Leptin regulates serotonin synthesis by brainstem neurons in adult mice; serotonin then acts on arcuate neurons to inhibit food intake via Creb.
Abstract
Recent evidence indicates that leptin regulates appetite and energy expenditure, at least in part by inhibiting serotonin synthesis and release from brainstem neurons. To demonstrate that this pathway works postnatally, we used a conditional, brainstem-specific mouse CreERT2 driver to show that leptin signals in brainstem neurons after birth to decrease appetite by inhibiting serotonin synthesis. Cell-specific gene deletion experiments and intracerebroventricular leptin infusions reveal that serotonin signals in arcuate nuclei of the hypothalamus through the Htr1a receptor to favor food intake and that this serotonin function requires the expression of Creb, which regulates the expression of several genes affecting appetite. Accordingly, a specific antagonist of the Htr1a receptor decreases food intake in leptin-deficient but not in Htr1a−/− mice. Collectively, these results establish that leptin inhibition of serotonin is necessary to inhibit appetite postnatally and provide a proof of principle that selective inhibition of this pathway may be a viable option to treat appetite disorders.
Leptin is a vertebrate-specific, adipocyte-derived hormone regulating, among other functions, appetite and energy expenditure after its signaling in the brain (Flier and Elmquist, 1997; Friedman and Halaas, 1998; Spiegelman and Flier, 2001). Numerous studies in the last 16 yr aimed at drawing a precise map of the circuitry used by leptin signaling in the brain to fulfill its functions (Elmquist et al., 1997; Takeda et al., 2002; Balthasar et al., 2004, 2005; Gao and Horvath, 2008; Scott et al., 2009; Yadav et al., 2009; Grill, 2010). After the lead provided by chemical lesion experiments and expression studies of the leptin receptor, the majority of these studies assumed that leptin signals in hypothalamic neurons to regulate appetite and energy expenditure (Elmquist, 2000; Elmquist et al., 2005; Coll et al., 2007). Surprisingly, however, cell-specific deletion experiments of the leptin receptor in various hypothalamic neurons have failed to affect appetite or energy expenditure in mice fed a normal chow, which is something leptin deficiency achieves (Balthasar et al., 2004; Dhillon et al., 2006). These data raised the hypothesis that leptin may act elsewhere in the brain to affect appetite. Consistent with this hypothesis, a combination of cellular, electrophysiological, and genetic experiments revealed that leptin inhibits appetite in mice by decreasing the synthesis in and release from brainstem neurons of serotonin, which, after its binding to the Htr1a and Htr2b receptors, favors appetite (Srinivasan et al., 2008; Yadav et al., 2009). Thus, providing an explanation for the lack of effect on appetite of the cell-specific deletion of the leptin receptor in hypothalamic neurons, these experiments indicated that leptin acts outside the hypothalamus, in the brainstem, as an inhibitor of the synthesis of an activator of appetite, serotonin.
The prominent role ascribed to brain serotonin in this model of leptin regulation of appetite raises several questions. First, because it is based, in part, on cell-specific gene deletion of the leptin receptor, it needs to be verified through the use of a Cre driver whose activity is even more exquisitely limited to serotonergic neurons of the brainstem. Second (and this is a more important issue), one needs to demonstrate that this regulatory loop takes place during adult life. Third, if indeed leptin inhibits appetite by preventing synthesis and release of serotonin, one needs to identify the neurons on which serotonin acts and to decipher, at least in part, the transcriptional events elicited by serotonin in these neurons. Lastly, an implication of this model is that pharmacologically inhibiting serotonin signaling should affect appetite in mice lacking leptin. Testing this latter issue is critical not only to formally demonstrate that leptin inhibits appetite by inhibiting serotonin synthesis but also because it could pave the way for novel therapeutic avenues to treat obesity.
This study addressed these four issues. Specifically, we provide additional evidence, based on the use of a tamoxifen-inducible CreERT2 driver whose expression is restricted to Tph2-expressing neurons, that leptin signaling in brainstem neurons does affect appetite postnatally. We show that it is through its expression in arcuate neurons of the hypothalamus that the Htr1a receptor favors appetite and that it does so by recruiting the transcription factor CREB. Lastly, using a pharmacological approach, we demonstrate that a specific antagonist of Htr1a decreases food intake in leptin-deficient mice and weakens their obesity.
RESULTS
Characterization of an inducible Tph2-CreERT2 mouse model
To establish that leptin signals in brainstem neurons to control appetite in mice fed a normal chow and that this pathway is operating after birth, we used mice in which the conditional CreERT2 was inserted at the ATG in a bacterial artificial chromosome clone containing the entire mouse Tph2, the gene encoding the initial enzyme in brainstem serotonin synthesis. In this construct, expression of the Cre recombinase should be under the control of Tph2-regulating elements and therefore expressed in serotonergic neurons of the brainstem only (Fig. 1 A). To ascertain the cell-specific activity of the regulatory elements contained in this bacterial artificial chromosome, we crossed Tph2-CreERT2 transgenic mice with Rosa26R mice (Soriano 1999). In this mouse model, the β-Galactosidase reporter gene, containing a floxed transcriptional blocker cassette inserted between the transcription start site and the ATG, is placed downstream of the Rosa26 promoter. Thus, β-galactosidase can only be expressed after Cre-mediated deletion of the transcriptional blocking cassette. After treatment of 6-wk-old mice with tamoxifen (1 mg/20 g body weight [BW] successively over 5 d), β-galactosidase staining showed that this construct drives gene expression in serotonergic neurons of the brainstem (stained in blue) but not in hypothalamic or any other neurons (Fig. S1). Thus, phenotypes developed by mutant mice generated using this Tph2-CreERT2 transgene should be explained by the lack of expression of this gene in serotonergic neurons.
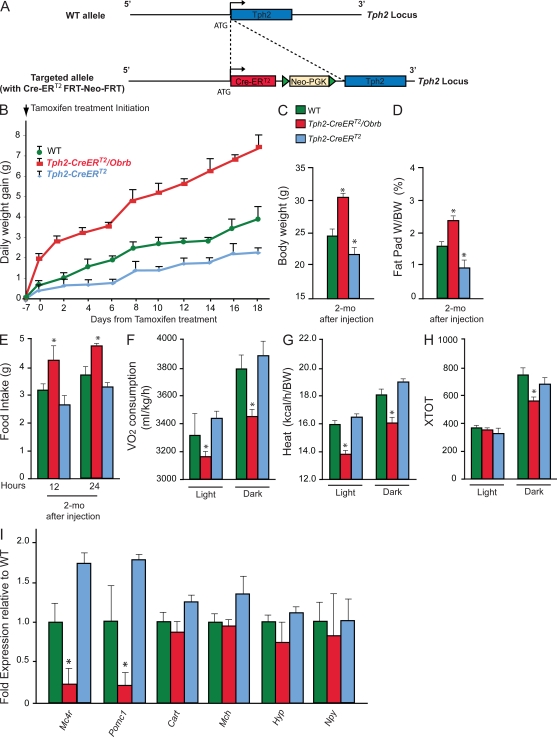
Figure 1.
Leptin signaling in serotonergic neurons of the raphe nuclei. (A) Schematic drawing of the Tph2-CreERT2 construct. CreERT2 cassette was placed under the control of Tph2 regulatory elements by insertion at the ATG of the Tph2 locus. (B) Measurement of daily weight gain (grams) over 3 wk in WT, Tph2-CreERT2, and Tph2-CreERT2 Obrb mice (n = 3 for each group). The measurement started 7 d after the first tamoxifen injection. (C–H) BW (grams; n = 3 for each group; C), fat pad weight (fat pad weight/BW; n = 3 for each group; D), food intake (grams/day; n = 3 for each group; E), and (F–H) energy expenditure analysis in WT, Tph2-CreERT2, and Tph2-CreERT2 Obrb mice 2 mo after tamoxifen injection (n = 3 for each group). Volume of oxygen consumption VO2 (milliliters/kilogram/hour; F), heat production (kilocalories/hour/BW; G), and locomotor activity (XTOT; H) are shown. (I) Quantitative PCR analysis of Mc4r, Pomc-1, Cart (Cocaine and amphetamine regulated transcript), Mch (Melanin-concentrating hormone receptor 1), and Hyp (hypocretin) in WT (n = 4), Tph2-CreERT2 (n = 3), and Tph2-CreERT2Obrb (n = 4) hypothalami 2 mo after tamoxifen injection. Data shown are representative of two independent experiments. For all experiments: *, P < 0.05 (Student’s t test). Error bars represent SEM.
Postnatal deletion of the leptin receptor in serotonergic neurons affects appetite and energy expenditure
We next used this Tph2-CreERT2 transgenic mouse to delete Obrb in serotonergic neurons of the brainstem after birth. For that purpose, we performed daily tamoxifen (1 mg/20 g BW) injections over 5 d in 6-wk-old Tph2-CreERT2 and Tph2-CreERT2; Obrbf/f. Tph2-CreERT2; Obrbf/f mice gained significantly more weight than control littermates (Fig. 1 B). 6 wk after the end of this tamoxifen treatment, appetite (+32%), BW (+33%), and fat pad weight (+35%) were significantly increased, whereas energy expenditure was significantly decreased in Tph2-CreERT2; Obrbf/f mice compared with WT or Tph2-CreERT2 mice that were heterozygous for Tph2 inactivation and therefore mildly anorexic (Fig. 1, C–H; Yadav et al., 2009). To demonstrate molecularly that the deletion of Obrb selectively in Tph2-expressing neurons after birth could affect appetite, we measured expression of genes such as pro-opiomelanocortin-α (Pomc-1) and melanocortin receptor 4 (Mc4r) that contribute to the regulation of appetite (Huszar et al., 1997; Cowley et al., 2001; Balthasar et al., 2005; Coll et al., 2007) and whose expression in the hypothalamus is decreased by the absence of leptin signaling in serotonergic neurons (Coll et al., 2007; Yadav et al., 2009). Consistent with their decreased appetite, expression of Pomc-1 and Mc4r was increased in hypothalami of Tph2-Cre; Obrbf/f mice (Fig. 1 I). Collectively, these results indicate that leptin signaling in serotonergic neurons of the brainstem is necessary to regulate appetite and energy expenditure in adult mice.
Ablation of Htr1a and Htr2b in Pomc-expressing neurons of the arcuate nuclei decreases appetite
Next we asked where in the brain serotonin signals through the Htr1a receptor to regulate appetite. Because neurons of the arcuate nuclei of the hypothalamus are implicated in the regulation of appetite and energy expenditure (Cowley et al., 2001; Pinto et al., 2004; Balthasar et al., 2005; Coll et al., 2007), we asked whether it is through its expression in these neurons that the Htr1a receptor regulates appetite.
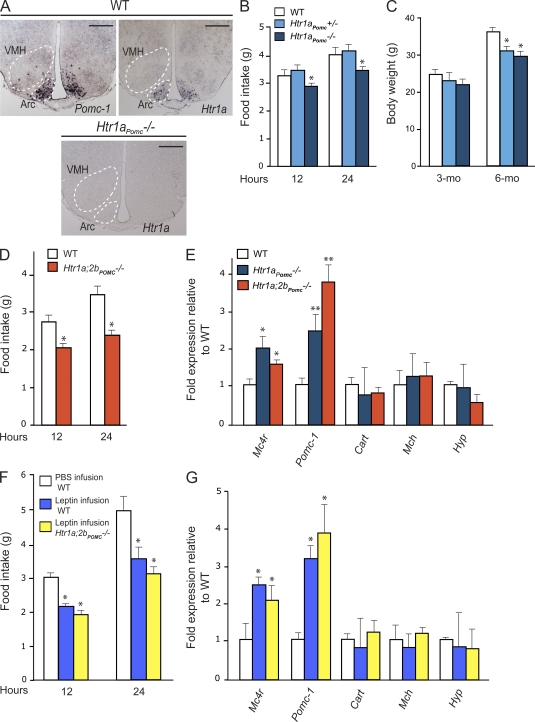
To address this question, we crossed mice harboring a floxed allele of Htr1a with Pomc-Cre transgenic mice that express Cre in Pomc-expressing neurons of the arcuate nuclei (Balthasar et al., 2004). In situ hybridization analysis verified that Htr1a expression in arcuate neurons was completely ablated in Htr1aPomc−/− mice (Fig. 2 A). We should point out that Pomc1 is also expressed in the pituitary gland and caudal brainstem (Salbert et al., 1992). 3-mo-old Htr1aPomc−/− mice demonstrated a significant reduction in food intake (−19%) that led to a significant decrease in BW (−20%; Fig. 2, B and C). As Htr2b, another serotonin receptor affecting appetite, is also expressed in Pomc-expressing neurons (Yadav et al., 2009), we generated mutant mice lacking both Htr1a and Htr2b in Pomc-expressing neurons (Htr1a;2bPomc−/− mice). In these double mutant mice, appetite was significantly lower (−36%) than a mere additive effect of the two mutations would have predicted (Fig. 2 D). Moreover, we observed an up-regulation of Pomc-1 and Mc4r expression in Htr1aPomc−/− and Htr1a;2bPomc−/− mice hypothalami, providing a molecular mechanism whereby the deletion of Htr1a and of Htr1a and Htr2b from Pomc-expressing neurons could affect appetite (Fig. 2 E). Collectively, these results establish that serotonin signals in Pomc-expressing neurons through Htr1a and Htr2b to favor appetite.
Figure 2.
Brain-derived serotonin regulates appetite through the Htr1a and Htr2b receptors expressed in arcuate neurons. (A) Expression analysis of Htr1a in WT and Htr1aPomc−/− hypothalamus by in situ hybridization compared with Pomc-1 in arcuate (Arc) nuclei (outlined by dashed line; experiment was performed using three different brains for each group). Bars, 500 µm. (B) Measurement of food intake (grams) over 12 and 24 h in WT, Htr1aPomc+/−, and Htr1aPomc−/− mice (n = 6 for each group). (C) BW (grams) analysis in WT, Htr1aPomc+/−, and Htr1aPomc−/− mice at 3 and 6 mo of age (n = 6 for each group). (D) Measurement of food intake (grams) over 12 and 24 h in WT (n = 6) and Htr1a;2bPomc−/− (n = 4) mice. (E) Quantitative PCR analysis of Mc4r, Pomc-1, Cart (Cocaine and amphetamine regulated transcript), Mch (Melanin-concentrating hormone receptor 1), and Hyp (hypocretin) in WT (n = 8), Htr1aPomc−/− (n = 4), and Htr1a;2bPomc−/− (n = 4) hypothalami. Data shown are representative of three independent experiments. (F and G) Measurement of food intake (grams) over 12 and 24 h in WT (F) and quantitative PCR analysis of gene controlling appetite in WT mice infused with vehicle (n = 6) and WT (n = 6) and Htr1a;2bPomc−/− (n = 6) mice infused by leptin (4 ng/h) for 7 d (G). Data shown are representative of two independent experiments. For all experiments: *, P < 0.05; **, P < 0.01 (Student’s t test). Error bars represent SEM.
Leptin anorexigenic function requires serotonin signaling in arcuate neurons of the hypothalamus
Is serotonin signaling in neurons of the arcuate nuclei necessary for leptin regulation of appetite? If this is the case, leptin should not affect appetite in mice lacking serotonin signaling in hypothalamic neurons. To address this question, we infused leptin (4 ng/h) in the third ventricle of WT and Htr1a;2bPomc−/− for 10 d and then measured food intake and expression of genes affecting appetite. We did not observe any difference in Mc4r and Pomc-1 gene expression and food intake in Htr1a;2bPomc−/− hypothalami after leptin infusion (+32%) compared with untreated Htr1a;2bPomc−/− (+36%; Fig. 2, F and G). These data confirmed that leptin acts as an inhibitor of serotonin signaling in arcuate neurons of the hypothalamus to inhibit food intake.
Creb expression in arcuate neurons of the hypothalamus is necessary for serotonin regulation of appetite
Htr1a is a Gs protein–coupled receptor signaling through the cAMP-PKA–dependent pathway. The main transcription factor downstream of this pathway is CREB, which mediates other homeostatic functions of serotonin (Yadav et al., 2008; Oury et al., 2010). Thus, we asked whether CREB, through its expression in neurons of the arcuate nuclei, was also involved in serotonin’s regulation of appetite.
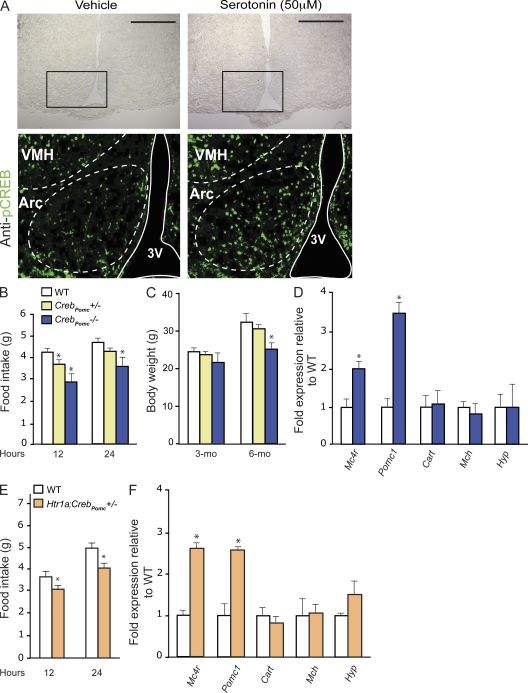
To address this question, we first used hypothalamic explant cultures. Immunofluorescence of phosphorylated CREB (p-CREB) showed that serotonin treatment of these explants increased CREB phosphorylation in arcuate neurons (Fig. 3 A). To establish in vivo that CREB mediates serotonin regulation of appetite through its expression in arcuate neurons, we generated mice lacking Creb in Pomc-expressing neurons (CrebPomc−/− mice). CrebPomc−/− mice showed a significant reduction in food intake (−31%), BW (−20%), and normal energy expenditure (Fig. 3, B and C; and Fig. S2). Furthermore, expression of genes inhibiting food intake such as Mc4r and Pomc-1 was significantly increased in CrebPomc−/− hypothalami (Fig. 3 D). These data indicate that CREB signaling in the Pomc-expressing neurons regulates food intake in part by favoring Mc4r and Pomc-1 expression.
Figure 3.
Creb expression in arcuate neurons is necessary for brain-derived serotonin regulation of appetite. (A) Analysis of CREB phosphorylation by immunofluorescence using p-CREB (S133) antibody. Immunofluorescence was performed on coronal sections of WT hypothalamic explants previously treated with vehicle or 50 µM serotonin for 30 min (n = 5 each). Brightfield images of hypothalamic sections (top) are shown; the black boxes delimit the frame for the immunofluorescence analysis shown below. Arcuate nuclei (Arc) and the third ventricle (3V) are outlined by dashed and solid lines, respectively. Data shown are representative of three independent experiments. VMH, ventromedial hypothalamus nucleus. Bars, 500 µm. (B and C) Measurement of food intake (grams) for a period of 12 and 24 h (B) and BW (grams) in 3- and 6-mo-old WT, CrebPomc+/−, and CrebPomc−/− mice (n = 6 for each group; C). Figures show combined data from two independent experiments. (D) Quantitative PCR analysis of Mc4r, Pomc-1, Cart (Cocaine and amphetamine regulated transcript), Mhc (Melanin-concentrating hormone receptor 1), and Hyp (hypocretin) in WT and CrebPomc−/− hypothalami (n = 6 for each group). Data shown are representative of two independent experiments. (E) Measurement of food intake (grams) over a period of 12 and 24 h in WT (n = 4) and Htr1a;CrebPomc+/− (n = 5) mice. (F) Quantitative PCR analysis of Mc4r, Pomc-1, Cart (Cocaine and amphetamine regulated transcript), Mhc (Melanin-concentrating hormone receptor 1), and Hyp (hypocretin) in WT (n = 4) and Htr1a;CrebPomc−/− (n = 5) hypothalami. Data shown are representative of two independent experiments. For all experiments: *, P < 0.05 (Student’s t test). Error bars represent SEM.
To establish that this function of CREB occurs after serotonin signaling in arcuate neurons, we generated compound heterozygous mice lacking one copy of Creb and one copy of Htr1a in Pomc-expressing neurons of the arcuate nuclei. These mice also showed decrease of appetite (−23% after 24 h), increase of Mc4r and Pomc-1 expression, and normal energy expenditure (Fig. 3, E and F; and Fig. S3), thereby providing strong support to the hypothesis that CREB is a transcriptional effector of serotonin’s regulation of appetite.
CREB regulates expression of genes involved in the control of appetite in arcuate neurons
Next we asked whether CREB regulates other genes besides Mc4r and Pomc-1 that could account for the orexigenic function of serotonin. To that end, we isolated hypothalami of WT and CrebPomc−/− mice and performed a microarray experiment.
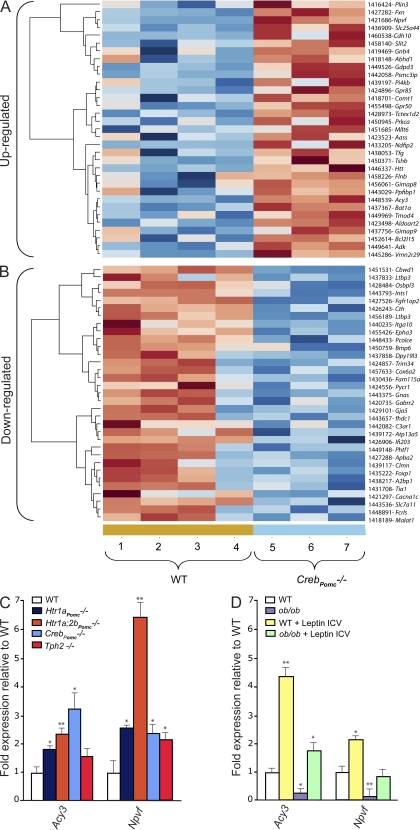
Among large numbers of genes whose expression was altered by the absence of Creb in Pomc-expressing neurons (Fig. 4, A and B), we focused on two genes whose expression was up-regulated in CrebPomc−/− hypothalami. Those are neuropeptide VF (Npvf), which has been shown to induce satiety (Cline et al., 2008; Cline and Sliwa, 2009), and aspartoacyclase (aminocyclase 3 [Acy3]; Gordon, 2001; Velinov et al., 2008; Caliebe et al., 2010), which encodes an enzyme that is inactivated in patients with aspartoacylase deficiency that have increased appetite. One and four canonical binding sites for CREB are present in the Acy3 and Npvf promoter, respectively (Fig. S4). We verified by real-time PCR that expression of these two genes was increased in CrebPomc−/− hypothalami (Fig. 4, C and D). Adding further evidence that these genes are downstream effectors of the serotonin orexigenic function, we also observed an increase of their expression in hypothalami of Tph2−/−, Htr1aPomc−/−; Htr1a;2bPomc−/−, and CrebPomc−/− mice (Fig. 4, C and D). Moreover, the fact that the expression of Npvf and Acy3 was strongly decreased in ob/ob hypothalami, whereas acute leptin intracerebroventricular injection in these mice increased their expression, demonstrates that Npvf and Acy3 are downstream of leptin signaling in the brain. Collectively, these data establish that under the control of leptin and serotonin, CREB transcriptional activity in arcuate neurons affects the expression of genes that have been shown to modulate appetite.
Figure 4.
Microarray experiment comparing the expression of genes in hypothalami of WT mice and of mice lacking Creb only in arcuate neurons. (A and B) Heat map of the microarray analysis of three WT hypothalami versus hypothalami of four mice lacking Creb only in arcuate neurons (CrebPomc−/− mice). Genes significantly (P < 0.05) up (A)- or down-regulated (B) in the hypothalamus of CrebPomc−/− mice compared with WT littermates are shown. These genes are ordered by fold change from maximum to the threshold of 1.20. The genes down- or up-regulated are listed in the last column. (C) Quantitative PCR analysis of Acy3 and Npvf expression in hypothalami of WT (n = 8), Htr1a Pomc−/− (n = 6), Htr1a;2bPomc−/− (n = 6), CrebPomc−/− (n = 6), and Tph2−/− (n = 5) mice at 12 wk of age. Data shown are representative of three independent experiments. (D) Quantitative PCR analysis of Acy3 and Npvf expression in the hypothalami of WT and ob/ob mice after acute intracerebroventricular (ICV) injection with vehicle or 2 µg leptin (WT, n = 4; ob/ob, n = 6; WT + leptin ICV, n = 4; and ob/ob + leptin ICV, n = 4). Figure shows combined data from two independent experiments. For all experiments: *, P < 0.05; **, P < 0.01 (Student’s t test). Error bars represent SEM.
Pharmacological targeting of Htr1a receptor decreases appetite in mice
If serotonin favors appetite through the Htr1a receptor, inhibition of serotonin signaling through this receptor should decrease appetite in WT mice. Moreover, if leptin inhibits appetite by decreasing serotonin synthesis and release, this compound should decrease appetite in ob/ob mice that are leptin deficient. This last point is needed to validate the notion that leptin decreases appetite by inhibiting serotonin signaling in the hypothalamus and to show that, if achievable, inhibition of serotonin signaling could be an alternative strategy in the treatment of obesity.
To address these questions, we used a small molecule antagonizing serotonin signaling through the Htr1a receptor only because we have been unable so far to obtain a small molecule antagonizing signaling through Htr2b. This molecule (LY426965) has high affinity for the Htr1a receptor (Ki = 4.66 nM) and 20-fold or greater selectivity over other serotonin and nonserotonin receptor subtypes (Fig. 5 A; Rasmussen et al., 2000). That appetite and Mc4r and Pomc-1 expression were not decreased in Htr1aPomc−/− mice treated with LY426965 strongly suggests that this compound acts as a specific inhibitor of Htr1a signaling (Fig. S5).
Figure 5.
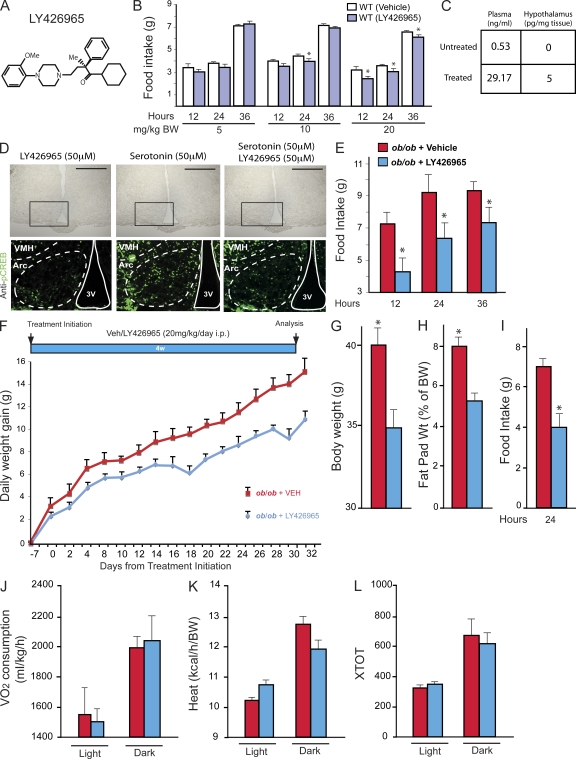
Pharmacological targeting of Htr1a receptors in mice. (A) Organic structure of the Htr1a antagonist LY426965. (B) Measurement of food intake (grams) over a period of 12, 24, and 36 h after single i.p. injection of 5, 10, or 20 mg/kg BW of vehicle or LY426965 compound in 12-wk-old WT mice (n = 6 for each group). Figure shows combined data from two independent experiments. (C) Measurement by mass spectrometry of LY426965 compound concentration in plasma (nanograms/milliliter) and hypothalamus (picograms/milligrams of tissue) of untreated and treated mice (n = 4 for each group). (D) Analysis of CREB phosphorylation by immunofluorescence using p-CREB (S133) antibody. Immunofluorescence was performed on coronal sections of WT hypothalamic explants previously treated with LY426965 compound, 50 µM serotonin, or LY426965 compound + 50 µM serotonin for 30 min. Brightfield images of hypothalamic sections (top) are shown; the black boxes delimit the frame for the immunofluorescence analysis shown below. Arcuate nuclei (Arc) and the third ventricle (3V) are outlined by dashed and solid lines, respectively (data shown are representative of four independent experiments). VMH, ventromedial hypothalamus nucleus. Bars, 500 µm. (E) Measurement of food intake (grams) over a period of 12, 24, and 36 h after single i.p. injection of 20 mg/kg BW of vehicle or LY426965 compound in 12-wk-old ob/ob mice (ob/ob + vehicle, n = 6; ob/ob + LY426965, n = 6). Data shown are representative of two independent experiments. (F) Daily weight gain (grams) after daily i.p. injection of vehicle or LY426965 compound (20 mg/kg/d). Measurement started 7 d after the first i.p. injection (ob/ob + vehicle, n = 3; ob/ob + LY426965, n = 5). (G–I) BW (grams; G), fat pad weight (percentage of BW; H), and food intake (grams; I) over a period of 24 h in ob/ob mice after 4 wk of daily injection with vehicle or LY426965 compound (20 mg/kg/d; ob/ob + vehicle, n = 3; ob/ob + LY426965, n = 5). Data shown are representative of two independent experiments. (J–L) Energy expenditure analysis after 4 wk of i.p. daily injection with vehicle or LY426965 compound (20 mg/kg/d) in ob/ob mice (ob/ob + vehicle, n = 3; ob/ob + LY426965, n = 3). Volume of oxygen consumption VO2 (milliliter/kilogram/hour; J), heat production (kilocalorie/hour/BW; K), and locomotor activity (XTOT; L) are shown. For all experiments: *, P < 0.05 (Student’s t test). Error bars represent SEM.
To evaluate LY426965 efficacy in inhibiting signaling through Htr1a receptors in the regulation of appetite, we fed 12-wk-old C57BL/6J mice with either vehicle or LY426965 at doses ranging from 1 to 20 mg/kg BW and observed a dose-dependent decrease in appetite (Fig. 5 B). This decrease in food intake reached 77% of the control values when using 20 mg/kg of the compound (Fig. 5 B). We verified that LY426965 could reach the hypothalamus by performing mass spectrometric analysis of mice hypothalami after i.p. administration of the compound. We could detect, in treated mice, LY426865 in hypothalamic tissue at a concentration of 5 pg/mg when exposed to a plasma concentration of ∼29.2 ng/ml (Fig. 5 C).
To demonstrate that LY426865 can act on arcuate neurons of the hypothalamus, we treated hypothalamic explants obtained from WT mice with either vehicle or 50 µM LY426865 in the presence or absence of 50 µM serotonin for 30 min. Immunofluorescence showed an increase of phosphorylation of CREB in arcuate neurons of the hypothalamus after treatment with serotonin, whereas CREB phosphorylation did not change when LY426865 alone was added to the medium (Fig. 5 D).
To determine whether LY426965 could decrease appetite in ob/ob mice, we administered 4-wk-old ob/ob mice with a single dose of LY426965 (0.4 mg/20 g BW) and measured food intake 12, 24, and 36 h later. Food intake in LY426965-treated animals was 20–25% lower than in vehicle-treated mice at all time points analyzed, demonstrating that inhibition of signaling through Htr1a in ob/ob mice can reduce appetite (Fig. 5 E). That this compound did not fully rescue the appetite phenotype caused by the absence of leptin is consistent with the notion that serotonin also decreases appetite by signaling through Htr2b receptor. Given the success of this initial experiment, we then asked whether LY426965 could rescue, even partially, the hyperphagia of ob/ob mice if administered chronically. For that purpose, 4-wk-old ob/ob mice were administered daily with a 20-mg/kg BW dose of LY426965 for 4 wk. LY426965-mediated suppression of Htr1a receptor signaling significantly decreased the obesity phenotype of ob/ob mice (−27%), whereas energy expenditure was not changed (Fig. 5, G–L). This result is consistent with the notion that one mechanism whereby leptin inhibits appetite is by decreasing serotonin synthesis and release from brainstem neurons and signaling trough the Htr1a receptor (Yadav et al., 2009).
DISCUSSION
The idea that leptin may not act directly in the hypothalamus to regulate appetite and energy metabolism was surprising. As a result, this finding needed to be verified and, if possible, its therapeutic potential explored. In all experiments, our purpose was to study WT or mutant mice fed a normal chow. Our rationale for this choice was based on the fact that leptin anorexigenic function was discovered in mice fed this diet.
A first question, which is inherent to every cell-specific deletion experiment, is to rule out, as much as possible, that the effect observed is not caused by a spurious gene deletion in a cell type other than the one under study. To address this concern as thoroughly as possible, we used here a different Cre driver than the one we used initially (Yadav et al., 2009). Two tryptophan hydroxylase isoforms, Tph1 and Tph2, act as rate-limiting enzymes in serotonin synthesis. The expression of the two isoforms is mutually exclusive; Tph1 is abundantly expressed in the pineal gland and enterochromaffin cells, and Tph2 is specifically expressed in serotonergic brainstem neurons of the raphe nuclei. To drive Cre expression, we used here the regulatory elements of the Tph2 gene that encode the initial enzyme in the biosynthesis of serotonin in the brainstem. Thus, by definition, Cre should be expressed in serotonergic neurons of the brainstem, although Tph2 has been shown to be expressed in other parts of the brain (Walther and Bader, 2003; Patel et al., 2004). We note that this Tph2-CreERT2 driver mouse does not express Cre at a detectable level in any cell type other than brainstem neurons. That the use of this Cre driver to delete Obrb caused a severe hyperphagia phenotype provides more rigorous evidence supporting the notion that leptin signals in brainstem neurons to regulate appetite and energy expenditure. We remain aware that these results do not rule out the possibility that leptin may signal in additional neurons to inhibit appetite in mice fed a normal chow. Further experiments are required to identify such a neuronal population.
A second question generated by our previous results was to determine whether leptin uses this serotonin-dependent pathway during development only or also after birth (Yadav et al., 2009). To address this question, we were fortunate that the activity of the Tph2-CreERT2 driver we used here is inducible by tamoxifen. This allowed us to show that leptin uses this pathway in mice that are at least 6 wk old, thus ruling out that it is only active during embryonic or perinatal development. Together, these two sets of experiments, because of the exquisite specificity of expression of Cre and its postnatal activation, support the notion that leptin signals in brainstem neurons to regulate appetite and energy expenditure.
If we now look at how serotonin itself favors appetite, we first established that it occurs in arcuate neurons and that CREB is one important transcriptional mediator of this function, although we cannot rule out that others exists. A microarray analysis identified at least two genes besides Mc4r and Pomc-1: Npvf and Acy3, whose expression is up-regulated in absence of CREB or serotonin signaling or by leptin acute injection and is down-regulated in the absence of leptin signaling. Neuropeptide VF was recently described as a neuropeptide that induces hypothalamus associated-satiety in chicks (Cline et al., 2008; Cline and Sliwa, 2009), and this study strongly suggests for the first time that Npvf can also regulate appetite in mice. The second gene, Acy3, encodes an enzyme, aspartoacyclase, that is mutated in patients with an autosomal recessive disease called Canavan disease or aspartoacyclase disease, which is characterized by an increase in appetite. These two genes were not known to be affected by leptin signaling in the brain. We cannot exclude at the present time that CREB regulates the expression of other genes in arcuate neurons that are involved in the control of appetite. In that respect, we note that CREB affects the expression of γ-aminobutyric acid receptors or other genes implicated in the regulation of appetite (Kalra et al., 1999; Berthoud and Morrison, 2008; Dietrich and Horvath, 2009). In vivo experiments are now needed to determine the importance of these two genes in the regulation of appetite by serotonin and leptin.
The last point we wanted to explore was whether we could take advantage of the wealth of pharmacological information surrounding serotonin signaling in the brain to devise a strategy to decrease appetite in leptin-deficient mice. This would not only be a verification of the importance of serotonin downstream of leptin signaling in the brain but could also be viewed as a proof of principle experiment in the ongoing search for additional means to inhibit appetite in morbid obesity. To address this point, we could rely only on a small molecule inhibitor of Htr1a, one of the two serotonin receptors mediating the orexigenic function of serotonin. As a result, any positive results we could obtain would only be partial. This being properly acknowledged, our results indicate that inhibiting serotonin signaling in the brain decreases appetite in leptin-deficient mice. An implication of our results is that antagonist of serotonin signaling should not effect on the appetite of Pomc1−/− and Mc4r−/− mice.
Altogether, the experiments presented in this study (a) establish that inhibition of serotonin synthesis is a mechanism used by leptin to regulate appetite in mice, (b) identify additional leptin-sensitive genes involved in the regulation of appetite, and (c) provide an initial proof of principle that targeting serotonin in the hypothalamus is a viable option to treat hyperphagic diseases.
MATERIALS AND METHODS
Mice generation
To generate mice lacking Htr1a, Htr2b, and Creb in Pomc-expressing neurons, flox/+ mice were crossed with Pomc-Cre mice (provided by B. Lowell, Beth Israel Deaconess Medical Center, Boston, MA), and their progeny was intercrossed to obtain Htr1aPomc−/−, Htr2bPomc−/−, Htr1a;2bPomc−/−, and CrebPomc−/− mice. To generate mice lacking Obrb in serotonergic neurons of the brainstem after birth, Obrb flox/+ mice were crossed with Tph2-CreERT2; Obrb flox/+, and the progeny were injected daily with tamoxifen (1 mg/20 g BW) for 5 d at 6 wk of age. All of these mice were in the mixed genetic background as follows: Htr1aPomc−/−, Htr2bPomc−/−, and CrebPomc−/− mice (129sv: 75%; C57BL/6J: 25%), Htr1a;2bPomc−/− (129sv: 87.5%; C57BL/6J: 12.5%), and Tph2-CreERT2; Obrbf/f mice (129sv: 12.5%; C57BL/6J: 87.5%). WT C57BL/6J and ob/ob mice were obtained from The Jackson Laboratory. All experiments were conducted according to Columbia University Guidelines for the Animal Use and Care of laboratory mice. Mice were bred and maintained in our animal facility according to institutional guidelines with protocols approved by the Animal Studies Committee of Columbia University.
Molecular experiments
RNA isolation, cDNA preparation, and real-time PCR analysis were performed according to standard protocols using oligonucleotides from QIAGEN. Genotypes of all mice were determined by PCR.
Histology
Mice were anesthetized and perfused transcardially with ice-cold saline followed by 4% paraformaldehyde (PFA). Brains were dissected, fixed in 4% PFA overnight at 4°C, cryoprotected by overnight immersion in 20% sucrose, embedded in Shandon Cryomatrix (Thermo Fisher Scientific), and frozen at −80°C. 20-µm cryostat sections were cut in the coronal plane. In situ hybridization and β-galactosidase staining were performed according to standard procedures in Yadav et al. (2009).
Hypothalamic explants
Brains were dissected in cold artificial cerebrospinal fluid solution and left in the same solution for 30 min before being sectioned at 500 µm using a chopper at the level of the arcuate nuclei of the hypothalamus (from bregma −1.22 to −1.70 mm). The resulting slices were incubated in artificial cerebrospinal fluid for 1 h before treatment.
Immunofluorescence
Immunofluorescence analysis of CREB phosphorylation in hypothalamus was performed after 30-min treatment with PBS, 50 µM serotonin, or 50 µM serotonin + 50 µM LY426965 on 500-µm brain slices of WT mice. After treatment, brain slices were fixed in 4% PFA overnight at 4°C. 20-µm cryostat sections were cut in the coronal plane, blocked in donkey serum, and then incubated in p-CREB (S133) rabbit antibody (87G3; Cell Signaling Technology) for 24 h at 4°C. Sections were rinsed and incubated with a donkey anti–rabbit antibody (1:1,000; Cy2; Jackson ImmunoResearch Laboratories, Inc.).
Microarray analysis
RNA from micro-dissected WT and CrebPomc1−/− mice hypothalami were extracted using TRIZOL and analyzed using GeneChip Mouse Genome 430 2.0 Array (Affymetrix). Data analyses were performed by Precision Biomarker using Expression Console software (Affymetrix) and normalized by robust multi-array average expression measure. The criteria for increased or decreased gene expression were P < 0.05. The heat map is a false color image derived from the selected gene expression values with a dendrogram added to the left side and to the top. Dendrograms are based on hierarchical clustering. Vertical lines indicate the distance between samples (columns) or genes (rows). The color red indicates higher relative expression as compared with blue.
Intracerebroventricular leptin infusion or injection
Animals were anesthetized and placed on a stereotaxic instrument (model 51600; Stoelting). The skin covering the head was then cut, and the calvaria were exposed. A hole was drilled upon bregma using a 28-gauge needle. A 28-gauge needle cannula (brain infusion kit II; Alzet) was then implanted into the hole reaching the third cerebral ventricle according to the following coordinates: bregma, −0.3 anteroposterior; 3 mm ventral (0 point bregma). Leptin infusions were made according to (Ducy et al., 2000). For acute injections, PBS or 2 µg leptin was injected with a Hamilton syringe into the third cerebral ventricle. Brains were removed (after 4 h for the acute injection), and the hypothalamus was carefully dissected.
Experimental regimen for food intake measurement in WT and mutant animals
Animals were individually housed in metabolic cages under 12-h light/12-h dark conditions with ad libitum feeding. Measurements were performed after a minimum of 4 d of acclimatization to the housing conditions. Control and mutant mice were separated into individual cages 1 d before the experiment. Food consumption was determined by weighing the powdered chow every 12 h for 36 h.
Treatment with LY426965
Two different experimental regimens were used to assess the effect of LY426965 on appetite in WT and ob/ob mice.
Experiment I: acute dose response of LY426965 in WT mice.
3-mo-old C57BL/6J inbred female mice were used in these experiments. Animals were separated into individual cages 1 d before the experiment. LY426965 was dissolved in water and injected i.p. according to the BW of the mouse at doses of 1, 5, 10, and 20 mg/kg BW 2 h before the beginning of the dark cycle. Control animals received the same volume of vehicle. Food intake was measured every 12 h for 36 h after giving one dose of the antagonist.
Experiment II: chronic treatment of WT and ob/ob mice with LY426965.
1-mo-old WT and ob/ob mice were divided into different groups and injected i.p. with LY426965 (20 mg/kg BW) dissolved in water and diluted in saline (final concentration 0.9%) once a day, 2 h before the beginning of the dark cycle, for 4 wk. BW was recorded every day, and the dose of drug or vehicle was adjusted accordingly. At the end of the experiment, all mice were subjected to measurement of food intake every 12 h over a period of 36 h.
Physiological measurements
Oxygen consumption (VO2) and respiratory exchange ratio were measured by an indirect calorimetry method using a six-chamber Oxymax system (Columbus Instruments). Mice were individually housed in the chamber and fed ad libitum. After 30-h acclimation to the apparatus, data for 24-h measurement were collected and analyzed as recommended by the manufacturer.
Quantification of LY426965 in plasma and hypothalamic tissue
LY426965 was quantified using 200 µl of plasma or 15 mg of hypothalamic tissue. The tissue was homogenized in 100 µl of saline using a 1-ml dounce homogenizer. The homogenizer was rinsed with 100 µl saline and pooled with the homogenate. Proteins from plasma and hypothalamus homogenates were precipitated with 800 µl acetonitrile. The tubes were vortexed for 60 s. After centrifugation (6,000 g for 6 min), the supernatant was transferred to a Waters maximum recovery vial. The supernatant was evaporated with nitrogen and resolubilized with 200 µl of 50% methanol. 25 µl of each sample was injected onto a high-performance liquid chromatographer (Aliance 2795; Waters) with an Atlantis 2.1 × 50 mm C18 3-µm column (35°C; Waters) with the initial conditions 0.3 ml/min, 55% of 20 mM ammonium formate, 0.1% formic acid (solvent A), and 45% acetonitrile with 0.1% formic acid (solvent B). The initial conditions were held for 1 min. Solvent B was increased to 62% over the next 1.6 min and then to 80% over the next 3.4 min, after which time the system was re-equilibrated to the initial conditions for 2 min (total run time: 8 min).
The LY426965 was detected with a mass spectrometer (Micromass Quattro Micro; Waters) with positive electrospray ionization. LY426965 was quantified using multiple reactions monitoring of the +H ion with the transition 435.3 to 243.1. LY426965 spiked plasma was used to create a standard curve, which was linear from 1 to 250 ng/ml. Quantification of LY426965 of both plasma and hypothalamic tissue was calculated relative to the spiked plasma standard curve. The mass spectrometry conditions were as follows: capillary, 3.0 kv; cone, 50 V; source temperature, 120°C; desolvation temperature, 400°C; desolvation gas flow, 500 l/h; cone gas flow, 50 l/h; collision energy, 30 V.
Statistical analysis
Results are given as means ± standard deviations. Statistical analysis was performed by Student’s t test. In all panels in Figs. 1–5 and Figs. S1–S5: *, P < 0.05 versus WT or control.
Online supplemental material
Fig. S1 shows the specificity of an inducible Tph2-CreERT2 mouse model to drive the expression of the Cre recombinase selectively in serotonergic neurons of the brainstem. Fig. S2 shows that selective inactivation of Creb Pomc-expressing neurons of the arcuate neurons does not affect the energy expenditure. Fig. S3 shows that the selective inactivation of one copy of Creb and one copy of Htr1a in Pomc-expressing neurons of the arcuate nuclei does not affect the energy expenditure. Fig. S4 shows the canonical binding sites for CREB present in the Acy3 and Npvf regulatory elements. Fig. S5 shows that appetite and Mc4r and Pomc-1 expression were not decreased in Htr1aPomc−/− mice treated with LY426965. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101940/DC1.
Acknowledgments
We thank Dr. Bradford Lowell and Eli Lilly and Company for providing us with the Pomc1-Cre transgenic mice and the Htr1a antagonist LY426965, respectively.
This work was supported by grants from the March of Dimes foundation (#1-FY08-354; to G. Karsenty) and National Institutes of Health (to G. Karsenty and V.K. Yadav), a Clinical and Translation Science Award grant (UL1 RR024156-03), a Gideon and Sevgi Rodan Fellowship from the International Bone and Mineral Society (to V.K. Yadev), and a Human Frontier Science Program Fellowship (to F. Oury).
The authors have no conflicting financial interest.
Footnotes
Abbreviations used:
- BW
- body weight
- p-CREB
- phosphorylated CREB
- PFA
- paraformaldehyde
References
- Balthasar N., Coppari R., McMinn J., Liu S.M., Lee C.E., Tang V., Kenny C.D., McGovern R.A., Chua S.C., Jr, Elmquist J.K., Lowell B.B. 2004. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 42:983–991 10.1016/j.neuron.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T., Ferreira M., Tang V., McGovern R.A., Kenny C.D., et al. 2005. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 123:493–505 10.1016/j.cell.2005.08.035 [DOI] [PubMed] [Google Scholar]
- Berthoud H.R., Morrison C. 2008. The brain, appetite, and obesity. Annu. Rev. Psychol. 59:55–92 10.1146/annurev.psych.59.103006.093551 [DOI] [PubMed] [Google Scholar]
- Caliebe A., Vater I., Plendl H., Gesk S., Siebert R., Cremer F.W., Klein-Hitpass L. 2010. A 439 kb-sized homozygous deletion in 17p13.3 leading to biallelic loss of the ASPA as cause of Canavan disease detected by SNP-array analysis. Mol. Genet. Metab. 99:184–185 10.1016/j.ymgme.2009.10.011 [DOI] [PubMed] [Google Scholar]
- Cline M.A., Sliwa L.N. 2009. Neuropeptide VF-associated satiety involves mu and kappa but not delta subtypes of opioid receptors in chicks. Neurosci. Lett. 455:195–198 10.1016/j.neulet.2009.03.029 [DOI] [PubMed] [Google Scholar]
- Cline M.A., Bowden C.N., Calchary W.A., Layne J.E. 2008. Short-term anorexigenic effects of central neuropeptide VF are associated with hypothalamic changes in chicks. J. Neuroendocrinol. 20:971–977 10.1111/j.1365-2826.2008.01749.x [DOI] [PubMed] [Google Scholar]
- Coll A.P., Farooqi I.S., O’Rahilly S. 2007. The hormonal control of food intake. Cell. 129:251–262 10.1016/j.cell.2007.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley M.A., Smart J.L., Rubinstein M., Cerdán M.G., Diano S., Horvath T.L., Cone R.D., Low M.J. 2001. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 411:480–484 10.1038/35078085 [DOI] [PubMed] [Google Scholar]
- Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V., Kenny C.D., Christiansen L.M., White R.D., Edelstein E.A., et al. 2006. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 49:191–203 10.1016/j.neuron.2005.12.021 [DOI] [PubMed] [Google Scholar]
- Dietrich M.O., Horvath T.L. 2009. GABA keeps up an appetite for life. Cell. 137:1177–1179 10.1016/j.cell.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Ducy P., Amling M., Takeda S., Priemel M., Schilling A.F., Beil F.T., Shen J., Vinson C., Rueger J.M., Karsenty G. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 100:197–207 10.1016/S0092-8674(00)81558-5 [DOI] [PubMed] [Google Scholar]
- Elmquist J.K. 2000. Anatomic basis of leptin action in the hypothalamus. Front. Horm. Res. 26:21–41 10.1159/000061020 [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Ahima R.S., Maratos-Flier E., Flier J.S., Saper C.B. 1997. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 138:839–842 10.1210/en.138.2.839 [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Coppari R., Balthasar N., Ichinose M., Lowell B.B. 2005. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J. Comp. Neurol. 493:63–71 10.1002/cne.20786 [DOI] [PubMed] [Google Scholar]
- Flier J.S., Elmquist J.K. 1997. Energetic pursuit of leptin function. Nat. Biotechnol. 15:20–21 10.1038/nbt0197-20 [DOI] [PubMed] [Google Scholar]
- Friedman J.M., Halaas J.L. 1998. Leptin and the regulation of body weight in mammals. Nature. 395:763–770 10.1038/27376 [DOI] [PubMed] [Google Scholar]
- Gao Q., Horvath T.L. 2008. Neuronal control of energy homeostasis. FEBS Lett. 582:132–141 10.1016/j.febslet.2007.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N. 2001. Canavan disease: a review of recent developments. Eur. J. Paediatr. Neurol. 5:65–69 10.1053/ejpn.2001.0467 [DOI] [PubMed] [Google Scholar]
- Grill H.J. 2010. Leptin and the systems neuroscience of meal size control. Front. Neuroendocrinol. 31:61–78 10.1016/j.yfrne.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R., Gu W., Kesterson R.A., Boston B.A., Cone R.D., et al. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 88:131–141 10.1016/S0092-8674(00)81865-6 [DOI] [PubMed] [Google Scholar]
- Kalra S.P., Dube M.G., Pu S., Xu B., Horvath T.L., Kalra P.S. 1999. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20:68–100 10.1210/er.20.1.68 [DOI] [PubMed] [Google Scholar]
- Oury F., Yadav V.K., Wang Y., Zhou B., Liu X.S., Guo X.E., Tecott L.H., Schutz G., Means A.R., Karsenty G. 2010. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 24:2330–2342 10.1101/gad.1977210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P.D., Pontrello C., Burke S. 2004. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol. Psychiatry. 55:428–433 10.1016/j.biopsych.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Pinto S., Roseberry A.G., Liu H., Diano S., Shanabrough M., Cai X., Friedman J.M., Horvath T.L. 2004. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 304:110–115 10.1126/science.1089459 [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Calligaro D.O., Czachura J.F., Dreshfield-Ahmad L.J., Evans D.C., Hemrick-Luecke S.K., Kallman M.J., Kendrick W.T., Leander J.D., Nelson D.L., et al. 2000. The novel 5-Hydroxytryptamine(1A) antagonist LY426965: effects on nicotine withdrawal and interactions with fluoxetine. J. Pharmacol. Exp. Ther. 294:688–700 [PubMed] [Google Scholar]
- Salbert G., Chauveau I., Bonnec G., Valotaire Y., Jego P. 1992. One of the two trout proopiomelanocortin messenger RNAs potentially encodes new peptides. Mol. Endocrinol. 6:1605–1613 10.1210/me.6.10.1605 [DOI] [PubMed] [Google Scholar]
- Scott M.M., Lachey J.L., Sternson S.M., Lee C.E., Elias C.F., Friedman J.M., Elmquist J.K. 2009. Leptin targets in the mouse brain. J. Comp. Neurol. 514:518–532 10.1002/cne.22025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70–71 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Spiegelman B.M., Flier J.S. 2001. Obesity and the regulation of energy balance. Cell. 104:531–543 10.1016/S0092-8674(01)00240-9 [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Sadegh L., Elle I.C., Christensen A.G., Faergeman N.J., Ashrafi K. 2008. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab. 7:533–544 10.1016/j.cmet.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L., Armstrong D., Ducy P., Karsenty G. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell. 111:305–317 10.1016/S0092-8674(02)01049-8 [DOI] [PubMed] [Google Scholar]
- Velinov M., Zellers N., Styles J., Wisniewski K. 2008. Homozygosity for mutation G212A of the gene for aspartoacylase is associated with atypical form of Canavan’s disease. Clin. Genet. 73:288–289 10.1111/j.1399-0004.2007.00934.x [DOI] [PubMed] [Google Scholar]
- Walther D.J., Bader M. 2003. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 66:1673–1680 10.1016/S0006-2952(03)00556-2 [DOI] [PubMed] [Google Scholar]
- Yadav V.K., Ryu J.H., Suda N., Tanaka K.F., Gingrich J.A., Schütz G., Glorieux F.H., Chiang C.Y., Zajac J.D., Insogna K.L., et al. 2008. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 135:825–837 10.1016/j.cell.2008.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav V.K., Oury F., Suda N., Liu Z.W., Gao X.B., Confavreux C., Klemenhagen K.C., Tanaka K.F., Gingrich J.A., Guo X.E., et al. 2009. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 138:976–989 10.1016/j.cell.2009.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]