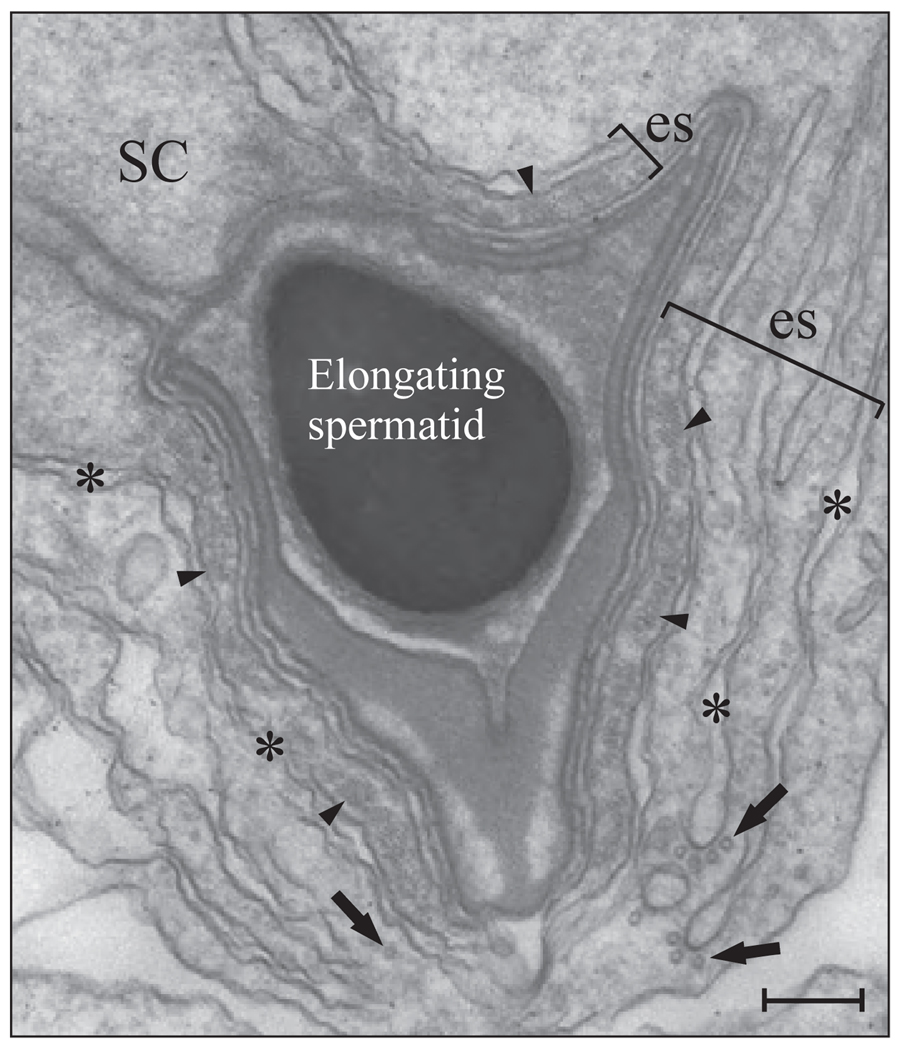
Apical ectoplasmic specialization in the adult rat testis. Electron micrograph of the apical ectoplasmic specialization (es, see brackets) between a Sertoli cell (SC) and elongating spermatid consisting of hexagonally arranged bundles of actin filaments (see arrowheads) sandwiched between the Sertoli cell membrane and flattened cisternae of endoplasmic reticulum (see asterisks). Also shown are microtubules (see arrows). Bar, 0.4 µm.
The primary function of the apical ectoplasmic specialization is to prevent premature release of spermatids into the tubule lumen because no other type of junction is present between Sertoli cells and elongating/elongated spermatids from steps 8 to 19 (Russell, 1993b) (Fig. 6). In an interesting study, which quantified the physical adhesive strength of the apical ectoplasmic specialization present between Sertoli cells and step 8 spermatids, it was found that a greater force was required to disrupt the apical ectoplasmic specialization versus the desmosome-like junction present between Sertoli cells and pre–step-8 spermatids (Wolski et al., 2005). Although these results reveal that the apical ectoplasmic specialization confers stable and robust cell adhesion, this structure was the primary target of adjudin because its function was compromised before that of the desmosome-like junction. These findings illustrate that the testis-specific ectoplasmic specialization is an ideal target for male contraceptive development. On the other hand, the primary function of the basal ectoplasmic specialization, which is present between adjacent Sertoli cells, is to contribute to the integrity of the blood-testis barrier (Setchell, 1980, 1998; Russell, 1993b) (Fig. 3).
So far, a growing list of proteins has been shown to underlie ectoplasmic specialization function including α6β1 integrin (Palombi et al., 1992; Salanova et al., 1995; Mulholland et al., 2001), laminin (Yan and Cheng, 2006), actin (Vogl et al., 1993), α-actinin (Russell and Goh, 1988), myosin VIIA (Hasson et al., 1997), fimbrin (Grove and Vogl, 1989), espin (Bartles et al., 1996), vinculin (Grove et al., 1990; Pfeiffer and Vogl, 1991), phosphorylated c-Src (Wong et al., 2005), paxillin (Mulholland et al., 2001), gelsolin (Guttman et al., 2002), cadherin (Lee et al., 2004), ILK (Mulholland et al., 2001), phosphorylated FAK (Siu et al., 2003), testin (Grima et al., 1998), and Keap1 (Itoh et al., 1999; Velichkova et al., 2002) (Fig. 1). As previously discussed, many of these proteins, such as vinculin, paxillin and ILK, are constituents of the focal contact (Brown and Turner, 2004; Legate et al., 2006; Lo, 2006; Ziegler et al., 2006). This property makes the ectoplasmic specialization unique and an excellent target for male contraceptive research, especially because focal contacts have never been identified in the testis. It should also be noted that many of these proteins localize to both the basal and apical ectoplasmic specialization but some are restricted to (or predominantly located at) either the apical (e.g., β1 integrin, nectin-3, phosphorylated Src-Tyr416, and phosphorylated FAK-Tyr397) or basal ectoplasmic specialization [e.g., N-cadherin, β-catenin, c-Src, and FAK] (Ozaki-Kuroda et al., 2002; Siu et al., 2003; Mruk and Cheng, 2004b; Lee and Cheng, 2005; Wong et al., 2005).
1. Integrin-Laminin Multiprotein Complex
In addition to the cadherin-catenin and nectin-afadin multiprotein complexes previously discussed, the integrin-laminin complex also mediates cell adhesion in the seminiferous epithelium. For example, the α6β1 integrin-laminin-333 (also known as laminin α3β3γ3) complex is generally found between cells and the extracellular matrix, but in the testis it is present between Sertoli and elongating/elongated spermatids at the apical ectoplasmic specialization (Salanova et al., 1995, 1998; Siu and Cheng, 2004b; Yan and Cheng, 2006; Yan et al., 2007) (Fig. 1). This is the only multiprotein complex that is unique to the apical ectoplasmic specialization because cadherin-catenin and nectin-afadin multiprotein complexes are also found at the basal ectoplasmic specialization. For this reason, it is very important that the biology and regulation of the integrin-laminin complex be better understood in light of the fact that it seems to be an excellent “druggable” target for male contraceptive development. In particular, α6β1 integrin was restricted to Sertoli cells, whereas laminin-333 was present on the surface of elongating/elongated spermatids (Yan and Cheng, 2006). Associated with this adhesion complex in the testis is a list of adaptor and signaling proteins. They include vinculin (Grove et al., 1990; Pfeiffer and Vogl, 1991), espin (Bartles et al., 1996), c-Src (Siu et al., 2003; Yan and Cheng, 2006), FAK, phosphorylated FAK-Tyr397 (Siu et al., 2003), paxillin, and ILK (Mulholland et al., 2001) (Fig. 1). However, the identity of the integral membrane protein that physically anchors the laminin complex to elongating/elongated spermatids to mediate cell adhesion is not yet known because laminin does not have a transmembrane domain. Moreover, except for c-Src, which was shown to coimmunoprecipitate with laminin-333 (Yan and Cheng, 2006), the identities of the cytoplasmic proteins that interact with laminin in elongating/elongated spermatids are not known. It is noteworthy that a recent study showed that β3 integrin, which participates in the formation of anchoring junctions, physically interacts with nectin-3 in NIH3T3 cells (Sakamoto et al., 2006). Given that nectin-3 is present at the apical ectoplasmic specialization and β3 integrin is expressed highly in the testis (Le Gat et al., 2003), the integrin-laminin multiprotein complex may facilitate Sertoli-spermatid adhesion by interacting with nectin-3. Thus far, there exists only a single report on the localization of β3 integrin in the testis, which seemed to be restricted to Leydig cells (Merono et al., 2002), but this is an interesting possibility that should be examined further through well designed functional studies.
Alternatively, adhesion conferred by the α6β1 integrin-laminin-333 complex may not be mediated via a conventional mechanism of protein-protein interactions. It should be noted that elongating/elongated spermatids are differentiated cells possessing densely packed nuclear chromatin with virtually no transcriptional activity (de Kretser and Kerr, 1988). Because of this, the level of laminin expression by elongating/elongated spermatids may not be stoichiometrically equivalent to the level of α6β1 integrin expression by Sertoli cells, even though these two proteins probably interact with each other as ligand and receptor. If the expression levels of these two proteins are highly divergent, then they may not contribute equally to integrin-laminin-mediated cell adhesion, which is essential for spermatogenesis and fertility. We speculate cautiously that laminin-333 may be acting as a “glue” to link integrin to the germ cell surface indirectly, in turn facilitating adhesion between elongating/elongated spermatids and Sertoli cells. Moreover, the ability of laminin-333 to mediate cell adhesion in a functional in vitro assay has never been demonstrated even though the integrin-laminin complex is known to confer adhesion between cells and the basement membrane in other epithelia. It is also possible that α6β1 integrin-laminin-333 association is needed for the recruitment of focal contact proteins such as phosphorylated FAK-Tyr397, Src, and ILK to the apical ectoplasmic specialization (Yan and Cheng, 2006) and, in this way, the integrin-laminin complex would participate in cell adhesion indirectly. Thus, this would make the ectoplasmic specialization a unique type of anchoring junction, one that has adopted features of the focal contact, which is known to facilitate migration of cells on the extracellular matrix.
D. Regulation of Anchoring Junctions in the Testis
1. Phosphatases and Kinases
In mammalian cells such as Sertoli and germ cells, ~30% of proteins are phosphoproteins (Denu and Dixon, 1998). Kinases and phosphatases, which either phosphorylate (activate) or dephosphorylate (deactivate) proteins, respectively, regulate the overall phosphoprotein content within cells. This is critical to virtually all cellular processes including differentiation, growth, development, apoptosis, tumorigenesis, germ cell meiosis, and anchoring junction dynamics (Cheng and Mruk, 2002; Mruk and Cheng, 2004b; Wolgemuth et al., 2004; Janssens et al., 2005; Stoker, 2005; Wong and Cheng, 2005; Burridge et al., 2006; Wu, 2007).
A phosphatase is an enzyme that removes a phosphate group (PO4) from Tyr, Ser, Thr, and/or His residues of a target protein by hydrolyzing phosphoric acid monoesters into a phosphate ion to yield an amino acid residue with a free hydroxyl group. This action is the opposite of a kinase, which attaches a phosphate group to either Tyr, Ser, Thr, and/or His residues of a target protein by using ATP. Protein phosphatases are classified into different types based on their substrate specificity: 1) Tyr-, 2) Ser/Thr-, 3) His-, and 4) dual-specificity (e.g., Tyr and Ser/Thr) phosphatases (Barford, 1996; Zhang, 2002). Ser/Thr-specific phosphatases are further subdivided into 1) PP1; 2) PP2A, 2B, 2C; 3) PP4; and 4) PP5. Likewise, protein kinases are defined as 1) Tyr-, 2) Ser/Thr-, 3) His-, 4) Asp/Glu-, and 5) mixed-specificity kinases, and these classifications describe the protein’s site of phosphorylation. The best-studied kinases are protein tyrosine kinases, of which there are two types: 1) receptor [e.g., Janus kinase (JAK)] and 2) nonreceptor [e.g., c-Src, FAK, and extracellular signal-regulated kinase (ERK)].
The significance of phosphatases and kinases in anchoring junction dynamics in the testis has only recently begun to be explored, and most of these studies have used specific inhibitors against different phosphatases and kinases to understand ectoplasmic specialization dynamics. For instance, Y-27632, a specific Rho kinase (ROCK, an effector of Rho GTPase) inhibitor, was shown to delay and partially block adjudin-mediated germ cell loss from the seminiferous epithelium (Lui et al., 2003), suggesting that the actin cytoskeleton is one of the targets of adjudin in the testis. Additional studies have shown that adhesion of germ cells in the seminiferous epithelium is also regulated by the ERK signaling cascade (Xia and Cheng, 2005; Xia et al., 2006a). ERK, a nonreceptor protein tyrosine kinase of the mitogen-activated protein (MAP) kinase family, was shown to associate largely with Sertoli cells and spermatocytes in the basal compartment (Mizrak et al., 2007). Weak ERK immunoreactivity was also detected at the apical ectoplasmic specialization between Sertoli cells and early elongating spermatids, but phosphorylated ERK-Thr202/Tyr204 localization was found predominantly between Sertoli cells and spermatids at stage 8 and beyond in the rat (Wong et al., 2005; Xia and Cheng, 2005). As such, phosphorylated ERK has been hypothesized to mediate restructuring of the apical ectoplasmic specialization, and to regulate spermatid movement and release. Note that the localization patterns of ERK and phosphorylated ERK-Thr202/Tyr204 (Xia and Cheng, 2005; Mizrak et al., 2007) are remarkably similar to the localization patterns of FAK and phosphorylated FAK-Tyr397, because the immunoreactivity of FAK was also restricted largely to the blood-testis barrier but that of phosphorylated FAK-Tyr397 was restricted to the apical ectoplasmic specialization (Siu et al., 2003). These results seemingly suggest that two forms of a specific protein kinase (i.e., phosphorylated and unphosphorylated) are needed to regulate closely related cellular functions in Sertoli cells, such as junction restructuring that occurs at the apical ectoplasmic specialization and blood-testis barrier, which are present at opposite ends of the Sertoli cell.
In another series of complementary studies, an increase in Tyr phosphorylation of β-catenin was detected after suppression of the intratesticular androgen level, which resulted in the disassembly of anchoring junctions present between Sertoli cells and spermatids at step 8 and beyond. This was probably mediated by interactions between myotubularin-related protein 2 (a phosphatidylinositol 3-phosphatase) and c-Src (Xia et al., 2005b; Zhang et al., 2005). These findings are consistent with the general concept that phosphorylation of transmembrane proteins (e.g., cadherins, nectins, and integrins), as well as their associated adaptor proteins (e.g., catenins and afadin), leads to loss of cell adhesion (Gumbiner, 2000; Kikyo et al., 2000; Mruk and Cheng, 2004b). This is because phosphorylated proteins move away from anchoring junctions and relocate to the basolateral region of epithelial cells (Yap et al., 1997; Denker and Nigam, 1998; Cheng and Mruk, 2002; Mruk and Cheng, 2004b). A recent study reported that T-cadherin [an unusual member of the cadherin superfamily that is anchored to the plasma membrane via a glycosyl phosphatidylinositol moiety (Vestal and Ranscht, 1992)] is phosphorylated and that this phosphorylated form is subjected to proteasomal degradation (Bai et al., 2007).
Src, a protein tyrosine kinase of the transforming gene of the Rous sarcoma virus, is composed of the following family members: 1) c-Src, 2) Fyn, 3) c-Yes, 4) Fgr, 5) Lyn, 6) Hck, 7) Lck, 8) Blk, and 9) Yrk. The Src protein family is important largely in growth factor signaling (Abram and Courtneidge, 2000), but selected members are also known to regulate other aspects of cell function such as anchoring junction dynamics during spermatogenesis (Bjorge et al., 2000; Courtneidge, 2002; Lee and Cheng, 2004; Abram and Lowell, 2007; Kanda et al., 2007; Rivera and Olivera, 2007; Schenone et al., 2007). Studies have shown that c-Src is expressed by both Sertoli and germ cells (Lee and Cheng, 2005) and that it associates with the apical ectoplasmic specialization via 1) the integrin multiprotein complex (Siu et al., 2005; Wong et al., 2005), 2) myotubularin-related protein 2 (Zhang et al., 2005), and 3) laminin-333 (Yan and Cheng, 2006) (Fig. 1). c-Src also interacts with actin and tubulin (Siu et al., 2005) and was recently reported to regulate actin-and tubulin-based cytoskeleton dynamics (Head et al., 2006; Perez-Moreno and Fuchs, 2006; Tehrani et al., 2007), thereby facilitating cell adhesion and movement. Furthermore, phosphorylated c-Src-Tyr416 was shown to localize predominantly to the apical ectoplasmic specialization in early stage VIII of the epithelial cycle before spermiation (Wong et al., 2005), suggesting that Src is important in the restructuring of the ectoplasmic specialization that occurs before spermiation. It would be interesting to determine in future studies whether cellular events such as spermiation and germ cell movement can be affected when the function of this important kinase is disrupted specifically in the testis and more importantly, whether this can lead to infertility.
2. Cytokines and Growth Factors
The participation of cytokines in the restructuring of tight junctions in different epithelia and endothelia including the testis has been studied extensively (Walsh et al., 2000; Cheng and Mruk, 2002; Mruk and Cheng, 2004b; Xia et al., 2005a). However, because this subject is outside the scope of this review, it is not discussed in detail herein. In this section, we consider briefly how cytokines affect the dynamics of anchoring junctions. The general consensus is that cytokines can affect the function of anchoring junctions by several mechanisms: 1) down-regulation of expression (transcriptional repression)/steady-state protein levels, 2) altering protein-protein interactions, 3) affecting the localization of proteins, 4) protein phosphorylation 5) restructuring actin and microtubule cytoskeletons, 6) internalization of integral membrane proteins, and 7) proteolysis. Some of these cytokine-mediated mechanisms are discussed below.
Cadherins are target proteins of tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and interleukin-1β (IL-1β) in multiple epithelia and endothelia, and this includes Sertoli cells in the testis (Karmakar and Das, 2004; Angelini et al., 2006; Li et al., 2006; Ogata et al., 2007). These cytokines were shown to reduce cadherin steady-state mRNA and/or protein levels, leading to adherens junction disassembly. Similar results were recently published from our laboratory in which a decrease in N-cadherin after intratesticular injection of TNF-α was reported (Li et al., 2006). Indeed, recent studies illustrate that cytokines reduce cadherin expression via transcriptional regulation. For example, TGFβ1-induced down-regulation of E-cadherin expression in lens epithelial cells was shown to be mediated via the transcription factor Slug (Choi et al., 2007), whereas E-cadherin function in TGF-β1-treated MDCK cells was regulated via Snail and lymphoid enhancer binding factor 1 transcription factors (Peinado et al., 2003; Medici et al., 2006). Proteolytic degradation of cell adhesion proteins that occurred after treatment of cells with various cytokines has also been reported as a means to effectively reduce protein levels in different cell types, thereby regulating cell adhesion. Treatment of tumor cells with hepatocyte growth factor (HGF) increased the extracellular cleavage of E-cadherin and reduced the level of E-cadherin (Lee et al., 2007). In addition, cytokines such as TGF-β have also been shown to directly affect the production of proteases (Marcet-Palacios et al., 2007), and this may affect cell adhesion indirectly. For instance, Sertoligerm cell junction disassembly induced by cadmium chloride, an environmental toxicant, was associated with a surge in TGF-β2 and TGF-β3, as well as a concomitant increase in cathepsin L (Wong et al., 2004). Besides cadherin, nectin was shown to be proteolytically cleaved in response to HGF treatment in MDCK cells (Tanaka et al., 2002), but there exist no reports detailing the transcriptional regulation of nectin after treatment with cytokines or growth factors.
Cytokines can also alter the phosphorylation status of cell adhesion proteins, and this often leads to changes in protein localization (e.g., endocytosis) and/or protein-protein interactions. For example, treatment of endothelial cells with TNF-α in vitro resulted in the phosphorylation of VE-cadherin, β- and γ-catenin, and p120 catenin (Angelini et al., 2006). Other cytokines, such as fibroblast growth factor, caused dissociation of the cadherin-β-catenin complex and recruited β-catenin into the nucleus (Halama et al., 2001). Likewise, the interaction between E-cadherin and β-catenin decreased after HGF treatment (Lee et al., 2007). Numerous in vitro studies have also demonstrated that cytokines can cause integral membrane proteins such as E-cadherin and integrin to be internalized into endocytic vesicles, resulting in compromised cell adhesion. For instance, TGF-β-mediated morphogenesis, which occurs during vertebrate gastrulation, resulted in cadherin internalization (Ogata et al., 2007). In addition, a similar endocytic trafficking mechanism was recently shown to exist in TGF-β2-treated Sertoli cells having functional cell junctions (Yan et al., 2008), illustrating that this cytokine facilitates the disassembly of Sertoli cell adherens junctions to enable germ cells to traverse the blood-testis barrier.
Finally, cytokines such as IL-1, TNF-α, and TGF-β can regulate the function of adherens junctions by affecting the cytoskeleton directly. For example, TNF-α was shown to disrupt actin microfilaments and microtubules (Domnina et al., 2002, 2004), whereas TGF-β1 affected microtubule dynamics in endothelial cells (Birukova et al., 2005). In this context, it is interesting to note that intratesticular administration of IL-1α was shown to perturb Sertoli cell actin dynamics without significantly affecting the steady-state levels of cadherin and catenin, resulting in the depletion of germ cells from the seminiferous epithelium (Sarkar et al., 2008). From these examples, it is clear that cytoskeletal integrity is required for anchoring junction function. The next logical step would be to expand the latter study and to investigate the mechanism underlying actin cytoskeleton reorganization.
Taken collectively, these studies illustrate that cytokines elicit a diverse range of effects to affect different parameters of cell adhesion depending on the cytokine and cell system being examined. This information may be helpful in the research and development of innovative approaches for male contraception. For instance, studies have shown that TGF-β3 activates p38 MAP kinase possibly to open the blood-testis barrier to facilitate the passage of leptotene spermatocytes at stages VIII to XI of the seminiferous epithelial cycle (Lui et al., 2001; Wong et al., 2004; Xia et al., 2006a). This cascade of events only takes place when TGF-β receptor I (TβRI) binds simultaneously to two adaptors: 1) CD2-associated protein (CD2AP) and 2) TAK1-binding protein 1 to form a multiprotein complex with TGF-β3 (Xia et al., 2006a). On the other hand, if TGF-β3 and TβRI bind to CD2AP only, then ERK is activated instead of p38 MAP kinase (Xia et al., 2006a). This compromises Sertoli-germ cell adhesion without affecting blood-testis barrier dynamics. Moreover, the importance of ERK in the testis is supported by another study, which demonstrated that testosterone can mediate its effects on Sertoli-germ cell adhesion via the Src–ERK signaling cascade (Cheng et al., 2007) instead of the classic androgen receptor-mediated pathway (Fix et al., 2004). Indeed, testosterone is known to play a critical function in the regulation of the blood-testis barrier and anchoring junctions in the testis (O’Donnell et al., 2000; Meng et al., 2005). Thus, a specific inhibitor, antagonist or agonist that would prevent formation of the TGF-β3–TβRI–CD2AP–TAK1-binding protein 1 multiprotein complex may be an interesting approach to nonhormonal male contraceptive development. However, the major challenge of this approach would be to specifically activate ERK in the testis and not in other organs, because ERK is a MAP kinase known to have numerous physiological functions (Helfand et al., 2005; Wilkie, 2007; Wu, 2007).
3. Small GTPases
There are five major classes of small GTPases: 1) Rho, 2) Ras, 3) Rab, 4) Arf, and 5) Ran. Each subfamily is functionally unique with important roles in processes such as signal transduction, cell junction dynamics, cell polarity, cytoskeleton dynamics, endocytosis/exocytosis, cell cycle, apoptosis, and protein transport (Etienne-Manneville and Hall, 2002; Fransson et al., 2003; Mruk et al., 2005; Joseph, 2006; Bustelo et al., 2007; Gillingham and Munro, 2007; Inoue and Randazzo, 2007). Generally speaking, GTPases function as molecular switches by cycling between active (GTP-bound) and inactive (GDP-bound) conformations. This cyclical activation and inactivation is facilitated by three types of regulators: GDP/GTP exchange factor (GEF), GTPase-activating protein (GAP), and GDP dissociation inhibitor. In their GTP-bound conformations, GTPases bind to specific effector proteins that facilitate downstream signaling and cell function. For additional background information on GTPases in general, readers are encouraged to refer to the following reviews: Olkkonen and Stenmark (1997), Takai et al. (2001), Stork (2003), Schwartz (2004), Bos (2005), DerMardirossian and Bokoch (2005), Mruk et al. (2005), D’Souza-Schorey and Chavrier (2006), Grosshans et al. (2006), Joseph (2006), Sabe et al. (2006), Buchsbaum (2007), Bustelo et al. (2007), and Stewart (2007). Readers are also encouraged to refer to original research and review articles that discuss atypical Rho GTPases, which are not discussed herein but are an area of recent interest for many investigators (Fransson et al., 2003; Shutes et al., 2006; Aspenström et al., 2007; Chuang et al., 2007).
Rac1, RhoA, and Cdc42 are the best-studied members of the Rho family, and we discuss them in this review article because they play essential roles in regulating cell adhesion. Both Rac1 and Cdc42 have been shown to localize to sites of cell contact, and disrupting cell adhesion resulted in the translocation of Rac1 to the cytosol (Kuroda et al., 1997; Takaishi et al., 1997; Nakagawa et al., 2001). In agreement with these results, overexpression of dominant-active Rac1 in MDCK cells caused E-cadherin and β-catenin to concentrate at sites of cell contact (Takaishi et al., 1997), clearly illustrating the importance of Rho GTPases in adherens junction function. Nevertheless, both Rac1 and Cdc42 have been reported to regulate E-cadherin activity through the effector IQGAP (Kuroda et al., 1998; Fukata et al., 1999; Briggs and Sacks, 2003), and three IQGAP isoforms that differ in tissue distribution and function have been identified in mammals (Brown and Sacks, 2006; Brandt and Grosse, 2007; Wang et al., 2007). In addition to cadherin, nectin was also shown to be involved in the localization of IQGAP at the adherens junction (Katata et al., 2003). In the testis, IQGAP1 was found to be a negative regulator of Sertoli-germ but not Sertoli-Sertoli cell adhesion. IQGAP1, the best-characterized member of the IQGAP family, is produced by both Sertoli and germ cells (Lui et al., 2005). Transiently perturbing Sertoligerm cell adhesion in vitro with the use of EGTA, for instance, resulted in IQGAP binding to β-catenin. This, in turn, interfered with its ability to bind to N-cadherin and induced the loss of cell adhesion (Lui et al., 2005). However, in cocultures with intact adherens junctions, IQGAP associated with Cdc42 (Lui et al., 2005). All three IQGAP isoforms have previously been reported to be present in the testis (Wang et al., 2007) (Fig. 1). Moreover, both Cdc42 and RhoB have been found to localize at the apical ectoplasmic specialization (Chapin et al., 2001; Lui et al., 2003), illustrating that they are important regulators of Sertoli-spermatid adhesion. Taken collectively, these results demonstrate that adhesion between Sertoli and germ cells in the testis is regulated distinctly from adhesion between Sertoli cells. If these differences in cell adhesion can be understood better, one can selectively perturb adhesion of germ cells to Sertoli cells without significantly affecting adhesion between Sertoli cells.
Rap is a Ras GTPase that has been shown to regulate actin dynamics, cell adhesion, junction formation (but not maintenance) and cell polarity (Knox and Brown, 2002; Hogan et al., 2004; Schwamborn and Puschel, 2004; Bos, 2005). For instance, Rap1 was shown to regulate E-cadherin-based cell adhesion, and inhibition of Rap1 in MDCK cells resulted in the loss of E-cadherin from the cell surface and disassembly of cell junctions (Hogan et al., 2004; Price et al., 2004). There is also evidence to suggest the existence of cross talk between Rap1 and Rho GTPases. Two separate studies have reported that Rap1 is required for the activation of Rac (Maillet et al., 2003) and Cdc42 (Hogan et al., 2004). Rap1 was also shown to interact directly with Vav2 and Tiam1 (Arthur et al., 2004), two Rac GEFs, as well as with IQGAP (Jeong et al., 2007). In addition to these examples, Rap1 also interacts with DOCK4 (Yajnik et al., 2003), as well as with C3G (Dupuy et al., 2005) and PDZ-GEF1/PDZ-GEF2 (de Rooij et al., 1999; Kuiperij et al., 2003), Rap GEFs that associate directly with cell adhesion proteins (Hogan et al., 2004; Sakurai et al., 2006). It is noteworthy that RNA silencing of DOCK4 was shown to perturb cell adhesion (Yajnik et al., 2003). Of these Rap1-interacting proteins, DOCK4 (Yajnik et al., 2003) and Tiam1, as well as Tiam2 (Habets et al., 1995; Chiu et al., 1999), have been shown to be present in the testis.
Rap1 is a critical regulator of integrin-based cell adhesion. Integrins that specifically associate with actin seem to be regulated by Rap1, but not those that associate with intermediate filaments (Bos et al., 2003; Enserink et al., 2004; Bos, 2005). The mechanism underlying these interactions is not completely understood, but Rap1-GTP-interacting adaptor molecule has been shown to play a critical role in linking Rap1 to integrins (Lafuente et al., 2004). Rap1-GTP-interacting adaptor molecule also interacts with profilin and Ena/VASP proteins (Lafuente et al., 2004), known regulators of actin dynamics (Krause et al., 2003; Kwiatkowski et al., 2003; Witke, 2004). In short, additional studies are needed in the future to elucidate the roles of GTPases, such as RhoA and Rap1, in spermatogenesis, especially in light of the fact that transgenic mice expressing an inactive Rap1 mutant were subfertile (Aivatiadou et al., 2007). Although experimental evidence is presently lacking in the testis, these findings thus illustrate that it is possible that Rap1 is a key regulator of the α6β1 integrin-laminin 333 multiprotein complex at the apical ectoplasmic specialization.
4. Proteases and Protease Inhibitors
Proteases are enzymes that participate in an array of physiological processes, and they are classified into six broad groups based on their catalytic mechanism of substrate hydrolysis: 1) serine, 2) cysteine, 3) threonine, 4) aspartic, 5) glutamic (not yet identified in mammals), and 6) metalloproteases. Their activity is under the regulation of different signaling cascades and is tightly controlled (Turk, 2006). In this section, we limit our discussion to a unique family of proteases with dual functions in protein cleavage and cell adhesion, the members of which are expressed predominantly in the testis (Seals and Courtneidge, 2003). Additional background information on proteases can be found in the following review articles: Evans and Turner (2007), Stoka et al. (2007), Ghersi (2008), Hildenbrand et al. (2008), and Ramsay et al. (2008).
A disintegrin and a metalloprotease domain (ADAM) comprises a family of unique transmembrane proteins that function both as proteases and adhesion molecules (Primakoff and Myles, 2000; Seals and Courtneidge, 2003; White, 2003; Blobel, 2005; Huovila et al., 2005). The metalloprotease domain of ADAMs has been shown to induce ectodomain shedding (a specialized type of limited proteolysis) of various membrane-bound proteins (e.g., cytokines and growth factors, cytokine and growth factor receptors, and adhesion molecules) in vitro and in vivo, whereas the disintegrin and cysteine-rich domains have adhesive properties in that they interact with integrins on the opposing cell surface (Seals and Courtneidge, 2003; White, 2003). Selected ADAM proteins have also been reported to degrade ECM proteins (Millichip et al., 1998; Alfandari et al., 2001; Martin et al., 2002; White, 2003) to facilitate cell migration. On the other hand, the cytoplasmic tails are divergent and contain several potential phosphorylation sites, as well as binding sites for Src homology region 3 domain-containing proteins (Seals and Courtneidge, 2003), which may ultimately regulate the ability of an ADAM to cleave a specific substrate. It is noteworthy that ADAMs 9 (Weskamp et al., 1996), 12 (Kang et al., 2000; Suzuki et al., 2000), 13 (Cousin et al., 2000), and 15 (Poghosyan et al., 2002) associate with c-Src. ADAM activity is limited by a family of protease inhibitors known as tissue inhibitor of metalloproteases (Handsley and Edwards, 2005; Huovila et al., 2005; Malemud, 2006).
Surprisingly, 2 to 4% of cell surface proteins are subjected to ectodomain shedding, which specifically results in the generation of two fragments: 1) a soluble extracellular fragment containing homophilic binding sites and 2) a significantly shorter membrane-bound fragment containing transmembrane and cytoplasmic domains. Ectodomain shedding essentially disrupts cell adhesion and supports cell movement (Arribas and Borroto, 2002). In terms of cell adhesion, E- and N-cadherin, γ-protocadherin, nectin-1 and -4, and desmoglein-2 have all been shown to be cleaved specifically in their ectodomains by ADAM proteins (Kim et al., 2002; Tanaka et al., 2002; Fabre-Lafay et al., 2005; Haas et al., 2005; Maretzky et al., 2005; Reiss et al., 2005, 2006; Bech-Serra et al., 2006). Surprisingly, integrins only serve as ligands for ADAMs (Seals and Courtneidge, 2003; White, 2003); they have not been reported to be cleaved by members of the ADAM protein family. Nevertheless, shedding of the cadherin ectodomain by ADAM10 has been reported to affect the subcellular localization (Ito et al., 1999; Maretzky et al., 2005; Reiss et al., 2005), and possibly the phosphorylation status, of β-catenin (Takahashi et al., 1997). Perhaps it is not surprising that different studies have localized at least three separate ADAMs specifically to the site of cell-cell contact. By immunofluorescence, ADAM10 was detected at the basolateral plasma membrane in MDCK cells, colocalizing with β-catenin but not with ZO-1 (Wild-Bode et al., 2006). Likewise, ADAM15 colocalized with VE-cadherin in monolayer cultures of human umbilical vein endothelial cells and Chinese hamster ovary cells (Ham et al., 2002), and ADAM9 was shown to interact with E-cadherin in HT29 cells by coimmunoprecipitation (Hirao et al., 2006). In this respect, it is interesting to mention that overexpression of ADAM9 prevented degradation of the E-cadherin ectodomain and apparently facilitated its recycling to the plasma membrane (Hirao et al., 2006).
In terms of protease research, this is an active area of research in the testis, and several ADAMs are either exclusively or predominantly expressed in the testis (Cho et al., 1998; Zhu et al., 1999; Evans, 2001; Seals and Courtneidge, 2003). One of the best-studied ADAMs is fertilin, a heterodimer of fertilin α (ADAM1) and fertilin β (ADAM2) present on the sperm surface (Evans, 2001). Fertilin has been shown to play an important role in fertilization, especially in sperm-egg adhesive interactions (Evans, 2001). Although mice lacking ADAM2 are viable and healthy, they were shown to be infertile (Cho et al., 1998). Likewise, male mice lacking ADAM3 (cyritestin) were also infertile (Shamsadin et al., 1999; Nishimura et al., 2001), illustrating that ADAMs are critical to spermatogenesis and fertility.
IV. Targeting Anchoring Junctions in the Testis for Male Contraceptive Development
A. Background
Adjudin, a potential nonhormonal male contraceptive, is a derivative of lonidamine [1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid], which was developed as an anticancer drug (Silvestrini et al., 1984). Initial studies with lonidamine demonstrated that oral administration to adult rats (50–200 mg/kg b.wt.) induced depletion of germ cells from the seminiferous epithelium, resulting in infertility (Lobl et al., 1979; De Martino et al., 1981; Lobl et al., 1981). At the ultrastructural level, lonidamine was shown to noticeably cause retraction of the apical cytoplasm in Sertoli cells (Lobl et al., 1979; De Martino et al., 1981; Marcante et al., 1981; Malorni et al., 1992). Shortly thereafter, it became clear that this drug targets Sertoli cell stress fibers by disrupting actin dynamics (Malorni et al., 1992), thereby resulting in changes in Sertoli cell morphology, such as vacuolization and germ cell loss from the seminiferous epithelium, instead of targeting dividing germ cells. Although the antispermatogenic effects of lonidamine in the testis were interesting, it was not developed into a male contraceptive because its effects in the testis were shown to be irreversible, and its use was associated with hepatoand nephrotoxicity.
In an ardent attempt to identify novel and reversible antispermatogenetic agents having minimal side-effects, adjudin, formerly known as Angelini Francesco (AF)-2364 or 1-(2,4)-dichlorobenzyl-1H-indazole-3-carbohydrazide (Fig. 7), was identified and selected from more than a dozen newly synthesized analogs of lonidamine. Similar to lonidamine, adjudin was shown to perturb adhesion between Sertoli and germ cells (Cheng et al., 2001; Grima et al., 2001). Note that cell adhesion was not compromised in other organs when this drug was administered orally, or by intraperitoneal or intramuscular injection, perhaps because these organs lack the apical ectoplasmic specialization and induced a significant surge in the steady-state level of testin at the apical ectoplasmic specialization (Table 1) (Cheng et al., 2005a). The effects of adjudin on the testis were shown to be relatively rapid. By ~6.5 h after treatment, half of all tubules examined microscopically showed signs of damage, namely the appearance of elongating/elongated spermatids in the seminiferous tubule lumen (Chen et al., 2003b). This was followed by the detachment of round spermatids and spermatocytes by 3 to 6 days after treatment (Chen et al., 2003b), and sloughed germ cells were found in the epididymal lumen (Fig. 8). After detailed morphological analyses, adjudin was ultimately shown to perturb adhesion between Sertoli and most, but not all, germ cells. Adhesion between Sertoli cells and spermatogonia and between Sertoli cells and some primary spermatocytes, however, was largely unaffected (Cheng et al., 2001; Grima et al., 2001; Lee et al., 2004) (Fig. 9). Although it is not entirely clear whether adjudin’s initial antispermatogenic effects in the testis are stage-dependent, it does seem that elongating/elongated spermatids at stages VII to VIII are the first germ cells to deplete the seminiferous epithelium. Nevertheless, once the epididymal sperm reserve was depleted (Fig. 8), infertility was attained by ~30 days after administration of a single oral dose of adjudin at 50 mg/kg b.wt. (Cheng et al., 2005a). At this dose, neither kidney nor liver function was compromised (Cheng et al., 2001; Grima et al., 2001). In addition, serum FSH, LH, and testosterone levels were not significantly different from control rats, illustrating that the hypothalamic-pituitarytesticular axis was not disrupted (Grima et al., 2001; Mruk and Cheng, 2004a). Other morphological manifestations of adjudin action in the testis include: 1) retraction of the Sertoli cell cytoplasm, 2) formation of large vacuoles, 3) presence of multinucleated germ cells, and 4) occasional relocation of Sertoli cell nuclei to a higher position within the seminiferous epithelium (Cheng et al., 2001; Grima et al., 2001; Mruk et al., 2006).
FIG. 7.
Chemical structure of adjudin. Adjudin is a derivative of lonidamine [1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid], an anticancer drug (Silvestrini et al., 1984).
TABLE 1. Molecular targets of adjudin in the adult rat testis.
Many of the results summarized in this table have been validated in a recent article (Xia et al., 2007) using gene profiling to identify genes in which the steady-state levels changed during adjudin-mediated germ cell loss from the seminiferous epithelium. Readers are encouraged to refer to the original research articles and to the Gene Express Omnibus Data Repository website (http://www.ncbi.nih.gov/geo) for additional information.
| Effects on Target Protein | |||||||
|---|---|---|---|---|---|---|---|
| Junction type | Integral Membrane Protein |
Adaptor | Kinase | GTPase | Signaling Protein | Proteases and Protease Inhibitors |
Reference(s) |
| Apical ectoplasmic specialization | Testin ↑ | Cheng et al., 2001; Grima et al., 2001 | |||||
| β1-Integrin ↑ | p130Cas ↑ ; Paxillin ↑ | PI-3K ↑ ; p-FAK ↑ | Siu et al., 2003 | ||||
| GSK⇄; p-GSK ↑ ; p-PKG ↑ ; PAK ↓ | ERK⇄; p-ERK ↑ ; PLCγ ↓ | Siu et al., 2005 | |||||
| Rab8B ↓ | Lau and Mruk, 2003 | ||||||
| Rab4A ↑ | Mruk et al., 2007 | ||||||
| β2-Integrin ↑ | ROCK1 ↑ ; LIMK1 ↑ | RhoB ↑ | Lui et al., 2003 | ||||
| E-Cadherin ↑ | Lee et al., 2003 | ||||||
| Nectin-3 ↓ | Afadin ↓ | Lee et al., 2004 | |||||
| Fer kinase ↑ | Chen et al., 2003b | ||||||
| Laminin γ3 ↓ | MMP-2 ↑ ; TIMP-2 ↑ | Siu and Cheng, 2004b | |||||
| c-Src ↑ ; Csk ↑ ; CSK2⇄ | Lee and Cheng, 2005 | ||||||
| Basal ectoplasmic specialization | N-Cadherin ↑ | α-Catenin ↑ ; β-Catenin ↑ ; p120 Catenin ↑ | Chen et al., 2003b; Lee et al., 2003; Yan and Cheng, 2005 | ||||
| N-Cadherin ↑ | PKG ↑ | sGC ↑ | Sarkar et al., 2006 | ||||
| N-Cadherin ↑ | WASP ↓ ; Axin ↓ ; Zyxin ↓ | Lee et al., 2004 | |||||
| PKG ↑ ; PKA ↓ | NOS-2 ↑ ; NOS-3⇄ | Lee et al., 2005 | |||||
| ERK⇄; p-ERK ↑ ; p38⇄; p-p38⇄ | TGF-β3 ↑ | Xia and Cheng, 2005 | |||||
| CD2AP ↓ ; TAB1 ↓ | Xia et al., 2006a | ||||||
| Tight junction | Occludin ↑ | ZO-1 ↑ | Mruk and Cheng, 2004b | ||||
| JAM-A ↑ | Yan and Cheng, 2005 | ||||||
| Occludin ↑ | ZO-1 ↑ ; Dynamin-2 ↓ | Lie et al., 2006 | |||||
| Claudin-3 ↑ | Testin ↑ | α2-MG ↑ ; Cathepsin L ↑ | Xia et al., 2007 | ||||
↑, up-regulation; ↓, down-regulation; ⇄, no change; BTB, blood-testis barrier; CD2AP, CD2-associated protein; CK2, casein kinase 2; Csk, carboxyl-terminal Src kinase; p-ERK, phosphorylated (activated) ERK; GSK, glycogen synthase kinase; p-GSK, phosphorylated GSK; JAM, junctional adhesion molecule; LIMK1, lin-11 isl-1 mec3 kinase 1; p38, p38 mitogen-activated protein kinase; p-p38, phosphorylated p38 mitogen-activated protein kinase; α2-MG, α2-macroglobulin; MMP-2, matrix metalloprotease-2; NOS, nitric-oxide synthase; p130Cas, Crk-associated protein (a 130-kDa protein encoded by Crkas gene); PAK, p21-activated kinase; PKB, protein kinase B; PKG, protein kinase G; PLCγ, phospholipase C γ, ROCK1, Rho-associated protein kinase 1; Src, a protein tyrosine kinase of the transforming gene of the Rous sarcoma virus; TAB1, TAK1-binding protein 1; TAK1, TGF-β activating kinase; TIMP-2, tissue inhibitor of metalloproteases-2.
FIG. 8.
A study to examine the effects of adjudin in the epididymis. A single oral dose of adjudin at 50 mg/kg b.wt. was administered to adult rats. On days 2, 4, and 8, epididymides were removed, dissected into three segments [caput (initial segment), corpus (middle segment), and cauda (final segment)] and processed for histological analysis after hematoxylin & eosin staining using paraffin sections. By days 2 and 4 after treatment, immature germ cells such as spermatocytes and early spermatids (see arrowheads in B) were detected in the caput (A–C), but by day 8, these cells had depleted this segment of the epididymis (D). On days 2 and 4, normal epididymal spermatozoa were found in the corpus (E) and cauda (F), and apparently their function was unaffected by adjudin, because all animals remained fertile until the epididymis became devoid of all spermatozoa by ~30 days after treatment (Cheng et al., 2005a). By day 8, however, immature germ cells were seen in the cauda mixed together with normal epididymal spermatozoa (G). In addition, adjudin did not affect adhesion between epididymal epithelial cells. Bars, A and C to G, 120 µm; B, 60 µm.
FIG. 9.
Ultrastructural changes in the seminiferous epithelium of the adult rat testis after treatment of animals with a single oral dose of adjudin at 50 mg/kg b.wt. This is an electron micrograph of the seminiferous epithelium, and Sertoli and germ cells, namely spermatogonia (sg), are seen lying on top of the tunica propria (tp). The blood-testis barrier (see boxed area) appears to be normal 1 week after administration of adjudin. However, many intercellular spaces (see asterisks) are seen between a pachytene spermatocyte (sp) and a Sertoli cell, illustrating loss of adhesion. ld, lipid droplet; n, Sertoli cell nucleus. Bar, 6 µm.
B. Adjudin Efficacy, Dosing, and Reversibility of Antifertility Effects
More than 20 different treatment regimens (including oral, intramuscular, and intraperitoneal administration routes at different dosings) were tested in adult rats over the course of a decade to illustrate the efficacy and reversibility of adjudin as a male contraceptive (Cheng et al., 2001, 2005a; Grima et al., 2001). Based on these studies, there are several important findings regarding the potential suitability of adjudin as a nonhormonal male contraceptive. First, this drug perturbed Sertoligerm cell adhesion, which resulted in the depletion of most germ cells except spermatogonia and some primary spermatocytes from the seminiferous epithelium without affecting epididymal sperm. Second, elongating/elongated spermatids were the first germ cells to deplete the epithelium after administration of a single dose of adjudin at 50 mg/kg b.wt., followed shortly thereafter by round spermatids and spermatocytes (Chen et al., 2003b). This seemingly illustrates that the apical ectoplasmic specialization is one of the primary targets of adjudin. However, it is likely that desmosome-like and gap junctions were also affected. Third, adjudin-induced infertility in adult rats was shown to be effective after administration of two consecutive doses at 35–50 mg/kg b.wt. given 1 week apart by gavage or intraperitoneal or intramuscular injection (Cheng et al., 2005a). Infertility was attained by ~4 weeks after treatment because it took this long for the sperm reserve in the epididymis to be exhausted, as noted by standard mating studies. By ~8 to 10 weeks, germ cells had gradually repopulated the epithelium and complete fertility was restored by 12 weeks. This is consistent with the amount of time needed for spermatogonia to divide and differentiate into spermatozoa, which is 58 days in the rat (LeBlond and Clermont, 1952; de Kretser and Kerr, 1988; Clermont et al., 1993). Fourth, all animals responded equally well to adjudin treatment and absolutely no deaths were reported during the course of this study, including all the FDA-mandated toxicity studies conducted by licensed toxicologists (Mruk et al., 2006). These findings, taken together with repeated dosing studies (Cheng et al., 2005a), illustrate that the antifertility effects of adjudin are effective and reversible in rats.
C. Bioavailability, Tissue Distribution, and Metabolic Clearance of Adjudin
Less than 10% of adjudin is absorbed by the gastrointestinal tract in adult rats after oral administration, clearly revealing low bioavailability. For the relatively small percentage of adjudin that is absorbed, it distributes equally among all organs examined, including the testis, epididymis, prostate, liver, kidney, and brain (Cheng et al., 2005a). This illustrates that adjudin was not specifically taken up by any particular organ. Moreover, when the metabolic clearance of this compound was estimated in blood samples obtained from adult rats after administration of [3H]adjudin via the jugular vein, it was estimated to be ~5.5 to 6 h (Fig. 10), revealing rapid clearance from the systemic circulation. This was consistent with clearance data obtained after the administration of [3H]adjudin by gavage (Cheng et al., 2005a).
FIG. 10.
Estimation of the half-time of disappearance of [3H]adjudin in adult rats after i.v. administration. The half-time of disappearance of adjudin was estimated in adult rats (~300 g b.wt., n = 3 per time point) after i.v. administration of [3H]adjudin. In brief, [3H]adjudin, [indazole-5,7-3H(N)]-1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, was purchased (PerkinElmer Life and Analytical Sciences, Waltham, MA). The purity of [3H]adjudin was confirmed by 1) high-performance liquid chromatography using a Zorbax C18 reversed-phase column, which had an identical retention time when both 3H-labeled and unlabeled adjudin were injected onto the column simultaneously or separately with the eluents monitored by UV absorbance at 210 nm or spectrophotometry using a β-counter, 2) mass spectrometry, and 3) elemental analysis. The t1/2 of adjudin in the systemic circulation of adult rats was estimated by injecting ~3 × 106 cpm of [3H]adjudin in a sample volume of 50 µl of phosphate-buffered saline via the jugular vein. An aliquot of blood was collected from the tail vein from each rat in this experiment at 0.5, 1, 3.5, 7, 10, 24, 48, 76, and 120 h after administration of [3H]adjudin and was allowed to clot. Serum was obtained by centrifugation for radioactivity determination. The t1/2 was determined using nonlinear least-squares curve-fitting techniques to fit [3H]adjudin levels in blood as a function of time to a multiexponential function consisting of one to four terms of the following equation: Y(t) = ΣAie−Bit, where Y(t) is the response variable (in this case, the level of [3H]adjudin obtained in the blood sample). Data were fitted using a computer program based on code implementing the Marquardt algorithm to minimize χ2 (Bevington, 1969). Because data exhibited nonuniform variation, they were weighted as 1/σ2, where σ was determined from samples of three different animals at each time point. The number of exponentials fitted to experimental data was determined as the number that minimized χ2. Estimates were obtained for the parameters σAi and σBi, as well as their uncertainties Ai and Bi, which were derived from the diagonal terms in the error matrix generated during the fitting procedure. Note that the estimates for the parameter uncertainties did not take into account covariance terms and, as a result, tended to be underestimated. However, this analysis yielded the best estimate on the disappearance of [3H]adjudin from the systemic circulation after i.v. administration.
D. Acute and Subchronic Toxicity
Licensed toxicologists have completed a battery of FDA-mandated toxicity studies for adjudin, and these results are summarized in Table 2 (Mruk et al., 2006). Although acute toxicity tests conducted in mice and rats, as well as standard mutagenicity and genotoxicity tests performed in bacterial and eukaryotic cells, have shown adjudin to be safe for further development (Mruk et al., 2006), the margin between safety and efficacy was narrow in adult rats. For instance, treatment of adult rats (n = 10 for male rats and n = 10 for female rats) with adjudin at 50 mg/kg b.wt. by gavage for 29 consecutive days showed signs of liver inflammation and skeletal muscle atrophy in 3 of 10 male rats in a subchronic toxicity study (Table 2), but no mortalities were detected in 20 animals. These results suggest that prolonged use of adjudin by humans, which would span several decades, might result in adverse affects. Note that these adverse effects were not detected in female animals [see Supplemental Information: “Subchronic Toxicity Report” in Mruk et al. (2006)]. Although a reason for this has yet to be determined, gender differences that are in part under hormonal control can affect the ability of some organs, such as the liver, to metabolize certain drugs (Morris et al., 2003). Taken collectively, these results illustrate that the margin between safety and efficacy would have to be widened significantly in order for adjudin to become a male contraceptive for human use.
TABLE 2. Results of adjudin toxicity study.
Results of standard toxicity studies on adjudin performed by licensed toxicologists according to FDA guidelines. [Adapted and reproduced from Mruk DD, Wong CH, Silvestrini B, and Cheng CY (2006) A male contraceptive targeting germ cell adhesion. Nat Med 12:1323–1328. Copyright © 2006 Nature Publishing Group. Used with permission.]
| Test | Regimen and Dosing | Administration Route | Species | Results |
|---|---|---|---|---|
| Acute toxicity study | 100 mg/kg b.wt. (1 dose) | Intraperitoneal | Mouse; males (n = 3) | Negative |
| 1000 mg/kg b.wt. (1 dose) | Intraperitoneal | Mouse; males (n = 3) | Negative | |
| Acute toxicity study | 2000 mg/kg b.wt. (1 dose) | Oral | Rat; males (n = 5) and females (n = 5) | Negative |
| Bacterial mutation assay | 0.586–18.8 µg/plate | In vitro | Salmonella typhimurium andEscherichia coli | Negative in both species |
| Mammalian erythrocyte micronucleus test | 500, 1000, and 2000 mg/kg b.wt. (1 dose) | Oral | Mouse; males (n = 5) and females (n = 5) | Negative in all three dosings |
| Mammalian chromosome aberration test | 6.25–100 µg/ml | In vitro | CHO cells | Negative in inducing numerical chromosome aberrations; positive in inducing structural chromosome aberrations |
| 29-Day subchronic toxicity study | 50 mg/kg b.wt. every day for 29 days | Oral | Rat; male (n = 10) and female (n = 10) | Negative in female; positive in male |
E. Mechanism of Adjudin Action in the Testis: The Current Model
The effects of adjudin on Sertoli-germ cell adhesion have been well documented in both in vitro and in vivo studies (Cheng et al., 2001, 2005a; Grima et al., 2001; Chen et al., 2003b; Lau and Mruk, 2003; Lee et al., 2003; Lui et al., 2003; Siu et al., 2003; Mruk et al., 2006; Wolski et al., 2006) (Table 1), and these reports have shown that adjudin exerts its effects on Sertoli-germ cell adhesion by activating two separate but related signaling cascades. It was found that integrin and RhoB were activated within hours of adjudin treatment (Lui et al., 2003; Siu et al., 2003). In the case of RhoB, this activated ROCK→LIMK1→cofilin, which resulted in germ cell detachment (Lui et al., 2003). Other studies using adjudin, in conjunction with inhibitors to block specific protein kinases or antibodies against signaling molecules, have shown that ectoplasmic specialization dynamics are also regulated by the integrin→pFAK→phosphatidylinositol 3-kinase→p130Cas→ERK MAP kinase signaling pathway (Siu et al., 2003). These findings are significant because they illustrate for the first time that defined signaling cascades regulate anchoring junctions in the testis. These reports, taken together with additional ongoing studies from this laboratory, suggest essentially that adjudin mediates its affects in the seminiferous epithelium in part by affecting actin cytoskeleton dynamics in the Sertoli cell. However, other signaling pathways are also likely to be involved, because blocking ROCK activity with a specific inhibitor, Y-27632, could delay, but not completely prevent, adjudin-mediated germ cell loss from epithelium (Lui et al., 2003).
Based on results summarized in Table 1, the primary cellular target of adjudin in the seminiferous epithelium is the apical ectoplasmic specialization, because several constituent proteins were induced after treatment. Although the precise molecular target(s) of adjudin at this site is presently unknown, the integrin-laminin multiprotein complex seems to be a likely candidate. Indeed, the argument exists that integrin and laminin are components of the ECM; if this is the case, then adjudin should affect adhesion in all organs. In the testis, however, the α6β1 integrin-laminin-333 complex is present specifically at the apical ectoplasmic specialization. While the complete molecular architecture of this complex is not yet known, it is likely to associate with key testis-specific proteins because the ectoplasmic specialization is unique in structure, function, and regulation. This may explain the lack of subchronic toxicity detected in female rats (Table 2) and the lack of cell detachment observed in other organs, such as the kidney, liver, heart, and epididymis when adjudin was used at a dose that effectively depleted germ cells from the seminiferous epithelium (Cheng et al., 2001, 2005a; Grima et al., 2001). In summary, we have come to better understand the regulation of the apical ectoplasmic specialization in the testis through the use of adjudin as an in vivo model to study anchoring junction dynamics. The reverse is also true; we have a very good grasp of adjudin’s mechanism of action in the testis because we understand, at least in part, the structure and regulation of the apical ectoplasmic specialization.
F. Current Status of Adjudin Research
As discussed previously, oral administration of adjudin for 29 consecutive days resulted in adverse effects (e.g., liver inflammation and skeletal muscle atrophy) in a small subset of male animals (Mruk et al., 2006). To bypass these effects, which would preclude adjudin from being developed into a male contraceptive for human use, this drug would have to be delivered directly to the testis. To achieve this, adjudin was conjugated to a recombinant FSH mutant protein, which served as its “carrier” to the testis (Mruk et al., 2006), because FSH receptors are restricted to Sertoli cells in the testis (Heckert and Griswold, 2002; Walker and Cheng, 2005; Hermann and Heckert, 2007) (Fig. 11). It should be noted that appropriate modifications were made in the FSH mutant to remove its hormonal activity by mutating several of its glycosylation sites on the corresponding α and β subunits (Mruk et al., 2006). These modifications did not affect its receptor binding ability. Using this approach, infertility was induced in rats when ~0.5 µg/kg b.wt. adjudin was administered intraperitoneally (Mruk et al., 2006). These antifertility results, which were comparable with animals receiving adjudin orally at 50 mg/kg b.wt. (50 µg/kg b. wt. adjudin-FSH conjugate), represent a significant increase in efficacy when the two treatment regimens are compared (Fig. 11).
FIG. 11.
Effects of the adjudin-FSH mutant conjugate on the testis, kidney, liver, and small intestine. Adjudin-FSH mutant conjugate (50 µg containing ~0.5 µg of adjudin/kg b.wt.) was administered to adult rats (~300 g b.wt., n = 8 per time point) intraperitoneally via a 28-gauge needle. Rats were sacrificed at 2, 4, 6, and 12 weeks thereafter for histological analysis by hematoxylin & eosin staining. A, cross-section of the testis from normal rats (control) without treatment. B, by 2 weeks after treatment, almost all elongating/elongated spermatids were depleted from the seminiferous epithelium. C, by 4 weeks, ~98% of the tubules examined were devoid of spermatids and spermatocytes, and the tubule diameter was reduced by ~35%. D, magnified view of the boxed area in C, showing a tubule that contained only Sertoli cells, spermatogonia, and a few spermatocytes. E, by 6 weeks, germ cells began to repopulate the seminiferous epithelium. F, by 12 weeks, ~90% of tubules were indistinguishable from those of the control testes. G to I, cross-sections of kidney (G), liver (H), and small intestine (I) 4 weeks after treatment. No histological changes were detected in these organs at this time or at 6 or 12 weeks after treatment versus control rats. In addition, no histological changes were detected in skeletal muscle versus control rats at 4, 6, or 12 weeks after treatment (data not shown). Bars in A to C and E to I, 150 µm; D, 40 µm. J, changes in testes weights (organ pair) after treatment. K, summary of the fertility test (n = 4 rats) results. The fertility efficacy of control rats was arbitrarily set at 100%. [Reproduced from Mruk DD, Wong CH, Silvestrini B, and Cheng CY (2006) A male contraceptive targeting germ cell adhesion. Nat Med 12:1323–1328. Copyright © 2006 Nature Publishing Group. Used with permission.]
Although the adjudin-FSH conjugate was shown to effectively induce infertility in adult rats, its administration required injection, one of the least acceptable methods of contraceptive drug administration for humans. Transdermal patches offer many advantages including patient acceptability and ease of use. However, the use of transdermal patches for drug delivery is extremely limited because this methodology does not allow large molecules to pass through the skin’s barrier, which is on average ~10 µm thick. Indeed, permeation enhancers (presently, two types exist: 1) small polar solvents and 2) amphiphilic compounds containing a polar and a hydrophobic head) have increased the number of drugs that can be delivered transdermally because they act on the epidermis to alter the composition of lipids and decrease barrier resistance, thereby facilitating drug entry (Vávrová et al., 2005; Babita et al., 2006). Permeation enhancers may also affect the dynamics of the desmosome, as well as intermediate filament proteins such as keratin. However, due to the relatively large molecular mass of FSH (40 kDa), transdermal patches are not likely to be useful in the delivery of the adjudin-FSH conjugate to the testis unless a permeation enhancer can be successfully incorporated into the formulation to facilitate the transport of the conjugate across the skin. In addition, it is not yet clear whether the conformational folding of the FSH mutant protein would prohibit its entry through the skin, even if a permeation enhancer was successfully included in the formulation.
However, a modification of the transdermal patch technology may show clinical promise, and this line of research is being pursued actively in our laboratory. It is known that successful spermatogenesis requires that the temperature of the testis be ~2 to 3°C lower than the temperature of the body at 35°C versus 37.8°C, respectively. To maintain this homeostasis, heat carried to the testis by the arterial blood is efficiently cooled by countercurrent heat exchange in the spermatic cord with testicular venous blood. Moreover, blood within veins located on the surface of the testis is cooled by loss of heat through scrotal skin, which is especially thin with little subcutaneous fat. Finally, the spermatic artery becomes extensively coiled and branched once it leaves the inguinal canal, and this increased surface area also contributes to efficient heat exchange (Setchell, 1978; Ohtsuka, 1984; Turner et al., 1996; Setchell and Breed, 2006). Given the unique microvasculature of the testis and the sophisticated mechanism of heat exchange and loss, topical application of an adjudin-permeation enhancer gel formulation may pass through the thin scrotal skin and enter the underlying vasculature surrounding the testis to elicit antifertility effects locally (Alberti et al., 2005). It is likely that adverse effects on the liver and muscle would be reduced significantly because the testis would be the first-pass target organ.
Perhaps another promising approach to deliver the adjudin-FSH conjugate would be needle-free injection. This recent methodology can be used to painlessly deliver a relatively small volume of drug (usually 0.2–1 ml) within a few hundredths of a second either subcutaneously, intramuscularly, or intradermally (Baxter and Mitragotri, 2006; Arora et al., 2007). More importantly, needle-free injection offers more reliable depth of penetration and more efficient use of the drug, thereby requiring the use of significantly less drug and lowering costs.
Finally, protein transduction domains (PTDs) are cationic peptides that deliver molecular cargo such as proteins and small molecules across the plasma membrane. PTDs enter cells independently of receptors or transporter proteins, and they seem to be internalized by endocytosis rather than crossing the plasma membrane (Drin et al., 2003; Fuchs and Raines, 2004, 2006). For instance, Vectocell peptides (Diatos Pharmaceuticals, Paris, France) are a family of cell-penetrating peptides originating from heparin binding proteins. They interact with glycosaminoglycans present on the cell surface or the extracellular matrix and can internalize proteins of up to 200 kDa. However, there are a number of important disadvantages associated with this approach. First, PTDs generally penetrate tissues poorly, although tissue penetration is improved when smaller peptides are used. Second, PTDs tend to have short lifetimes in the circulation, and this can result in low tissue accumulation and reduced drug-mediated effects. Insertion of a tissue-specific cleavage site between the PTD and adjudin-FSH conjugate may lead to increased accumulation of the adjudin-FSH conjugate in the testis. Third, entry of a protein into a cell would most likely require that this protein be unfolded and then quickly refolded in the cytoplasm for activity, but this criterion may not apply to the FSH mutant protein because the FSH receptor is known to internalize its ligand via transcytosis (Vu Hai et al., 2004). Moreover, use of this drug delivery technology requires injection, because PTDs cannot cross the skin’s barrier effectively. Thus, linking the adjudin-FSH conjugate to a PTD such as Vectocell to deliver adjudin specifically to the testis is not a viable option versus injection unless this formulation is administered via nasal inhalation or some other feasible approach.
G. Additional Comments
As discussed previously, adjudin has poor bioavailability, and factors that may play a role in adjudin’s low bioavailability include poor absorption from the gastrointestinal tract, degradation or metabolism of the drug before absorption, and hepatic first-pass effect. In addition, transporter proteins are known to function in tissue defense by regulating drug absorption, distribution, and elimination from the body. For example, the low bioavailability of some drugs, such as paclitaxel (an anticancer drug), has been linked to the presence of P-glycoprotein (an ATP-dependent efflux transporter of the MDR/TAP subfamily, also known as MDR1) in the apical membrane of epithelial cells in the intestine (Meerum Terwogt et al., 1999; Stephens et al., 2002). P-glycoprotein functions essentially as a gatekeeper to limit or prevent the entry of certain drugs past blood-tissue barriers, such as those present in the brain, testis, and placenta, thereby reducing systemic blood levels of the drug (Thiebaut et al., 1987; Cordon-Cardo et al., 1989). Its function in renal tubules and hepatocyte canalicular membranes, on the other hand, is to rapidly clear drugs from the systemic circulation. Although drug transporters such as P-glycoprotein have been shown to reduce drug toxicity, they also decrease the efficacy of oral drugs, often requiring that the dose of drug needed to reach a required effect be increased. In the testis, P-glycoprotein is expressed abundantly by Sertoli cells and late spermatids but not by spermatocytes and spermatogonia (Melaine et al., 2002; Bart et al., 2004), illustrating that the blood-testis barrier has the ability to prevent drugs that would perturb postmeiotic germ cell development from entering into the seminiferous epithelium.
Blood-tissue barriers also express different transporter proteins with basolateral localizations such as multidrug resistance-associated protein 1 (MRP1), a member of the ATP-binding cassette superfamily, but these transporters tend to pump drugs into cells, leading to increased drug uptake and increased toxicity (Liang and Aszalos, 2006). It remains unknown how adjudin enters cells; uptake of this drug was not restricted to any particular organ, so it may simply diffuse through the lipid bilayer of plasma membranes because it is a small hydrophobic molecule (molecular weight 335). In the testis, adjudin may also diffuse through adjacent Sertoli cells and cross the blood-testis barrier to affect cell adhesion. Note that besides P-glycoprotein, MRP1 and another drug transporter, known as Abcb8, were shown to be present in the testis (Wijnholds et al., 1998; Bart et al., 2004; Melaine et al., 2006), but whether they participate in the transport of adjudin into or out of (in the case of P-glycoprotein) Sertoli cells in the testis is unknown. In vitro and in vivo studies are now under way to address these outstanding questions.
V. Concluding Remarks
Herein, we have discussed the biology, regulation, and physiological significance of anchoring junctions in the testis. Throughout this review, we have attempted to stress that loss of Sertoli-germ cell adhesion is a novel approach for male contraceptive development. In this respect, we have highlighted several important areas of investigation that should be pursued in future studies, because they seem particularly relevant to understanding Sertoli-germ cell adhesion in the testis. The merging of scientific disciplines and the use of new methods such as RNA silencing will greatly assist investigators in determining the functional significance of many molecules critical to Sertoli-germ cell adhesion in the testis. With any luck, these studies will also establish whether these molecules are “druggable” targets for nonhormonal male contraceptive development in the future. In addition, continued research and development relating to drug delivery in general should offer more feasible options to specifically deliver compounds to the testis. Although in this review we focused on Sertoli-germ cell adhesion as an approach to nonhormonal male contraceptive development, other ongoing studies in the field should not be ignored. On this note, it is important to point out that other contraceptive compounds that are known to induce germ cell loss from the seminiferous epithelium of laboratory rats and primates have also been actively investigated as potential male contraceptives. These compounds include CDB-4022, whose antispermatogenic effects and mechanism of action in the testis seem to be very similar to that of adjudin (Hild et al., 2001, 2004, 2007; Koduri et al., 2008). Nonetheless, the studies summarized in this review should provide a good framework for investigators in the field who may seek to examine whether Sertoli-germ cell adhesion is compromised by other nonhormonal contraceptives in the future. Although it will take several years, if not decades, for a safe, effective, and reversible nonhormonal male contraceptive to reach the consumer market, the wait is worthwhile given the predicted increase in the world’s population.
Acknowledgments
Studies in the authors’ laboratory were supported in part by grants from the National Institutes of Health (National Institute of Child Health and Human Development U54-HD029990, Project 5; U01-HD045908; R03-HD051512) and the Contraceptive Research and Development (CONRAD) Program (Consortium for Industrial Collaboration in Contraceptive Research, CIG-01-72, CIG-01-74, CIG-96-05A/B).
Footnotes
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; CDB-4022, [4aS,5R,9bS]-2-ethyl-2,3,4,4a,5,9b-hexahydro-8-iodo-7-methyl-5-[4-carbomethoxyphenyl]-1H-indeno[1,2-c]pyridinehydrochloride; adjudin, 1-(2,4-chlorobenzyl)-1H-indazole-3-carbohydrazide; ZO-1, zonula occludens-1; ARVCF, Armadillo repeat gene deleted in velo-cardio-facial syndrome; WASP, Wiskott-Aldrich syndrome protein; ADIP, afadin dilute domain-interacting protein; APC, adenomatous polyposis coli; PDZ, postsynaptic density 95/disc-large/zona occludens; BP, bullous pemphigoid; ILK, integrin-linked kinase; FAK, focal adhesion kinase; ECM, extracellular matrix; ERK, extracellular signal-regulated kinase; MAP, mitogen-activated protein; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; IL-1β, interleukin-1β; MDCK, Madin-Darby canine kidney; HGF, hepatocyte growth factor; CD2AP, CD2-associated protein; GEF, GDP/GTP exchange factor; GAP, GTPase-activating protein; IQGAP, IQ motif containing GTPase-activating protein; ADAM, A disintegrin and a metalloprotease domain; PTD, protein transduction domain.
REFERENCES
- Abram CL, Courtneidge SA. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Riazuddin S, Wilcox ER. The molecular genetics of Usher syndrome. Clin Genet. 2003;63:431–444. doi: 10.1034/j.1399-0004.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- Aivatiadou E, Mattei E, Ceriani M, Tilia L, Berruti G. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant. Mol Biol Cell. 2007;18:1530–1542. doi: 10.1091/mbc.E06-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti I, Grenier A, Kraus H, Carrara DN. Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug delivery. Expert Opin Drug Deliv. 2005;2:935–950. doi: 10.1517/17425247.2.5.935. [DOI] [PubMed] [Google Scholar]
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. Garland, New York: 1994. Cell junctions, cell adhesion, and the extracellular matrix; pp. 949–1009. [Google Scholar]
- Alfandari D, Cousin H, Gaultier A, Smith K, White JM. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr Biol. 2001;11:918–930. doi: 10.1016/s0960-9822(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Amory JK, Page ST, Bremner WJ. Drug insight: recent advances in male hormonal contraception. Nat Clin Pract Endocrinol Metab. 2006;2:32–41. doi: 10.1038/ncpendmet0069. [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ. p120ctn: a nexus for contexual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13:604–610. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Baird DT. Male contraception. Endocr Rev. 2002;23:735–762. doi: 10.1210/er.2002-0002. [DOI] [PubMed] [Google Scholar]
- Andra K, Nikolic B, Stocher M, Drenckhahn D, Wiche G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev. 1998;12:3442–3451. doi: 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, Passaniti A, Hasday JD, Goldblum SE. TNF-α increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–L1245. doi: 10.1152/ajplung.00109.2006. [DOI] [PubMed] [Google Scholar]
- Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- Arora A, Hakim I, Baxter J, Rathnasingham R, Srinivasan R, Fletcher DA, Mitragotri S. Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets. Proc Natl Acad Sci U S A. 2007;104:4255–4260. doi: 10.1073/pnas.0700182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas J, Borroto A. Protein ectodomain shedding. Chem Rev. 2002;102:4627–4637. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada M, Irie K, Morimoto K, Yamada A, Ikeda W, Takeuchi M, Takai Y. ADIP, a novel afadin- and α-actinin-binding protein localized at cell-cell adherens junctions. J Biol Chem. 2003;278:4103–4111. doi: 10.1074/jbc.M209832200. [DOI] [PubMed] [Google Scholar]
- Asada M, Irie K, Yamada A, Takai Y. Afadin- and α-actinin-binding protein ADIP directly binds α’-COP, a subunit of the coatomer complex. Biochem Biophys Res Commun. 2004;321:350–354. doi: 10.1016/j.bbrc.2004.06.143. [DOI] [PubMed] [Google Scholar]
- Aspenström P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res. 2007;313:3673–3679. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Babita K, Kumar V, Rana, Jain S, Tiwary AK. Thermotropic and spectroscopic behavior of skin: relationship with percutaneous permeation enhancement. Curr Drug Deliv. 2006;3:95–113. doi: 10.2174/156720106775197466. [DOI] [PubMed] [Google Scholar]
- Bacallao R, Antony C, Karsenti E, Stelzer E, Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989;109:2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Datta J, Jacob ST, Ghoshal K. Treatment of PC12 cells with nerve growth factor induces proteasomal degradation of T-cadherin that requires tyrosine phosphorylation of its cadherin domain. J Biol Chem. 2007;282:27171–27180. doi: 10.1074/jbc.M700691200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- Bart J, Holleman H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, Pgp, BCRP, MRP1 and MRP2 in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Bartles JR, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109:1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- Baxter J, Mitragotri S. Needle-free liquid injections: mechanisms and applications. Expert Rev Med Devices. 2006;3:565–574. doi: 10.1586/17434440.3.5.565. [DOI] [PubMed] [Google Scholar]
- Beall SA, Boekelheide K, Johnson KJ. Hybrid GPCR/cadherin (Celsr) proteins in rat testis are expressed with cell type specificity and exhibit differential Sertoli cell-germ cell adhesion activity. J Androl. 2005;26:529–538. doi: 10.2164/jandrol.05003. [DOI] [PubMed] [Google Scholar]
- Bech-Serra JJ, Santiago-Josefat B, Esselens C, Saftig P, Baselga J, Arribas J, Canals F. Proteomic identification of desmoglein-2 and activated leukocyte cell adhesion molecule as substrates of ADAM17 and ADAM10 by difference gel electrophoresis. Mol Cell Biol. 2006;26:5086–5095. doi: 10.1128/MCB.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shaul Y, Ophir I. Tight junctions and proteases. In: Cereijido M, Anderson J, editors. Tight Junctions. New York: CRC Press; 2001. pp. 459–482. [Google Scholar]
- Bevington PR. Data Reduction and Error Analysis for the Physical Sciences. New York: McGraw-Hill; 1969. [Google Scholar]
- Bienz M. β-Catenin: a pivot between cell adhesion and Wnt signaling. Curr Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD. Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J. 2007;21:2285–2293. doi: 10.1096/fj.06-7518com. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajian V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-β1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–947. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–5635. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Boguslavsky S, Grosheva I, Landau E, Shtutman M, Cohen M, Arnold K, Feinstein E, Geiger B, Bershadsky A. p120 Catenin regulates lamellipodial dynamics and cell adhesion in cooperation with cortactin. Proc Natl Acad Sci U S A. 2007;104:10882–10887. doi: 10.1073/pnas.0702731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolós V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Corcoran CM, Stappenbeck TS, Green KJ. Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell-cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J Cell Biol. 1996;134:985–1001. doi: 10.1083/jcb.134.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Godsel LM, Corcoran CM, Park JK, Hatzfeld M, Kowalczyk AP, Green KJ. Plakophilin 1 interferes with plakoglobin binding to desmoplakin, yet together with plakoglobin promotes clustering of desmosomal plaque complexes at cell-cell borders. J Cell Sci. 2001;114:727–738. doi: 10.1242/jcs.114.4.727. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Bos JL, de Bruyn K, Enserink JM, Kuiperij B, Rangarajan S, Rehmann H, Riedl J, de Rooij J, van Mansfeld F, Zwartkruis F. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003;31:83–86. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- Bouchard MJ, Dong Y, McDermott BM, Jr, Lam DH, Brown KR, Shelanski M, Bellve AR, Racaniello VR. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell-cell adherens junctions. Mol Cell Biol. 2000;20:2865–2873. doi: 10.1128/mcb.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RS, Cowin P, Brown AM. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep. 2007;8:1019–1023. doi: 10.1038/sj.embor.7401089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Aszodi A, Hellebrand H, Berna A, Fassler R, Brandau O. Genomic organization of profilin-III and evidence for a transcript expressed exclusively in the testis. Gene. 2002;283:219–225. doi: 10.1016/s0378-1119(01)00855-1. [DOI] [PubMed] [Google Scholar]
- Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–249. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Buchert M, Poon C, King JA, Baechi T, D’Abaco G, Hollande F, Hovens CM. AF-6/s-afadin is a dual residency protein and localizes to a novel subnuclear compartment. J Cell Physiol. 2007;210:212–223. doi: 10.1002/jcp.20853. [DOI] [PubMed] [Google Scholar]
- Buchsbaum RJ. Rho activation at a glance. J Cell Sci. 2007;120:1149–1152. doi: 10.1242/jcs.03428. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K. Focal contacts: transmembrane links between the extracellular matrix and cytoskeleton. BioEssays. 1989;10:104–108. doi: 10.1002/bies.950100403. [DOI] [PubMed] [Google Scholar]
- Burridge K, Sastry SK, Sallee JL. Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell matrix adhesion. J Biol Chem. 2006;281:15593–15596. doi: 10.1074/jbc.R500030200. [DOI] [PubMed] [Google Scholar]
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. BioEssays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz S, Stappert J, Weissig H, Kemler R. Plakoglobin and β-catenin: distinct but closely related. Science. 1992;257:1142–1144. doi: 10.1126/science.257.5073.1142-a. [DOI] [PubMed] [Google Scholar]
- Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Calkins CC, Hoepner BL, Law CM, Novak MR, Setzer SV, Hatzfeld M, Kowalczyk AP. The Armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J Biol Chem. 2003;278:1774–1783. doi: 10.1074/jbc.M205693200. [DOI] [PubMed] [Google Scholar]
- Caron E. Regulation of Wiskott-Aldrich syndrome protein and related molecules. Curr Opin Cell Biol. 2002;14:82–87. doi: 10.1016/s0955-0674(01)00298-8. [DOI] [PubMed] [Google Scholar]
- Carreau S, Bourguiba S, Delalande C, Silandre D, Said L, Galeraud-Denis I, Lambard S. Estrogen: roles in spermatogenesis. Curr Med Chem Immunol Endocrinol Metab Agents. 2008;8:59–65. [Google Scholar]
- Castaño J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, Garcia de Herreros A, Dunach M. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Wine RN, Harris MW, Borchers CH, Haseman JK. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl. 2001;22:1030–1052. doi: 10.1002/j.1939-4640.2001.tb03444.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kojima S, Borisy GG, Green KJ. p120 Catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003a;163:547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YM, Lee NP, Mruk DD, Lee WM, Cheng CY. Fer kinase/Fer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003b;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MK, Lee NP, Lui WY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005a;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, Saso L, Leone MG, Palmery M, Mruk D. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in Sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- Cheng X, Den Z, Koch PJ. Desmosomal cell adhesion in mammalian development. Eur J Cell Biol. 2005b;84:215–223. doi: 10.1016/j.ejcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Chitaev NA, Leube RE, Troyanovsky RB, Eshkind LG, Franke WW, Troyanovsky SM. The binding of plakoglobin to desmosomal cadherins: patterns of binding sites and topogenic potential. J Cell Biol. 1996;133:359–369. doi: 10.1083/jcb.133.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CY, Leng S, Martin KA, Kim E, Gorman S, Duhl DM. Cloning and characterization of T-cell lymphoma invasion and metastasis (TIAM2), a novel guanine nucleotide exchange factor related to TIAM1. Genomics. 1999;61:66–73. doi: 10.1006/geno.1999.5936. [DOI] [PubMed] [Google Scholar]
- Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Fertilization defects in sperm function from mice lacking fertilin β. Science. 1998;281:1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Joo CK. Transforming growth factor-β1 represses E-cadherin production via Slug expression in lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2708–2718. doi: 10.1167/iovs.06-0639. [DOI] [PubMed] [Google Scholar]
- Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dulfour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YY, Valster A, Coniglio SJ, Backer JM, Symons M. The atypical Rho family GTPase Wrch-1 regulates focal adhesion formation and cell migration. J Cell Sci. 2007;120:1927–1934. doi: 10.1242/jcs.03456. [DOI] [PubMed] [Google Scholar]
- Chung NP, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- Chung SS, Lee WM, Cheng CY. Study on the formation of specialized inter-Sertoli cell junctions in vitro. J Cell Physiol. 1999;181:258–272. doi: 10.1002/(SICI)1097-4652(199911)181:2<258::AID-JCP8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Oko R, Hermo L. Cell biology of mammalian spermatogenesis. In: Desjardins C, Ewing LL, editors. Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993. pp. 332–376. [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of β-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell CJ. The Sertoli cells of the sexually mature dog. Anat Rec. 1977;185:133. (Abstract) [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge SA. Role of Src in signal transduction pathway. Biochem Soc Trans. 2002;30:11–17. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Cousin H, Gaultier A, Bleux C, Darribere T, Alfandari D. PACSIN2 is a regulator of the metalloprotease/disintegrin ADAM 13. Dev Biol. 2000;227:197–210. doi: 10.1006/dbio.2000.9871. [DOI] [PubMed] [Google Scholar]
- Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Dancing in and out of the nucleus: p120ctn and the transcription factor Kaiso. Biochim Biophys Acta. 2007;1773:59–68. doi: 10.1016/j.bbamcr.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. The catenin p120ctn interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds RC. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de França LR, Ghosh S, Ye SJ, Russell LD. Surface and surface-to-volume relationships of the Sertoli cell during the cycle of the seminiferous epithelium in the rat. Biol Reprod. 1993;49:1215–1228. doi: 10.1095/biolreprod49.6.1215. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- De Martino C, Malcorni W, Bellocci M, Floridi A, Marcante ML. Effects of AF1312 TS and lonidamine on mammalian testis. A morphological study. Chemotherapy. 1981;27 Suppl 2:27–42. doi: 10.1159/000238043. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- de Rooij J, Boenink NM, van Triest M, Cool RH, Wittinghofer A, Bos JL. PDZ-GEF1, a guanine nucleotide exchange factor specific for Rap1 and Rap2. J Biol Chem. 1999;274:38125–38130. doi: 10.1074/jbc.274.53.38125. [DOI] [PubMed] [Google Scholar]
- Deguchi M, Iizuka T, Hata Y, Nishimura W, Hirao K, Yao I, Kawabe H, Takai Y. Papin. A novel multiple PSD-95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related Armadillo repeat protein/δ-catenin and p0071. J Biol Chem. 2000;275:29875–29880. doi: 10.1074/jbc.M005384200. [DOI] [PubMed] [Google Scholar]
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- Denu JM, Dixon JE. Protein tyrosine phosphatases: mechanisms of catalysis and regulation. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Domnina LV, Ivanova OY, Cherniak BV, Skulachev VP, Vasiliev JM. Effects of the inhibitors of dynamics of cytoskeletal structures on the development of apoptosis induced by tumor necrosis factor. Biochemistry (Mosc) 2002;67:737–746. doi: 10.1023/a:1016336421582. [DOI] [PubMed] [Google Scholar]
- Domnina LV, Ivanova OY, Pletjushkina OY, Fetisova EK, Chernyak BV, Skulachev VP, Vasiliev JM. Marginal blebbing during the early stages of TNF-induced apoptosis indicates alteration in actomyosin contractility. Cell Biol Int. 2004;28:471–475. doi: 10.1016/j.cellbi.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. α-Catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- Dupuy AG, L’Hoste S, Cherfils J, Camonis J, Gaudriault G, de Gunzburg J. Novel Rap1 dominant-negative mutants interfere selectively with C3G and Epac. Oncogene. 2005;24:4509–4520. doi: 10.1038/sj.onc.1208647. [DOI] [PubMed] [Google Scholar]
- Dusek RL, Godsel LM, Green KJ. Discriminating roles of desmosomal cadherins: beyond desmosomal adhesion. J Dermatol Sci. 2007;45:7–21. doi: 10.1016/j.jdermsci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Eger A, Stockinger A, Wiche G, Foisner R. Polarisation-dependent association of plectin with desmoplakin and the lateral submembrane skeleton in MDCK cells. J Cell Sci. 1997;110:1307–1316. doi: 10.1242/jcs.110.11.1307. [DOI] [PubMed] [Google Scholar]
- El-Amraoui A, Petit C. Usher I syndrome: unravelling the mechanisms that underlie the cohesion of the growing hair bundle in inner ear sensory cells. J Cell Sci. 2005;118:4593–4603. doi: 10.1242/jcs.02636. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, Tasken K. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the α3β1 integrin but not the α6β4 integrin. J Biol Chem. 2004;279:44889–44896. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Evans JP. Fertilin β and other ADAMs as integrin ligands: insights into cell adhesion and fertilization. BioEssays. 2001;23:628–639. doi: 10.1002/bies.1088. [DOI] [PubMed] [Google Scholar]
- Evans JS, Turner MD. Emerging functions of the calpain superfamily of cysteine proteases in neuroendocrine secretory pathways. J Neurochem. 2007;103:849–859. doi: 10.1111/j.1471-4159.2007.04815.x. [DOI] [PubMed] [Google Scholar]
- Fabre-Lafay S, Garrido-Urbani S, Reymond N, Goncalves A, Dubreuil P, Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-α-converting enzyme (TACE)/ADAM-17. J Biol Chem. 2005;280:19543–19550. doi: 10.1074/jbc.M410943200. [DOI] [PubMed] [Google Scholar]
- Fanto M, McNeill H. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- Fawcett DW. Ultrastructure and function of the Sertoli cell. In: Hamilton DW, Greep RO, editors. Handbook of Physiology. Washington, DC: American Physiological Society; 1975. pp. 21–25. [Google Scholar]
- Fawcett DW, Leak LV, Heidger PM. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105–122. [PubMed] [Google Scholar]
- Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R, Leichtfried FE, Herrmann H, Small JV, Lawson D, Wiche G. Cytoskeleton-associated plectin: in situ localization, in vitro reconstitution, and binding to immobilized intermediate filament proteins. J Cell Biol. 1988;106:723–733. doi: 10.1083/jcb.106.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R, Traub P, Wiche G. Protein kinase A- and protein kinase C-regulated interaction of plectin with lamin B and vimentin. Proc Natl Acad Sci U S A. 1991;88:3812–3816. doi: 10.1073/pnas.88.9.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Borrmann CM, Grund C, Pieperhoff S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol. 2006;85:69–82. doi: 10.1016/j.ejcb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- Franz CM, Ridley AJ. p120 Catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279:6588–6594. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SM, Raines RT. Internalization of cationic peptides: the road less (or more?) traveled. Cell Mol Life Sci. 2006;63:1819–1822. doi: 10.1007/s00018-006-6170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, Maekawa M, Kojima N, Senoo H, Toshimori K, Momoi T. Oligo-astheno-teratozoospermia in mice lacking RA175/TSLC1/ SynCAM/IGSF4A, a cell adhesion molecule in the immunoglobulin superfamily. Mol Cell Biol. 2006;26:718–726. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsura Y, Yonehara S, Fujisawa H, Kikuchi A, et al. Cdc42 and Rac1 regulate the interaction of IQGAP1 with β-catenin. J Biol Chem. 1999;274:26044–26050. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Irie K, Nakanishi H, Takekuni K, Kawakatsu T, Ikeda W, Yamada A, Katata T, Honda T, Sato T, et al. Involvement of nectin in the localization of junctional adhesion molecule at tight junctions. Oncogene. 2002;21:7642–7655. doi: 10.1038/sj.onc.1205875. [DOI] [PubMed] [Google Scholar]
- Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol. 1998;143:2009–2022. doi: 10.1083/jcb.143.7.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts D, Fontao L, Nievers MG, Schaapveld RQ, Purkis PE, Wheeler GN, Lane EB, Leigh IM, Sonnenberg A. Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J Cell Biol. 1999;147:417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghersi G. Roles of molecules involved in epithelial/mesenchymal transition during angiogenesis. Front Biosci. 2008;13:2335–2355. doi: 10.2741/2848. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Signal transduction by the α6β4 integrin: charting the path between laminin binding and nuclear events. J Cell Sci. 1996;109:1165–1171. doi: 10.1242/jcs.109.6.1165. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- Goldman EE. Die aussere ind inner Sekretion des gesunden und kranken Organisimus im Lichte der “vitalen Farbung.”. Beitr Klin Chirurg. 1909;64:192–265. [Google Scholar]
- Goossens S, Janssens B, Bonne S, de Rycke R, Braet F, van Hengel J, van Roy F. A unique and specific interaction between αT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J Cell Sci. 2007a;120:2126–2136. doi: 10.1242/jcs.004713. [DOI] [PubMed] [Google Scholar]
- Goossens S, Janssens B, Vanpoucke G, De Rycke R, Van Hengel J, Van Roy F. Truncated isoform of mouse αT-catenin is testis-restricted in expression and function. FASEB J. 2007b;21:647–655. doi: 10.1096/fj.06-6066com. [DOI] [PubMed] [Google Scholar]
- Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1:208–216. doi: 10.1038/35043032. [DOI] [PubMed] [Google Scholar]
- Gregor M, Zeold A, Oehler S, Marobela KA, Fuchs P, Weigel G, Hardie DG, Wiche G. Plectin scaffolds recruit energy-controlling AMP-activated protein kinase (AMPK) in differentiating myofibres. J Cell Sci. 2006;119:1864–1875. doi: 10.1242/jcs.02891. [DOI] [PubMed] [Google Scholar]
- Grima J, Cheng CY. Testin induction: the role of cyclic 3′,5′-adenosine monophosphate/protein kinase A signaling in the regulation of basal and lonidamine-induced testin expression by rat Sertoli cells. Biol Reprod. 2000;63:1648–1660. doi: 10.1095/biolreprod63.6.1648. [DOI] [PubMed] [Google Scholar]
- Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol. 1992;89:127–140. doi: 10.1016/0303-7207(92)90219-v. [DOI] [PubMed] [Google Scholar]
- Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–1508. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- Grima J, Wong CC, Zhu LJ, Zong SD, Cheng CY. Testin secreted by Sertoli cells is associated with the cell surface, and its expression correlates with the disruption of Sertoli-germ cell junctions but not the inter-Sertoli tight junction. J Biol Chem. 1998;273:21040–21053. doi: 10.1074/jbc.273.33.21040. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove BD, Pfeiffer DC, Allen S, Vogl AW. Immunofluorescence localization of vinculin in ectoplasmic (“junctional”) specializations of rat Sertoli cells. Am J Anat. 1990;188:44–56. doi: 10.1002/aja.1001880106. [DOI] [PubMed] [Google Scholar]
- Grove BD, Vogl AW. Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J Cell Sci. 1989;93:309–323. doi: 10.1242/jcs.93.2.309. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cadherins: a family of Ca2+-dependent adhesion molecules. Trends Biochem Sci. 1988;13:75–76. doi: 10.1016/0968-0004(88)90040-0. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–403. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Guttman JA, Janmey P, Vogl AW. Gelsolin - evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J Cell Sci. 2002;115:499–505. doi: 10.1242/jcs.115.3.499. [DOI] [PubMed] [Google Scholar]
- Guttman JA, Mulholland DJ, Vogl AW. Plectin is concentrated at intercellular junctions and at the nuclear surface in morphologically differentiated rat Sertoli cells. Anat Rec. 1999;254:418–428. doi: 10.1002/(SICI)1097-0185(19990301)254:3<418::AID-AR13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Haas IG, Frank M, Veron N, Kemler R. Presenilin-dependent processing and nuclear function of γ-protocadherins. J Biol Chem. 2005;280:9313–9319. doi: 10.1074/jbc.M412909200. [DOI] [PubMed] [Google Scholar]
- Habets GG, van der Kammen RA, Stam JC, Michiels F, Collard JG. Sequence of the human invasion-inducing TIAM1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene. 1995;10:1371–1376. [PubMed] [Google Scholar]
- Halama T, Groger M, Pillinger M, Staffler G, Prager E, Stockinger H, Holnthoner W, Lechleitner S, Wolff K, Petzelbauer P. Platelet endothelial cell adhesion molecule-1 and vascular endothelial cadherin cooperatively regulate fibroblast growth factor-induced modulations of adherens junction function. J Invest Dermatol. 2001;116:110–117. doi: 10.1046/j.1523-1747.2001.00176.x. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Ham C, Levkau B, Raines EW, Herren B. ADAM15 is an adherens junction molecule whose surface expression can be driven by VE-cadherin. Exp Cell Res. 2002;279:239–247. doi: 10.1006/excr.2002.5606. [DOI] [PubMed] [Google Scholar]
- Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115:849–860. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. Decisions, decisions: β-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234–237. doi: 10.1016/j.tcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hasson T, Walsh J, Cable J, Mooseker MS, Brown SD, Steel KP. Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton. 1997;37:127–138. doi: 10.1002/(SICI)1097-0169(1997)37:2<127::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M. Plakophilins: multifunctional proteins or just regulators of desmosomal adhesion? Biochim Biophys Acta. 2007;1773:69–77. doi: 10.1016/j.bbamcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Green KJ, Sauter H. Targeting of p0071 to desmosomes and adherens junctions is mediated by different protein domains. J Cell Sci. 2003;116:1219–1233. doi: 10.1242/jcs.00275. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Haffner C, Schulze K, Vinzens U. The function of plakophilin 1 in desmosome assembly and actin filament organization. J Cell Biol. 2000;149:209–222. doi: 10.1083/jcb.149.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M, Nachtsheim C. Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: evidence for a subfamily of closely related proteins. J Cell Sci. 1996;109:2767–2778. doi: 10.1242/jcs.109.11.2767. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- Heckert LL, Griswold MD. The expression of the follicle-stimulating hormone receptor in spermatogenesis. Recent Prog Horm Res. 2002;57:129–148. doi: 10.1210/rp.57.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand BT, Chou YH, Shumaker DK, Goldman RD. Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 2005;15:568–570. doi: 10.1016/j.tcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Heckert LL. Transcriptional regulation of the FSH receptor: new perspectives. Mol Cell Endocrinol. 2007;260–262:100–108. doi: 10.1016/j.mce.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrenknecht K. Cadherins. In: Horton MA, editor. Molecular Biology of Cell Adhesion Molecules. New York: John Wiley & Sons; 1996. pp. 45–70. [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein α-catenin is a vinculin homologue. Proc Natl Acad Sci U S A. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H, Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem. 1987;262:1320–1325. [PubMed] [Google Scholar]
- Hess RA. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol Reprod. 1990;43:525–542. doi: 10.1095/biolreprod43.3.525. [DOI] [PubMed] [Google Scholar]
- Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Bahr J. Oestrogen, its receptors and function in the male reproductive tract - a review. Mol Cell Endocrinol. 2001;178:29–38. doi: 10.1016/s0303-7207(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Hess RA, Franca LR. Structure of the Sertoli cell. In: Skinner MK, Griswold MD, editors. Sertoli Cell Biology. New York: Elsevier Academic Press; 2005. pp. 19–40. [Google Scholar]
- Hess RA, Schaeffer DJ, Eroschenko VP, Keen JE. Frequency of the stages in the cycle of the seminiferous epithelium in the rat. Biol Reprod. 1990;43:517–524. doi: 10.1095/biolreprod43.3.517. [DOI] [PubMed] [Google Scholar]
- Hieda Y, Nishizawa Y, Uematsu J, Owaribe K. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J Cell Biol. 1992;116:1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild SA, Attardi BJ, Reel JR. The ability of a gonadotropin-releasing hormone antagonist, acyline, to prevent irreversible infertility induced by the indenopyridine, CDB-4022, in adult male rats: the role of testosterone. Biol Reprod. 2004;71:348–358. doi: 10.1095/biolreprod.103.026989. [DOI] [PubMed] [Google Scholar]
- Hild SA, Marshall GR, Attardi BJ, Hess RA, Schlatt S, Simorangkir DR, Ramaswamy S, Koduri S, Reel JR, Plant TM. Development of l-CDB-4022 as a nonsteroidal male oral contraceptive: induction and recovery from severe oligospermia in the adult male cynomolgus monkey (Macaca fascicularis) Endocrinology. 2007;148:1784–1796. doi: 10.1210/en.2006-1487. [DOI] [PubMed] [Google Scholar]
- Hild SA, Reel JR, Larner JM, Blye RP. Disruption of spermatogenesis and Sertoli cell structure and function by the indenopyridine CDB-4022 in rats. Biol Reprod. 2001;65:1771–1779. doi: 10.1095/biolreprod65.6.1771. [DOI] [PubMed] [Google Scholar]
- Hildenbrand R, Gandhari M, Stroebel P, Marx A, Allgayer H, Arens N. The urokinase system - role in cell proliferation and apoptosis. Histol Histopathol. 2008;23:227–236. doi: 10.14670/HH-23.227. [DOI] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Hirao T, Nanba D, Tanaka M, Ishiguro H, Kinugasa Y, Doki Y, Yano M, Matsuura N, Monden M, Higashiyama S. Overexpression of ADAM9 enhances growth factor-mediated recycling of E-cadherin in human colon cancer cell line HT29 cells. Exp Cell Res. 2006;312:331–339. doi: 10.1016/j.yexcr.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger LJ, Ward B, Duffield B, Zachwieja J, Jallal B. The transmembrane receptor protein tyrosine phosphatase DEP1 interacts with p120ctn. Oncogene. 2002;21:7067–7076. doi: 10.1038/sj.onc.1205858. [DOI] [PubMed] [Google Scholar]
- Holthöfer B, Windoffer R, Troyanovsky S, Leube RE. Structure and function of desmosomes. Int Rev Cytol. 2007;264:65–163. doi: 10.1016/S0074-7696(07)64003-0. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- Hosking CR, Ulloa F, Hogan C, Ferber EC, Figueroa A, Gevaert K, Birchmeier W, Briscoe J, Fujita Y. The transcriptional repressor Glis2 is a novel binding partner for p120 catenin. Mol Biol Cell. 2007;18:1918–1927. doi: 10.1091/mbc.E06-10-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House CM, Frew IJ, Huang HL, Wiche G, Traficante N, Nice E, Catimel B, Bowtell DD. A binding motif for Siah ubiquitin ligase. Proc Natl Acad Sci U S A. 2003;100:3101–3106. doi: 10.1073/pnas.0534783100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O. Structure and function of desmosomal proteins and their role in development and disease. Cell Mol Life Sci. 2003;60:1872–1890. doi: 10.1007/s00018-003-3050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol. 1994;127:2061–2069. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ichii T, Takeichi M. p120 Catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells. 2007;12:827–839. doi: 10.1111/j.1365-2443.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells. 2006;11:1125–1132. doi: 10.1111/j.1365-2443.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic. 2007;8:1465–1475. doi: 10.1111/j.1600-0854.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, Van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian LM, Bundy LM, Sealy L, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie K, Shimizu K, Sakisaka T, Ikeda W, Takai Y. Roles and modes of action of nectins in cell-cell adhesion. Semin Cell Dev Biol. 2004;15:643–656. doi: 10.1016/j.semcdb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S, Tomita K, Mimori T, Saya H. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of β-catenin from cell-cell contacts. Oncogene. 1999;18:7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, McCall IC, Babbin B, Samarin SN, Nusrat A, Parkos CA. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol. 2006;7:12. doi: 10.1186/1471-2121-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa I, Nishizawa M, Tomono Y, Ohtakara K, Takahashi T, Inagaki M. ERBIN associates with p0071, an armadillo protein, at cell-cell junctions of epithelial cells. Genes Cells. 2002;7:475–485. doi: 10.1046/j.1365-2443.2002.00533.x. [DOI] [PubMed] [Google Scholar]
- Jamora C, Fuchs E. Intercellular adhesion, signaling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Janecki A, Jakubowiak A, Steinberger A. Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: in vitro model of the blood-testis barrier. Endocrinology. 1991;129:1489–1496. doi: 10.1210/endo-129-3-1489. [DOI] [PubMed] [Google Scholar]
- Janssens B, Goossens S, Staes K, Gilbert B, Van Hengel J, Colpaert C, Bruyneel E, Mareel M, Van Roy F. αT-catenin: a novel tissue-specific β-cateninbinding protein mediating strong cell-cell adhesion. J Cell Sci. 2001;114:3177–3188. doi: 10.1242/jcs.114.17.3177. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J, Van Hoof C. PP2A: the expected tumor suppressor. Curr Opin Genet Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Jaulin-Bastard F, Arsanto JP, Le Bivic A, Navarro C, Vely F, Saito H, Marchetto S, Hatzfeld M, Santoni MJ, Birnbaum D, et al. Interaction between erbin and a catenin-related protein in epithelial cells. J Biol Chem. 2002;277:2869–2875. doi: 10.1074/jbc.M109652200. [DOI] [PubMed] [Google Scholar]
- Jefferson JJ, Leung CL, Liem RK. Plakins: goliaths that link cell junctions and the cytoskeleton. Nat Rev Mol Cell Biol. 2004;5:542–553. doi: 10.1038/nrm1425. [DOI] [PubMed] [Google Scholar]
- Jeong HW, Li Z, Brown MD, Sacks DB. IQGAP1 binds to Rap1 and modulates its activity. J Biol Chem. 2007;282:20752–20762. doi: 10.1074/jbc.M700487200. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Isenberg G. Interaction of α-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981;78:3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Boekelheide K. Dynamic testicular adhesion junctions are immunologically unique. II. Localization of classic cadherins in rat testis. Biol Reprod. 2002;66:992–1000. doi: 10.1095/biolreprod66.4.992. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Patel SR, Boekelheide K. Multiple cadherin superfamily members with unique expression profiles are produced in rat testis. Endocrinology. 2000;141:675–683. doi: 10.1210/endo.141.2.7334. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Zecevic A, Kwon EJ. Protocadherin α3 acts at sites distinct from classic cadherins in rat testis and sperm. Biol Reprod. 2004;70:303–312. doi: 10.1095/biolreprod.103.021758. [DOI] [PubMed] [Google Scholar]
- Jones C, Chen P. Planar cell polarity signaling in vertebrates. BioEssays. 2007;29:120–132. doi: 10.1002/bies.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. BioEssays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Joseph J. Ran at a glance. J Cell Sci. 2006;119:3481–3484. doi: 10.1242/jcs.03071. [DOI] [PubMed] [Google Scholar]
- Kanda S, Miyata Y, Kanetake H, Smithgall TE. Non-receptor protein-tyrosine kinases as molecular targets for antiangiogenic therapy. Int J Mol Med. 2007;20:113–121. [PubMed] [Google Scholar]
- Kang Q, Cao Y, Zolkiewska A. Metalloprotease-disintegrin ADAM12 binds to the SH3 domain of Src and activates Src tyrosine kinase in C2C12 cells. Biochem J. 2000;352:883–892. [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Das C. Modulation of ezrin and E-cadherin expression by IL-1β and TGF-β1 in human trophoblasts. J Reprod Immunol. 2004;64:9–29. doi: 10.1016/j.jri.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Katata T, Irie K, Fukuhara A, Kawakatsu T, Yamada A, Shimizu K, Takai Y. Involvement of nectin in the localization of IQGAP1 at the cell-cell adhesion sites through the actin cytoskeleton in Madin-Darby canine kidney cells. Oncogene. 2003;22:2097–2109. doi: 10.1038/sj.onc.1206255. [DOI] [PubMed] [Google Scholar]
- Kaufmann U, Zuppinger C, Waibler Z, Rudiger M, Urbich C, Martin B, Jockusch BM, Eppenberger H, Starzinski-Powitz A. The armadillo repeat region targets ARVCF to cadherin-based cellular junctions. J Cell Sci. 2000;113:4121–4135. doi: 10.1242/jcs.113.22.4121. [DOI] [PubMed] [Google Scholar]
- Kausalya PJ, Phua DC, Hunziker W. Association of ARVCF with zonula occludens (ZO)-1 and ZO-2: binding to PDZ-domain proteins and cell-cell adhesion regulate plasma membrane and nuclear localization of ARVCF. Mol Biol Cell. 2004;15:5503–5515. doi: 10.1091/mbc.E04-04-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil R, Wolf A, Huttelmaier S, Hatzfeld M. Beyond regulation of cell adhesion: local control of RhoA at the cleavage furrow by the p0071 catenin. Cell Cycle. 2007;6:122–127. doi: 10.4161/cc.6.2.3741. [DOI] [PubMed] [Google Scholar]
- Keilhack H, Hellman U, Van Hengel J, Van Roy F, Godovac-Zimmermann J, Bohmer FD. The protein-tyrosine phosphatase SHP-1 binds to and dephosphorylates p120 catenin. J Biol Chem. 2000;275:26376–26384. doi: 10.1074/jbc.M001315200. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Ketema M, Wilhelmsen K, Kuikman I, Janssen H, Hodzic D, Sonnenberg A. Requirements for the localization of nesprin-3 at the nuclear envelope and its localization with plectin. J Cell Sci. 2007;120:3384–3394. doi: 10.1242/jcs.014191. [DOI] [PubMed] [Google Scholar]
- Kikyo M, Matozaki T, Kodama A, Kawabe H, Nakanishi H, Takai Y. Cell-cell adhesion-mediated tyrosine phosphorylation of nectin-2δ, an immunoglobulin-like cell adhesion molecule at adherens junctions. Oncogene. 2000;19:4022–4028. doi: 10.1038/sj.onc.1203744. [DOI] [PubMed] [Google Scholar]
- Kim DY, MacKenzie Ingano LA, Kovacs DM. Nectin-1α, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/γ-secretase-like cleavage. J Biol Chem. 2002;277:49976–49981. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- Kim L, Wong TW. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol Cell Biol. 1995;15:4553–4561. doi: 10.1128/mcb.15.8.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–1288. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, Burgeson RE, Champliaud MF. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koduri S, Hild SA, Pessaint L, Reel JR, Attardi BJ. Mechanism of action of l-CDB-4022, a potential nonhormonal male contraceptive, in the seminiferous epithelium of the rat testis. Endocrinology. 2008;149:1850–1860. doi: 10.1210/en.2007-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeser J, Troyanovsky SM, Grund C, Franke WW. De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp Cell Res. 2003;285:114–130. doi: 10.1016/s0014-4827(03)00016-8. [DOI] [PubMed] [Google Scholar]
- Koster J, Kuikman I, Kreft M, Sonnenberg A. Two different mutations in the cytoplasmic domain of the integrin β4 subunit in nonlethal forms of epidermolysis bullosa prevent interaction of β4 with plectin. J Invest Dermatol. 2001;117:1405–1411. doi: 10.1046/j.0022-202x.2001.01567.x. [DOI] [PubMed] [Google Scholar]
- Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Bornslaeger EA, Norvell SM, Palka H, Green KJ. Desmosomes: intercellular adhesive junctions specialized for attachment of intermediate filaments. Int Rev Cytol. 1999;185:237–302. doi: 10.1016/s0074-7696(08)60153-9. [DOI] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- Kuiperij HB, de Rooij J, Rehmann H, van Triest M, Wittinghofer A, Bos JL, Zwartkruis FJ. Characterization of PDZ-GEFs, a family of guanine nucleotide exchange factors specific for Rap1 and Rap2. Biochim Biophys Acta. 2003;1593:141–149. doi: 10.1016/s0167-4889(02)00365-8. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Fujii K, Nakamura T, Izawa I, Kaibuchi K. Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem Biophys Res Commun. 1997;240:430–435. doi: 10.1006/bbrc.1997.7675. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, et al. Role of IQGAP, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski AV, Gertler FB, Loureiro JJ. Function and regulation of Ena/VASP proteins. Trends Cell Biol. 2003;13:386–392. doi: 10.1016/s0962-8924(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Lau AS, Mruk DD. Rab8B GTPase and junction dynamics in the testis. Endocrinology. 2003;144:1549–1563. doi: 10.1210/en.2002-220893. [DOI] [PubMed] [Google Scholar]
- Laura RP, Witt AS, Held HA, Gerstner R, Deshaves K, Koehler MF, Kosik KS, Sidhu SS, Lasky LA. The erbin PDZ domain binds with high affinity and specificity to the carboxyl termini of δ-catenin and ARVCF. J Biol Chem. 2002;277:12906–12914. doi: 10.1074/jbc.M200818200. [DOI] [PubMed] [Google Scholar]
- Le Gat L, Gogat K, Van Den Berghe L, Brizard M, Kobetz A, Marchant D, Abitbol M, Menasche M. The β3 integrin gene is expressed at high levels in the major haematopoietic and lymphoid organs, vascular system, and skeleton during mouse embryo development. Cell Commun Adhes. 2003;10:129–140. [PubMed] [Google Scholar]
- LeBlond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lech MM, Ostrowaska L. Risk of cancer development in relation to oral contraception. Eur J Contracept Reprod Health Care. 2006;11:162–168. doi: 10.1080/13625180600815706. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J Cell Biol. 2007;176:147–154. doi: 10.1083/jcb.200609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Choi EY, Hyun MS, Jang BI, Kim TN, Kim SW, Song SK, Kim JH, Kim JR. Association with extracellular cleavage of E-cadherin mediated by MMP-7 with HGF-induced in vitro invasion in human stomach cancer cells. Eur Surg Res. 2007;39:208–215. doi: 10.1159/000101452. [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update. 2004;10:349–369. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- Lee NP, Mruk D, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- Lee NP, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich Syndrome Protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Lee NP, Mruk DD, Wong CH, Cheng CY. Regulation of Sertoli-germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS)/cGMP/protein kinase G (PRKG)/β-catenin (CATNB) signaling pathway: an in vitro and in vivo study. Biol Reprod. 2005;73:458–471. doi: 10.1095/biolreprod.105.040766. [DOI] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH, and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Wahl JK, III, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136:919–934. doi: 10.1083/jcb.136.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY. Tumor necrosis factor-α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- Liang XJ, Aszalos A. Multidrug transporters as drug targets. Curr Drug Targets. 2006;7:911–921. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- Lie PP, Xia W, Wang CQ, Mruk DD, Yan HH, Wong CH, Lee WM, Cheng CY. Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood-testis barrier in adult rat testes. J Endocrinol. 2006;191:571–586. doi: 10.1677/joe.1.06996. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8:808–819. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to β-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Liu J, Stevens J, Rote CA, Yost HJ, Hu YZ, Neufeld KL, White RL, Matsunami N. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Lo SH. Focal adhesions: what’s new inside. Dev Biol. 2006;294:280–291. doi: 10.1016/j.ydbio.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Lobl TJ, Bardin CW, Gunsalus GL, Musto NA. Effects of lonidamine (AF 1890) and its analogues on follicle-stimulating hormone, testosterone and rat androgen binding protein concentrations in the rat and rhesus monkey. Chemotherapy. 1981;27 Suppl 2:61–76. doi: 10.1159/000238046. [DOI] [PubMed] [Google Scholar]
- Lobl TJ, Forbes AD, Kirton KT, Wilks JW. Characterization of the exfoliative antispermatogenic agent 1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid in the rhesus monkey. Arch Androl. 1979;3:67–77. doi: 10.3109/01485017908985051. [DOI] [PubMed] [Google Scholar]
- Lopez LM, Grimes DA, Schulz KF. Nonhormonal drugs for contraception in men: a systematic review. Obstet Gynecol Surv. 2005;60:746–752. doi: 10.1097/01.ogx.0000182905.71077.13. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- Lui WY, Mruk DD, Cheng CY. Interactions among IQGAP1, Cdc42, and the cadherin/catenin protein complex regulate Sertoli-germ cell adherens junction dynamics in the testis. J Cell Physiol. 2005;202:49–66. doi: 10.1002/jcp.20098. [DOI] [PubMed] [Google Scholar]
- Lunter PC, Wiche G. Direct binding of plectin to Fer kinase and negative regulation of its catalytic activity. Biochem Biophys Res Commun. 2002;296:904–910. doi: 10.1016/s0006-291x(02)02007-7. [DOI] [PubMed] [Google Scholar]
- Lurie G, Thompson P, McDuffie KE, Carney ME, Terada KY, Goodman MT. Association of estrogen and progestin potency of oral contraceptives with ovarian carcinoma risk. Obstet Gynecol. 2007;109:597–607. doi: 10.1097/01.AOG.0000255664.48970.e6. [DOI] [PubMed] [Google Scholar]
- Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, Kusugami K, Sekido Y. Plakoglobin (γ-catenin) has TCF/LEF family-dependent transcriptional activity in β-catenin-deficient cell line. Oncogene. 2004;23:964–972. doi: 10.1038/sj.onc.1207254. [DOI] [PubMed] [Google Scholar]
- Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and α-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualc’h F. Crosstalk between Rap1 and Rac regulates secretion of sAPPα. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Malorni W, Meschini S, Matarrese P, Arancia G. The cytoskeleton as a subcellular target of the antineoplastic drug lonidamine. Anticancer Res. 1992;12:2037–2045. [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, et al. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Takahashi K, Satoh K, Nishioka H, Mizoguchi A, Takai Y. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J Cell Biol. 1999;144:1001–1017. doi: 10.1083/jcb.144.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcante ML, Natali PG, Floridi A, De Martino C. Effects of AF 1312/TS and lonidamine on cultured Sertoli cells. Chemotherapy. 1981;27 Suppl 2:43–49. doi: 10.1159/000238044. [DOI] [PubMed] [Google Scholar]
- Marcet-Palacios M, Ulanova M, Duta F, Puttagunta L, Munoz S, Gibbings D, Radomski M, Cameron L, Mayers I, Befus AD. The transcription factor Wilms Tumor 1 regulates matrix metalloproteinase-9 through a nitric oxide-mediated pathway. J Immunol. 2007;179:256–265. doi: 10.4049/jimmunol.179.1.256. [DOI] [PubMed] [Google Scholar]
- Marcozzi C, Burdett ID, Buxton RS, Magee AI. Coexpression of both types of desmosomal cadherin and plakoglobin confers strong intercellular adhesion. J Cell Sci. 1998;111:495–509. doi: 10.1242/jcs.111.4.495. [DOI] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner DJ, Anastasiadis P, Keilhack H, Bohmer FD, Wang J, Reynolds AB. Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem. 2001;276:28006–28013. doi: 10.1074/jbc.M102443200. [DOI] [PubMed] [Google Scholar]
- Mariner DJ, Wang J, Reynolds AB. ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120ctn in E-cadherin complexes. J Cell Sci. 2000;113:1481–1490. doi: 10.1242/jcs.113.8.1481. [DOI] [PubMed] [Google Scholar]
- Martin J, Eynstone LV, Davies M, Williams JD, Steadman R. The role of ADAM15 in glomerular mesangial cell migration. J Biol Chem. 2002;277:33683–33689. doi: 10.1074/jbc.M200988200. [DOI] [PubMed] [Google Scholar]
- Martinez-Rico C, Pincet F, Perez E, Thiery JP, Shimizu K, Takai Y, Dufour S. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J Biol Chem. 2005;280:4753–4760. doi: 10.1074/jbc.M412544200. [DOI] [PubMed] [Google Scholar]
- Mary SS, Charrasse M, Meriane F, Comunale P, Travo P, Blangy A, Gauthier-Rouviere C. Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol Biol Cell. 2002;13:285–301. doi: 10.1091/mbc.01-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915–926. doi: 10.1016/s1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Park J. Developmental functions of the p120-catenin subfamily. Biochim Biophys Acta. 2007;1773:17–33. doi: 10.1016/j.bbamcr.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Meçse G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- Medici D, Hay ED, Goodenough DA. Cooperation between Snail and LEF-1 transcription factors is essential for TGF-β1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerum Terwogt JM, Malingre MM, Beijnen JH, ten Bokkel Huinink WW, Rosing H, Koopman FJ, van Tellingen O, Swart M, Schellens JH. Co-administration of oral cyclosporin A enables oral therapy with paclitaxel. Clin Cancer Res. 1999;5:3379–3384. [PubMed] [Google Scholar]
- Melaine N, Lienard MO, Dorval I, Le Goascogne C, Lejeune H, Jegou B. Multidrug resistance genes and P-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biol Reprod. 2002;67:1699–1707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- Melaine N, Satie AP, Lassurquere J, Desmots S, Jegou B, Samson M. Molecular cloning of several rat ABC transporters including a new ABC transporter, Abcb8, and their expression in rat testis. Int J Androl. 2006;29:392–399. doi: 10.1111/j.1365-2605.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel AR, Kroemker M, Bubeck P, Ronsiek M, Nikolai G, Jockusch BM. Characterization of an F-actin-binding domain in the cytoskeletal protein vinculin. J Cell Biol. 1994;126:1231–1240. doi: 10.1083/jcb.126.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The α6β4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Merono A, Lucena C, Lopez A, Garrido JJ, Perez de LL, Llanes D. Immunohistochemical analysis of β3 integrin (CD61): expression in pig tissues and human tumors. Histol Histopathol. 2002;17:347–352. doi: 10.14670/HH-17.347. [DOI] [PubMed] [Google Scholar]
- Millichip MI, Dallas DJ, Wu E, Dale S, McKie N. The metallo-disintegrin ADAM10 (MADM) from bovine kidney has type IV collagenase activity in vitro. Biochem Biophys Res Commun. 1998;245:594–598. doi: 10.1006/bbrc.1998.8485. [DOI] [PubMed] [Google Scholar]
- Miravet S, Piedra J, Miro F, Itarte E, Garcia de Herreros A, Dunach M. The transcriptional factor Tcf-4 contains different binding sites for β-catenin and plakoglobin. J Biol Chem. 2002;277:1884–1891. doi: 10.1074/jbc.M110248200. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Nectin and nectin-like molecules: biology and pathology. Am J Nephrol. 2007;27:590–604. doi: 10.1159/000108103. [DOI] [PubMed] [Google Scholar]
- Mizrak SC, Renault-Mihara F, Parraga M, Bogerd J, van de Kant HJ, Lopez-Casas PP, Paz M, del Mazo J, de Rooij DG. Phosphoprotein enriched in astrocytes-15 is expressed in mouse testis and protects spermatocytes from apoptosis. Reproduction. 2007;133:743–751. doi: 10.1530/REP-06-0281. [DOI] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, Kriz W, Holzman LB. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J. 2004;23:3769–3779. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Yagi T. Protocadherin family: diversity, structure and function. Curr Opin Cell Biol. 2007;19:584–592. doi: 10.1016/j.ceb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Morris ME, Lee HJ, Predko LM. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol Rev. 2003;55:229–240. doi: 10.1124/pr.55.2.1. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004a;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004b;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Lau AS, Conway AM. Crosstalk between Rab GTPases and cell junctions. Contraception. 2005;72:280–290. doi: 10.1016/j.contraception.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Lau AS, Sarkar O, Xia W. Rab4A GTPase-catenin interactions are involved in cell junction dynamics in the testis. J Androl. 2007;28:742–754. doi: 10.2164/jandrol.106.002204. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- Mueller S, Rosenquist TA, Takai Y, Bronson RA, Wimmer E. Loss of nectin-2 at Sertoli-spermatid junctions leads to male infertility and correlates with severe spermatozoan head and midpiece malformation, impaired binding to the zona pellucida, and oocyte penetration. Biol Reprod. 2003;69:1330–1340. doi: 10.1095/biolreprod.102.014670. [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. Drosophila p120 catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol. 2003;160:433–449. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007;178:323–335. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic B, MacNulty E, Mir B, Wiche G. Basic amino acid residue cluster within nuclear targeting sequence motif is essential for cytoplasmic plectin-vimentin network junctions. J Cell Biol. 1996;134:1455–1467. doi: 10.1083/jcb.134.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin β. Dev Biol. 2001;233:204–213. doi: 10.1006/dbio.2001.0166. [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Buridge K, Kreft B. p120 Catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber UA, Schäfer SG, Schmidt A, Koch PJ, Franke WW. The widespread human desmocollin Dsc2 and tissue-specific patterns of synthesis of various desmocollin subtypes. Eur J Cell Biol. 1995;66:69–74. [PubMed] [Google Scholar]
- O’Donnell L, Stanton PG, Bartles JR, Robertson DM. Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat. Biol Reprod. 2000;63:99–108. doi: 10.1095/biolreprod63.1.99. [DOI] [PubMed] [Google Scholar]
- Ogata S, Morokuma J, Hayata T, Kolle G, Niehrs C, Ueno N, Cho KW. TGF-β signaling-mediated morphogenesis: modulation of cell adhesion via cadherin endocytosis. Genes Dev. 2007;21:1817–1831. doi: 10.1101/gad.1541807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita H, Takai Y. Nectins and nectin-like molecules: roles in cell adhesion, polarization, movement and proliferation. IUBMB Life. 2006;58:334–343. doi: 10.1080/15216540600719622. [DOI] [PubMed] [Google Scholar]
- Ohno H, Hirabayashi S, Iizuka T, Ohnishi H, Fujita T, Hata Y. Localization of p0071-interacting proteins, plakophilin-related armadillo-repeat protein-interacting protein (PAPIN) and ERBIN, in epithelial cells. Oncogene. 2002;21:7042–7049. doi: 10.1038/sj.onc.1205852. [DOI] [PubMed] [Google Scholar]
- Ohtsuka A. Microvascular architecture of the pampiniform plexus-testicular artery system in the rat: a scanning electron microscope study of corrosion casts. Am J Anat. 1984;169:285–293. doi: 10.1002/aja.1001690305. [DOI] [PubMed] [Google Scholar]
- Okumura M, Uematsu J, Hirako Y, Nishizawa Y, Shimizu H, Kido N, Owaribe K. Identification of the hemidesmosomal 500 kDa protein (HD1) as plectin. J Biochem (Tokyo) 1999;126:1144–1150. doi: 10.1093/oxfordjournals.jbchem.a022560. [DOI] [PubMed] [Google Scholar]
- Olkkonen VM, Stenmark H. Role of Rab GTPases in membrane traffic. Int Rev Cytol. 1997;176:1–85. doi: 10.1016/s0074-7696(08)61608-3. [DOI] [PubMed] [Google Scholar]
- Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y. Cooperative roles of PAR-3 and afadin in the formation of adherens and tight junctions. J Cell Sci. 2007;120:2352–2365. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- Ooshio T, Irie K, Morimoto K, Fukuhara A, Imai T, Takai Y. Involvement of LMO7 in the association of two cell-cell adhesion molecules, nectin and E-cadherin, through afadin and α-actinin in epithelial cells. J Biol Chem. 2004;279:31365–31373. doi: 10.1074/jbc.M401957200. [DOI] [PubMed] [Google Scholar]
- Osmanagic-Myers S, Wiche G. Plectin-RACK1 (receptor for activated C kinase) scaffolding: a novel mechanism to regulate protein kinase C activity. J Biol Chem. 2004;279:18701–18710. doi: 10.1074/jbc.M312382200. [DOI] [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Panorchan P, Thompson MS, Davis KJ, Tseng Y, Konstantopoulos K, Wirtz D. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J Cell Sci. 2006;119:66–74. doi: 10.1242/jcs.02719. [DOI] [PubMed] [Google Scholar]
- Park JL, Ji H, Jun S, Gu D, Hikasa H, Sokol SY, McCrea PD. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: distinct catenin subfamilies promote Wnt signals. Dev Cell. 2006a;11:683–695. doi: 10.1016/j.devcel.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Park S, Gwak J, Cho M, Song T, Won J, Kim DE, Shin JG, Oh S. Hexachlorophene inhibits Wnt/β-catenin pathway by promoting Siah-mediated β-catenin degradation. Mol Pharmacol. 2006b;70:960–966. doi: 10.1124/mol.106.024729. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, ZEB and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- Peinado H, Quintanilla M, Cano A. Transforming growth factor β-1 induces Snail transcription factor in epithelial cell lines. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-Catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer DC, Vogl AW. Evidence that vinculin is co-distributed with actin bundles in ectoplasmic (“junctional”) specializations of mammalian Sertoli cells. Anat Rec. 1991;231:89–100. doi: 10.1002/ar.1092310110. [DOI] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castaño J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincemail J, Vanbelle S, Gaspard U, Collette G, Haleng J, Cheramy-Bien JP, Charlier C, Chapelle JP, Giet D, Albert A, et al. Effect of different contraceptive methods on the oxidative stress status in women aged 40–48 years from the ELAN study in the province of Liege, Belgium. Hum Reprod. 2007;22:2335–2343. doi: 10.1093/humrep/dem146. [DOI] [PubMed] [Google Scholar]
- Plöen L, Setchell BP. Blood-testis barriers revisited: a homage to Lennart Nicander. Int J Androl. 1992;15:1–4. doi: 10.1111/j.1365-2605.1992.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Poghosyan Z, Robbins SM, Houslay MD, Webster A, Murphy G, Edwards DR. Phosphorylation-dependent interactions between ADAM15 cytoplasmic domain and Src family protein-tyrosine kinases. J Biol Chem. 2002;277:4999–5007. doi: 10.1074/jbc.M107430200. [DOI] [PubMed] [Google Scholar]
- Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin-and actin-binding sites of α-catenin. J Biol Chem. 2002;277:18868–18874. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure and mechanism of cadherins and catenin in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- Prokhortchouk A, Hendrich B, Jorgensen H, Ruzov A, Wilm M, Georgiev G, Bird A, Prokhortchouk E. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 2001;15:1613–1618. doi: 10.1101/gad.198501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AJ, Reid JC, Velasco G, Quigley JP, Hooper JD. The type II transmembrane serine protease matriptase-2: identification, structural features, enzymology, expression pattern and potential roles. Front Biosci. 2008;13:569–579. doi: 10.2741/2702. [DOI] [PubMed] [Google Scholar]
- Redies C, Vanhalst K, Roy F. δ-Protocadherins: unique structures and functions. Cell Mol Life Sci. 2005;62:2840–2852. doi: 10.1007/s00018-005-5320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Haas IG, Schulte M, Ludwig A, Frank M, Saftig P. Regulated ADAM10-dependent ectodomain shedding of γ-protocadherin C3 modulates cell-cell adhesion. J Biol Chem. 2006;281:21735–21744. doi: 10.1074/jbc.M602663200. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and β-catenin nuclear signaling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren HP, Russell LD. Quantitation of Sertoli cell-germ cell desmosome gap junctions in relation to meiotic divisions in the male rat. Tissue Cell. 1992;24:565–573. doi: 10.1016/0040-8166(92)90072-f. [DOI] [PubMed] [Google Scholar]
- Reynolds AB. p120-Catenin: past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezniczek GA, de Pereda JM, Reipert S, Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between β4 subunit and plectin at multiple molecular sites. J Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Takai Y. Interactions of the cell adhesion molecule nectin with transmembrane and peripheral membrane proteins for pleiotropic functions. Cell Mol Life Sci. 2008;65:253–263. doi: 10.1007/s00018-007-7290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the β subunit of G proteins. Proc Natl Acad Sci U S A. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with α-catenin, β-catenin, and plakoglobin. J Biol Chem. 1995;270:5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Watt FM. The plakin family: versatile organizers of cytoskeletal architecture. Curr Opin Genet Dev. 1997;7:392–397. doi: 10.1016/s0959-437x(97)80154-2. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke WW, et al. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol. 1996;135:215–225. doi: 10.1083/jcb.135.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977a;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977b;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977c;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- Russell LD. Sertoli-germ cell interrelations: a review. Gamete Res. 1980;3:179–202. [Google Scholar]
- Russell LD. Form, dimensions, and cytology of mammalian Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, FL: Cache River Press; 1993a. pp. 1–37. [Google Scholar]
- Russell LD. Morphological and functional evidence for Sertoli-germ cell relationships. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, FL: Cache River Press; 1993b. pp. 365–390. [Google Scholar]
- Russell LD. Role in spermiation. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, FL: Cache River Press; 1993c. pp. 269–303. [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- Russell LD, Gardner RJ, Weber JE. Reconstruction of a type-B configuration monkey Sertoli cell: size, shape, and configurational and specialized cell-to-cell relationships. Am J Anat. 1986;175:73–90. doi: 10.1002/aja.1001750108. [DOI] [PubMed] [Google Scholar]
- Russell LD, Goh JC. Localization of actinin in the rat testis: preliminary observations. In: Parvinen M, Huhtaniemi I, Pelliniemi LJ, editors. Development and Function of the Reproductive Organs VII Ares-Serono Symposia Series. New York: Raven Press; 1988. pp. 237–244. [Google Scholar]
- Russell LD, Lee IP, Ettlin RA, Peterson RN. Development of the acrosome and alignment, elongation and entrenchment of spermatids in procarbazine-treated rats. Tissue Cell. 1983;15:615–626. doi: 10.1016/0040-8166(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int J Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- Sabe H, Onodera Y, Mazaki Y, Hashimoto S. ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr Opin Cell Biol. 2006;18:558–564. doi: 10.1016/j.ceb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Sacco PA, McGranahan TM, Wheelock MJ, Johnson KR. Identification of plakoglobin domains required for association with N-cadherin and α-catenin. J Biol Chem. 1995;270:20201–20206. doi: 10.1074/jbc.270.34.20201. [DOI] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Iwai K, Ciechanover A, Geiger B, Ben-Ze’ev A. Differential interaction of plakoglobin and β-catenin with the ubiquitin-proteasome system. Oncogene. 2000;19:1992–2001. doi: 10.1038/sj.onc.1203519. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Ogita H, Hirota T, Kawakatsu T, Fukuyama T, Yasumi M, Kanzaki N, Ozaki M, Takai Y. Interaction of integrin avβ3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J Biol Chem. 2006;281:19631–19644. doi: 10.1074/jbc.M600301200. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol. 2007;19:593–602. doi: 10.1016/j.ceb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Takai Y. Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol. 2004;16:513–521. doi: 10.1016/j.ceb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod. 1998;58:371–378. doi: 10.1095/biolreprod58.2.371. [DOI] [PubMed] [Google Scholar]
- Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- Santoro G, Romeo C, Impellizzeri P, Cutroneo G, Micali A, Trimarchi F, Gentile C. Immunofluorescence distribution of actin-associated proteins in human seminiferous tubules of adolescent testes, normal and pathologic. J Endocrinol Invest. 2000;23:369–375. doi: 10.1007/BF03343740. [DOI] [PubMed] [Google Scholar]
- Sarkar O, Mathur PP, Cheng CY, Mruk DD. Interleukin-1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod. 2008;78:445–454. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar O, Xia W, Mruk DD. Adjudin-mediated junction restructuring in the seminiferous epithelium leads to displacement of soluble guanylate cyclase from adherens junctions. J Cell Physiol. 2006;208:175–187. doi: 10.1002/jcp.20651. [DOI] [PubMed] [Google Scholar]
- Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2006;281:5288–5299. doi: 10.1074/jbc.M510070200. [DOI] [PubMed] [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of the immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Schäfer SG, Koch PJ, Franke WW. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res. 1994;211:391–399. doi: 10.1006/excr.1994.1103. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schenone S, Manetti F, Botta M. SRC inhibitors and angiogenesis. Curr Pharm Des. 2007;13:2118–2128. doi: 10.2174/138161207781039580. [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci. 2004;7:923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Rho signaling at a glance. J Cell Sci. 2004;117:5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- Scott JA, Yap AS. Cinderella no longer: α-catenin steps out of cadherin’s shadow. J Cell Sci. 2006;119:4599–4605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Seidel B, Braeg S, Adler G, Wedlich D, Menke A. E- and N-cadherin differ with respect to their associated p120ctn isoforms and their ability to suppress invasive growth in pancreatic cancer cells. Oncogene. 2004;23:5532–5542. doi: 10.1038/sj.onc.1207718. [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Lawson D, Wiche G. Immunolocalization of the intermediate filament-associated protein plectin at focal contacts and actin stress fibers. Eur J Cell Biol. 1992;59:138–147. [PubMed] [Google Scholar]
- Setchell BP. The Mammalian Testis. Ithaca, New York: Cornell University Press; 1978. The scrotum and thermoregulation; pp. 90–108. [Google Scholar]
- Setchell BP. The functional significance of the blood-testis barrier. J Androl. 1980;1:3–10. [Google Scholar]
- Setchell BP. Blood-testis barrier. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. New York: Academic Press; 1998. pp. 375–381. [Google Scholar]
- Setchell BP, Breed WG. Anatomy, vasculature, and innervation of the male reproductive tract. In: Neill JD, editor. The Physiology of Reproduction. New York: Academic Press; 2006. pp. 771–825. [Google Scholar]
- Setchell BP, Waites GM. The blood-testis barrier. In: Hamilton DW, Greep RO, editors. The Handbook of Physiology. Baltimore: Williams and Wilkens; 1975. pp. 143–172. [Google Scholar]
- Shamsadin R, Adham IM, Nayernia K, Heinlein UA, Oberwinkler H, Engel W. Male mice deficient for germ cell cyritestin are infertile. Biol Reprod. 1999;61:1445–1451. doi: 10.1095/biolreprod61.6.1445. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Fukunaga Y, Ikenouchi J, Nagafuchi A. Defining the roles of β-catenin and plakoglobin in LEF/T-cell factor-dependent transcription using β-catenin/plakoglobin-null F9 cells. Mol Cell Biol. 2008;28:825–835. doi: 10.1128/MCB.02375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, Satoh K, Takeuchi M, Imai T, Monden M, et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem. 2003;278:35421–35427. doi: 10.1074/jbc.M305387200. [DOI] [PubMed] [Google Scholar]
- Shutes A, Berzat AC, Chenette EJ, Cox AD, Der CJ. Biochemical analyses of the Wrch atypical Rho family GTPases. Methods Enzymol. 2006;406:11–26. doi: 10.1016/S0076-6879(06)06002-2. [DOI] [PubMed] [Google Scholar]
- Silvestrini B, Palazzo G, De Gregorio M. Lonidamine and related compounds. Prog Med Chem. 1984;21:110–135. [PubMed] [Google Scholar]
- Simcha I, Shtutman M, Salomon D, Zhurinsky J, Sadot E, Geiger B, Ben-Ze’ev A. Differential nuclear translocation and transactivation potential of β-catenin and plakoglobin. J Cell Biol. 1998;141:1433–1448. doi: 10.1083/jcb.141.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin H, O’Donnell H, DasGupta R, Halford S, St Jore B, Puech A, Parimoo S, Morrow B, Skoultchi A, Weissman SM, et al. Identification of a new human catenin gene family member (ARVCF) from the region deleted in velo-cardio-facial syndrome. Genomics. 1997;41:75–83. doi: 10.1006/geno.1997.4627. [DOI] [PubMed] [Google Scholar]
- Siu MK, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004a;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- Siu MK, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin protein complex in the regulation of ectoplasmic specialization dynamics in the testis. Biol Reprod. 2004b;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- Siu MK, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1 integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Skop AR, Liu HZ, Yates J, III, Meyer BJ, Herald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- South AP, Wan H, Stone MG, Dopping-Hepenstal PJ, Purkis PE, Marshall JF, Leigh IM, Eady RA, Hart IR, McGrath JA. Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability. J Cell Sci. 2003;116:3303–3314. doi: 10.1242/jcs.00636. [DOI] [PubMed] [Google Scholar]
- Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Parton RG, Yap AS. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci. 2006;119:1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr Opin Cell Biol. 1999;11:554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Steinberger E. Hormonal control of mammalian spermatogenesis. Physiol Rev. 1971;51:1–22. doi: 10.1152/physrev.1971.51.1.1. [DOI] [PubMed] [Google Scholar]
- Stephens RH, O’Neill CA, Bennett J, Humphrey M, Henry B, Rowland M, Warhurst G. Resolution of P-glycoprotein effects on drug permeability using intestinal tissues from mdr1a−/− mice. Br J Pharmacol. 2002;135:2038–2046. doi: 10.1038/sj.bjp.0704668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- Stoka V, Turk V, Turk B. Lysosomal cysteine cathepsins: signaling pathways in apoptosis. Biol Chem. 2007;388:555–560. doi: 10.1515/BC.2007.064. [DOI] [PubMed] [Google Scholar]
- Stoker AW. Protein tyrosine phosphatases and signaling. J Endocrinol. 2005;185:19–33. doi: 10.1677/joe.1.06069. [DOI] [PubMed] [Google Scholar]
- Stork PJ. Does rap1 deserve a bad Rap? Trends Biochem Sci. 2003;28:267–275. doi: 10.1016/S0968-0004(03)00087-2. [DOI] [PubMed] [Google Scholar]
- Strumane K, Bonnomet A, Stove C, Vandenbroucke R, Nawrocki-Raby B, Bruyneel E, Mareel M, Birembaut P, Berx G, Van Roy F. E-cadherin regulates human Nanos1, which interacts with p120ctn and induces tumor cell migration and invasion. Cancer Res. 2006;66:10007–10015. doi: 10.1158/0008-5472.CAN-05-3096. [DOI] [PubMed] [Google Scholar]
- Surace EI, Strickland A, Hess RA, Gutmann DH, Naughton CK. Tslc1 (nectin-like molecule-2) is essential for spermatozoa motility and male fertility. J Androl. 2006;27:816–825. doi: 10.2164/jandrol.106.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kadota N, Hara T, Nakagami Y, Izumi T, Takenawa T, Sabe H, Endo T. Meltrin α cytoplasmic domain interacts with SH3 domains of Src and Grb2 and is phosphorylated by v-Src. Oncogene. 2000;19:5842–5850. doi: 10.1038/sj.onc.1203986. [DOI] [PubMed] [Google Scholar]
- Svitkina TM, Verkhovsky AB, Borisy GG. Plectin side-arms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Suzuki K, Tsukatani Y. Induction of tyrosine phosphorylation and association of β-catenin with EGF receptor upon tryptic digestion of quiescent cells at confluence. Oncogene. 1997;15:71–78. doi: 10.1038/sj.onc.1201160. [DOI] [PubMed] [Google Scholar]
- Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, Takai Y. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 2003;278:5497–5500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Irie K, Hirota T, Sakisaka T, Nakanishi H, Takai Y. Ectodomain shedding of nectin-1α by SF/HGF and TPA in MDCK cells. Biochem Biophys Res Commun. 2002;299:472–478. doi: 10.1016/s0006-291x(02)02681-5. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Takeichi M. Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J Cell Biol. 2004;165:517–528. doi: 10.1083/jcb.200403006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya S, Yamamoto T, Kano K, Kawano Y, Iwamatsu A, Tsuchiya T, Tanaka K, Kanai-Azuma M, Wood SA, Mattick JS, et al. The Ras target AF-6 is a substrate of the fam deubiquitinating enzyme. J Cell Biol. 1998;142:1053–1062. doi: 10.1083/jcb.142.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J, Rai T, Tanaka Y, Takei Y, Nakata T, Hirasawa M, Kulkarni AB, Hirokawa N. The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat Cell Biol. 2005;7:474–482. doi: 10.1038/ncb1249. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120ctn from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda M, Kadokawa Y, Kurahashi H, Marunouchi T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76:130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- Tselepis C, Chidgey M, North A, Garrod D. Desmosomal adhesion inhibits invasive behavior. Proc Natl Acad Sci U S A. 1998;95:8064–8069. doi: 10.1073/pnas.95.14.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- Turner TT, Caplis L, Miller DW. Testicular microvascular blood-flow: alteration after Leydig cell eradication and ischemia but not experimental varicocele. J Androl. 1996;17:239–248. [PubMed] [Google Scholar]
- Uitto J, Richard G, McGrath JA. Diseases of epidermal keratins and their linker proteins. Exp Cell Res. 2007;313:1995–2009. doi: 10.1016/j.yexcr.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Vaid KS, Guttman JA, Singaraja RR, Vogl AW. A kinesin is present at unique Sertoli/spermatid adherens junctions in rat and mouse testes. Biol Reprod. 2007;77:1037–1048. doi: 10.1095/biolreprod.107.063735. [DOI] [PubMed] [Google Scholar]
- Van Hengel J, Vanhoenacker P, Staes K, Van Roy F. Nuclear localization of the p120ctn armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc Natl Acad Sci U S A. 1999;127:7980–7985. doi: 10.1073/pnas.96.14.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vávrová K, Zbytovska J, Hrabalek A. Amphiphilic transdermal permeation enhancers: structure-activity relationships. Curr Med Chem. 2005;12:2273–2291. doi: 10.2174/0929867054864822. [DOI] [PubMed] [Google Scholar]
- Velichkova M, Guttman J, Warren C, Eng L, Kline K, Vogl AW, Hasson T. A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil Cytoskeleton. 2002;51:147–164. doi: 10.1002/cm.10025. [DOI] [PubMed] [Google Scholar]
- Vestal DJ, Ranscht B. Glycosyl phosphatidylinositol-anchored T-cadherin mediates calcium-dependent homophilic cell adhesion. J Cell Biol. 1992;119:451–461. doi: 10.1083/jcb.119.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367:235–241. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. BioEssays. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Redenbach DM, Grove BD. Sertoli cell cytoskeleton. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 39–86. [Google Scholar]
- Vogl AW, Soucy LJ. Arrangement and possible function of actin filament bundles in ectoplasmic specializations of ground squirrel Sertoli cells. J Cell Biol. 1985;100:814–825. doi: 10.1083/jcb.100.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu Hai MT, Lescop P, Loosfelt H, Ghinea H. Receptor-mediated transcytosis of FSH through the rat testicular microvasculature. Biol Cell. 2004;96:133–144. doi: 10.1016/j.biolcel.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Waibler Z, Schafer A, Starzinski-Powitz A. mARVCF cellular localisation and binding to cadherins is influenced by the cellular context but not by alternative splicing. J Cell Sci. 2001;114:3873–3884. doi: 10.1242/jcs.114.21.3873. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Koami H, Ariga H, Kobayashi D, Sai Y, Tsuji A, Yamamoto M, Iseki S. Expression and functional characterization of the adhesion molecule spermatogenic immunoglobulin superfamily in the mouse testis. Biol Reprod. 2003;68:1755–1763. doi: 10.1095/biolreprod.102.012344. [DOI] [PubMed] [Google Scholar]
- Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- Walsh SV, Hopkins AM, Nusrat A. Modulation of tight junction structure and function by cytokines. Adv Drug Deliv Rev. 2000;41:303–313. doi: 10.1016/s0169-409x(00)00048-x. [DOI] [PubMed] [Google Scholar]
- Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N, et al. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci. 2007;120:567–577. doi: 10.1242/jcs.03356. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, Van Roy F, Adamson ED, Takeichi M. α-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon WC, Salmon ED. Feedback interactions between cell-cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol Biol Cell. 2000;11:2471–2483. doi: 10.1091/mbc.11.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JE, Russell LD, Wong V, Paterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. Vinculin is part of the cadherin-catenin junctional complex: complex formation between α-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Kratzschmar J, Reid MS, Blobel CP. MDC9, a widely expressed cellular disintegrin containing cytoplasmic SH3 ligand domains. J Cell Biol. 1996;132:717–726. doi: 10.1083/jcb.132.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KJ. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Whittock NV, Bower C. Genetic evidence for a novel human desmosomal cadherin, desmoglein 4. J Invest Dermatol. 2003;120:523–530. doi: 10.1046/j.1523-1747.2003.12113.x. [DOI] [PubMed] [Google Scholar]
- Wiche G, Krepler R, Artlieb U, Pytela R, Denk H. Occurence and immunolocalization of plectin in tissues. J Cell Biol. 1983;97:887–901. doi: 10.1083/jcb.97.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnholds J, Scheffer GL, van der Valk M, van de Valk P, Beijnen JH, Scheper RJ, Borst P. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med. 1998;188:797–808. doi: 10.1084/jem.188.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild-Bode C, Fellerer K, Kugler J, Haass C, Capell A. A basolateral sorting signal directs ADAM10 to adherens junctions and is required for its function in cell migration. J Biol Chem. 2006;281:23824–23829. doi: 10.1074/jbc.M601542200. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie AO. Cancer drugs to treat birth defects. Nat Genet. 2007;39:1057–1059. doi: 10.1038/ng0907-1057. [DOI] [PubMed] [Google Scholar]
- Wine RN, Chapin RE. Adhesion and signaling proteins spatiotemporally associated with spermiation in the rat. J Androl. 1999;20:198–213. [PubMed] [Google Scholar]
- Witcher LL, Collins R, Puttagunta S, Mechanic SE, Munson M, Gumbiner BM, Cowin P. Desmosomal cadherin binding domains of plakoglobin. J Biol Chem. 1996;271:10904–10909. doi: 10.1074/jbc.271.18.10904. [DOI] [PubMed] [Google Scholar]
- Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14:461–469. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Wolf A, Keil R, Gotzl O, Mun A, Schwarze K, Lederer M, Huttelmaier S, Hatzfeld M. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat Cell Biol. 2006;8:1432–1440. doi: 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- Wolgemuth DJ, Lele KM, Jobanputra V, Salzar G. The A-type cyclins and the meiotic cell cycle in mammalian germ cells. Int J Androl. 2004;27:192–199. doi: 10.1111/j.1365-2605.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- Wolski KM, Mruk DD, Cameron DF. The Sertoli-spermatid junctional complex adhesion strength is affected in vitro by Adjudin. J Androl. 2006;27:790–794. doi: 10.2164/jandrol.106.000422. [DOI] [PubMed] [Google Scholar]
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- Wong CC, Chung SS, Grima J, Zhu LJ, Mruk D, Lee WM, Cheng CY. Changes in the expression of junctional and nonjunctional complex component genes when inter-Sertoli tight junctions are formed in vitro. J Androl. 2000;21:227–237. [PubMed] [Google Scholar]
- Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Wong CH, Xia W, Lee NP, Mruk DD, Lee WM, Cheng CY. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–1204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- Wong V, Russell LD. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am J Anat. 1983;167:143–161. doi: 10.1002/aja.1001670202. [DOI] [PubMed] [Google Scholar]
- Wu GS. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev. 2007;26:579–585. doi: 10.1007/s10555-007-9079-6. [DOI] [PubMed] [Google Scholar]
- Wu JC, Gregory CW, DePhilip RM. Expression of E-cadherin in immature rat and mouse testis and in rat Sertoli cell cultures. Biol Reprod. 1993;49:1353–1361. doi: 10.1095/biolreprod49.6.1353. [DOI] [PubMed] [Google Scholar]
- Xia W, Cheng CY. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: an in vivo study. Dev Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring during spermatogenesis - a lesson to learn from the testis. Cytokine Growth Factor Rev. 2005a;16:469–493. doi: 10.1016/j.cytogfr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006a;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Lee WM, Cheng CY. Unraveling the molecular targets pertinent to junction restructuring events during spermatogenesis using the Adjudin-induced germ cell depletion model. J Endocrinol. 2007;192:563–583. doi: 10.1677/JOE-06-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Wong CH, Lee NP, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model. J Cell Physiol. 2005b;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- Xia X, Carnahan RH, Vaughan MH, Wildenberg GA, Reynolds AB. p120 Serine and threonine phosphorylation is controlled by multiple ligand-receptor pathways but not cadherin ligation. Exp Cell Res. 2006b;312:3336–3348. doi: 10.1016/j.yexcr.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta. 2007;1773:8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Xu G, Craig AW, Greer P, Miller M, Anastasiadis PZ, Lilien J, Balsamo J. Continuous association of cadherin with β-catenin requires the non-receptor tyrosine-kinase Fer. J Cell Sci. 2004;117:3207–3219. doi: 10.1242/jcs.01174. [DOI] [PubMed] [Google Scholar]
- Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. N-cadherin and Cx43α1 gap junctions modulates mouse neural crest cell motility via distinct pathways. Cell Commun Adhes. 2001;8:321–324. doi: 10.3109/15419060109080746. [DOI] [PubMed] [Google Scholar]
- Yajnik V, Paulding C, Sordella R, McClatchey AI, Saito M, Wahrer DC, Reynolds P, Bell DW, Lake R, van den Heuvel S, et al. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell. 2003;112:673–684. doi: 10.1016/s0092-8674(03)00155-7. [DOI] [PubMed] [Google Scholar]
- Yamada A, Fujita N, Sato T, Okamoto R, Ooshio T, Hirota T, Morimoto K, Irie K, Takai Y. Requirement of nectin, but not cadherin, for formation of claudinbased tight junctions in annexin II-knockdown MDCK cells. Oncogene. 2006a;25:5085–5102. doi: 10.1038/sj.onc.1209525. [DOI] [PubMed] [Google Scholar]
- Yamada A, Irie K, Hirota T, Ooshio T, Fukuhara A, Takai Y. Involvement of the annexin II-S100A10 complex in the formation of E-cadherin-based adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2005a;280:6016–6027. doi: 10.1074/jbc.M408215200. [DOI] [PubMed] [Google Scholar]
- Yamada D, Yoshida M, Williams YN, Fukami T, Kikuchi S, Masuda M, Maruyama T, Ohta T, Nakae D, Maekawa A, et al. Disruption of spermatogenic cell adhesion and male infertility in mice lacking TSLC1/IGSF4, an immunoglobulin superfamily cell adhesion molecule. Mol Cell Biol. 2006b;26:3610–3624. doi: 10.1128/MCB.26.9.3610-3624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005b;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci U S A. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1 integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008 doi: 10.1096/fj.06-070342. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004;279:9512–9521. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Tachibana K, Nakanishi H, Yamamoto Y, Irie K, Mandai K, Nagafuchi A, Monden M, Takai Y. α-Catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin. Mol Biol Cell. 2001;12:1595–1609. doi: 10.1091/mbc.12.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wong CH, Xia WJ, Mruk DD, Lee NP, Lee WM, Cheng CY. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-β-catenin protein complex, which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology. 2005;146:1268–1284. doi: 10.1210/en.2004-1194. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Horiguchi Y, Ueda M, Yoshiki T, Imamura S. 1-2B7B: monoclonal antibody reacting to the 120 kDa polypeptide component of human epidermal hemidesmosomes. Arch Dermatol Res. 1991;283:310–316. doi: 10.1007/BF00376619. [DOI] [PubMed] [Google Scholar]
- Zhang ZY. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol. 2002;42:209–234. doi: 10.1146/annurev.pharmtox.42.083001.144616. [DOI] [PubMed] [Google Scholar]
- Zhu GZ, Lin Y, Myles DG, Primakoff P. Identification of four novel ADAMs with potential roles in spermatogenesis and fertilization. Gene. 1999;234:227–237. doi: 10.1016/s0378-1119(99)00208-5. [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze’ev A. Plakoglobin and β-catenin: protein interactions, regulation, and biological roles. J Cell Sci. 2000;113:3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Zondag GC, Moolenaar WH. Receptor protein tyrosine phosphatases: involvement of cell-cell interactions and signaling. Biochimie. 1997;79:477–483. doi: 10.1016/s0300-9084(97)82739-3. [DOI] [PubMed] [Google Scholar]