Human B1 cells consist of CD20+CD27+CD43+CD70− cells bearing a skewed B cell receptor repertoire, and are present in umbilical cord and adult peripheral blood.
Abstract
B1 cells differ in many ways from conventional B cells, most prominently in the production of natural immunoglobulin, which is vitally important for protection against pathogens. B1 cells have also been implicated in the pathogenesis of autoimmune dyscrasias and malignant diseases. It has been impossible to accurately study B1 cells during health and illness because the nature of human B1 cells has not been successfully defined. This has produced controversy regarding the existence of human B1 cells. Here, we determined the phenotype of human B1 cells by testing sort-purified B cell fractions for three fundamental B1 cell functions based on mouse studies: spontaneous IgM secretion, efficient T cell stimulation, and tonic intracellular signaling. We found that a small population of CD20+CD27+CD43+ cells present in both umbilical cord and adult peripheral blood fulfilled these criteria and expressed a skewed B cell receptor repertoire. These B cells express little or no surface CD69 and CD70, both of which are markedly up-regulated after activation of CD20+CD27−CD43− (naive) and CD20+CD27+CD43− (memory) B cells. This work identifies human B1 cells as CD20+CD27+CD43+CD70−. We determined that the proportion of B1 cells declines with age, which may contribute to disease susceptibility. Identification of human B1 cells provides a foundation for future studies on the nature and role of these cells in human disease.
B lineage expression of the 67-kD pan–T-cell antigen CD5 was first detected on the surface of certain human and murine malignancies 30 yr ago, and was subsequently identified on a subset of normal B cells in both species (Kantor and Herzenberg, 1993; Morris and Rothstein, 1994; Hardy and Hayakawa, 2001). In mice, CD5 expression identifies a distinct B cell lineage, termed B1, which manifests unique ontological, anatomical, and functional characteristics. In contrast to conventional B2 cells, murine B1 cells derive from CD19+B220− progenitors, appear early in development, and preferentially locate to coelomic cavities (Herzenberg, 2000; Berland and Wortis, 2002; Rothstein, 2002; Dorshkind and Montecino-Rodriguez, 2007). Most importantly, B1 cells differ functionally from B2 cells by spontaneously secreting “natural” Ig that is generated in the absence of specific immunization and which accounts for most of the resting IgM, and a large portion of the resting IgA, found in normal serum (Sidman et al., 1986; Forster and Rajewsky, 1987; Ishida et al., 1992; Kroese et al., 1993). This B1 cell–derived natural Ig differs from B2 cell–derived antibody because it is more germline-like as a result of minimal N-region addition and somatic hypermutation—and is broadly reactive, autoreactive, and repertoire-selected (Forster et al., 1988; Hayakawa and Hardy, 1988; Hardy et al., 1989; Pennell et al., 1989; Gu et al., 1990). Natural Ig is vitally important in the early defense against bacterial and viral infections (Briles et al., 1981; Boes et al., 1998; Ochsenbein et al., 1999; Baumgarth et al., 2000; Haas et al., 2005), and may play a role in a wide variety of diseases through recognition of self-antigens and binding of cellular debris (Binder and Silverman, 2005). In addition, B1 cells differ functionally from B2 cells in efficiently presenting antigen to T cells (Zhong et al., 2007) and in displaying evidence of tonic signaling (Karras et al., 1997; Wong et al., 2002; Holodick et al., 2009b) in the “resting” state in the absence of specific stimulation. Somewhat akin to anergic B cells, B1 cells are relatively nonresponsive to B cell receptor (BCR) engagement (Morris and Rothstein, 1993; Wong et al., 2002). Whereas B1 cells have been considered to be self-renewing, and thus self-perpetuating, in adult animals (Hayakawa et al., 1986; Kantor et al., 1995), recent evidence suggests that new bone marrow emigrants are continually added to the B1 cell pool (Duber et al., 2009; Holodick et al., 2009a).
A subpopulation of CD5-expressing B cells is found in various human tissues; these CD5+ B cells are capable of autoantibody production, and the number of such CD5+ B cells is expanded in some autoimmune diseases (Plater-Zyberk et al., 1985; Taniguchi et al., 1987; Burastero et al., 1988; Dauphinee et al., 1988). The significance of these findings vis a vis B1 cells is uncertain, however, because it is not clear that CD5 is a durable marker of the B1 cell population across species. Not only is CD5 expressed on B2 cell populations in the human system (including transitional, prenaive, and activated B cells), but in other mammals CD5 is nondiscriminatory (Freedman et al., 1989; Raman and Knight, 1992; Sims et al., 2005; Wilson and Wilkie, 2007; Lee et al., 2009). Further, both CD5− and CD5+ B cells can produce IgM autoantibodies (Casali and Notkins, 1989; Kasaian et al., 1992; Mackenzie et al., 1991). As a result, there has been much controversy regarding whether B1 cells exist at all in Homo sapiens, and if so, how human B1 cells might be characterized. Resolution of this problem is of great importance, because a full understanding of the relationship between B1 cells and diseases ranging from autoimmune dyscrasias to lymphoid malignancies, and the development of therapeutics to enhance natural Ig, depends on elucidating identifying features that will allow human B1 cells to be readily enumerated and functionally evaluated in clinical situations. To address this issue, we screened various phenotypically defined populations from umbilical cord and adult peripheral blood for key characteristics of the well-studied murine B1 cell population, specifically, spontaneous IgM secretion, efficient T cell stimulation, and tonic intracellular signaling. Using these functional criteria, we identified the phenotypic profile for human B1 cells as CD20+CD27+CD43+CD70−. We determined that some characteristics attributed to human memory B cells identified on the basis of CD27 expression are actually produced by (CD27+) B1 cells.
RESULTS
In the murine system, B1 cell progenitors are more abundant in fetal hematopoietic tissues than in adult hematopoietic tissues, and B1 cells emerge before the bulk of B2 cell production occurs (Montecino-Rodriguez et al., 2006). Following this paradigm, we first examined umbilical cord blood specimens for spontaneous IgM secretion by ELISPOT assay, which we used for initial screening, and then looked for efficient T cell stimulation and tonic intracellular signaling. We isolated B cells that were CD20+ to avoid CD19+CD20− plasmablasts that might secrete IgM.
Umbilical cord blood contains CD20+CD27+CD43+ B cells
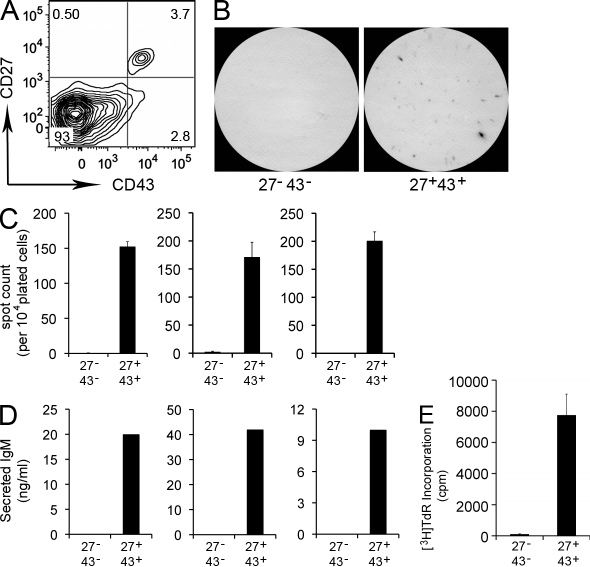
Initially, we sort-purified CD20+ B cells from umbilical cord blood samples, and then tested for IgM secretion in 3-h ELISPOT assays. We found that unstimulated cord blood B cells spontaneously generated IgM-containing ELISPOTs (unpublished data). To phenotypically characterize the Ig-secreting population, we examined various CD20+ cord blood populations defined by known B cell surface antigens. Among these populations, we unexpectedly identified a small subset of CD27+ cells, ranging from 3 to 11% of CD20+ B cells. Similar numbers of CD27+ cord blood B cells were detected by a variety of different anti-CD27 immunofluorescent reagents (unpublished data), and expression of CD27 mRNA coincident with expression of CD27 surface antigen was verified by real-time PCR conducted on sort-purified CD20+CD27+ and CD20+CD27− cord blood cells (unpublished data). These CD20+CD27+ cord blood B cells uniquely express CD43, a well-described marker for murine B1 cells (Wells et al., 1994), whereas CD20+CD27− cord blood B cells are CD43− (Fig. 1 A). Thus, CD20+ cord blood B cells segregate into two populations, CD27+CD43+ (amounting to 6.1 ± 1.1%; mean ± SEM; n = 13) and CD27−CD43− (amounting to 93.9 ± 1.1%).
Figure 1.
Umbilical cord blood CD20+CD27+CD43+ B cells spontaneously secrete IgM and efficiently stimulate T cells. (A) Umbilical cord blood mononuclear cells were stained with immunofluorescent antibodies and evaluated by flow cytometry. The contour plot displays expression of CD27 and CD43 by gated CD20+ cells. Results shown represent 1 of 13 separate cord blood samples. (B) Sort-purified CD20+CD27−CD43− (27−43−) and CD20+CD27+CD43+ (27+43+) cord blood B cells were plated at 1 × 104 cells per well, incubated for 3 h at 37°C, and analyzed for IgM secretion by ELISPOT. Images shown are representative of three separate experiments on three different cord blood samples each done in triplicate. (C) Enumeration of ELISPOT results displayed as mean values for triplicate wells, with error bars indicating the SEM. Each bar graph indicates an individual experiment on a separate cord blood sample. (D) Sort-purified CD20+CD27−CD43− and CD20+CD27+CD43+ cord blood B cells were cultured for 5 d, after which supernatants were evaluated for secreted IgM by ELISA. Each bar graph indicates an individual experiment on a separate cord blood sample. (E) Sort purified and irradiated CD20+CD27−CD43− (27−43−) and CD20+CD27+CD43+ (27+43+) cord blood B cells were evaluated for the ability to drive allogeneic T cell proliferation as measured by tritiated thymidine incorporation for 8 h at the end of 5 d co-cultures. Data shown are representative of three separate experiments on three different cord blood samples each done in triplicate. Mean cpm values are displayed with error bars indicating SEM.
Umbilical cord blood CD20+CD27+CD43+ B cell Igs express few mutations
In the human system, CD27 expression is generally considered to mark memory B cells (Agematsu et al., 2000), which suggests incongruity in the identification of CD27+ B cells in cord blood that is obtained at birth before exogenous antigen exposure. To clarify the nature of umbilical cord blood CD20+CD27+CD43+ B cells, we examined the mutational status of Ig amplified from single cells, as described in Materials and methods. In contrast to the large number of somatic mutations present in adult CD27+ B cell–derived antibodies (Klein et al., 1998), we found that all cord blood B cells express antibodies with very low levels of somatic mutation, both CD20+CD27+CD43+ and CD20+CD27−CD43− B cells (Table I, Fig. S1 and Tables S1 and S2). All cord blood CD27+CD43+ B cell Ig sequences contained N region additions at the V–D and D–J junctions, much like CD27−CD43− B cell Ig sequences (Table I) reflecting the presence of terminal deoxynucleotidyl transferase throughout ontogeny in the human system (Asma et al., 1986). Among cord blood B cells, CD27 expression does not correlate with increased Ig mutational status and does not correspond to memory B cell status, and thus is presumably indicative of another B cell population.
Table I.
Umbilical cord blood CD20+CD27+CD43+ B cell immunoglobulins express few mutations
| B cell populations | Total mutations (no. of bases) | VH mutations (no. of bases) | N1 additions (no. of bases) | N2 additions (no. of bases) | CDR3 length (no. of bases) |
| B1: CD20+CD27+CD43+ | 2.0 ± 0.3 | 0.14 ± 0.05 | 6.8 ± 1.2 | 3.8 ± 0.7 | 14.5 ± 0.7 |
| B2: CD20+CD27−CD43− | 1.9 ± 0.4 | 0.08 ± 0.06 | 6.4 ± 0.8 | 4.6 ± 0.8 | 14.8 ± 0.7 |
Number of cells analyzed: B1, n = 43; B2, n = 25. Mean number ± SEM.
Umbilical cord blood CD20+CD27+CD43+ B cells spontaneously secrete IgM
We sort-purified cord blood CD20+ B cells and found that CD27 expression (and synonymous CD43+ expression) neatly and completely separated cord blood B cells that spontaneously secrete IgM from those that do not, as shown by ELISPOT (Fig. 1 B,C) and ELISA (Fig. 1 D) analyses. Thus, a small population of CD20+CD27+CD43+ B cells, amounting on average to <10% of total B cells, is responsible for spontaneous IgM secretion in this tissue.
Umbilical cord blood CD20+CD27+CD43+ B cells efficiently stimulate T cells
To determine the extent to which IgM-secreting umbilical cord blood CD20+CD27+CD43+ B cells express other characteristics derived from murine B1 cell studies, we examined the efficiency of CD4+ T cell allogeneic stimulation produced by irradiated B cell populations, assessed by thymidine incorporation. We found that CD20+CD27+CD43+ B cells strongly stimulated T cell proliferation over a 5-d period, whereas CD20+CD27−CD43− B cells had little effect (Fig. 1 E). Thus, not only do CD20+CD27+CD43+ B cells efficiently stimulate T cells, but this functional characteristic is completely restricted to the CD27+CD43+ B cell population.
Umbilical cord blood CD20+CD27+CD43+ B cells exhibit tonic intracellular signaling
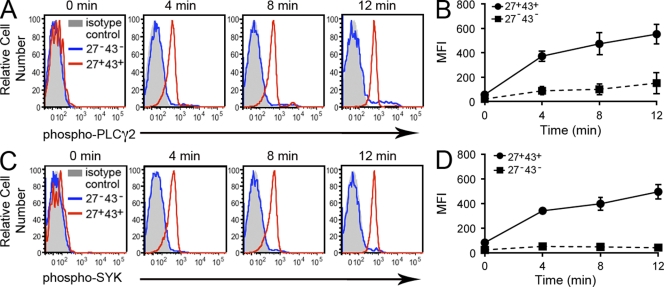
To further evaluate the extent to which IgM-secreting, T cell–stimulating umbilical cord blood CD20+CD27+CD43+ B cells express other murine B1 cell characteristics, we examined tonic intracellular signaling by phosphoflow analysis, in which tyrosine phosphorylation of phospholipase C gamma 2 (PLC-γ2) and spleen tyrosine kinase (Syk) was readily visualized by intracellular immunofluorescent staining after inhibition of phosphatase activity. Untreated B cells showed little evidence of phosphorylated PLC-γ2 or phosphorylated Syk. Within minutes of phosphatase inhibition, we found that CD20+CD27+CD43+ B cells expressed substantial levels of phosphorylated PLC-γ2 and phosphorylated Syk, which increased further with time, whereas the levels of phosphorylated PLC-γ2 and phosphorylated Syk in CD20+CD27−CD43− B cells did not change (Fig. 2). Thus, the small population of CD20+CD27+CD43+ cord blood B cells displays tonic intracellular signaling that is apparent when phosphatase activity is inhibited, whereas the majority of cord blood B cells that are negative for CD27 expression do not.
Figure 2.
Umbilical cord blood CD20+CD27+CD43+ B cells exhibit tonic intracellular signaling. (A) Cord blood mononuclear cells were unexposed (0) or were exposed to pervanadate for 4, 8, and 12 min and then fixed, permeabilized and stained for surface antigens and intracellular phosphorylated PLC-γ2 with specific immunofluorescent antibodies. Because isotype control antibody staining by “fluorescence minus one” did not vary between the two cell populations being studied, only a single control tracing is shown. Results for one of three comparable experiments are shown. (B) Mean values of mean fluorescence intensity (MFI) are shown (with error bars indicating the SEM) for intracellular phosphorylated PLC-γ2 staining at various time points from three separate umbilical cord blood samples. (C) Cord blood mononuclear cells were unexposed (0) or were exposed to pervanadate for 4, 8, or 12 min, and then fixed, permeabilized, and stained for surface antigens and intracellular phosphorylated Syk with specific immunofluorescent antibodies. Results for one of three comparable experiments are shown. (D) Mean values of MFI are shown (with error bars indicating the SEM) for intracellular phosphorylated Syk staining at various time points from three separate umbilical cord blood samples.
In sum, on the basis of spontaneous IgM secretion, efficient T cell stimulation, tonic intracellular signaling, and low level Ig somatic mutation, CD20+CD27+CD43+ cord blood B cells appear to fulfill the functional criteria for B1 cell status.
Adult peripheral blood CD20+CD27+CD43+ B cells spontaneously secrete IgM
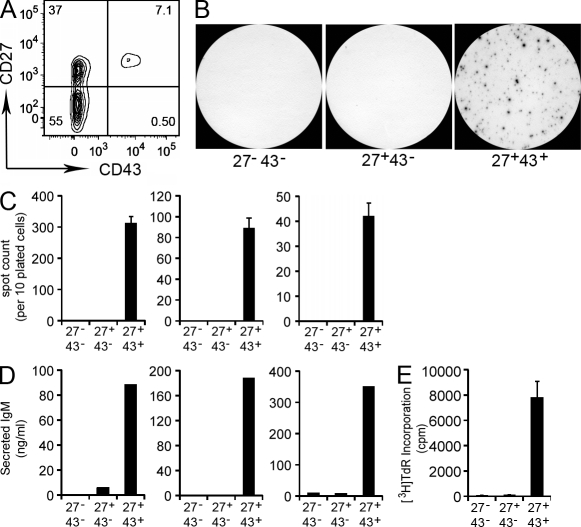
Following the strategy outlined for umbilical cord blood B cells, we sort-purified CD20+ B cells from adult peripheral blood samples, and then tested for IgM secretion in 3-h ELISPOT assays. We found that unstimulated adult B cells spontaneously generated IgM-containing ELISPOTs, and that IgM secretion segregated with CD27 expression (unpublished data). We then extrapolated from our umbilical cord blood studies, in which CD43 expression coincided with CD27 expression on a nonmemory B cell population, to separate adult peripheral blood CD20+CD27+ B cells according to CD43 expression. In so doing, CD27 and CD43 defined 3 distinct populations of CD20+ B cells: CD27+CD43+ (12.7 ± 1.6% of total CD20+ B cells; mean ± SEM; n = 25), CD27+CD43− (19.6 ± 2.2%), and CD27−CD43− (67.7 ± 2.4%; Fig. 3 A), the first of which parallels the phenotype of IgM-secreting cord blood B cells. Indeed, when tested by ELISPOT (Fig. 3, B and C) and ELISA (Fig. 3 D), we found that only the small CD20+CD27+CD43+ population from adult blood spontaneously secreted IgM, recapitulating the results obtained with the comparable cord blood B cell population. Further, CD20+CD27+CD43− and CD20+CD27−CD43− B cells were negative for spontaneously secreted IgM, even when 10-fold more of these cells were plated on ELISPOT as compared with CD20+CD27+CD43+ B cells (unpublished data).
Figure 3.
Adult peripheral blood CD20+CD27+CD43+ B cells spontaneously secrete IgM and efficiently stimulate T cells. (A) Adult peripheral blood mononuclear cells were stained with immunofluorescent antibodies and evaluated by flow cytometry. The contour plot displays expression of CD27 and CD43 by gated CD20+ cells. Results shown represent 1 of 25 separate peripheral blood samples. (B) Sort-purified CD20+CD27−CD43− (27−43−), CD20+CD27+CD43− (27+43−), and CD20+CD27+CD43+ (27+43+) adult peripheral blood B cells were plated at 1 × 104 cells per well, incubated for 3 h at 37°C, and analyzed for IgM secretion by ELISPOT. Images shown are representative of three separate experiments on three different adult blood samples each done in triplicate. (C) Enumeration of ELISPOT results displayed as mean values for triplicate wells, with error bars indicating SEM. Each bar graph indicates an individual experiment on a separate adult blood sample. (D) Sort-purified CD20+CD27+CD43+, CD20+CD27+CD43−, and CD20+CD27−CD43− adult blood B cells were cultured for 5 d, after which supernatants were evaluated for secreted IgM by ELISA. Each bar graph indicates an individual experiment on a separate adult blood sample. (E) Sort-purified and irradiated CD20+CD27−CD43− (27−43−), CD20+CD27+CD43− (27+43−), and CD20+CD27+CD43+ (27+43+) adult peripheral blood B cells were evaluated for the ability drive allogeneic T cell proliferation as measured by tritiated thymidine incorporation for 8 h at the end of 5-d cultures. Data shown are representative of three separate experiments on three different blood samples each done in triplicate. Mean cpm values are displayed with error bars indicating SEM.
Adult peripheral blood CD20+CD27+CD43+ B cells efficiently stimulate T cells
To determine the extent to which IgM-secreting CD20+CD27+CD43+ adult peripheral blood B cells express other B1 characteristics (and recapitulate the features of cord blood CD20+CD27+CD43+ B cells), we examined the efficiency of allogeneic CD4+ T cell stimulation produced by irradiated B cell populations. We found that CD20+CD27+CD43+ B cells strongly stimulated T cell proliferation, whereas CD20+CD27+CD43− B cells, and CD20+CD27−CD43− B cells, had little effect (Fig. 3 E). In particular, CD20+CD27+CD43+ B cells exceeded CD20+CD27+CD43− B cells by >50-fold in the ability to stimulate T cell proliferation. Thus, in comparison with the bulk of adult peripheral B cells that do not express CD43, only the small population of CD20+CD27+CD43+ B cells efficiently stimulates T cells.
Adult peripheral blood CD20+CD27+CD43+ B cells exhibit tonic intracellular signaling
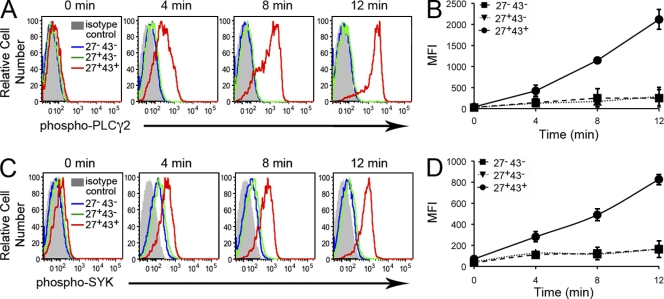
To further evaluate IgM-secreting, T cell–stimulating adult peripheral blood B cells, we examined tonic intracellular signaling by phosphoflow analysis of phosphorylated PLC-γ2 and Syk, as described earlier. Untreated B cells showed little evidence of phosphorylated PLC-γ2 or phosphorylated Syk. As with cord blood B cells, within minutes of phosphatase inhibition we found that CD20+CD27+CD43+ B cells expressed substantial levels of phosphorylated PLC-γ2 and phosphorylated Syk, whereas the levels of phosphorylated PLC-γ2 and phosphorylated Syk in CD20+CD27+CD43− and CD20+CD27−CD43− B cells changed little (Fig. 4). Thus, the small population of CD20+CD27+CD43+ adult blood B cells, and only that population, displays tonic intracellular signaling.
Figure 4.
Adult peripheral blood CD20+CD27+CD43+ B cells exhibit tonic intracellular signaling. (A) Adult blood mononuclear cells were unexposed (0) or were exposed to pervanadate for 4, 8, or 12 min, and then fixed, permeabilized, and stained for surface antigens and intracellular phosphorylated PLC-γ2 with specific immunofluorescent antibodies. Because isotype control antibody staining by “fluorescence minus one” did not vary between the three cell populations under study, only a single control tracing is shown. Results for one of three comparable experiments are shown. (B) Mean values of MFI are shown (with error bars indicating the SEM) for intracellular phosphorylated PLC-γ2 staining at various time points from three separate adult blood samples. (C) Adult blood mononuclear cells were unexposed (0) or were exposed to pervanadate for 4, 8, or 12 min, and then fixed, permeabilized, and stained for surface antigens and intracellular phosphorylated Syk with specific immunofluorescent antibodies. Results for one of three comparable experiments are shown. (D) Mean values of MFI are shown (with error bars indicating the SEM) for intracellular phosphorylated Syk staining at various time points from three separate adult peripheral blood samples.
In sum, because CD20+CD27+CD43+ adult blood B cells reproduce the characteristics of CD20+CD27+CD43+ cord blood B cells in manifesting three key B1 cell functional characteristics, CD27 and CD43 identify a B cell population throughout ontogeny that represents the human equivalent of murine B1 cells. Additional surface antigens displayed by CD20+CD27+CD43+ B cells (as single peak expression with >90% positive) are as follows: IgD, CD19, CD21, CD44, CD45RB, and HLA-DR (Fig. S2).
CD20+CD27+CD43+ B1 cells display two typical B1 cell specificities
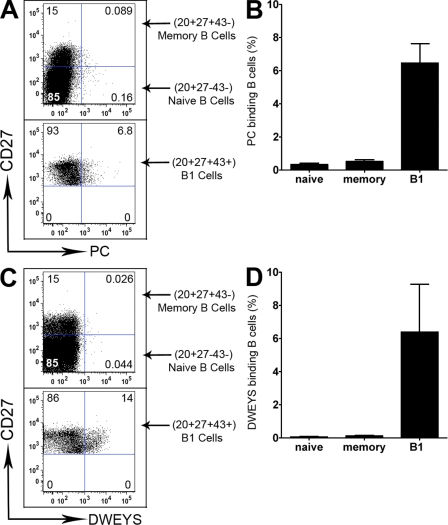
The production of particular antimicrobial and autoantibody specificities is a well-described characteristic of murine B1 cells (Hayakawa et al., 1984) and it has been reported that autoreactive Ig is preferentially produced by human CD5+ B cells (Casali and Notkins, 1989), although it has also been reported that human CD5− B cells produce autoantibodies (Mackenzie et al., 1991; Kasaian et al., 1992). Because the repertoire of human B1 cells might well differ from murine B1 cells in terms of pathogenic microbes and self-antigens in view of the tens of millions of years that have passed since murine and human evolution diverged, we did not initially use specificity criteria based on the mouse system to identify human B1 cells. However, having determined the phenotype of human B1 cells, it was of interest to examine whether, as in murine B1 cells, the repertoire of CD20+CD27+CD43+ B1 cells is weighted in favor of antimicrobial and autoantibody specificities. To address this issue, we exposed human adult peripheral blood mononuclear cells to phosphorylcholine (PC; Lalor and Morahan, 1990) and to aspartate-tryptophan-glutamate-tyrosine-serine (DWEYS) tetramer DNA mimetope (Shirai et al., 1991; Jacobi et al., 2009) and determined the level of binding to CD20+CD27+CD43+ (B1), CD20+CD27+CD43− (memory), and CD20+CD27−CD43− (naive) B cells, as described in Materials and methods. We found that only B1 cells, and not memory or naive B cells, contained a substantial number of PC-binding and mimetope-binding antigen receptors (Fig. 5). Thus, human B1 cells are similar to murine B1 cells in displaying a skewed antigen receptor repertoire as indicated by preferential expression of anti-PC and anti-DNA specificities.
Figure 5.
CD20+CD27+CD43+ B1 cells display two typical B1 cell specificities. Adult peripheral blood mononuclear cells were immunofluorescently stained with specific antibodies and with either PC-BSA-fluorescein (A and B) or biotinylated DWEYS tetramer plus PE-streptavidin (C and D), as described in the Materials and methods. Representative results are shown in A and C for three different B cell populations (CD20+CD27+CD43+ B1 cells, CD20+CD27+CD43− memory B cells, and CD20+CD27−CD43− naive B cells). (top) Gated CD20+CD43− cells; (bottom) gated CD20+CD27+CD43+ cells. Aggregate results are shown in B and D, with the proportion of B cells that bound antigen displayed on the Y axis as mean ± SEM, n = 4.
CD20+CD5+ B cells are not the same as CD20+CD27+CD43+ B1 cells
Inasmuch as human CD5+ B cells have been reported to express autoreactive specificities, our results raise the question of the relationship between CD5+ B cells and CD27+CD43+ B1 cells. To address this issue, we immunofluorescently stained and analyzed adult peripheral blood CD20+ B cells for CD5, CD27, and CD43. We found that CD20+CD27+CD43+ B cells were largely CD5+, with ∼75 ± 2.5% (mean ± SEM; n = 46) expressing CD5 (Fig. 6 and Fig. S2 B). However, CD20+CD27+CD43+ B1 cells comprised only a minority (34 ± 3.0%) of CD20+CD5+ B cells, so that approximately 2/3 of CD20+CD5+ B cells were outside the CD20+CD27+CD43+ B1 cell pool. Thus, B cell positivity for CD5 captures approximately 3/4, but misses approximately 1/4, of human CD20+CD27+CD43+ B1 cells, and includes many B cells that are not B1 cells, consistent with reports that CD5 marks prenaive, transitional, and activated B cell populations.
Figure 6.
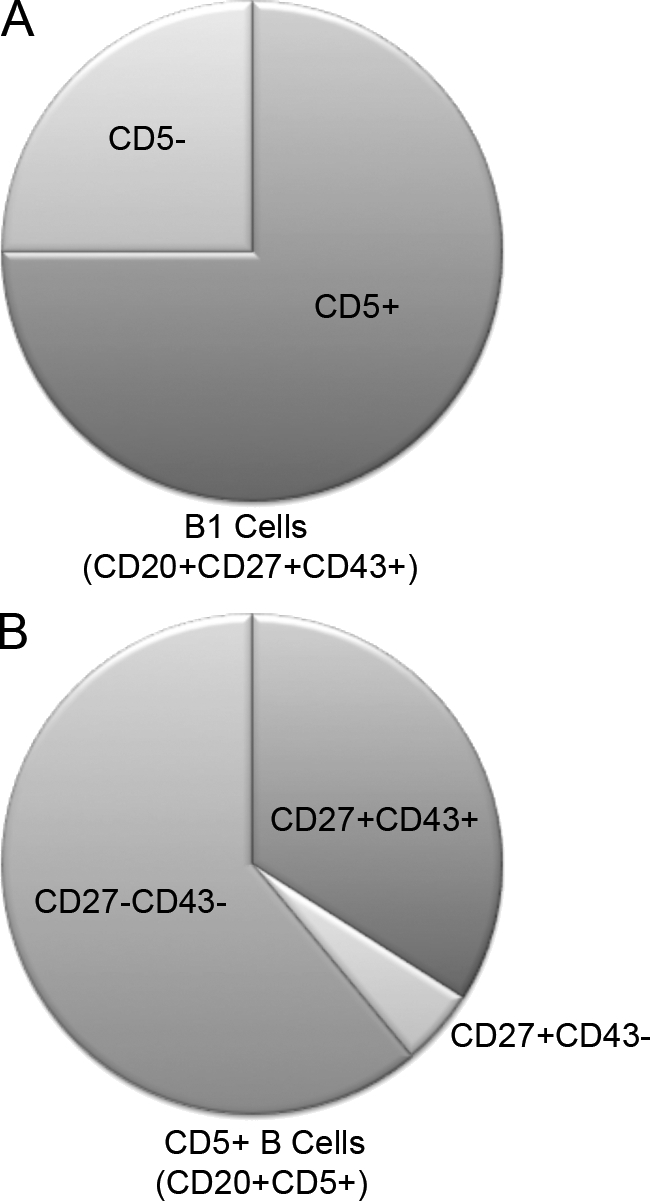
CD20+CD5+ B cells are not the same as CD20+CD27+CD43+ B1 cells. Adult peripheral blood mononuclear cells were immunofluorescently stained with specific antibodies. The proportion of CD20+CD27+CD43+ B1 cells that express CD5 (75 ± 2.5%; n = 46) is shown in A; the proportions of CD20+CD5+ B cells that phenotype as CD20+CD27+CD43+ (34 ± 3.0%; n = 46), CD20+CD27+CD43− (4.8 ± 0.41%), and CD20+CD27−CD43− (61 ± 3.1%) are shown in B. Representative flow cytometry data are shown in Fig. S2.
CD20+CD27+CD43+ B1 cells decline with age in normal individuals
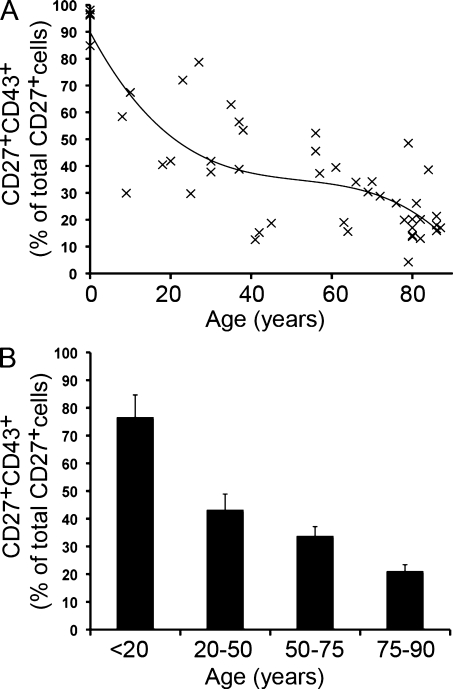
To evaluate the variation in B cell populations among normal individuals and with advancing age, we screened 6 umbilical cord and 43 adult peripheral blood samples obtained from normal volunteers. As expected, the fraction of B cells expressing CD27 that lack CD43 (CD20+CD27+CD43−) increased from very few in cord blood to >50% in the sixth through eighth decades as (true) memory B cells accumulated. We found, conversely, that the fraction of CD27+ B cells expressing CD43 (CD20+CD27+CD43+) declined from nearly 100% in cord blood to <20%, on average, in the eighth decade and older (Fig. 7). We used various regression models to examine this trend; the cubic model had a higher R-squared than the quadratic model and the line so generated is shown. The same data were separately evaluated by comparing samples from individuals under age 20, between 20 and 50, between 50 and 75, and between 75 and 90 years of age. This comparison shows a progressive loss of CD43+ B cells among CD20+CD27+ B cells with increasing age, amounting to more than fourfold between the youngest and oldest age groups.
Figure 7.
CD20+CD27+CD43+ B1 cells decline with age in normal individuals. (A) Cord blood (n = 6) and peripheral blood (n = 43) samples were stained with immunofluorescent antibodies and evaluated by flow cytometry. CD20+CD27+CD43+ B cells are expressed as a percentage of the total number of CD20+CD27+ cells at the ages indicated. The line through the datapoints represents a cubic regression curve fit (R-squared = 0.070494). (B) Mean values for the proportion of CD43+ B cells among CD20+CD27+ B cells are shown for the indicated age intervals with error bars indicating the SEM.
Expression of CD69 and CD70 differentiates B1 cells from activated memory and naive B cells
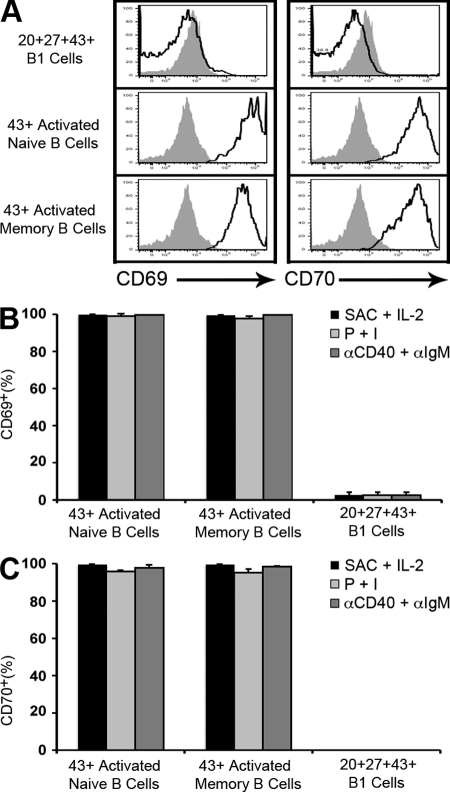
Although in the murine system CD43 marks B1 cells and only appears on mature B2 cells during terminal differentiation (Gulley et al., 1988), we considered the possibility that CD43 might be inducible in the human system, much like CD5 (Freedman et al., 1989; Cong et al., 1991). In such an instance, CD20+CD27+CD43+ B cell populations could contain an unknown number of activated B cells in addition to B1 cells. To address this possibility, we cultured CD20+CD43− adult peripheral blood B cells (both CD27+ and CD27−), with medium alone, or with LPS, CpG, anti-IgM, Staphylococcus aureus Cowan strain 1 (SAC) plus IL-2, PMA plus ionomycin, and anti-CD40 plus anti-IgM, for 4 d and reassessed expression of CD43. Although many stimuli, including BCR engagement with anti-IgM failed to up-regulate CD43 expression on previously CD43− B cells, we found that combinations of SAC plus Il-2, PMA plus ionomycin, and anti-CD40 plus anti-IgM did up-regulate expression. However, these activated memory and naive B cells differed from unstimulated B1 cells in two important ways. First, activated, CD20+CD43+ fractions of previously CD43− memory (CD20+CD27+CD43−) and CD43− naive (CD20+CD27−CD43−) B cells failed to spontaneously secrete IgM as detected by ELISPOT assay (unpublished data), and thus cannot account for this key B1 cell attribute. Second, activated, CD43+ fractions of previously CD43− memory and CD43− naive B cells expressed markedly elevated levels of CD69 and CD70 for each of the CD43-inducing stimuli; such high levels of CD69 and CD70 were not found on unstimulated B1 cells that are instead CD69lo/− and CD70− (Fig. 8). In fact, for all forms of CD43-inducing stimulation, CD43+ activated memory (n = 3) and naive B cells (n = 3) were >99% positive for CD69 and >97% positive for CD70, whereas B1 cells (n = 3) were <3% positive for CD69 and <1% positive for CD70.
Figure 8.
Expression of CD69 and CD70 differentiates B1 cells from activated memory and naive B cells. (A) CD20+CD27+CD43− memory and CD20+CD27−CD43− naive B cells were sort-purified from adult peripheral blood and were cultured with SAC (0.005%) plus IL-2 (5 ng/ml) for 4 d, after which surface expression of CD43, CD69, and CD70 was determined by immunofluorescent staining and flow cytometry. Unstimulated CD20+CD27+CD43+ B1 cells were immunofluorescently stained and analyzed for CD69 and CD70 at the same time. CD69 and CD70 expression is displayed for CD43+ B cells. Results are shown for one of three separate samples. Fluorescence minus one isotype control staining is shown in solid gray for each population. (B and C) Memory and naive B cells from adult blood were cultured for 4 d with SAC plus IL-2, PMA at 100 ng/ml plus ionomycin at 400 ng/ml (P + I), or anti-CD40 at 10 µg/ml plus anti-IgM at 7 µg/ml (αCD40 plus αIgM), and immunofluorescently stained, after which surface expression of CD43, CD69, and CD70 was determined. Unstimulated CD20+CD27+CD43+ B1 cells were immunofluorescently stained and analyzed at the same time. The proportion of CD43+ B cells that stained positively for CD69 (B) and for CD70 (C) is shown. Mean values for three separate adult blood samples for each population are shown along with error bars indicating SEM.
Although the levels of CD69/CD70 expression on B1 cells and activated memory and naive B cells are essentially nonoverlapping, indicating that they represent distinct B cell populations, we repeated functional assays with adult peripheral blood B1 cells in which the topmost 5% of B1 cells in terms of CD70 staining density were excluded (thereby insuring that no activated B cells with high CD70 expression would be present). We found no difference in IgM secretion, T cell stimulation, and tonic signaling in comparing CD20+CD27+CD43+CD70− B cells with CD20+CD27+CD43+ B cells (unpublished data). Thus, the properties of human B1 cells cannot be accounted for by contaminating activated B2 cells.
Human B1 cells are not contained within the CD20− B cell population
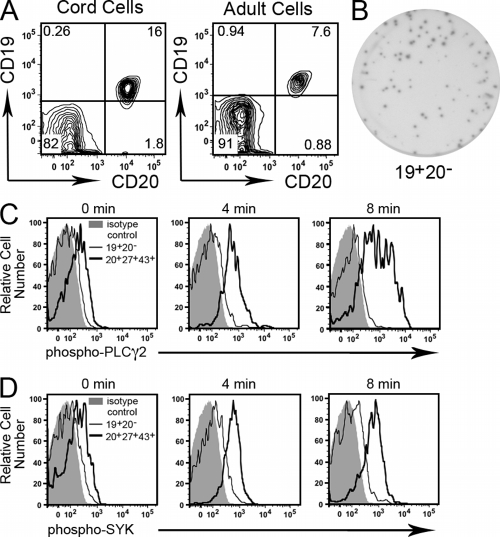
In identifying human B1 cells we focused on CD20+ B cells to avoid the few circulating terminally differentiating (CD20−) plasma cells that have been reported in peripheral blood samples and that can secrete Ig (Radbruch et al., 2006). Umbilical cord blood lacks CD19+CD20− B cells (Fig. 9 A), and so it is clear that no version of human B1 cells exists among putative CD20− B cells in cord blood because CD20− B cells do not exist in cord blood. However, in adult peripheral blood ∼1% of CD19+ B cells are CD20−. This very small number, less than 1/10 the number of CD20+CD27+CD43+ B1 cells, suggests that if a version of human B1 cells exists among CD19+CD20− B cells in adult blood it would constitute a very small fraction of total B1 cells. To determine whether CD19+CD20− B cells from adult blood meet the functional criteria established for B1 cell status, we examined spontaneous IgM secretion and tonic intracellular signaling (whereas insufficient CD19+CD20− B cells were obtained to test T cell stimulation). We found that, as expected, CD19+CD20− B cells from adult blood generated IgM-containing ELISPOTs (Fig. 9 B). In fact, the frequency and size of ELISPOTs produced by CD19+CD20− B cells was much greater than found in the ELISPOTs produced by CD20+CD27+CD43+ B cells from either cord or adult blood. Importantly, however, we found little or no increase in either phosphorylated PLC-γ2 or phosphorylated Syk among CD19+CD20− B cells after phosphatase inhibition, in contrast to the marked increase observed in CD20+CD27+CD43+ B1 cells (Fig. 9, C and D). CD19+CD20− B cells fail to display tonic intracellular signaling, and thus fail to express a key feature of B1 cells. Therefore, as functionally defined, human B1 cells do not exist to a measurable extent within the CD19+CD20− B cell population in adult blood.
Figure 9.
Human B1 cells are not contained within the CD20− B cell population. (A) Umbilical cord blood and adult peripheral blood mononuclear cells were stained with immunofluorescent antibodies and evaluated by flow cytometry. Expression of CD20 and CD19 is displayed. Representative results for one of three cord blood and one of three adult blood samples are shown. (B) CD19+CD20− B cells were sort-purified from adult blood and plated at 800 cells per well, incubated for 1 h at 37°C, and analyzed for IgM secretion by ELISPOT. The image shown is representative of three separate experiments on three different adult blood samples. (C) Adult blood CD19+CD20− B cells (thin black lines) and CD20+CD27+CD43+ B1 cells (thick black lines) were treated with the phosphatase inhibitor pervanadate. At the indicated times, B cells were immunofluorescently stained and phosphorylation of PLC-γ2 (C) and Syk (D) was analyzed as described in the legend to Fig. 4. Results for one of three comparable experiments are shown.
DISCUSSION
The identity and even existence of human B1 cells has been in doubt for many years because of the absence of known cell surface markers for this population. This contrasts with the murine system, in which the use of CD5 to identify B1 cells has led to a rich accumulation of information concerning the unique developmental and behavioral characteristics of this B cell subset. We have now examined three foundational features elucidated through studies of murine B1 cells and applied them to the human system by working in reverse to discover the surface antigens expressed on human B cells that exhibit these index functional characteristics. By evaluating spontaneous IgM secretion, efficient T cell stimulation, and tonic intracellular signaling, in conjunction with somatic hypermutation and repertoire skewing, we determined that human B1 cells are CD20+ B cells that coexpress CD27 and CD43 in both umbilical cord blood and in adult peripheral blood. The results are very clear cut in that not only do CD20+CD27+CD43+ cells express each of three key functional characteristics, but other CD20+ B cell populations examined directly ex vivo do not express even one. Overall, this represents a major step forward in the translation of what is known about B1 cell physiology and pathology from animal models to the human condition, and will provide the means to correctly determine the number and function of B1 cells in various disease states and in normal individuals at various stages of life.
It is important to note that the CD20+CD27+CD43+ cells we identified as human B1 cells are not in any way related to differentiated follicular B cells that might secrete IgM, such as plasma cells or plasmablasts, for the following reasons: human B1 cells expressed CD20 (which is lost during differentiation at the plasmablast stage; Jego et al., 2001); human B1 cells did not express CD138 (which is acquired by differentiated plasma cells; Klein and Dalla-Favera, 2007); and neither CD20 loss nor CD138 acquisition occurred during culture of human B1 cells with IL-6 for 5 d (unpublished data). Further, B1 cells are not contained within the very small excluded CD20− population because CD19+CD20− B cells showed no evidence of increased phosphorylated PLC-γ2 or phosphorylated Syk upon phosphatase inhibition; thus, they fail the test of tonic intracellular signaling and do not qualify as B1 cells according to the threefold functional criteria established for this study. For this reason, in addition to the many other differences between CD20− plasma cells and CD20+ B cells (Horst et al., 2002; Hogerkorp and Borrebaeck, 2006; Radbruch et al., 2006), there is no evidence to suggest that any significant number of human B1 cells reside within the CD20− B cell population.
It is also important to note that the CD20+CD27+CD43+ cells we identified as human B1 cells are not contaminated with, and are not confused by, activated memory (CD27+CD43−) or naive (CD27−CD43−) B cells. Although CD43 is associated with B cell differentiation, and not activation, in the murine system (Gulley et al., 1988), in the human system this surface antigen is up-regulated in previously CD43− B cells by some, but not all, activating stimuli, and so must be considered an “activation antigen.” In this sense, CD43 behaves much like CD5, which is also induced by activating stimuli (Freedman et al., 1989; Cong et al., 1991; but which, however, is still used effectively to delineate murine B1 cells). Further, activated B cells stimulate T cells and exhibit tonic signaling, and so B1 cells are not differentiated from activated B cells by these two functional criteria. In this two-way comparison, these assays represent qualifying criteria that candidate B cells must meet to be considered B1 cells, whereas in other two-way comparisons these functional tests serve to distinguish B1 cells (e.g., IgM-secreting CD20+ B1 cells versus IgM-secreting CD20− B cells). Despite these points of similarity (CD43 expression, T cell stimulation, and tonic signaling), activated but not differentiated CD20+ memory and naive B cells fail to spontaneously secrete IgM. That rules out the possibility that CD20+CD27+CD43+ B cells are nothing more than activated B cells. Moreover, the CD20+CD27+CD43+ B cells that we have defined as B1 cells are not contaminated by activated B cells as far as can be determined because activated memory (CD20+CD27+CD43−) or naive (CD20+CD27−CD43−) B cells that up-regulate CD43 also express markedly elevated levels of CD69 and CD70, whereas B1 cells do not express (CD70), or express very low levels (CD69), of both of these antigens. In fact, the profile of CD69 and CD70 expression by B1 cells is essentially devoid of cells with high expression, and thus it is highly unlikely that activated B cells are intermixed with the CD20+CD27+CD43+ B1 cell pool. This is consistent with current estimates that very, very few activated B cells actually circulate in the peripheral blood at any given time in normal individuals. Thus, when we excluded from CD20+CD27+CD43+ B cells those that expressed the topmost 5% staining density for CD70 during sort-purification (thereby insuring that no activated B cells with high CD70 expression would be present), we found no difference in IgM secretion, T cell stimulation, or tonic signaling in comparing CD20+CD27+CD43+CD70− B cells with CD20+CD27+CD43+ B cells. Although there is no evidence that CD20+CD27+CD43+ human B1 cells contain activated B cells, and, in fact, there is clear evidence that this is not the case, an additional requirement that sort-purified B1 cells express no more than low levels of CD69 and/or CD70 provides redundancy to ensure that activated memory and naive B cells are not present.
Because only 0.5–3% of input CD20+CD27+CD43+ B1 cells were positive on ELISPOT assay, it may be that another cell type, albeit not activated B cells, could be intermixed with this population, particularly in view of ELISPOT results on murine B1 cells, which detect almost an order of magnitude greater proportion of secreting cells (Tumang et al., 2005; Holodick et al., 2010). It seems more likely, however, that the ELISPOT assay is not detecting all secreting B1 cells, inasmuch as detection depends on several variables, including the affinities of capture and detecting antibodies, the rate of Ig secretion, and the duration of ELISPOT incubation. We intentionally used a brief period of incubation so that results reflect in vivo events to the greatest extent possible. Importantly, the low proportion of ELISPOT positive human B1 cells cannot be attributed to secretion of another isotype, inasmuch as >90% of CD20+CD27+CD43+ adult peripheral blood B1 cells (Fig. S2) and 100% of umbilical cord blood B1 cells (unpublished data) express IgD. It remains possible that the circulating pool of human B1 cells differs from B1 cells that are tissue-resident, and in that respect ELISPOT assays have not been performed on murine peripheral blood B1 cells. Still, results on tonic intracellular signaling reflect a single-cell population, and no other CD20+ B cell population showed even minimal secretion, so despite the small proportion of B1 cells that are positive on ELISPOT assay we suggest that this most likely represents a single type of B cell in which the test for Ig secretion is imperfectly sensitive.
As defined by CD20, CD27, and CD43, human B1 cells are for the most part CD5+, although some are CD5 negative. In preliminary experiments we found these two subpopulations to be equivalent in IgM secretion, T cell stimulation, and tonic signaling (unpublished data) and the existence of CD20+CD27+CD43+CD5− B1 cells may explain previous reports that CD5− B cells are capable of producing autoreactive and polyreactive antibodies. Importantly, CD5 positivity encompasses many B cells that are not B1 as defined herein, consistent with previous reports that follicular B cells at various stages of development and activated B cells express CD5. Thus, isolating B1 cells as CD20+CD27+CD43+ provides a much more refined and discrete population as compared with the very heterogeneous nature of CD5+ B cells.
The phenotypic composition of B1 cells has already led to additional insights regarding human B cell populations. First, whereas peripheral (cord) blood at birth would seem to be necessarily devoid of memory B cells, as has been previously reported (Maurer et al., 1990; Agematsu et al., 1997), our results indicate that a population of CD27+ B1 cells is, in fact, present, as has been suggested (Shi et al., 2005), furthering the notion that CD27 is not an immutable indicator of memory B cell status, as also suggested by its presence on developing B cells (Nilsson et al., 2005). Second, whereas adult peripheral blood memory B cells have been reported to efficiently stimulate T cells (Good et al., 2009), our results indicate that this function is actually contributed by B1 cells contained within the CD27+ population and not by memory B cells, per se. In other words, CD20+CD27+ B cells represent a heterogeneous grouping that includes CD27+CD43+ B1 cells and CD27+CD43− “true” memory B cells. Thus, the features ascribed to memory B cells must now be reevaluated, and functional studies of CD27+ B cells should proceed only after depletion of CD27+CD43+ B1 cells to ensure a true memory B cell population.
The confusion between CD27+CD43+ B1 cells and CD27+ memory B cells may be particularly acute with respect to so-called “IgM memory” B cells. Like B1 cells, IgM memory B cells express both IgM and CD27; however, as noted above in respect to all memory B cells, IgM memory B cells and B1 cells are two distinct populations separable by CD43 expression. IgM memory B cells lack the functional features of B1 cells; this is clear from the complete failure of CD27+CD43− B cells to secrete IgM, stimulate T cells, or exhibit tonic signaling, even though more than half of these B cells are IgM+. Thus, IgM memory B cells, constituting a substantial portion of CD27+ non-B1 cells, lack the characteristics of B1 cells and are completely separable from them. Notably, it has been reported that IgM memory B cells are responsible for controlling infections produced by Streptococcus pneumoniae and other encapsulated organisms (Kruetzmann et al., 2003); however, the population of IgM memory B cells studied in previous work presumably included B1 cells because prior investigations used CD27 to identify memory B cells. Instead, it may be that (CD27+CD43+) B1 cells are responsible for producing anti-PC antibody in the human system as they are in the murine system, a notion supported by our finding that CD20+CD27+CD43+ B1 cells are repertoire-skewed to preferentially recognize PC in comparison to CD20+CD27+CD43− memory B cells and CD20+CD27−CD43− naive B cells (Fig. 5). This again emphasizes the importance of distinguishing B1 cells from memory B cells so that characteristics of the former not be attributed to the latter.
The number of human CD20+CD27+CD43+ B1 cells found in the peripheral circulation was small, and this raises the possibility that human B1 cells may be primarily located in a reservoir other than the peripheral circulation. This is, in fact, the situation with mouse B1 cells, which are located primarily in coelomic cavities and the spleen. The report that anti-Streptococcus pneumoniae IgM memory B cells are generated in the spleen (Kruetzmann et al., 2003) is reminiscent of previous reports on mouse B1 cells indicating they also require the spleen for development (Wardemann et al., 2002), and again raises the possibility that at least some B cells previously described as IgM memory B cells may actually be human B1 cells.
Regardless of the relationship between human B1 cells and IgM memory B cells, the number of CD27+ B cells declines with advancing age (Shi et al., 2005), and our results indicate that the fraction of CD43+ B1 cells within the CD20+CD27+ population declines even more precipitously, particularly in the very old age group. Still, preliminary results suggest that the proportion of IgD+ B1 cells changes little with age and remains above 90%. Collectively, however, it is likely that B1 cell protective IgM natural antibody also declines with age, and this may explain, at least in part, the susceptibility of aged individuals to overwhelming infection by encapsulated organisms.
It has been conjectured for many years that the normal counterpart for malignant CD5-expressing chronic lymphocytic leukemia cells lies in human B1 cells; however, the identity of such cells has not been known up until now. With the characteristics of human B1 cells in hand, several similarities between normal human B1 cells and malignant chronic lymphocytic leukemia (CLL) cells, at least of the poor prognosis type, are evident. For example, both are CD20+CD27+CD43+CD70−; most normal B1 cells express CD5, as do malignant CLL cells; and, both express relatively nonmutated Ig (Damle et al., 1999; Hamblin et al., 1999; Jung et al., 2003). In addition, we have found that normal human B1 cells ZAP-70 and ILT3 (unpublished data) like CLL cells (Best et al., 2006; Colovai et al., 2007). And, in respect to pathophysiology, the chronically activated phenotype of normal B1 cells may predispose to malignant transformation. Thus, in regard to CLL, in some respects we may be back to the future that was postulated 30 yr ago; to wit, that the normal counterpart cell for, and the target for malignant tranformation by, CLL is the human B1 cell, only now the true identity of the normal counterpart is known. This will, in turn, provide the means to carry out informative experiments to elucidate the nature of CLL neoplasia by comparing malignant CLL cells with the proper corresponding non-transformed B1 cells.
In summary, we have found that a small subset of human peripheral CD20+ B cells, specifically expressing CD27 and CD43 and present in both umbilical cord and adult peripheral blood, recapitulates key functional characteristics of murine B1 cells and for this reason is here denoted as the human B1 cell population. Identification of this population carries important implications for studying the normal behavior of B1 cells, for probing the production of natural Ig, for clarifying the functions previously ascribed to memory B cells, for determining the origin of chronic lymphocytic leukemia, and for evaluating the pathophysiology of autoimmune dyscrasias.
MATERIALS AND METHODS
Donors and samples.
Adult peripheral blood samples were obtained by venipuncture of adult volunteers after obtaining informed consent in accordance with the Declaration of Helsinki. Additional samples in the form of leukopacks were obtained from the New York Blood Center on the day of donation. Anonymous umbilical cord blood samples were obtained from the Tissue Donation Program at The Feinstein Institute for Medical Research. This study was approved by, and all samples were obtained in accordance with, the Institutional Review Board of the North Shore-LIJ Health System.
Processing.
All blood samples were treated in a similar manner and processed promptly upon receipt. Mononuclear cells were obtained by density gradient separation using lymphocyte separation medium (Cellgro). Except as otherwise noted, mononuclear cells were then washed and resuspended in RPMI 1640 (Cellgro) containing 10% fetal calf serum plus 2 mM l-glutamine, 10 mM Hepes, pH 7.25, 100 U/ml penicillin, and 100 µg/ml streptomycin.
B cell enrichment.
For some experiments B cells were enriched by CD19+ selection using the EasySep Human CD19+ B Cell magnetic bead selection kit (StemCell Technologies) according to the manufacturer’s instructions.
Flow cytometry analysis and cell sorting.
Enriched B cells and mononuclear cells were sort-purified on an Influx instrument (BD) after immunofluorescent staining, as described in the Results. In all experiments displayed, except in Fig. 9, CD20+ cells were studied. Flow cytometric analysis of immunofluorescently stained cells was performed on a LSR-II instrument (BD).
BCR specificity.
Adult peripheral blood mononuclear cells were immunofluorescently stained and exposed to PC-BSA-fluorescein and biotinylated DWEYS tetramer. The biotinylation of the DWEYS tetramer was performed using the Miltenyi Biotec one step biotinylation kit according to the manufacturer’s instructions. After 30 min in the dark and on ice, the cells were washed and labeled with PE-streptavidin. Cells were then analyzed by flow cytometry.
ELISPOT.
Ig secretion was determined by ELISPOT assay as previously described (Tumang et al., 2005), using MultiScreen-IP plates (Millipore) coated with goat anti–human IgM (SouthernBiotech), and blocked with 5% bovine serum albumin (Sigma-Aldrich). In brief, 10,000 sort-purified B cells were cultured in 100 µl RPMI medium (supplemented with 10% FCS for 3 h at 37°C, after which plates were treated with alkaline phosphatase-conjugated anti–human IgM antibody (SouthernBiotech) and developed with 5-bromo-4-chloro-3-indoyl phosphate/p-NBT chloride substrate (KPL). Ig secreting cells were enumerated with Phoretix Expression software (NonLinear Dynamics) after plates were scanned.
ELISA.
Ig secretion was determined by ELISA assay, as previously described (Hastings et al., 2006). In brief, sort-purified B cells were cultured for 5 d at 1 × 106 per ml in RPMI medium (supplemented as above). Supernatants were evaluated using anti-IgM coated plates (Bethyl Laboratories) and concentrations were determined with a standard curve.
Allogeneic stimulation.
Naive CD4+ T cells were negatively selected from PBMC using the EasySep human naive CD4+ T cell magnetic bead selection kit (StemCell Technologies) according to the manufacturer’s instructions and were co-cultured at a ratio of 2:1 with sort-purified, irradiated (4,000 rads) B cells (50,000) in 0.2 ml in RPMI medium in triplicate wells of 96-well round bottom plates. Cultures were pulsed with 0.75 µCi [3H]thymidine for the last 8 h of 5-d cultures, and counts per minute (cpm) were determined by scintillation counting.
Phosphoflow analysis.
Mononuclear cells were analyzed after phosphatase inhibition, as previously described (Holodick et al., 2009b). In brief, cells were treated with sodium pervanadate for varying periods of time, after which they were fixed with paraformaldehyde, permeabilized with methanol, and stained for surface antigens and intracellular phosphorylated proteins with specific immunofluorescent antibodies. Flow cytometric analysis was performed using a LSR-II instrument.
Single cell Ig sequencing.
Individual cells were sort-purified onto a 48-well Ampligrid (Beckman Coulter) and Ig sequences were PCR-amplified in a semi-nested approach as previously described (Holodick et al., 2009a), using primers designed for human Ig gene transcripts (Wang and Stollar, 2000). Products were sequenced (GENEWIZ), and sequences were analyzed using the International ImMunoGeneTics Information System.
Reagents.
Sodium orthovanadate was obtained from MP Biomedicals; one-step pcr and gel extraction kits were obtained from Qiagen; LPS and PMA were obtained from Sigma-Aldrich; CpG was obtained from Invitrogen; ionomycin and SAC were obtained from Calbiochem; anti-IgM was obtained from Southern Biotech; recombinant IL-2 and anti-CD40 were obtained from R&D Systems; recombinant IL-6 was obtained from BD; and fluorescently labeled antibodies (anti–CD20-APC-Cy7, anti–CD27-V450, anti–CD43-FITC, anti–CD70-PE, anti–CD5-PE-Cy7, anti–-IgD-PE, anti–CD19-PE, anti–CD21-PE, anti–CD44-PE, anti–HLA-DR-PE, anti-CD45RB, anti–phospho-PLC-gamma-2-A647, and anti–phospho-Syk-A647) were obtained from BD. Anti–CD69-PE was obtained from Beckman Coulter. Anti–CD43-APC was obtained from eBioscience. PC-BSA-fluorescein was obtained from Biosearch Technologies. DWEYS tetramer protein was a generous gift from B. Diamond (The Feinstein Institute for Medical Research, Manhasset, NY). PE-Streptavidin was obtained from Biomeda.
Online supplemental material.
Fig. S1 shows that umbilical cord blood B1 cells display preferential heavy chain variable gene usage. Fig. S2 shows that adult peripheral blood B1 cells express CD19, CD21, IgD, CD44, and CD45RB, but not all B1 cells express CD5. Table S1 shows actual sequences of Ig genes from umbilical cord B1 cells. Table S2 shows actual sequences of Ig genes from umbilical cord naive B cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101499/DC1.
Acknowledgments
The authors gratefully acknowledge the Tissue Donation Program at The Feinstein Institute for Medical Research and the New York Blood Center for assistance in obtaining umbilical cord and adult peripheral blood samples. We thank Dr. B. Diamond for generously providing DWEYS.
This work was supported in part by grants from the Donald and Barbara Zucker Foundation and the National Institutes of Health.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BCR
- B cell receptor
- CLL
- chronic lymphocytic leukemia
- DWEYS
- aspartate-tryptophan-glutamate-tyrosine-serine
- PC
- phosphorylcholine
- PLC-γ2
- phospholipase C-γ2
- Syk
- spleen tyrosine kinase
- SAC
- Staphylococcus aureus Cowan strain 1
- MFI
- mean fluorescence intensity
References
- Agematsu K., Nagumo H., Yang F.C., Nakazawa T., Fukushima K., Ito S., Sugita K., Mori T., Kobata T., Morimoto C., Komiyama A. 1997. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur. J. Immunol. 27:2073–2079 10.1002/eji.1830270835 [DOI] [PubMed] [Google Scholar]
- Agematsu K., Hokibara S., Nagumo H., Komiyama A. 2000. CD27: a memory B-cell marker. Immunol. Today. 21:204–206 10.1016/S0167-5699(00)01605-4 [DOI] [PubMed] [Google Scholar]
- Asma G.E., van den Bergh R.L., Vossen J.M. 1986. Characterization of early lymphoid precursor cells in the human fetus using monoclonal antibodies and anti-terminal deoxynucleotidyl transferase. Clin. Exp. Immunol. 64:356–363 [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N., Herman O.C., Jager G.C., Brown L.E., Herzenberg L.A., Chen J. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280 10.1084/jem.192.2.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland R., Wortis H.H. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20:253–300 10.1146/annurev.immunol.20.100301.064833 [DOI] [PubMed] [Google Scholar]
- Best O.G., Ibbotson R.E., Parker A.E., Davis Z.A., Orchard J.A., Oscier D.G. 2006. ZAP-70 by flow cytometry: a comparison of different antibodies, anticoagulants, and methods of analysis. Cytometry B Clin. Cytom. 70:235–241 [DOI] [PubMed] [Google Scholar]
- Binder C.J., Silverman G.J. 2005. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin. Immunopathol. 26:385–404 10.1007/s00281-004-0185-z [DOI] [PubMed] [Google Scholar]
- Boes M., Prodeus A.P., Schmidt T., Carroll M.C., Chen J. 1998. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381–2386 10.1084/jem.188.12.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D.E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J. Exp. Med. 153:694–705 10.1084/jem.153.3.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burastero S.E., Casali P., Wilder R.L., Notkins A.L. 1988. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J. Exp. Med. 168:1979–1992 10.1084/jem.168.6.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Notkins A.L. 1989. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu. Rev. Immunol. 7:513–535 [DOI] [PubMed] [Google Scholar]
- Colovai A.I., Tsao L., Wang S., Lin H., Wang C., Seki T., Fisher J.G., Menes M., Bhagat G., Alobeid B., Suciu-Foca N. 2007. Expression of inhibitory receptor ILT3 on neoplastic B cells is associated with lymphoid tissue involvement in chronic lymphocytic leukemia. Cytometry B Clin. Cytom. 72:354–362 [DOI] [PubMed] [Google Scholar]
- Cong Y.Z., Rabin E., Wortis H.H. 1991. Treatment of murine CD5- B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int. Immunol. 3:467–476 10.1093/intimm/3.5.467 [DOI] [PubMed] [Google Scholar]
- Damle R.N., Wasil T., Fais F., Ghiotto F., Valetto A., Allen S.L., Buchbinder A., Budman D., Dittmar K., Kolitz J., et al. 1999. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 94:1840–1847 [PubMed] [Google Scholar]
- Dauphinée M., Tovar Z., Talal N. 1988. B cells expressing CD5 are increased in Sjögren’s syndrome. Arthritis Rheum. 31:642–647 (see comments) 10.1002/art.1780310509 [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Montecino-Rodriguez E. 2007. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat. Rev. Immunol. 7:213–219 10.1038/nri2019 [DOI] [PubMed] [Google Scholar]
- Düber S., Hafner M., Krey M., Lienenklaus S., Roy B., Hobeika E., Reth M., Buch T., Waisman A., Kretschmer K., Weiss S. 2009. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 114:4960–4967 10.1182/blood-2009-04-218156 [DOI] [PubMed] [Google Scholar]
- Förster I., Rajewsky K. 1987. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 17:521–528 10.1002/eji.1830170414 [DOI] [PubMed] [Google Scholar]
- Förster I., Gu H., Rajewsky K. 1988. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. EMBO J. 7:3693–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A.S., Freeman G., Whitman J., Segil J., Daley J., Nadler L.M. 1989. Studies of in vitro activated CD5+ B cells. Blood. 73:202–208 [PubMed] [Google Scholar]
- Good K.L., Avery D.T., Tangye S.G. 2009. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J. Immunol. 182:890–901 [DOI] [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. 1990. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 9:2133–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley M.L., Ogata L.C., Thorson J.A., Dailey M.O., Kemp J.D. 1988. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J. Immunol. 140:3751–3757 [PubMed] [Google Scholar]
- Haas K.M., Poe J.C., Steeber D.A., Tedder T.F. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 23:7–18 10.1016/j.immuni.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Hamblin T.J., Davis Z., Gardiner A., Oscier D.G., Stevenson F.K. 1999. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 94:1848–1854 [PubMed] [Google Scholar]
- Hardy R.R., Hayakawa K. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595–621 10.1146/annurev.immunol.19.1.595 [DOI] [PubMed] [Google Scholar]
- Hardy R.R., Carmack C.E., Shinton S.A., Riblet R.J., Hayakawa K. 1989. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J. Immunol. 142:3643–3651 [PubMed] [Google Scholar]
- Hastings W.D., Tumang J.R., Behrens T.W., Rothstein T.L. 2006. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur. J. Immunol. 36:1114–1123 10.1002/eji.200535142 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R. 1988. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu. Rev. Immunol. 6:197–218 [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Honda M., Herzenberg L.A., Steinberg A.D., Herzenberg L.A. 1984. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci. USA. 81:2494–2498 10.1073/pnas.81.8.2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Stall A.M., Herzenberg L.A., Herzenberg L.A. 1986. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur. J. Immunol. 16:1313–1316 10.1002/eji.1830161021 [DOI] [PubMed] [Google Scholar]
- Herzenberg L.A. 2000. B-1 cells: the lineage question revisited. Immunol. Rev. 175:9–22 10.1111/j.1600-065X.2000.imr017520.x [DOI] [PubMed] [Google Scholar]
- Högerkorp C.M., Borrebaeck C.A. 2006. The human CD77- B cell population represents a heterogeneous subset of cells comprising centroblasts, centrocytes, and plasmablasts, prompting phenotypical revision. J. Immunol. 177:4341–4349 [DOI] [PubMed] [Google Scholar]
- Holodick N.E., Repetny K., Zhong X., Rothstein T.L. 2009a. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur. J. Immunol. 39:2383–2394 10.1002/eji.200838920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodick N.E., Tumang J.R., Rothstein T.L. 2009b. Continual signaling is responsible for constitutive ERK phosphorylation in B-1a cells. Mol Immunol. 46:3029–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodick N.E., Tumang J.R., Rothstein T.L. 2010. Immunoglobulin secretion by B1 cells: differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur. J. Immunol. 40:3007–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst A., Hunzelmann N., Arce S., Herber M., Manz R.A., Radbruch A., Nischt R., Schmitz J., Assenmacher M. 2002. Detection and characterization of plasma cells in peripheral blood: correlation of IgE+ plasma cell frequency with IgE serum titre. Clin. Exp. Immunol. 130:370–378 10.1046/j.1365-2249.2002.02025.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H., Hastings R., Kearney J., Howard M. 1992. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J. Exp. Med. 175:1213–1220 10.1084/jem.175.5.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi A.M., Zhang J., Mackay M., Aranow C., Diamond B. 2009. Phenotypic characterization of autoreactive B cells—checkpoints of B cell tolerance in patients with systemic lupus erythematosus. PLoS One. 4:e5776 10.1371/journal.pone.0005776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G., Bataille R., Pellat-Deceunynck C. 2001. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood. 97:1817–1822 10.1182/blood.V97.6.1817 [DOI] [PubMed] [Google Scholar]
- Jung G., Eisenmann J.C., Thiébault S., Hénon P. 2003. Cell surface CD43 determination improves diagnostic precision in late B-cell diseases. Br. J. Haematol. 120:496–499 10.1046/j.1365-2141.2003.04071.x [DOI] [PubMed] [Google Scholar]
- Kantor A.B., Herzenberg L.A. 1993. Origin of murine B cell lineages. Annu. Rev. Immunol. 11:501–538 10.1146/annurev.iy.11.040193.002441 [DOI] [PubMed] [Google Scholar]
- Kantor A.B., Stall A.M., Adams S., Watanabe K., Herzenberg L.A. 1995. De novo development and self-replenishment of B cells. Int. Immunol. 7:55–68 10.1093/intimm/7.1.55 [DOI] [PubMed] [Google Scholar]
- Karras J.G., Wang Z., Huo L., Howard R.G., Frank D.A., Rothstein T.L. 1997. Signal transducer and activator of transcription-3 (STAT3) is constitutively activated in normal, self-renewing B-1 cells but only inducibly expressed in conventional B lymphocytes. J. Exp. Med. 185:1035–1042 (see comments) 10.1084/jem.185.6.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaian M.T., Ikematsu H., Casali P. 1992. Identification and analysis of a novel human surface CD5- B lymphocyte subset producing natural antibodies. J. Immunol. 148:2690–2702 [PMC free article] [PubMed] [Google Scholar]
- Klein U., Dalla-Favera R. 2007. Unexpected steps in plasma-cell differentiation. Immunity. 26:543–544 10.1016/j.immuni.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Klein U., Rajewsky K., Küppers R. 1998. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 188:1679–1689 10.1084/jem.188.9.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese F.G., Ammerlaan W.A., Kantor A.B. 1993. Evidence that intestinal IgA plasma cells in mu, kappa transgenic mice are derived from B-1 (Ly-1 B) cells. Int. Immunol. 5:1317–1327 10.1093/intimm/5.10.1317 [DOI] [PubMed] [Google Scholar]
- Kruetzmann S., Rosado M.M., Weber H., Germing U., Tournilhac O., Peter H.H., Berner R., Peters A., Boehm T., Plebani A., et al. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197:939–945 10.1084/jem.20022020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor P.A., Morahan G. 1990. The peritoneal Ly-1 (CD5) B cell repertoire is unique among murine B cell repertoires. Eur. J. Immunol. 20:485–492 10.1002/eji.1830200305 [DOI] [PubMed] [Google Scholar]
- Lee J., Kuchen S., Fischer R., Chang S., Lipsky P.E. 2009. Identification and characterization of a human CD5+ pre-naive B cell population. J. Immunol. 182:4116–4126 10.4049/jimmunol.0803391 [DOI] [PubMed] [Google Scholar]
- Mackenzie L.E., Youinou P.Y., Hicks R., Yuksel B., Mageed R.A., Lydyard P.M. 1991. Auto- and polyreactivity of IgM from CD5+ and CD5- cord blood B cells. Scand. J. Immunol. 33:329–335 10.1111/j.1365-3083.1991.tb01778.x [DOI] [PubMed] [Google Scholar]
- Maurer D., Holter W., Majdic O., Fischer G.F., Knapp W. 1990. CD27 expression by a distinct subpopulation of human B lymphocytes. Eur. J. Immunol. 20:2679–2684 10.1002/eji.1830201223 [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Leathers H., Dorshkind K. 2006. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7:293–301 10.1038/ni1301 [DOI] [PubMed] [Google Scholar]
- Morris D.L., Rothstein T.L. 1993. Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. J. Exp. Med. 177:857–861 10.1084/jem.177.3.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D.L., Rothstein T.L. 1994. CD5+ B (B-1) cells and immunity. Handbook of B and T Lymphocytes. Snow E.C., editor Academic Press, Inc., San Diego: 421-45 [Google Scholar]
- Nilsson A., de Milito A., Mowafi F., Winberg G., Björk O., Wolpert E.Z., Chiodi F. 2005. Expression of CD27-CD70 on early B cell progenitors in the bone marrow: implication for diagnosis and therapy of childhood ALL. Exp. Hematol. 33:1500–1507 10.1016/j.exphem.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Ochsenbein A.F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H., Zinkernagel R.M. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 286:2156–2159 10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- Pennell C.A., Mercolino T.J., Grdina T.A., Arnold L.W., Haughton G., Clarke S.H. 1989. Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur. J. Immunol. 19:1289–1295 10.1002/eji.1830190721 [DOI] [PubMed] [Google Scholar]
- Plater-Zyberk C., Maini R.N., Lam K., Kennedy T.D., Janossy G. 1985. A rheumatoid arthritis B cell subset expresses a phenotype similar to that in chronic lymphocytic leukemia. Arthritis Rheum. 28:971–976 10.1002/art.1780280903 [DOI] [PubMed] [Google Scholar]
- Radbruch A., Muehlinghaus G., Luger E.O., Inamine A., Smith K.G., Dörner T., Hiepe F. 2006. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6:741–750 10.1038/nri1886 [DOI] [PubMed] [Google Scholar]
- Raman C., Knight K.L. 1992. CD5+ B cells predominate in peripheral tissues of rabbit. J. Immunol. 149:3858–3864 [PubMed] [Google Scholar]
- Rothstein T.L. 2002. Cutting edge commentary: two B-1 or not to be one. J. Immunol. 168:4257–4261 [DOI] [PubMed] [Google Scholar]
- Shi Y., Yamazaki T., Okubo Y., Uehara Y., Sugane K., Agematsu K. 2005. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J. Immunol. 175:3262–3267 [DOI] [PubMed] [Google Scholar]
- Shirai T., Hirose S., Okada T., Nishimura H. 1991. CD5+ B cells in autoimmune disease and lymphoid malignancy. Clin. Immunol. Immunopathol. 59:173–186 10.1016/0090-1229(91)90016-4 [DOI] [PubMed] [Google Scholar]
- Sidman C.L., Shultz L.D., Hardy R.R., Hayakawa K., Herzenberg L.A. 1986. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 232:1423–1425 10.1126/science.3487115 [DOI] [PubMed] [Google Scholar]
- Sims G.P., Ettinger R., Shirota Y., Yarboro C.H., Illei G.G., Lipsky P.E. 2005. Identification and characterization of circulating human transitional B cells. Blood. 105:4390–4398 10.1182/blood-2004-11-4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi O., Miyajima H., Hirano T., Noguchi M., Ueda A., Hashimoto H., Hirose S., Okumura K. 1987. The Leu-1 B-cell subpopulation in patients with rheumatoid arthritis. J. Clin. Immunol. 7:441–448 10.1007/BF00915053 [DOI] [PubMed] [Google Scholar]
- Tumang J.R., Francés R., Yeo S.G., Rothstein T.L. 2005. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J. Immunol. 174:3173–3177 [DOI] [PubMed] [Google Scholar]
- Wang X., Stollar B.D. 2000. Human immunoglobulin variable region gene analysis by single cell RT-PCR. J. Immunol. Methods. 244:217–225 10.1016/S0022-1759(00)00260-X [DOI] [PubMed] [Google Scholar]
- Wardemann H., Boehm T., Dear N., Carsetti R. 2002. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195:771–780 10.1084/jem.20011140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells S.M., Kantor A.B., Stall A.M. 1994. CD43 (S7) expression identifies peripheral B cell subsets. J. Immunol. 153:5503–5515 [PubMed] [Google Scholar]
- Wilson S.M., Wilkie B.N. 2007. B-1 and B-2 B-cells in the pig cannot be differentiated by expression of CD5. Vet. Immunol. Immunopathol. 115:10–16 10.1016/j.vetimm.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Wong S.C., Chew W.K., Tan J.E., Melendez A.J., Francis F., Lam K.P. 2002. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells. J. Biol. Chem. 277:30707–30715 10.1074/jbc.M202460200 [DOI] [PubMed] [Google Scholar]
- Zhong X., Gao W., Degauque N., Bai C., Lu Y., Kenny J., Oukka M., Strom T.B., Rothstein T.L. 2007. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur. J. Immunol. 37:2400–2404 10.1002/eji.200737296 [DOI] [PubMed] [Google Scholar]