In the lungs of mice infected with influenza, the activity of cytotoxic T lymphocytes is modulated by the type of target cell encountered.
Abstract
Cytotoxic T lymphocytes (CTLs) play a prominent role in the resolution of viral infections through their capacity both to mediate contact-dependent lysis of infected cells and to release soluble proinflammatory cytokines and chemokines. The factors controlling these antiviral effector activities in vivo at infection sites are ill defined. Using a mouse model of influenza infection, we observed that the expression of CTL effector activity in the infected lungs is dictated by the target cell type encountered. CD45+ lung infiltrating inflammatory mononuclear cells, particularly CD11chi dendritic cells, trigger both CTL cytotoxicity and release of inflammatory mediators, whereas CD45− influenza-infected respiratory epithelial cells stimulate only CTL cytotoxicity. CTL proinflammatory mediator release is modulated by co-stimulatory ligands (CD80 and CD86) expressed by the CD45+ inflammatory cells. These findings suggest novel mechanisms of control of CTL effector activity and have potentially important implications for the control of excess pulmonary inflammation and immunopathology while preserving optimal viral clearance during respiratory virus infections.
CD8+ T cells are an important arm of the adaptive immune system and play a prominent role in the host response to infection with a variety of pathogenic microorganisms, most notably infections by viruses and certain intracellular bacteria. These T cells exit the thymus as naive small quiescent lymphocytes. Upon encounter with the relevant antigen (pathogen epitope), naive CD8+ T cells undergo a programmed process of activation, proliferation, and differentiation into effector cells (Lawrence et al., 2005). Effector CD8+ T cells are typically generated within secondary lymphoid organs (i.e., LNs draining sites of infection) and then migrate to extra lymphoid peripheral sites in response to homing signals and inflammatory stimuli produced by the pathogen (Lawrence et al., 2005). In response to encounter with the microorganism, effector CD8+ T cells use several distinct effector mechanisms to eliminate the pathogen, most notably elaboration of proinflammatory mediators (i.e., IFN-γ, TNF, and MIP-1α; La Gruta et al., 2007) and direct destruction of infected cells by perforin/granzyme and proapoptotic TNF receptor family–dependent mechanisms (Topham et al., 1997; Brincks et al., 2008).
Both the activation of naive T lymphocytes and the expression of effector activity by activated CD8+ (and CD4+) T lymphocytes usually requires engagement of the TCR by peptide–MHC class I complexes displayed on APCs (Mescher et al., 2007). This initial antigen-dependent signaling event can be modified by accessory signaling events involving direct T cell–APC contact such as co-stimulatory ligand–receptor interactions (Locksley et al., 2001; Sharpe and Freeman, 2002), as well as engagement of receptors on the responding T cells via soluble ligands such as cytokines (Mescher et al., 2007). Depending on the nature of the stimulus, engagement of the TCR and accessory signaling can result in a variety of outcomes for the responding T cell ranging from full activation/differentiation through to aborted activation and anergy (Mescher et al., 2007; Ream et al., 2010). Although the impact of the strength of signaling through the TCR and accessory interactions has been explored primarily during naive T cell activation (Locksley et al., 2001; Sharpe and Freeman, 2002), the expression of effector activity by fully differentiated effector T cells may likewise be regulated by the sum of antigen-dependent and accessory signaling events (Locksley et al., 2001; Sharpe and Freeman, 2002). Indeed, it has been demonstrated in vitro that there is a hierarchy of expression of effector activities by CD8+ T cells based on the strength of the antigenic stimulus to the CD8+ T cell (Valitutti et al., 1996; Hemmer et al., 1998; Gehring et al., 2007), although the in vivo significance of such a hierarchy is for the most part unknown.
Influenza virus is a major human pathogen that in its pandemic form has the potential to produce, on a global scale, severe infections of the respiratory tract, resulting in excess morbidity and mortality (Neumann et al., 2009). In most instances, influenza infection is restricted to the respiratory tract. Respiratory epithelial cells are the primary targets both for influenza virus replication (La Gruta et al., 2007) and for the host response to influenza infection (Hou and Doherty, 1995; Topham et al., 1997), as these CD45− cell types are, with rare exceptions, the only cell types capable of supporting productive virus infection (release of infectious virions from the infected cell). Other cell types (i.e., CD45+ mononuclear cells) can be infected by influenza but typically do not produce fully infectious virions (Hao et al., 2008; Manicassamy et al., 2010).
Severe lower respiratory tract influenza infection results in marked inflammation in the infected lungs (La Gruta et al., 2007). Although infection with influenza virus is lytic and usually results in the death of the infected cells (Fesq et al., 1994), there is a considerable body of evidence to suggest that the host immune response to infection, including the CD8+ T cell response, is a major contributor to the pulmonary inflammation and morbidity associated with infection and the process of virus clearance (Enelow et al., 1998; La Gruta et al., 2007). In particular, proinflammatory cytokines/chemokines released by innate and adaptive immune cells while acting to suppress the virus replication may also promote pulmonary inflammation and injury when produced in excess (Peper and Van Campen, 1995; Hussell et al., 2001; La Gruta et al., 2007; Sun and Metzger, 2008). Thus, understanding the factors regulating proinflammatory cytokine production and their relationship to the mechanism of virus clearance is important for a detailed understanding of influenza pathogenesis.
In this study, we evaluated the proinflammatory cytokine response of virus-specific effector CD8+ T cells responding in the infected lungs to sublethal influenza virus infection. We found that proinflammatory cytokine production by the effector CD8+ T cells was largely, if not exclusively, restricted to the T cells localized to the pulmonary interstitium. This antigen-driven proinflammatory cytokine production was dependent on T cell interaction with CD45+ (predominantly CD11c+) inflammatory cells infiltrating the infected lungs. Blockade of CD80 and CD86 co-stimulation at the time of effector CD8+ T cells migration into the lungs inhibited proinflammatory cytokine production by the T cells. In related experiments, we provide evidence both in vivo and in vitro that influenza-infected CD45− respiratory epithelial cells can be recognized and lysed by effector CD8+ T cells; however, this interaction does not trigger proinflammatory cytokine production by the responding T cells. The implications of these findings for influenza pathogenesis and CD8+ T cell function are discussed.
RESULTS
Kinetics of CD8+ T cell accumulation, in vivo effector activity, and virus clearance during experimental influenza infection
Infected respiratory epithelial cells are an important target of the immune response because these cell types support productive infection by influenza viruses; therefore, limiting infection of these cells is essential for virus clearance and recovery (Hou and Doherty, 1995; Topham et al., 1997). Because of the importance of these airway lining cells as targets for both the virus and effector CD8+ T cells, we analyzed the kinetics of influenza-specific CD8+ T cell accumulation simultaneously in the airspaces (cells overlaying the respiratory epithelium as collected in the bronchial alveolar lavage [BAL] fluid) and, in parallel, the subepithelial interstitial compartment (pulmonary interstitium) after sublethal experimental A/PR/8/34 influenza infection of BALB/c mice. We quantified the numbers of influenza-specific IFN-γ–secreting CD8+ T cells in these two lung compartments over time using the in vitro intracellular cytokine staining (ICCS) assay.
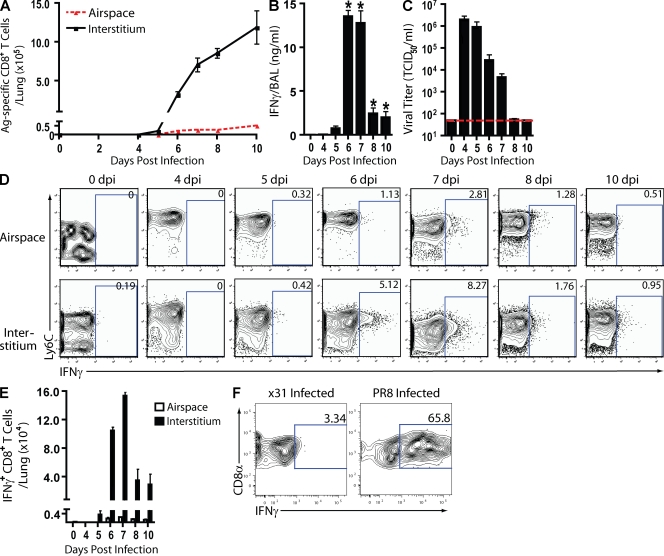
As Fig. 1 A demonstrates, virus-specific effector CD8+ T cells were first detectable in the respiratory tract between 5 and 6 d postinfection (dpi) and increased rapidly in both the airspaces and interstitium between 6 and 10 dpi, confirming earlier results from several laboratories (Tripp et al., 1995; Crowe et al., 2003; Lawrence and Braciale, 2004). The kinetics of release of the signature effector T cell proinflammatory cytokine, IFN-γ, into the BAL fluid during infection (Fig. 1 B) reflected the onset of effector T cell accumulation into the infected lungs (i.e., at 5 dpi) and reached maximum levels in the BAL fluid at 6 and 7 dpi. IFN-γ release into the BAL fluid dropped precipitously thereafter, commensurate with the clearance of infectious virus from the infected lungs (Fig. 1 C). In vivo T cell depletion analysis established CD8+ T cells as major contributors to IFN-γ production (Fig. S1 A) and virus clearance (Fig. S1 B). Comparable results were obtained in the analysis of infected C57BL/6 mice (unpublished data).
Figure 1.
Antigen-dependent CD8+ T cell IFN-γ protein production in situ. (A–F) A/PR/8-infected BALB/c mice were analyzed on the indicated dpi. (A) Airspace (BAL fluid) and interstitial (lung suspensions) cells were sampled and identified by IFN-γ production after in vitro restimulation with influenza-infected P815 cells. (B and C) IFN-γ protein (B) and viral titers (TCID50; C) in BAL fluid samples with horizontal line detection limit. (D and E) In situ CD8+ T cell IFN-γ protein production using in vivo ICCS assay at the indicated dpi. Representative flow profiles (D) and absolute numbers of activated (Ly6Chi) CD8+ T cells producing IFN-γ protein in situ (E) are shown. (A–E) Two to five independent experiments (n = 8–20 mice) as mean ± SEM are shown. Considered significant with respect to 0 dpi at *, P < 0.05. (F) Antigen dependence of IFN-γ production. CL-4 T cell (Thy1.1) congenic recipients (Thy1.2) were infected with A/PR/8 influenza virus. At 6 dpi, purified CD8+ cells (5 × 105) from recipient lung were transferred i.v. into 5 dpi X31- or PR8-infected congenic mice (Thy1.2) and evaluated with the in vivo ICCS assay 24 h later. Flow profiles of IFN-γ+ CL-4 T cells are representative of four independent experiments (n = 2/experiment).
CD8+ T cells expressing proinflammatory cytokines in response to influenza infection localize principally in the infected lung interstitial compartment, not in the airspaces
When the proinflammatory effector cytokine response (i.e., IFN-γ production) in the infected lungs was at its peak (Fig. 1 B), relatively few influenza-specific effector CD8+ T cells had accumulated in the infected lungs. The majority of these effector cells were localized to the pulmonary interstitium with 20–40-fold fewer effector CD8+ T cells localized to the airspace (Fig. 1 A). Because elimination of infected respiratory epithelial cells was essential for virus clearance but the majority of influenza-specific CD8+ T cells were localized to the interstitium at this time, we wanted to establish the contribution, if any, of these interstitial CD8+ T cells to the IFN-γ response detected in the BAL fluid. To explore this possibility, we used a modified version of the in vivo ICCS assay (Liu and Whitton, 2005) to identify and determine the kinetics of IFN-γ–secreting CD8+ T cells accumulating and responding in vivo. In brief, brefeldin A (BFA) was introduced into the respiratory tract intranasally (i.n.) 6 h before harvest of the infected lungs, followed by direct ex vivo analysis of intracellular IFN-γ accumulation by CD8+ T cells (see Fig. S2 for details of assay optimization).
Unexpectedly, we found that, throughout infection, a much larger fraction of in vivo IFN-γ–secreting CD8+ T cells was localized in the pulmonary interstitium than in the airspaces (Fig. 1 D and Fig. S2 D). At the time of peak IFN-γ release into the BAL fluid (6–7 dpi), the total number of in vivo IFN-γ+CD8+ T cells in the interstitium exceeded those in the airspaces by >100-fold (Fig. 1 E). It is also noteworthy that the numbers of CD8+ T cells scoring positive for IFN-γ in the in vivo ICCS assay over time (Fig. 1 E) directly paralleled the kinetics of IFN-γ release into the BAL fluid (Fig. 1 B). Comparable differences in the responsiveness of the interstitial and airspace CD8+ T cells were observed independent of the i.n. BFA dose administered (Fig. S2 B) and were also observed after i.n. administration of the protein transport inhibitor monensin (Fig. S2 C).
The aforementioned findings raised the possibility that the effector CD8+ T cells localized to the interstitium were the primary, if not exclusive, source of the IFN-γ released into the BAL fluid as they represented both the quantitatively larger fraction of effector CD8+ T cells in the infected lungs and were the major IFN-γ–secreting cells detected in the in vivo ICCS assay. Additionally, the data suggested that the effector CD8+ T cells localized to the airspaces may be poorly responsive to antigenic stimulation; however, when we examined the ability of CD8+ T cells isolated from the airspaces or interstitium to respond to antigenic stimulation in vitro in the ICCS assay, both T cell populations responded with comparable efficiency (Fig. S3, A and B). In keeping with these results, we found that after in vitro antigenic stimulation, both airway and interstitial CD8+ T cells could transiently up-regulate cell surface expression of CD107a/b, a surrogate for granule exocytosis–dependent cytotoxicity (Fig. S3 C; Betts et al., 2003). By these criteria, the effector CD8+ T cells in the two compartments were equally competent to respond to viral antigenic stimulation.
We were able to further confirm the findings obtained with the in vivo ICCS assay at the level of IFN-γ gene transcription. Using YETI mice, which express YFP driven off the IFN-γ promoter (Mayer et al., 2005), we evaluated IFN-γ gene transcription in tetramer+CD8+ T cells during the peak of proinflammatory cytokine release (6 dpi; Fig. S4 A). We found that consistent with the in vivo ICCS results, both the number and percentage of influenza polymerase tetramer+CD8+ T cells expressing YFP in the interstitium far exceeded those localized to the airspace (Fig. S4, B and C). We also noted apparent differences between interstitial and airway CD8+ T cells in the production of several other proinflammatory T cell cytokines/chemokines in vivo, notably TNF (unpublished data).
Proinflammatory cytokine production by interstitial virus-specific CD8+ T cells is dependent on specific antigenic stimulation
To further investigate the regulation of the proinflammatory cytokine IFN-γ by interstitial (and airspace)-localized, virus-specific CD8+ T cells, we performed adoptive transfer of purified CD8+ T cells from the TCR transgenic (tg) CL-4 mice into naive recipients. These TCR tg T cells are directed to a specific epitope in the hemagglutinin (HA) of A/PR/8 virus (HA533–541). After transfer of the T cells into Thy1 congenic recipients and subsequent infection of recipient mice with A/PR/8 virus, the frequency of IFN-γ–expressing CL-4 T cells in vivo was analyzed in the in vivo ICCS assay. Consistent with our observations with polyclonal CD8+ T cells, a large fraction (∼40–50%) of the CL-4 T cells isolated from the lung interstitium had produced IFN-γ protein in vivo at 6 dpi, whereas IFN-γ+ CL-4 T cells were not detected among cells isolated from the airspaces (Fig. S4 D).
To directly establish that IFN-γ production by the virus-specific effector CD8+ T cells in the interstitium was dependent on specific antigenic stimulation, effector CL-4 T cells isolated from the lungs of infected mice were transferred into either A/PR/8- or A/X31-infected recipients at 5 dpi, the time of peak virus titer and the initial onset of effector CD8+ T cell accumulation in the infected lungs (Fig. 1, B and C). 24 h later, effector CL-4 T cells transferred into the A/PR/8-infected recipient demonstrated robust IFN-γ synthesis in the in vivo ICCS assay. In stark contrast, effector CL-4 T cells transferred into mice infected with the antigenically distinct, mouse-adapted A/X31 virus, which lack the HA533–541 epitope recognized by the CL-4 T cells, showed minimal responsiveness, confirming the antigen dependence of proinflammatory cytokine production during the height of an influenza infection (Fig. 1 F).
Proinflammatory cytokine production by antiviral effector CD8+ T cells requires MHC class I expression only on CD45+ hematopoietic cells in the infected lung
The accumulating data from our analysis to this point suggested that it was virus-specific CD8+ T cells localized to the infected lung interstitium which exhibited in vivo antiviral effector activity at least as defined by antigen-dependent production of proinflammatory cytokines. Accordingly, effector CD8+ T cells encountering and engaging the infected respiratory epithelium in the airspaces, paradoxically, were contributing little to this effector T cell response. Infection of the mouse respiratory tract by influenza virus results not only in recruitment of effector CD8+ T cells to the infected lungs but also in a progressive influx of CD45+ inflammatory myeloid-derived cells into the pulmonary interstitium (La Gruta et al., 2007), which peaks at the time of effector T cell influx into the infected lungs. These considerations raised the possibility that the interaction of effector CD8+ T cells with CD45+ inflammatory cells within the interstitium, rather than with influenza-infected CD45− respiratory epithelial cells, accounted for preferential expression of effector activity by the interstitial virus-specific T cells.
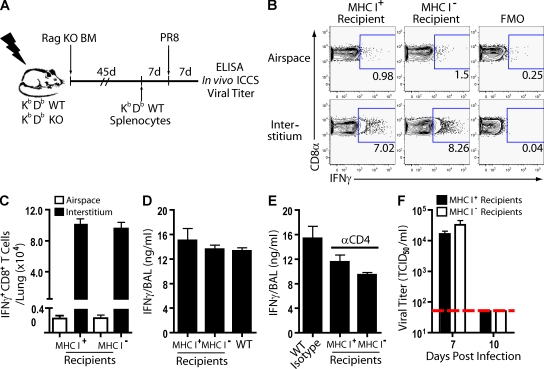
To further explore this possibility, we constructed BM chimeras in which irradiated MHC class Ia–deficient (and as controls irradiated MHC class Ia–sufficient WT) mice were reconstituted with MHC class I–sufficient BM from Rag1−/− donors (Fig. 2 A). Rag1−/− BM was used for reconstitution to avoid the development of MHC class Ib–restricted antiviral T cells (Swanson et al., 2008) and to assure that respiratory DCs expressing MHC class I would be present to serve as APCs during the induction of antiviral CD8+ T cell responses in both the experimental and control chimeras (GeurtsvanKessel et al., 2008; Kim and Braciale, 2009). After reconstitution, both sets of mice were MHC class I sufficient in the CD45+ hematopoietic compartment (including tissue-resident DCs, macrophages, and infiltrating inflammatory mononuclear cells in the infected lungs) and either MHC class I deficient or sufficient among CD45− cells (including the respiratory epithelium). These CD45−MHC class I− and CD45−MHC class I+ mice received, by adoptive transfer, WT splenocytes as a source of mature lymphocytes. The mice were subsequently infected, and T cell effector activity and antiviral responses were analyzed at 7 dpi (Fig. 2 A).
Figure 2.
Impact of MHC I–deficient respiratory epithelial cells on CD8+ T cell cytokine production after influenza infection. (A) Irradiation BM chimera schematic. Infected chimeric mice were examined at 7 dpi unless noted elsewhere. (B and C) In situ CD8+ T cell IFN-γ protein production using in vivo ICCS assay. Representative flow profiles (B) and absolute numbers of IFN-γ+CD8+ T cells in situ (C) are shown. (D) BAL fluid IFN-γ release. (E) Infected mice were depleted of CD4+ cells (3 dpi), and BAL fluid IFN-γ protein content was determined (7 dpi). (D and E) WT equals control infected mice. (F) Viral titers (TCID50) in BAL fluid at the indicated times postinfection with horizontal detection limit. (B–F) Four independent experiments (n = 8–12 mice) as mean ± SEM are shown. FMO, fluorescence minus one.
We analyzed the number and frequency of IFN-γ–secreting CD8+ T cells by the in vivo ICCS assay and IFN-γ release into the BAL fluid in infected chimeric mice. We found that the frequency (Fig. 2 B) and absolute number (Fig. 2 C) of IFN-γ+CD8+ T cells localized to the interstitium by the in vivo ICCS assay were comparable for CD45−MHC class I− and CD45−MHC class I+ mice. Equally important, the frequency (Fig. 2 B) and number (Fig. 2 C) of IFN-γ+CD8+ T cells in the airspaces were unaffected by the presence or absence of MHC class I expression by respiratory epithelial cells. In keeping with these findings, ELISA quantitation of IFN-γ protein in the BAL fluid revealed no difference in the ability of these mice to release the proinflammatory cytokine during an influenza infection (Fig. 2 D). The levels were comparable with those seen in nonirradiated controls at this point in infection. Furthermore, the level of IFN-γ in the BAL fluid of CD45−MHC class I− was not caused by a compensatory increase in IFN-γ production by responding antiviral CD4+ T cells, as administration of depleting α-CD4 antibody during infection reduced IFN-γ production by both CD45−MHC class I− and CD45−MHC class I+ mice to the same extent (Fig. 2 E). It is also noteworthy that infectious virus titers at 7 dpi were comparable in the lungs of CD45−MHC class I− and CD45−MHC class I+ mice and were statistically indistinguishable from those of unmanipulated WT infected animals (Fig. 2 F). Both chimeric mouse cohorts eliminated infectious virus by 10 dpi (Fig. 2 F). Equivalent viral elimination between the chimeric mice was not unexpected, though, considering the well documented combined contribution of effector CD4+ T cells and antiviral B cells as efficient mechanisms for the control of infectious virus titer in the absence of effector CD8+ T cell function (Topham et al., 1996; Topham and Doherty, 1998). Thus, deficiencies in triggering CD8+ T cell effector activity in CD45−MHC class I− mice would have minimal consequences on eventual viral clearance without additional manipulation.
Ablation of CD11chi mononuclear cells in the infected lungs inhibits proinflammatory cytokine production by antiviral CD8+ T cells
The aforementioned results supported the possibility that antiviral effector CD8+ T cells were engaging CD45+ cells in the inflamed pulmonary interstitium to trigger effector cytokine production. As demonstrated above, IFN-γ production by the effector CD8+ T cells required specific antigen recognition (Fig. 1 F). Accordingly, CD45+ inflammatory cells in the interstitium should contain viral antigen capable of being presented to effector CD8+ T cells and therefore serve as target cells (APCs). Indeed, we could detect influenza nucleocapsid protein (NP) in both CD45+ hematopoietic/inflammatory cells as well as CD45− cells (including respiratory epithelial cells) isolated from the infected lungs at the peak of inflammatory cytokine production (Fig. S5 A), suggesting that viral antigen was localized to both cell populations. However, the viral antigen displaying CD45+ cells and not the virus-infected, antigen-expressing CD45− respiratory epithelial cells trigger the proinflammatory cytokine response of effector CD8+ T cells.
Several different inflammatory cell subsets infiltrating the influenza-infected lungs (including DCs and Ly6Chi inflammatory macrophages) have been reported to have APC activity (Lin et al., 2008; McGill et al., 2008) and would be likely candidates to serve as target cells regulating the proinflammatory cytokine production by effector CD8+ T cells. These DC and macrophage subsets (CD45+, GR1int, CD11bhi, MHC class II+, inflammatory mononuclear cells) accumulate in large numbers at 6–7 dpi (Fig. S5 B), which is at the peak of expression of CD8+ T cell effector activity, and can take up and display influenza viral NP protein (Fig. S5 C). Importantly, these mononuclear cells within the inflamed lung interstitium express high levels of CD11c (Fig. S5 D). To assess the role of these CD11c+ inflammatory cells as APCs for effector CD8+ T cells, we evaluated the impact of depletion/ablation of this mononuclear cell population from the infected lungs on the production of IFN-γ by effector CD8+ T cells in vivo.
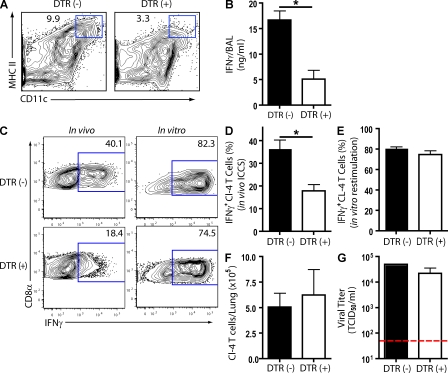
We used CD11c–diphtheria toxin receptor (DTR) mice to investigate the effect of depletion on CD8+ T cell proinflammatory cytokine production during an influenza infection. These mice express the primate DTR driven off of the CD11c promoter, and we have previously shown that i.n. administration of toxin efficiently depletes CD11chi cells from the respiratory tract (Kim and Braciale, 2009). Because toxin administration at the initiation of virus infection eliminates respiratory DCs and, as a consequence, profoundly inhibits the induction of the virus-specific effector CD8+ T cell response (Kim and Braciale, 2009), we delayed toxin administration until 5 dpi, which is 24 h before the peak of CD8+ T cell effector activity. Toxin administration led to a substantial reduction of CD11chi cells in DTR (+) but not in control DTR (−) mice at 6 dpi (Fig. 3 A). When we analyzed IFN-γ release into the BAL fluid after toxin administration, we found that depletion of CD11chi cells at the time of effector CD8+ T cell influx into the infected lungs markedly suppressed IFN-γ production 24 h later (Fig. 3 B), which was as expected if CD45+CD11chi cells were the primary APC for (trigger of) inflammatory cytokine/chemokine production by the T cells.
Figure 3.
The impact of acute CD11chi cell depletion on in vivo CD8+ T cell IFN-γ production. (A and B) Infected CD11c-DTR tg (+) and DTR non-tg (−) mice were treated with diphtheria toxin at 5 dpi. Representative flow profile of CD11chiMHC IIhi cell depletion in lung cell suspensions (6 dpi; A) and BAL fluid IFN-γ release (6 dpi; B) are shown. (C–F) Transferred tg CL-4 T cells into Thy-mismatched congenic CD11c-DTR (+) and DTR (−) mice. Infected mice received diphtheria toxin (5 dpi), and on 6 dpi, mice were interrogated for in vivo IFN-γ production by the in vivo ICCS assay (i.p. monensin). (C and D) Representative flow profiles (C) and percentage of IFN-γ+ tg CL-4 T cells in vivo (D) are shown. (C and E) IFN-γ production after in vitro restimulation with influenza-infected P815 cells. (E) Number of tg CL-4 T cells present in the total lung cell suspensions is shown. (G) Viral titers (TCID50) in sample BAL fluid with horizontal detection limit. (A–G) Two to four independent experiments (n = 8–16 mice) as mean ± SEM are shown. Considered a significant difference at *, P < 0.05.
Because after activation and migration to inflamed sites like the infected lungs some CD8+ T cells can express low levels of CD11c, CD8+ T cells from CD11c-DTR mice may also be depleted in the lungs after toxin administration (Bennett and Clausen, 2007). To further establish that elimination of CD11chi APCs from the infected lungs inhibits proinflammatory cytokine production by the responding CD8+ T cells, we analyzed the response of Thy-mismatched WT CL-4 tg T cells to influenza infection after adoptive transfer of the T cells into CD11c-DTR mice and toxin treatment at 5 dpi. We observed that the depletion of CD11chi cells was accompanied by an ∼50% reduction in the percentage of activated effector CL-4 T cells producing IFN-γ, as determined via the in vivo ICCS assay (Fig. 3, C and D). Depletion of the CD11chi cells at 5 dpi did not alter the activation state of the CD8+ T cells as ex vivo restimulation of the transferred CL-4 T cells resulted in the equivalent IFN-γ production by CL-4 T cells isolated from control and depleted mice at 6 dpi (Fig. 3, C and E). Furthermore, depletion of CD11chi cells did not have significant impact on the ability of CL-4 T cells to migrate/accumulate in the inflamed lung, as comparable numbers of CL-4 T cells were present in DTR (−) and DTR (+) mice at 6 dpi (Fig. 3 F). These results provide additional support for the concept that CD45+CD11c+ inflammatory mononuclear cells primarily residing in the pulmonary interstitium serve as the dominant APCs (and possibly a primary target) for the production of proinflammatory cytokines by antiviral effector CD8+ T cells in the influenza-infected lungs. Of particular note, the depletion of the CD45+CD11c+ APCs and the attendant decrease in IFN-γ production by the effector CD8+ T cells had minimal impact on the control of virus replication in the infected lungs (Fig. 3 G).
Efficient expression of the proinflammatory cytokines by effector CD8+ T cells requires co-stimulation
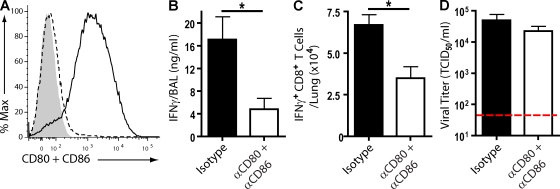
Engagement of the TCR is essential for activation of naive T cells as well as the expression of effector activity by activated T cells. Similarly, engagement of co-stimulatory receptors (e.g., CD28) by their requisite co-stimulatory ligands (e.g., CD80 and CD86) is also normally required for full T cell activation and differentiation, and in certain instances, these interactions have been demonstrated to modulate both T cell effector activity and the T cell response to pathogens (Locksley et al., 2001; Sharpe and Freeman, 2002). Because the co-stimulatory ligands CD80 and CD86 are expressed at high levels on CD45+CD11chi cells in the infected lung interstitium and only minimally expressed on CD45− lung cells, including the infected respiratory epithelium (Fig. 4 A), we wished to determine whether co-stimulatory ligand–receptor-dependent interaction between the CD45+ lung APCs and antiviral effector CD8+ T cells regulated IFN-γ production by the T cells.
Figure 4.
The impact of acute CD80 and CD86 blockade on in vivo T cell IFN-γ production during an influenza infection. (A) Lung suspensions were collected from 6 dpi C57BL/6 mice. Flow profile represents the expression of CD80 and CD86 on CD11chi cells (solid line; CD45+CD11b+CD11chi) and CD45− cells (dashed line) in comparison with isotype control antibody (shaded region). (B–D) A/PR/8-infected C57BL/6 mice received CD80 and CD86 blocking or isotype control antibody cocktail i.p. at 5 dpi. (B) BAL fluid IFN-γ release (6 dpi). (C) An in vivo ICCS assay (i.p. monensin) at 6 dpi. Graph depicts total lung CD8+ T cells producing IFN-γ protein in vivo. (D) Viral titers (TCID50) in sample BAL fluid with horizontal line detection limit. (A–D) Two independent experiments (n = 8 mice) as mean ± SEM are shown. Considered a significant difference at *, P < 0.05.
To evaluate this, C57BL/6 mice were infected with A/PR/8/34 virus, and at 5 dpi, a blocking CD80 and CD86 antibody cocktail was administered i.p. 24 h later, BAL fluid was collected and analyzed for IFN-γ production (Fig. 4 B). We observed that short-term acute CD80 and CD86 blockade at the time of effector CD8+ T cell influx into the infected lungs led to a significant decline in IFN-γ secretion. The reduction coincided with a corresponding reduction in IFN-γ–producing CD8+ T cells in vivo (Fig. 4 C). Of particular note, in vivo blockade of co-stimulatory receptor–ligand interactions between effector T cells and the CD45+ APC did not affect virus clearance from the infected lungs (Fig. 4 D). Comparable results were obtained after acute CD80 and CD86 blockade in influenza-infected BALB/c mice (Fig. S5, E–G). The capacity for CD8+ T cells to release IFN-γ was not altered in mice receiving acute administration of CD80 and CD86, as these cells responded equally well compared with CD8+ T cells from mice receiving isotype antibody when restimulated with influenza-infected CD45+ cells in vitro (Fig. S5 H). Altogether, these data suggest that CD80 and CD86 signaling was necessary for maximal proinflammatory cytokine production by effector CD8+ T cells responding to infection.
Effector CD8+ T cell interactions with infected CD45− respiratory epithelial cells signal for T cell–mediated cytolysis but not proinflammatory cytokine production
The aforementioned results raised the unexpected as well as unlikely possibility that the response of the virus-specific effector CD8+ T cells was directed primarily to CD45+CD11chi mononuclear cells and that infected respiratory epithelial cells were only a secondary target or perhaps not directly recognized. This latter possibility seemed unlikely, as suppression of virus replication in respiratory epithelial cells is critical for virus clearance and recovery (Hou and Doherty, 1995; Topham et al., 1997). In this connection, it is noteworthy that an earlier study implicated the expression of perforin and FAS cytotoxic pathways in CD8+ T cell–mediated control of virus replication in the respiratory tract (Topham et al., 1997). Accordingly, effector CD8+ T cell–dependent clearance of influenza-infected cells, in particular elimination of CD45−-infected respiratory epithelial cells, would be dependent on the operation of one or both of these cytolytic effector mechanisms.
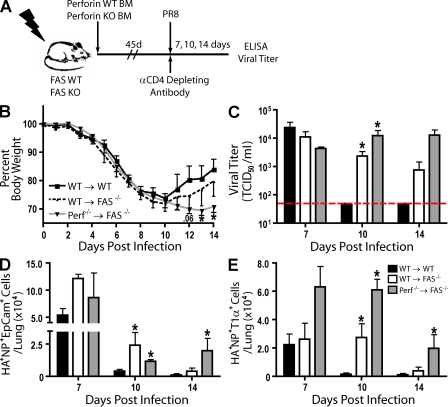
To reevaluate the contribution of perforin- and/or FAS-dependent cytotoxicity by effector CD8+ T cells on infectious virus clearance and the impact of these cytolytic effector mechanisms on the elimination of virally infected CD45− in vivo (Topham et al., 1997), we constructed BM chimeras in which irradiated FAS-deficient mice (and as controls irradiated FAS-sufficient WT mice) were reconstituted with BM from either WT or perforin-deficient mice (Fig. 5 A). This resulted in experimental mice that were selectively deficient in perforin expression in the effector CD8+ T cells or FAS expression selectively on CD45− respiratory epithelial cells or both. To examine the impact of effector CD8+ T cells exclusively on influenza virus clearance in vivo after reconstitution, experimental and control chimeric mice were depleted of CD4+ T cells (Fig. 5 A). This depletion effectively eliminated CD4+ antiviral activity as well as impacted the production of isotype-switched antibody. After infection, daily weights were obtained as a measure of systemic morbidity, and mice were evaluated for virus titer, infected cell numbers, and cytokine production at 7, 10, and 14 dpi.
Figure 5.
The impact of FAS and perforin deficiency on CD8+ T cell–mediated viral clearance during an influenza infection. (A) Irradiation BM chimera schematic. After reconstitution (≥45 d), mice were infected and depleted of CD4+ cells at 0 and 7 dpi. (B) Percent body weight loss (with respect to 0 dpi body weight) in chimeric mice. (C) Viral titers (TCID50) in sample BAL fluid from indicated dpi with horizontal line detection limit. (D and E) Absolute number of influenza-infected Ep-CAM+ (D) and T1α+ (E) epithelial cells (CD45− autofluorescent−HA+NP+) sample from lung suspensions at indicated dpi. (B–E) Four independent experiments (n = 8 mice) as mean ± SEM are shown. Considered a significant difference with respect to WT reconstituted control difference at given dpi (*, P < 0.05).
As expected, experimental and control of infected chimeric mice exhibited similar kinetics of initial weight reduction after sublethal i.n. infection, which was maximal at 7–10 dpi (Fig. 5 B). However, unlike WT chimeric mice (WT → WT), which exhibited the normal pattern of recovery and weight gain beyond 10 dpi, mice deficient in both perforin and FAS (Perf−/− → FAS−/−) failed to recover and sustained low body weight until the termination of the experiment (Fig. 5 B). Chimeric mice that were sufficient in perforin expression by CD8+ effector T cells but deficient in Fas expression on CD45− respiratory epithelial cells (WT → FAS−/−) exhibited a slightly delayed time course of weight gain and recovery (Fig. 5 B). These results correlated with the kinetics of virus clearance from the infected respiratory tract. WT → WT mice had, as expected, cleared infectious virus from the respiratory tract by 10 dpi, whereas significant virus titers were detected in both the WT → FAS−/− and Perf−/− → FAS−/− mice at this time point (Fig. 5 C). By 14 dpi, infectious virus was no longer at statistically elevated levels in WT → FAS−/− mice, but substantial virus titers were detected at this time in the infected Perf−/− → FAS−/− chimeras (Fig. 5 C).
We also examined the number of infected CD45− respiratory epithelial cells present over time in the lungs of these infected chimeric mice by flow cytometry. We focused on epithelial cells liberated from infected airways (identified by the expression of the cell surface marker epithelial cell adhesion molecule [Ep-CAM]; Kasper et al., 1995) and type 1 alveolar epithelial cells (identified by cell surface T1-α expression; Herold et al., 2008). Cells were identified as infected based on the simultaneous expression/detection of cell surface influenza HA and intracellular NP (Fig. S6, A and B). At 7 dpi, when infectious virus levels in the respiratory tracts of experimental and control infected chimeric animals were elevated (Fig. 5 C), virally infected respiratory epithelial cells were readily detected in both the experimental and control groups (Fig. 5, D and E). By 10 dpi, only very low numbers of residual infected cells were detected in the WT chimeric mice, but both WT → FAS−/− and Perf−/− → FAS−/− mice retained substantial numbers of infected cells (which is consistent with the elevated infectious virus titer in the respiratory tract of these mice at this time point; Fig. 5 C). Infected cell numbers in WT → WT mice decreased to near undetectable levels by 14 dpi, but Perf−/− → FAS−/− mice retained substantial numbers of infected cells of both epithelial cell types (Fig. 5, D and E), which is once again consistent with the data on residual virus titer in the lungs (Fig. 5 C). The number of infected epithelial cells in Perf−/− → FAS−/− mice did diminish over time, indicating that effector CD8+ T cells may eventually clear virus in the absence of perforin- or FAS-dependent mechanisms, suggesting that additional mechanisms may be in play (i.e., TNF-related apoptosis-inducing ligand [TRAIL]; Brincks et al., 2008).
A comparable pattern was observed for proinflammatory cytokine production (Fig. S6 C) and the presence of influenza antigen–expressing CD45+ cells (Fig. S6 D). Proinflammatory cytokine production by effector CD8+ T cells, as reflected in IFN-γ release into the BAL fluid, had almost ceased in WT → WT by 10 dpi (Fig. S6 C). This decline in proinflammatory cytokine secretion was also reflected in the diminished number of antigen-bearing CD45+ mononuclear cells in the lungs at 10 dpi (Fig. S6 D). In contrast, Perf−/− → FAS−/− mice continued to produce IFN-γ both at 10 dpi and at diminished levels out to 14 dpi (Fig. S6 C) and retained detectable numbers of residual antigen-bearing CD45+ cells in the lungs (Fig. S6 D).
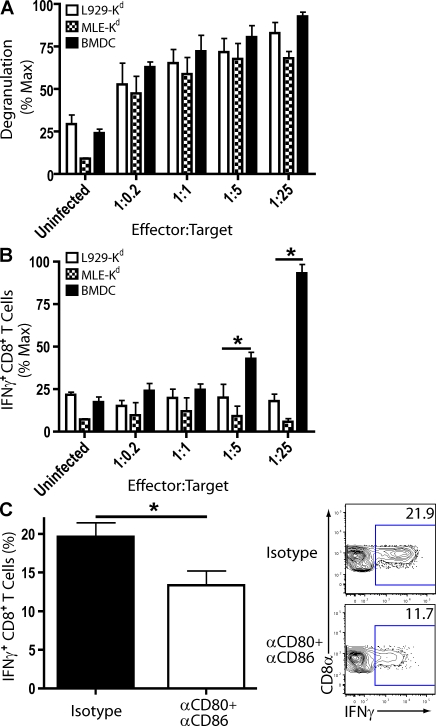
These data raised the intriguing possibility that CD8+ T cell engagement with antigen-bearing CD45+CD11chi cells can lead to both proinflammatory cytokine release and cytolysis, whereas interactions with the infected respiratory epithelium results in cell lysis without triggering proinflammatory cytokine release by effector CD8+ T cells. To determine whether the differential CD8+ T cell effector activity could be influenced by the infected target cell type, we co-cultured CD8+ T cell effectors isolated from the influenza-infected lungs with influenza-infected cell types representing hematopoietic (CD45+) and nonhematopoietic cell lineages (CD45−). After co-culture, we investigated transient up-regulation of CD107a/b as a marker for T cell cytolytic granule exocytosis (a measure of TCR engagement resulting in T cell cytolysis; Betts et al., 2003) and IFN-γ synthesis by ICCS (as a measure of TCR engagement proinflammatory cytokine production). The influenza-infected CD45− type II alveolar cell line MLE (mouse lung epithelial) and the infected L929 fibroblast cell line were as equally efficient in initiating CD8+ T cell degranulation as the infected CD45+ BM-derived DCs (BMDCs; Fig. 6 A). In marked contrast, only BMDCs were capable of triggering efficient IFN-γ production by the effector CD8+ T cells from the infected lungs (Fig. 6 B).
Figure 6.
CD8+ T cell effector activities upon co-culture with influenza-infected hematopoietic and nonhematopoietic cells. (A and B) Lung suspensions of infected BALB/c mice (8 dpi) were collected and pooled. CD8+ cells were positively selected via MACS bead separation. CD8+ cells (effectors) were co-cultured with influenza-infected cells (targets) in indicated effector/target ratios ± α-CD107a/b (FITC). CD8+ T cell degranulation (CD107a/b+; A) and IFN-γ–producing CD8+ T cells after co-culture (B) are shown. Percentage max is the proportion of effector activity with respect to maximum production of effector activity within one experiment. Four independent experiments (two to three pooled mice/experiment) as mean ± SEM are shown. (C) Lung suspensions of infected BALB/c mice (8 dpi) were co-cultured in a 1:1 ratio with influenza-infected BMDCs for 6 h in the presence of isotype or blocking CD80 and CD86 antibodies. Representative flow profiles (right) and percentage (left) of IFN-γ+CD8+ T cells after in vitro restimulation are shown. Two independent experiments (n = 5) as mean ± SEM are shown. A paired t test was used to test statistical significance. (A–C) Considered a significant difference at *, P < 0.05.
In view of the impact of CD80 and CD86 blockade in the infected lungs on proinflammatory cytokine production by effector CD8+ T cells in vivo (Fig. 4), we wanted to determine whether blockade of co-stimulatory receptor–ligand interactions in vitro also affected the ability of lung-derived effector CD8+ T cells to produce IFN-γ in response to stimulation by the infected CD45+ cells. CD8+ T cells were co-cultured with infected BMDCs in the presence of blocking antibodies to CD80 and CD86 co-stimulatory molecules. The presence of these blocking antibodies diminished the ability of BMDCs to stimulate T cell IFN-γ production (Fig. 6 C) while not affecting the ability of these target cells to trigger the granule exocytosis–cytolysis effector pathway (i.e., transient up-regulation of CD107a/b) by the CD8+ T cell effectors (not depicted).
DISCUSSION
In this study, we examined the effector CD8+ T cell response to influenza virus infection in the respiratory tract and the factors ultimately regulating it. This analysis focused on the kinetics of CD8+ T cell accumulation, the localization of effector CD8+ T cells within the infected lungs, and the localization of CD8+ T cell effector activity within the pulmonary interstitium and airspaces. We observed that proinflammatory cytokine production (e.g., IFN-γ production) by effector CD8+ T cells was detected at maximum levels during the early phase of effector CD8+ T cell recruitment into the infected lungs (i.e., 6–7 dpi). Effector cytokine release decreased with virus clearance. Quantitation of effector CD8+ T cells in the lung interstitium and in the airspaces (identified in collected BAL fluid) indicated that influenza-specific CD8+ T cell numbers in the interstitium far exceeded numbers in the airspaces, implicating interstitial effector CD8+ T cells as the major source of the proinflammatory cytokines liberated into the infected lungs. This concept received additional support from analysis of intracellular cytokine synthesis using the in vivo ICCS assay where, again, interstitial T cells were demonstrated to be the predominant, if not exclusive, proinflammatory cytokine producers. Using BM chimeras, we established that production of the proinflammatory cytokine IFN-γ by the effector CD8+ T cells was dependent on recognition of CD45+ BM-derived cells and did not require MHC class I recognition of the infected respiratory epithelium. Furthermore, depletion of CD11chi cells from the infected lung at the time of effector CD8+ T cell migration into the infected lung markedly decreased proinflammatory cytokine production by effector CD8+ T cells. In addition, the release of proinflammatory cytokines by effector CD8+ T cells was inhibited after blockade of the co-stimulatory ligands CD80 and CD86. Finally, we provide several lines of evidence that suggest that infected respiratory epithelium can be recognized and destroyed by effector CD8+ T cells but that this interaction does not lead to proinflammatory cytokine production by the effector CD8+ T cells.
A hallmark of the host response to influenza virus is the potent proinflammatory cytokine/chemokine response and the attendant inflammation in the respiratory tract elicited by infection. Effector molecules contributed by both innate and adaptive immune cells can contribute to this proinflammatory cytokine response. Although this proinflammatory response in the respiratory tract to influenza infection may aid recovery (Wiley et al., 2001), there is considerable evidence to suggest that this response may also be a major contributor to the pulmonary injury associated with influenza infection (Peper and Van Campen, 1995; Hussell et al., 2001; La Gruta et al., 2007; Sun et al., 2009) and may not be essential for virus clearance from the respiratory tract (Lukacher et al., 1984; Graham et al., 1993; Hussell et al., 2001). We found that proinflammatory cytokine production by the influenza-specific effector CD8+ T cells was dependent solely on the recognition of CD45+ (BM derived) cells infiltrating the infected lungs (Fig. 2). Several lines of evidence, most notably the in vivo ICCS assay, suggested that this interaction between the effector CD8+ T cells and the APCs leading to cytokine production was occurring predominantly, if not exclusively, within the pulmonary interstitium (Fig. 1). Cellular ablation analysis suggested that the predominant APCs in the infected lung interstitium express CD11c (Fig. 3) and appear in preliminary analyses to be inflammatory mononuclear cells of the DC lineage (not depicted). Of note, the ablation of this cell type in vivo did not affect infectious virus elimination from the infected lungs. Furthermore, although not formally evaluated in this study, we believe that the CD45+CD11chi interstitial APC likely presents processed viral antigen to the effector CD8+ T cells in vivo both by direct infection (Hao et al., 2008) and by uptake of viral antigen and presentation via exogenous processing pathways (Albert et al., 1998).
Respiratory epithelial cells are a critical target of influenza virus as these cells are the major cell type in the lungs that are productively infected by most type A influenza viruses (La Gruta et al., 2007). Thus, elimination of virus-infected respiratory epithelial cells (along with neutralization of infectious virions) is essential for recovery from infection. Our findings, both in vivo and in vitro, suggest that the encounter between effector CD8+ T cells and antigen-bearing CD45− cell types, such as the influenza-infected respiratory epithelium, results in minimal proinflammatory cytokine production; however, early analysis from our laboratory indicated that the interaction of effector CD8+ T cells with virus influenza-infected cells in the lungs was highly specific (Lukacher et al., 1984). In reprising and extending an earlier analysis using BM chimeras to evaluate the contribution of cytolytic effector mechanisms in virus clearance (Topham et al., 1997), we were able to confirm the importance of the perforin and Fas/FasL cytolysis mechanisms in virus clearance and to extend this analysis to formally demonstrate for the first time the dependence on these mechanisms for the elimination of influenza-infected respiratory epithelial cells (Fig. 5). These findings, along with our companion in vitro findings, reinforce the view that the infected respiratory epithelium is indeed recognized by effector CD8+ T cells, but this interaction selectively triggers only the activation of cytolytic effector machinery. It is noteworthy that the simultaneous elimination of both the perforin- and Fas/FasL-dependent cytolysis machinery serves to delay rather than prevent the elimination of virus-infected cells (Fig. 5). Thus, other cytolysis-dependent mechanisms (i.e., TRAIL–TRAIL-L interactions; Brincks et al., 2008) likely contribute to the overall cytolytic process used by effector CD8+ T cells to eliminate virally infected cells.
The requirement for co-stimulation in the activation of naive T cells (both CD4+ and CD8+ T cells) and in the differentiation of activated T cells into various types of effector cells has been well established from many model systems (Locksley et al., 2001; Sharpe and Freeman, 2002), including studies on the role of the co-stimulatory ligands in the induction of influenza-specific CD8+ T cell responses (Bertram et al., 2002; Humphreys et al., 2003; Vidric et al., 2005). In this study, we examined the impact of blockade of CD80 and CD86 on proinflammatory cytokine production by effector CD8+ T cells in the infected lungs, that is, after the influenza-specific CD8+ T cells had undergone activation, proliferation, and differentiation in the draining LNs (Lawrence and Braciale, 2004) and subsequently begun their migration to the site of infection (i.e., the infected lungs). We found that in vivo blockade of CD80 and CD86 resulted in markedly diminished proinflammatory cytokine production (i.e., IFN-γ) 24 h after co-stimulatory ligand blockade, both in BAL fluid IFN-γ and via the in vivo ICCS assay (Fig. 4). Importantly, this inhibition of co-stimulation of effector T cells had no effect on infectious virus titer in the infected lungs. Because the interstitial CD45+ cells (including CD11chi inflammatory mononuclear cells) express high levels of CD80 and CD86, whereas respiratory epithelial cells do not (Fig. 5 A), our results suggest that inhibition of co-stimulation through co-stimulatory ligand blockade acts to suppress proinflammatory cytokine production with minimal effect on the elimination of virus-infected respiratory epithelium. More importantly, our results as well as the findings of Humphreys et al. (2003) altering the host response to influenza infection by inhibiting OX-40–OX40-L interactions raised the possibility that we may be able to suppress excess proinflammatory cytokine responses and associated pulmonary injury without affecting virus clearance.
Our observation that CD45− cells, including respiratory epithelial cells, could trigger CD8+ T cell–mediated cytolysis but not proinflammatory cytokine production was initially unexpected but, in retrospect, not surprising. Studies over the past one to two decades have amply demonstrated a hierarchy of responses of T cells to antigen receptor engagement and co-stimulation based on strength of stimulus, with cell-mediated cytotoxicity representing one of the easiest effector responses to elicit, whereas responses like antigen-dependent T cell cytokine production require a stronger stimulus (Valitutti et al., 1996; Hemmer et al., 1998). Accordingly, influenza-infected CD45− respiratory epithelial cells, which express the requisite viral peptide–MHC complexes but do not express the relevant co-stimulatory ligands, would only stimulate the activation of perforin/FasL cytolytic machinery after TCR engagement on effector CD8+ T cells. Of note, because respiratory epithelial cells are reported to express molecules that can negatively regulate host immune/inflammatory responses (Stanciu et al., 2006; Mayer et al., 2008), we cannot exclude the possibility that, in vivo at least, negative signals delivered by infected respiratory epithelium can also act to limit the range of effector activities available to the CD8+ T cells entering the airspaces after TCR engagement.
In conclusion, in this study, we demonstrate that effector CD8+ T cell–mediated cytolysis-dependent elimination of virally infected cells (and associated virus clearance) and proinflammatory cytokine production by the T cells are differentially regulated in vivo. Cytolytic machinery can be activated by both antigen-bearing CD45+ and CD45− APCs (target cells). In contrast, in the influenza-infected lungs, only CD45+ (predominately CD11chi inflammatory mononuclear) APCs (target cells) can trigger proinflammatory cytokine production by effector CD8+ T cells. T cell–mediated proinflammatory cytokine production is, in part at least, dependent on co-stimulatory interactions mediated by CD80 and CD86. Blockade of CD80 and CD86 has no effect on virus elimination. These findings open up the possibility of therapeutic intervention in severe influenza infection by selectively inhibiting within the infected respiratory tract signaling events between effector T cells and APCs/target cells (i.e., CD45+ inflammatory mononuclear cells), which induce excess proinflammatory cytokine/chemokine responses without affecting T cell/target cell encounters resulting in virus elimination.
MATERIALS AND METHODS
Mice and infection.
Female BALB/c (H-2d) and C57BL/6 (H-2b) were purchased from the National Cancer Institute and The Jackson Laboratory. Tg mice expressing nonhuman primate DTR under the control of the mouse CD11c promotor (C.FVB-tgItgax-DTR/EGFP 57Lan/J, BALB/c background), Thy-1.1 clone 4 (CL-4) tg mice (BALB/c background), homozygous FASlpr mice (C57BL/6 background), perforin−/− mice (C57BL/6 background), and Rag1−/− mice (C57BL/6 background) were obtained from The Jackson Laboratory. IFN-γ reporter (YETI) mice (Mayer et al., 2005; C57BL/6 background) were a gift from M. Mohrs (Trudeau Institute, Saranac Lack, NY). Kb−/−Db−/− mice (Thy-1.1 C57BL/6 background) were a gift from A. Lukacher (Emory University, Atlanta, GA). To delete CD11chi cells, DTR tg mice were injected i.n. with 200 ng DTx/50 µl PBS (Sigma-Aldrich). All mice were housed in a pathogen-free environment and used at 8–14 wk of age for all experiments. All animal experiments were performed in accordance with protocols approved by the University of Virginia Animal Care and Use Committee. Type A influenza viruses A/PR/8/34 (H1N1) or A/X31 (H3N2) were grown in day 10 chicken embryo allantoic cavities as described previously (Lawrence and Braciale, 2004). Unless noted otherwise, mice were infected with 250 egg infectious doses (EID50) of A/PR/8/34 i.n. (corresponding to a 0.1 LD50 dose) or with 10,000 EID50 A/X31 i.n.
Preparation of tissue and single-cell suspension.
Mice were euthanized via cervical dislocation, and 1 ml BAL fluid was quickly collected (as described in BAL fluid cytokine determination). Lungs were perfused via the right ventricle of the heart with 3 ml PBS to remove blood lymphocytes from the vasculature. Subsequently, lung tissue was minced and enzymatically digested with type II collagenase (37°C for 30 min; Worthington), followed by passing through a steel screen. RBCs in the cell suspensions were lysed using ammonium chloride. Cells were counted using a hemacytometer after exclusion of dead cells using Trypan blue dye and resuspended at appropriate concentrations for each experiment.
Antibodies.
The following mAbs were purchased from BD or eBioscience (unless otherwise stated), as conjugated to FITC, Alexa-488, PE, PE-Cy7, PerCP-Cy5.5, APC, Alexa Fluor 647, APC–Alexa Fluor 780, or biotin: CD4 (GK1.5), CD4 (L3T4), CD8-α (53–6.7), CD11a (2D7), CD11b (M1/70), CD11c (HL3), CD19 (1D3), CD25 (PC61), CD43 (1B11), CD44 (IM7), CD45 (30-F11), CD49b (DX5), CD49d (R1-2), CD62L (MEL-14), CD69 (H1.2F3), CD80 (16-10A1), CD86 (GL-1), CD90.1 (OX-7), CD90.2 (53–2.1), CD107a (1D4B), CD107b (ABL-93), Gr-1 (RB6-8C5), SigLecF (E50-2440), F4/80 (C1:A3; Invitrogen), Ly6G (1A8), Ly6C (AL-21), Ep-CAM (G8.8; BioLegend), T1-α (clone 500; Abcam), H-2Kb (AF6-88.5), H-2Kd (SF1-1.1), I-Ad (AMS-32-1), MIP-1α (R&D Systems), IL-2 (18175A), IFN-γ (XMG1.2), TNF (MP6-XT22), HA (gift from J. Yewdell, National Institute of Allergy and Infectious Diseases, Bethesda, MD), influenza polymerase tetramer (Db; B16; Trudeau Institute), and isotype control antibodies. Anti–mouse CD16/32 used for Fc receptor blocking was isolated and purified in our laboratory. Anti–mouse influenza NP (H16) was isolated and purified in our laboratory. For biotinylated mAbs, samples were incubated with streptavidin–PerCp-Cy5.5 or -PE.
Flow cytometry analysis and intracellular staining.
Cells were suspended in FACS buffer containing PBS, 2% FBS, 10 mM EDTA, and 0.01% NaN3. Cells suspensions were blocked with anti–mouse CD16/32 and then incubated with specific mAbs or isotype/fluorescence minus one controls for 30 min at 4°C. Surface maker staining and ICCS were performed described previously (Kim and Braciale, 2009). Flow cytometry was performed on FACS Canto flow cytometers (BD), and data were analyzed using FlowJo (Tree Star, Inc.).
BAL fluid cytokine determination.
We obtained BAL fluid by making an incision in the trachea and subsequently flushing the airways three times with a single use of 1 ml sterile PBS. We spun down the BAL fluid–associated cells and collected supernatants for ELISA (BD). ELISA was performed according to the manufacturer’s manuals.
T cell depletion in vivo.
At noted dpi with influenza, we injected mice with 200 µg CD8-specific mAb (clone 2.43; BioExpress) and/or 500 µg CD4-specific mAb (clone GK1.5; BioExpress) i.p.
Viral titer.
We monitored lung viral titers via endpoint dilution assay and expressed them as tissue culture ID50 (TCID50). We incubated Madin-Darby canine kidney cells (American Type Culture Collection) with 10-fold dilutions of BAL fluid from influenza virus–infected mice in serum-free DME culture. After 3–4-d incubation at 37°C in a humidified atmosphere of 5% CO2, supernatants were collected and mixed with a half-volume of 1% chicken RBCs (University of Virginia Veterinary Facilities). Hemagglutination patterns were read thereafter, and TCID50 values were calculated.
In vitro CD8+ T cell restimulation assay.
For identification of antigen-specific polyclonal CD8+ T cells, single-cell suspensions from BALB/c mice or isolated CD8+ cells (see Adoptive Transfer for details) were co-cultured with infectious virus–pulsed P815 cells or BMDCs (10 multiplicity of infection) in a 1:1 ratio (unless indicated otherwise). We co-cultured cells for 6 h at 37°C in DME + 5% FCS in the presence of 1.6 µl ml−1 Golgi-Stop (BD). The percentage of CD8+ T cells stimulated to produce IFN-γ as determined by FACS analysis was used to calculate the number of total antigen-specific polyclonal CD8+ T cells. To measure the capacity of polyclonal CD8+ T cells to degranulate, single-cell suspensions from BALB/c mice were co-cultured with infectious virus–pulsed P815 cells (1 multiplicity of infection; or other noted cellular target) in a 1:1 ratio. We co-cultured cells for 6 h at 37°C in DME + 5% FCS in the presence of 1.6 µl ml−1 Golgi-Stop (BD) + α-CD107a/b (FITC; Betts et al., 2003). We generated BMDCs as previously described (Lutz et al., 1999). For CD80 and CD86 blockade, in vitro co-cultured cells were stimulated in media containing CD80-specific mAb (clone 16-10A1; Bio X Cell) + CD86-specific mAb (clone GL-1; Bio X Cell) or with isotype antibody (2 µg/ml). MLE-Kd cells were a gift from R. Enelow (Dartmouth Medical School, Hanover, NH).
In vivo intracellular cytokine synthesis assay.
Cytokine-producing cells in vivo were measured on a previously described protocol with modifications (Liu and Whitton, 2005). In brief, on the proscribed dpi, 50 µg BFA (Sigma-Aldrich) was administered i.n. or 500 µg monensin (Sigma-Aldrich) was administered i.p. 6 h later, we prepared lung single-cell suspensions in the presence of the respective protein transport inhibitor on ice. Tissues were not collagenase digested in this procedure. Cells were then fixed, permeabilized, and stained for intracellular cytokines as previously described (Kim and Braciale, 2009).
Irradiation and BM transplantation.
Mice were irradiated with 9.4 Gy and, within 24 h, i.v. injected with RBC-lysed BM cells (1–2 × 106) prepared from uninfected C57BL/6, Rag−/−, or perforin−/− mice.
Adoptive transfer.
For CD8+ T cell isolation, cell suspensions from spleen or lung were isolated by positive selection after incubation with anti-CD8 magnetic microbeads using MACS cell sorter (Miltenyi Biotec) in accordance with the manufacturer’s instruction. A total of 2 × 105 isolated splenic naive CL-4 tg T cells were i.v. injected into the tail vein of Thy-mismatched congenic mice at the indicated time points. For adoptively transferring T cell effectors, a total of 5 × 105 isolated CD8+ cells from a 6 dpi lung were i.v. injected into the tail vein of Thy-mismatched congenic mice at the indicated time points. Adoptively transferred cells were identified as FSCloSSCloCD45+ Thy1.2−Thy1.1+CD4−CD8+ in flow cytometric experiments. For reconstituting immune-deficient chimeric mice, 50 × 106 WT splenocytes were i.v. injected into the tail vein of Rag1−/−/MHC chimeric mice.
CD80 and CD86 blockade.
At noted dpi with influenza, we injected mice i.p. with 500 µg CD80-specific mAb (clone 16-10A1) and 500 µg CD86-specific mAb (clone GL-1) or with 1 mg of isotype antibody.
Statistics.
Unless otherwise noted, a two-tailed Mann-Whitney test was used to compare two treatment groups. Groups larger than two were analyzed with Kruskal-Wallis one-way analysis of variance test. These statistical analyses were performed using Prism3 software (for Macintosh; GraphPad Software, Inc.). Results are expressed as means ± SEM. Values of P < 0.05 were considered statistically significant.
Online supplemental material.
Fig. S1 shows the impact of T cell ablation on proinflammatory cytokine production and influenza virus clearance in the lung. Fig. S2 shows the optimization of the in vivo ICCS assay. Fig. S3 shows airspace and interstitial CD8+ T cell effector activity after in vitro restimulation. Fig. S4 shows YFP expression in airspace and interstitial CD8+ T cells in influenza-infected IFN-γ reporter mice and CD8+ T cell IFN-γ production in the adoptive transfer clone 4 model. Fig. S5 shows the migration, viral content, and CD11c expression of inflammatory mononuclear cells during influenza infection and the impact of acute CD80 and CD86 blockade on BALB/c T cell IFN-γ production during an influenza infection. Fig. S6 shows the impact of FAS and perforin deficiency on CD8+ T cell cytotoxicity and cytokine production. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101850/DC1.
Acknowledgments
We thank B. Small for excellent technical assistance and the members of the Braciale laboratory for insightful discussions. We also thank Dr. A. Lukacher and his laboratory for their technical assistance.
This study was supported by grants from the National Institutes of Health (RO1 AI-15608, RO1 AI-37293, RO1 HL-33391, and U-19 AI-83024) to T.J. Braciale and a National Institutes of Health training grant (T32 AI007496-13) to M. Hufford.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BAL
- bronchial alveolar lavage
- BFA
- brefeldin A
- BMDC
- BM-derived DC
- dpi
- day postinfection
- DTR
- diphtheria toxin receptor
- Ep-CAM
- epithelial cell adhesion molecule
- HA
- hemagglutinin
- ICCS
- intracellular cytokine staining
- i.n.
- intranasal(ly)
- NP
- nucleocapsid protein
- TCID50
- tissue culture ID50
- tg
- transgenic
- TRAIL
- TNF-related apoptosis-inducing ligand
References
- Albert M.L., Sauter B., Bhardwaj N. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89 10.1038/32183 [DOI] [PubMed] [Google Scholar]
- Bennett C.L., Clausen B.E. 2007. DC ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 28:525–531 10.1016/j.it.2007.08.011 [DOI] [PubMed] [Google Scholar]
- Bertram E.M., Lau P., Watts T.H. 2002. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 168:3777–3785 [DOI] [PubMed] [Google Scholar]
- Betts M.R., Brenchley J.M., Price D.A., De Rosa S.C., Douek D.C., Roederer M., Koup R.A. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 281:65–78 10.1016/S0022-1759(03)00265-5 [DOI] [PubMed] [Google Scholar]
- Brincks E.L., Katewa A., Kucaba T.A., Griffith T.S., Legge K.L. 2008. CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 181:4918–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S.R., Turner S.J., Miller S.C., Roberts A.D., Rappolo R.A., Doherty P.C., Ely K.H., Woodland D.L. 2003. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 198:399–410 10.1084/jem.20022151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enelow R.I., Mohammed A.Z., Stoler M.H., Liu A.N., Young J.S., Lou Y.H., Braciale T.J. 1998. Structural and functional consequences of alveolar cell recognition by CD8(+) T lymphocytes in experimental lung disease. J. Clin. Invest. 102:1653–1661 10.1172/JCI4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesq H., Bacher M., Nain M., Gemsa D. 1994. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology. 190:175–182 [DOI] [PubMed] [Google Scholar]
- Gehring A.J., Sun D., Kennedy P.T., Nolte-’t Hoen E., Lim S.G., Wasser S., Selden C., Maini M.K., Davis D.M., Nassal M., Bertoletti A. 2007. The level of viral antigen presented by hepatocytes influences CD8 T-cell function. J. Virol. 81:2940–2949 10.1128/JVI.02415-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel C.H., Willart M.A., van Rijt L.S., Muskens F., Kool M., Baas C., Thielemans K., Bennett C., Clausen B.E., Hoogsteden H.C., et al. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205:1621–1634 10.1084/jem.20071365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.B., Dalton D.K., Giltinan D., Braciale V.L., Stewart T.A., Braciale T.J. 1993. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J. Exp. Med. 178:1725–1732 10.1084/jem.178.5.1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X., Kim T.S., Braciale T.J. 2008. Differential response of respiratory dendritic cell subsets to influenza virus infection. J. Virol. 82:4908–4919 10.1128/JVI.02367-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer B., Stefanova I., Vergelli M., Germain R.N., Martin R. 1998. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J. Immunol. 160:5807–5814 [PubMed] [Google Scholar]
- Herold S., Steinmueller M., von Wulffen W., Cakarova L., Pinto R., Pleschka S., Mack M., Kuziel W.A., Corazza N., Brunner T., et al. 2008. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J. Exp. Med. 205:3065–3077 10.1084/jem.20080201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Doherty P.C. 1995. Clearance of Sendai virus by CD8+ T cells requires direct targeting to virus-infected epithelium. Eur. J. Immunol. 25:111–116 10.1002/eji.1830250120 [DOI] [PubMed] [Google Scholar]
- Humphreys I.R., Walzl G., Edwards L., Rae A., Hill S., Hussell T. 2003. A critical role for OX40 in T cell–mediated immunopathology during lung viral infection. J. Exp. Med. 198:1237–1242 10.1084/jem.20030351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussell T., Pennycook A., Openshaw P.J. 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31:2566–2573 [DOI] [PubMed] [Google Scholar]
- Kasper M., Behrens J., Schuh D., Müller M. 1995. Distribution of E-cadherin and Ep-CAM in the human lung during development and after injury. Histochem. Cell Biol. 103:281–286 10.1007/BF01457412 [DOI] [PubMed] [Google Scholar]
- Kim T.S., Braciale T.J. 2009. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 4:e4204 10.1371/journal.pone.0004204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta N.L., Kedzierska K., Stambas J., Doherty P.C. 2007. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 85:85–92 10.1038/sj.icb.7100026 [DOI] [PubMed] [Google Scholar]
- Lawrence C.W., Braciale T.J. 2004. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J. Immunol. 173:1209–1218 [DOI] [PubMed] [Google Scholar]
- Lawrence C.W., Ream R.M., Braciale T.J. 2005. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J. Immunol. 174:5332–5340 [DOI] [PubMed] [Google Scholar]
- Lin K.L., Suzuki Y., Nakano H., Ramsburg E., Gunn M.D. 2008. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J. Immunol. 180:2562–2572 [DOI] [PubMed] [Google Scholar]
- Liu F., Whitton J.L. 2005. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol. 174:5936–5940 [DOI] [PubMed] [Google Scholar]
- Locksley R.M., Killeen N., Lenardo M.J. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 104:487–501 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- Lukacher A.E., Braciale V.L., Braciale T.J. 1984. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J. Exp. Med. 160:814–826 10.1084/jem.160.3.814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M.B., Kukutsch N., Ogilvie A.L., Rössner S., Koch F., Romani N., Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 223:77–92 10.1016/S0022-1759(98)00204-X [DOI] [PubMed] [Google Scholar]
- Manicassamy B., Manicassamy S., Belicha-Villanueva A., Pisanelli G., Pulendran B., García-Sastre A. 2010. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc. Natl. Acad. Sci. USA. 107:11531–11536 10.1073/pnas.0914994107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.K., Bartz H., Fey F., Schmidt L.M., Dalpke A.H. 2008. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur. J. Immunol. 38:1689–1699 10.1002/eji.200737936 [DOI] [PubMed] [Google Scholar]
- Mayer K.D., Mohrs K., Crowe S.R., Johnson L.L., Rhyne P., Woodland D.L., Mohrs M. 2005. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-gamma production. J. Immunol. 174:7732–7739 [DOI] [PubMed] [Google Scholar]
- McGill J., Van Rooijen N., Legge K.L. 2008. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J. Exp. Med. 205:1635–1646 10.1084/jem.20080314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M.F., Popescu F.E., Gerner M., Hammerbeck C.D., Curtsinger J.M. 2007. Activation-induced non-responsiveness (anergy) limits CD8 T cell responses to tumors. Semin. Cancer Biol. 17:299–308 10.1016/j.semcancer.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G., Noda T., Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 459:931–939 10.1038/nature08157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper R.L., Van Campen H. 1995. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb. Pathog. 19:175–183 10.1006/mpat.1995.0056 [DOI] [PubMed] [Google Scholar]
- Ream R.M., Sun J., Braciale T.J. 2010. Stimulation of naive CD8+ T cells by a variant viral epitope induces activation and enhanced apoptosis. J. Immunol. 184:2401–2409 10.4049/jimmunol.0902448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A.H., Freeman G.J. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116–126 10.1038/nri727 [DOI] [PubMed] [Google Scholar]
- Stanciu L.A., Bellettato C.M., Laza-Stanca V., Coyle A.J., Papi A., Johnston S.L. 2006. Expression of programmed death-1 ligand (PD-L) 1, PD-L2, B7-H3, and inducible costimulator ligand on human respiratory tract epithelial cells and regulation by respiratory syncytial virus and type 1 and 2 cytokines. J. Infect. Dis. 193:404–412 10.1086/499275 [DOI] [PubMed] [Google Scholar]
- Sun J., Madan R., Karp C.L., Braciale T.J. 2009. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15:277–284 10.1038/nm.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Metzger D.W. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14:558–564 10.1038/nm1765 [DOI] [PubMed] [Google Scholar]
- Swanson P.A., II, Pack C.D., Hadley A., Wang C.R., Stroynowski I., Jensen P.E., Lukacher A.E. 2008. An MHC class Ib–restricted CD8 T cell response confers antiviral immunity. J. Exp. Med. 205:1647–1657 10.1084/jem.20080570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham D.J., Doherty P.C. 1998. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J. Virol. 72:882–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham D.J., Tripp R.A., Sarawar S.R., Sangster M.Y., Doherty P.C. 1996. Immune CD4+ T cells promote the clearance of influenza virus from major histocompatibility complex class II -/- respiratory epithelium. J. Virol. 70:1288–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham D.J., Tripp R.A., Doherty P.C. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197–5200 [PubMed] [Google Scholar]
- Tripp R.A., Hou S., McMickle A., Houston J., Doherty P.C. 1995. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J. Immunol. 154:6013–6021 [PubMed] [Google Scholar]
- Valitutti S., Müller S., Dessing M., Lanzavecchia A. 1996. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 183:1917–1921 10.1084/jem.183.4.1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidric M., Suh W.K., Dianzani U., Mak T.W., Watts T.H. 2005. Cooperation between 4-1BB and ICOS in the immune response to influenza virus revealed by studies of CD28/ICOS-deficient mice. J. Immunol. 175:7288–7296 [DOI] [PubMed] [Google Scholar]
- Wiley J.A., Cerwenka A., Harkema J.R., Dutton R.W., Harmsen A.G. 2001. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am. J. Pathol. 158:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]