Two-dimensional analysis reveals that peptide–MHC class II tetramers underestimate the frequency of cytokine-producing antigen-specific CD4+ T cells in polyclonal responses.
Abstract
T cell affinity for antigen initiates adaptive immunity. However, the contribution of low affinity cells to a response is unknown as it has not been possible to assess the entire affinity range of a polyclonal T cell repertoire. In this study, we used a highly sensitive two-dimensional binding assay to identify low affinity cells in polyclonal autoreactive and pathogen-reactive CD4+ T cell populations specific for myelin oligodendrocyte glycoprotein (MOG) and lymphocytic choriomeningitis virus (LCMV) antigens, respectively. Low affinity CD4+ T cells, below detection with peptide–major histocompatibility complex class II tetramers, were at least as frequent as high affinity responders and contributed significant effector cytokines in both primary antigen–specific responses. We further demonstrated that MOG- and LCMV-specific CD4+ T cells possessed similarly broad ranges in their affinities (>100-fold wide), only differing in the frequencies of low and high affinity cells. Thus, low as well as high affinity CD4+ T cells are critical effectors in autoimmune and pathogen-specific responses.
Determining the affinities of T cells in polyclonal responses is essential for understanding the outcome of cell-mediated immunity directed toward both foreign and self-antigens. The prevailing models of clonal selection and avidity maturation suggest that cells bearing the highest affinity TCRs for antigen are selectively expanded (Savage et al., 1999; Malherbe et al., 2004; Price et al., 2005), but the range in affinities of the T cells and the frequency of low affinity responders is unknown. The potential importance of low affinity T cells contributing to immunity is supported by the findings that monoclonal CD8+ T cells can proliferate to low affinity antigens (Zehn et al., 2009). In addition to foreign antigens, T cells specific for self-peptides that drive autoimmune disease could comprise another subset of low affinity cells as a result of tolerance mechanisms (Liu et al., 1995; Bouneaud et al., 2000; Zehn and Bevan, 2006). Although low affinity T cells may potentially contribute to responses to both foreign and self-antigens, it has been unclear how extensively low affinity T cells participate in polyclonal T cell responses where high affinity clonotypes are also present.
Insight into TCR affinity for antigen has been provided by three-dimensional and two-dimensional (2D) technologies, such as surface plasmon resonance, Förster resonance energy transfer, or micropipette-based assays (Alam and Gascoigne, 1998; Kersh et al., 1998; Huang et al., 2010; Huppa et al., 2010). However, to date, studies have only considered monoclonal TCRs and cannot reveal T cell frequency and breadth of affinities comprising an antigen-specific polyclonal population (Alam and Gascoigne, 1998; Kersh et al., 1998; Huang et al., 2010; Huppa et al., 2010). Peptide–MHC (pMHC) tetramers based on an enhanced TCR avidity via multivalent interactions provide the most valuable technique for detecting the frequency of antigen-specific T cells (Altman et al., 1996; Moon et al., 2007), yet the extent to which their limited avidity may preclude detection of low affinity T cells (Vollers and Stern, 2008; Wooldridge et al., 2009) is unknown. Developing sensitive and accurate measurements of T cell antigen reactivity is therefore of utmost importance to dissect the nature of the polyclonal T cell response.
Recently, we used a 2D-based affinity analysis, which measures TCR–pMHC binding in the cell membrane–anchored context (Huang et al., 2010; Huppa et al., 2010) to define a 1,000-fold range in affinities corresponding to response levels between a monoclonal CD8+ T cell and a panel of altered peptide ligands (Huang et al., 2010). In this study, we harnessed the sensitivity of the 2D binding assay to define the antigen-specific frequencies and affinities of two polyclonal IAb-restricted CD4+ T cell populations specific for a self-antigen, myelin oligodendrocyte glycoprotein (MOG)35–55, which induces experimental autoimmune encephalomyelitis (EAE; Mendel et al., 1995), and a foreign-antigen, glycoprotein (GP)61–80, the dominant T helper epitope for lymphocytic choriomeningitis virus (LCMV; Oxenius et al., 1995; Homann et al., 2001). The 2D analysis revealed significantly larger frequencies of antigen-reactive CD4+ T cells for both antigens as compared with pMHC II tetramers. Polyclonal T cell affinities were diverse, covering more than a 100-fold range of affinities, with a fraction identified as high affinity and tetramer positive and many as low affinity and tetramer negative. We defined the 2D affinity necessary for pMHC II tetramers to bind CD4+ T cells and found that the low affinity tetramer-negative CD4+ T cells contributed significantly to the effector cytokine response. The presence of low and high affinity T cells greatly expands the previously estimated frequencies of polyclonal CD4+ T cell populations in peak effector responses (Homann et al., 2001; Moon et al., 2007; Williams et al., 2008).
RESULTS
pMHC II tetramers underestimate the frequencies of polyclonal CD4+ T cells
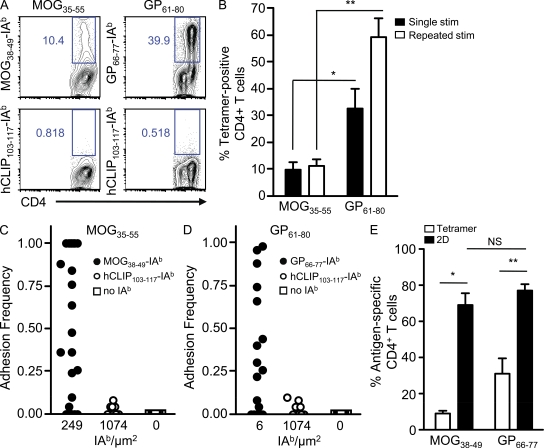
Polyclonal antigen-reactive CD4+ T cell frequencies are traditionally measured by pMHC II tetramers or functional experiments. To compare their responses, we generated in vitro polyclonal myelin- and viral-reactive CD4+ T cells (see Materials and methods) and investigated the response with MOG38–49-IAb and GP66–77-IAb tetramers encompassing the respective core CD4+ T cell epitopes (Fig. 1 A; Mendel et al., 1996; Homann et al., 2007; Sabatino et al., 2008). After 1 wk of stimulation in vitro, three times as many GP61–80 CD4+ T cells were identified by tetramer than MOG35–55 CD4+ T cells (32.4 ± 7.7% and 9.6 ± 2.8%, respectively; Fig. 1 B). Of interest, the tetramer-positive frequency of MOG35–55 CD4+ T cells remained relatively static (mean of 11.2 ± 2.4%) on repeated rounds of in vitro restimulation with antigen, whereas the tetramer-positive population specific for GP61–80 CD4+ T cells expanded to 59.2 ± 7.0% of the culture (Fig. 1 B). The tetramer data seemed strikingly low for the antigen-reactive MOG35–55 CD4+ T cells in particular and raised the possibility that the pMHC II tetramers were not detecting all antigen-specific CD4+ T cells.
Figure 1.
Tetramer versus 2D detection of polyclonal MOG35–55 and GP61–80 CD4+ T cells. (A) Polyclonal MOG35–55 and GP61–80 CD4+ T cells (after 1 wk in culture) were stained with the indicated tetramers in representative experiments. (B) MOG35–55 and GP61–80 CD4+ T cells were restimulated with antigen for 1 wk (single stimulation [stim]) or for a consecutive 4 wk (repeated stimulation), and the mean percentages ± SEM of tetramer-positive CD4+ T cells were measured in at least four independent experiments per group (*, P = 0.03; **, P = 0.0001). (C) Polyclonal MOG35–55 CD4+ T cells were tested for adhesion to the indicated concentrations (IAb/square micrometer) of MOG38–49-IAb, hCLIP103–117-IAb, and no IAb in a representative experiment. (D) Representative adhesion frequencies of polyclonal GP61–80 CD4+ T cells binding to GP66–77-IAb, hCLIP103–117-IAb, and no IAb. (E) The mean frequency ± SEM of antigen-specific binding by tetramer and 2D analysis was measured in parallel for polyclonal CD4+ T cells after 1 wk of passage in vitro (*, P = 0.0002; **, P = 0.0003). Data were based on four (MOG35–55) and three (GP61–80) independent experiments.
As an alternative measure of determining the frequency of antigen-specific CD4+ T cells, we used the micropipette adhesion frequency assay, which measures the 2D interactions of receptor–ligand interactions (Chesla et al., 1998; Huang et al., 2007, 2010). In this assay, a single T cell was brought in and out of contact with an RBC coated with pMHC II to yield an adhesion frequency (the percentage of adhesions out of the total number of contacts, as described in Materials and methods). After stimulation with antigen for 1 wk in vitro, polyclonal MOG35–55 and GP61–80 CD4+ T cells bound only with their respective antigens but not RBCs alone or irrelevant antigen (Fig. 1, C and D). 2D analysis revealed a range in adhesion frequencies, and antigen-specific CD4+ T cell frequencies were determined by the percentage of T cell binding above irrelevant antigen background (>0.1; see hCLIP103–117-IAb in Fig. 1, C and D). Surprisingly, the majority of the MOG35–55 CD4+ T cells were antigen specific (69.2 ± 6.0%), eightfold higher than the tetramer-based frequencies measured in parallel (8.7 ± 1.6%; Fig. 1 E). Moreover, the micropipette assay identified a similar wide range in adhesion frequencies and a 2.5-fold increase over tetramer-positive GP61–80 CD4+ T cells (76.9 ± 3.4% vs. 30.9 ± 8.8%; Fig. 1 E). Thus, 2D binding demonstrated that most CD4+ T cells in short-term cultures were antigen specific in contrast to the differences in frequency identified using pMHC II tetramer (Fig. 1 B).
pMHC II tetramer–negative CD4+ T cells are low affinity but elicit robust effector responses
The degree of tetramer binding is critically dependent on TCR avidity (Crawford et al., 1998; Savage et al., 1999; Fassò et al., 2000; Slifka and Whitton, 2001), a parameter determined by intrinsic TCR affinity (Vollers and Stern, 2008; Wooldridge et al., 2009) and TCR levels (Crawford et al., 1998; Mallone et al., 2005). However, MOG35–55 and GP61–80 CD4+ T cells expressed similar levels of surface TCRs (Fig. S1). To determine whether TCR affinity caused the deficiency in tetramer detection of polyclonal CD4+ T cells, we used the micropipette-based assay to define 2D affinities. The effective 2D affinity (AcKa, in µm4) of the T cell was derived from the adhesion frequency at equilibrium and expressed as a product of the two cells’ contact areas (Ac) and 2D affinity (Ka). The 2D affinities were derived at 5 s because binding of both T cell populations reached equilibrium within seconds of contact (Fig. S2). The adhesion frequency was dependent on the molecular densities of TCR and pMHC and can potentially detect a dynamic range of affinities spanning six orders of magnitude (10−2–10−8 µm4; Huang et al., 2010), with lower affinity T cells requiring increased pMHC densities.
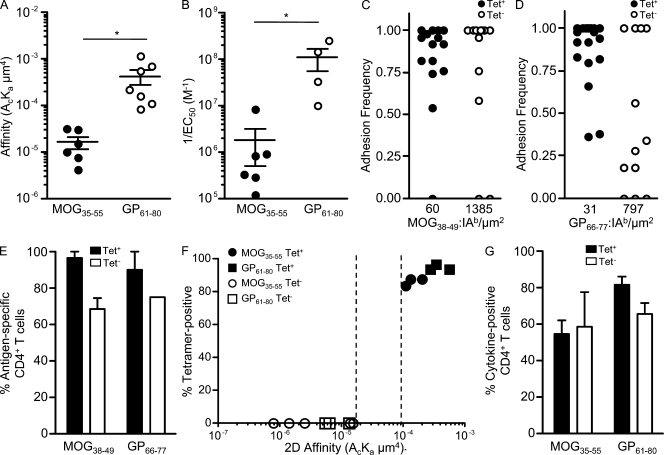
For comparable adhesion frequencies, GP61–80 CD4+ T cells (Fig. 1 D) required lower levels of antigen than many MOG35–55 CD4+ T cells (Fig. 1 C), which is indicative of overall higher affinity. Indeed, the population-averaged effective 2D affinity of GP61–80 CD4+ T cells was 26-fold higher than MOG35–55 CD4+ T cells (4.21 ± 1.48 × 10−4 µm4 and 1.63 ± 0.48 × 10−5 µm4, respectively; Fig. 2 A). Moreover, the effective 2D affinities paralleled the functional avidities (inverse of peptide concentration for half-maximal proliferation, 1/EC50) as GP61–80 CD4+ T cells had a 61-fold higher functional avidity than MOG35–55 CD4+ T cells (Fig. 2 B).
Figure 2.
2D affinity and antigen specificity of tetramer-positive and -negative CD4+ T cells. (A) The mean affinities ± SEM of polyclonal MOG35–55 and GP61–80 CD4+ T cells were based on 62 MOG35–55 CD4+ T cells (six independent experiments) and 54 GP61–80 CD4+ T cells (three independent experiments encompassing seven different antigen densities; *, P = 0.03). (B) The mean ± SEM functional avidities (1/EC50, based on proliferation) of polyclonal MOG35–55 (1.8 ± 1.3 × 106 M−1) and GP61–80 (1.1 ± 0.6 × 108 M−1) CD4+ T cells were based on six and four independent experiments, respectively (*, P = 0.04). (C and D) Representative adhesion frequencies of tetramer-positive and -negative sorted MOG35–55 (C) and GP61–80 (D) CD4+ T cells were performed at the indicated antigen densities. (E) The mean percentage ± SEM of antigen-specific 2D binding of tetramer-positive and -negative MOG35–55 and GP61–80 CD4+ T cells was based on three (MOG35–55) and two (GP61–80) independent experiments. (F) The mean 2D affinities of tetramer-positive and -negative MOG35–55 and GP61–80 CD4+ T cells were based on at least three independent experiments per group. The affinity threshold for tetramer binding is shown by the dotted lines, representing the lowest affinity for tetramer-positive CD4+ T cells (>1.10−4 µm4) and the highest affinity for tetramer-negative CD4+ T cells (<1.52 x 10−5 µm4). (G) The mean percentage ± SEM of cytokine-producing (IFN-γ and TNF) tetramer-positive and -negative MOG35–55 and GP61–80 CD4+ T cells was based on three and two independent experiments, respectively.
pMHC tetramers provide a useful tool for separating the polyclonal MOG35–55- and GP61–80-specific CD4+ T cells in two distinct populations based on reactivity to the tetramer (Fig. S3, A and B). Essentially all tetramer-positive CD4+ T cells (>94%) were detected by 2D binding, showing high adhesion frequencies at low antigen densities (Fig. 2, C–E). Importantly, the majority of tetramer-negative CD4+ T cells (68.6 ± 6.2% of MOG35–55 and 75.0 ± 0.0% of GP61–80) also bound to antigen in the micropipette assay (Fig. 2, C–E). Nevertheless, tetramer-negative MOG35–55 and GP61–80 CD4+ T cells had lower effective 2D affinities (6.67 ± 3.14 × 10−6 µm4 and 8.36 ± 2.49 × 10−6 µm4, respectively) than their tetramer-positive counterparts (1.48 ± 0.27 × 10−4 µm4 and 3.91 ± 0.83 × 10−4 µm4, respectively; Fig. 2 F). Taken as a whole, all tetramer-positive CD4+ T cells had an affinity >1.10 × 10−4 µm4, and all tetramer-negative CD4+ T cells had an affinity <1.52 × 10−5 µm4 (Fig. 2 F). We were unable to more precisely pinpoint the affinity threshold for tetramer binding as the measurements were based on the MOG35–55 and GP61–80 experimental means and because the intermediate tetramer-binding CD4+ T cells were excluded during cell sorting (Fig. S3, A and B). The presence of high and low affinity (henceforth broadly distinguished by the 2D affinity cutoff for tetramer binding) MOG35–55 and GP61–80 CD4+ T cells indicated that both populations were comprised of T cell clonotypes possessing a large (>100-fold), mostly overlapping span of affinities (7.92 × 10−7 to 2.01 × 10−4 µm4 for MOG35–55 and 5.37 × 10−6 to 5.53 × 10−4 µm4 for GP61–80; Fig. 2 F).
Despite their lower affinity, the tetramer-negative CD4+ T cells elicited robust effector functions as similar percentages of tetramer-positive and -negative MOG35–55 (54.6 ± 7.4% and 58.7 ± 19.1%, respectively) and GP61–80 (81.4 ± 4.8% and 65.6 ± 5.7%, respectively) CD4+ T cells produced TNF and/or IFN-γ after antigen stimulation (Fig. 2 G). Considering that tetramer-negative cells were not exclusively antigen reactive by 2D binding analysis, cytokine was produced at a higher frequency than in the tetramer-positive populations.
Low affinity tetramer-negative CD4+ T cells in the central nervous system (CNS) dominate during peak EAE
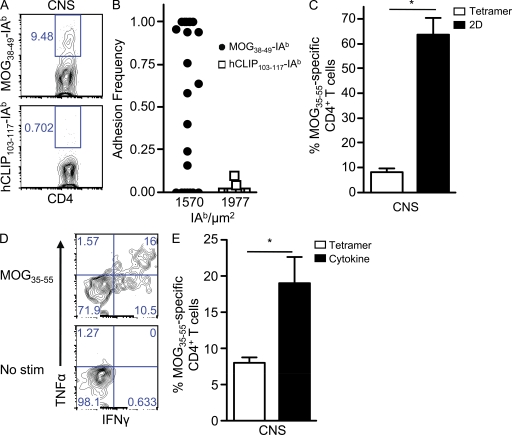
An added strength of the micropipette assay is that its increased sensitivity to antigen allows assessment of ex vivo polyclonal T cell responses. CD4+ T cells isolated from the CNS after EAE induction bound specifically to MOG38–49-IAb but not irrelevant antigen by both tetramer (Fig. 3 A) and 2D analysis (Fig. 3 B). The mean frequency of tetramer-positive MOG35–55 CD4+ T cells isolated from the CNS of mice with peak EAE symptoms was 8.2 ± 1.3% (Fig. 3 C), similar to previous findings (Korn et al., 2007; Sabatino et al., 2008). In parallel with the in vitro data, eightfold more CNS-infiltrating MOG35–55 CD4+ T cells were detected by 2D binding (63.6 ± 6.9%; Fig. 3 C). Moreover, the population-averaged effective 2D affinity of MOG35–55-specific CD4+ T cells from the CNS was 7.95 ± 2.77 × 10−6 µm4, which was comparable (2.1-fold lower) with the in vitro analysis (Fig. 2 A). This affinity level explains the low degree of tetramer staining as it was below the threshold for tetramer binding (Fig. 2 F). Thus, the majority of CD4+ T cells penetrating the CNS at the peak of EAE were in fact MOG35–55 specific.
Figure 3.
Dominance of proinflammatory low affinity myelin-reactive CD4+ T cells during EAE. (A and B) Representative tetramer (A) and 2D binding (B) of CNS-infiltrating CD4+ T cells to MOG38–49-IAb and hCLIP103–117-IAb. (C) The mean frequency ± SEM of MOG35–55-specific binding by tetramer and 2D analysis was based on three experiments performed in parallel, and CNS tissue was pooled together from 6–10 mice per experiment (*, P = 0.004). (D) Representative frequency of cytokine-producing (IFN-γ and TNF) CD4+ T cells isolated from the CNS during acute EAE after stimulation with MOG35–55 or no peptide (no stimulation [stim]). (E) The mean percentage ± SEM of CNS-infiltrating MOG35–55 CD4+ T cells was compared in parallel by MOG38–49-IAb tetramer and the percentage producing cytokine (IFN-γ and TNF) upon stimulation with MOG35–55 (no stimulation background subtracted) from two independent experiments (CNS tissue pooled from 12–24 mice; *, P = 0.04).
The presence of a large frequency of predominantly tetramer-negative MOG35–55 CD4+ T cells in the CNS during EAE suggested that low affinity myelin-reactive CD4+ T cells contributed to disease pathogenesis. To define the effector function of low affinity MOG35–55 CD4+ T cells in the CNS, we isolated CD4+ T cells from the CNS at the peak of EAE and assessed their ability to produce IFN-γ and TNF (Fig. 3 D). Approximately 2.4-fold more CD4+ T cells produced cytokine in response to MOG35–55 peptide stimulation than were detected by tetramer (19.0 ± 3.6% vs. 8.0 ± 0.7%, in parallel experiments; Fig. 3 E). Because not all antigen-specific CD4+ T cells produced cytokine (Fig. 2 G) and because the tetramer-negative MOG35–55 CD4+ T cells outnumbered their tetramer-positive counterparts by ∼8:1 (Fig. 3 C), this indicated that the majority of proinflammatory cytokine-secreting MOG35–55-specific CD4+ T cells were of low affinity.
High frequency of LCMV-specific tetramer-negative CD4+ T cells at the peak effector phase
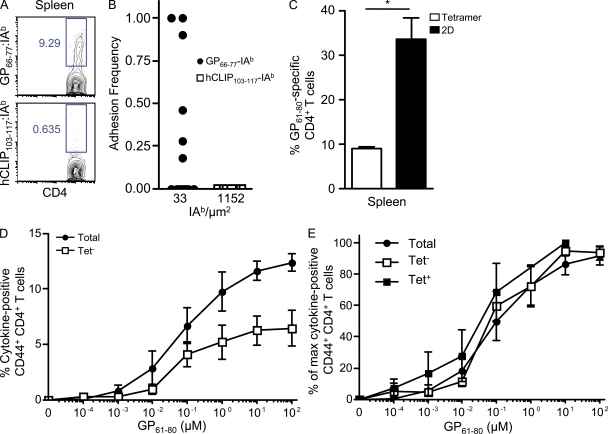
To measure ex vivo antiviral CD4+ T cells, we analyzed the GP61–80 LCMV-specific CD4+ T cell response at its peak, 8 d postinfection with LCMV Armstrong (Homann et al., 2001; Whitmire et al., 2006). CD4+ T cells from the spleen bound specifically to GP66–77-IAb by tetramer (Fig. 4 A) and 2D analysis (Fig. 4 B). Interestingly, although 8.9 ± 0.4% of CD4+ T cells from the spleen were detected by pMHC II tetramer, 33.7 ± 4.8% were in fact GP61–80 specific by 2D adhesion analysis (Fig. 4 C). This frequency closely matched the percentage (37.2%) of CD44+ CD4+ T cells that were induced by LCMV at day 8 postinfection. The mean effective 2D affinities (1.65 ± 0.79 × 10−4 µm4) of the GP61–80 CD4+ T cells in LCMV infection were again 2.6-fold less than in vitro GP61–80 CD4+ T cells (Fig. 2 A). Although viral-specific CD4+ T cells displayed an overall higher 2D affinity than myelin-specific CD4+ T cells, pMHC II tetramer nonetheless underestimated their frequency by fourfold.
Figure 4.
Low affinity viral-specific CD4+ T cells contribute significant effector responses during LCMV infection. (A and B) Representative tetramer (A) and 2D binding (B) of CD4+ T cells from the spleen at day 8 LCMV to GP66–77-IAb and hCLIP103–117-IAb. (C) The mean frequency ± SEM of GP61–80 CD4+ T cells in the spleen during LCMV infection by tetramer and 2D analysis was performed in parallel in three independent experiments (two spleens pooled per experiment; *, P = 0.007). (D and E) At day 8 postinfection, total CD44+ CD4+, tetramer-positive CD44+ CD4+, or tetramer-negative CD44+ CD4+ T cells were stimulated with the indicated concentrations of GP61–80 peptide, and the mean total ± SEM percentages of (D) and percentage of maximal ± SEM (E) cytokine-producing (IFN-γ and TNF) cells were assessed in four independent experiments (two to four spleens pooled per experiment).
To determine the contribution of low affinity T cells to overall effector function, splenocytes 8 d postinfection were sorted into GP66–77-IAb tetramer-positive and -negative CD4+ T cells and assessed for cytokine production (TNF and IFN-γ) in response to GP61–80 stimulation. In the absence of the GP66–77-IAb tetramer-positive CD4+ T cells, the tetramer-negative population produced approximately half of the total cytokines detected at all doses of antigen (Fig. 4 D). There was no significant difference (P = 0.80) in the EC50 values for cytokine production from the LCMV tetramer-positive or -negative or intact (i.e., unsorted) CD44+ CD4+ T cell populations (Fig. 4 E). The data were displayed as a percentage of maximal response because essentially all of the tetramer-positive CD4+ T cells were antigen reactive, whereas LCMV-specific CD4+ T cells comprised a third or less of the total and tetramer-negative CD4+ T cell populations. Thus, LCMV-specific, tetramer-negative CD4+ T cells were present at a greater frequency than the tetramer-positive population and produced a substantial fraction of the overall effector cytokine response.
DISCUSSION
T cells mount a response against antigens that is typically clonally diverse, but often experiments analyzing cell-mediated immunity must use monoclonal cells to facilitate analysis of cell fate, location, frequency, and affinity. Biophysical analyses of T cell affinity have almost exclusively focused on a single TCR species complicating extrapolation to polyclonal populations. The focus on one clonal TCR very likely limits our understanding into the range of affinities comprising the antigen-activated T cell population and prevents investigation into the distinct possibility that low affinity T cells appreciably participate in immunity (Gronski et al., 2004; Zehn et al., 2009). In this study, we applied a micropipette-based 2D binding technology to measure the affinities and frequencies of polyclonal CD4+ T cells by taking advantage of its capability over other measures to resolve low affinity monoclonal TCR–pMHC interactions (Huang et al., 2010). Beyond allowing analysis of polyclonal CD4+ T cell affinity, 2D analysis revealed significantly larger numbers of antigen-specific CD4+ T cells than can be quantified using pMHC II tetramers.
pMHC tetramers provide an important technique for quantifying the immune response and can be used to define a polyclonal population of antigen-specific precursor or effector T cells, yet their limited avidity inherently restricts the range of affinities that can be analyzed. Indeed, the ex vivo GP61–80 and MOG35–55 CD4+ T cell populations were four- and eightfold larger, respectively, than indicated by pMHC II tetramer. These differences between GP61–80- and MOG35–55-specific CD4+ T cells as prototypical foreign- and self-specific responses mirror other studies in which CD4+ T cells specific for foreign antigens (Homann et al., 2001; Moon et al., 2007) were more prevalent and easier to detect by tetramer than those specific for self-antigens (Gebe et al., 2003; Bischof et al., 2004; Falta et al., 2005; Korn et al., 2007). Regardless, pMHC II tetramer underestimated the frequency of MOG35–55 and GP61–80 CD4+ T cells, as the pMHC II tetramer staining was only accurate to the extent that the underlying T cell responses were dominated by high affinity T cells. It is currently unknown whether pMHC II tetramers are a sufficient surrogate for tracking CD4+ T cell responses. Interestingly, the CD4+ T cells possessed similar overlapping distributions of low to high affinities for MOG35–55 and GP61–80. In fact, the effective 2D affinities of tetramer-negative or -positive cells were essentially the same regardless of T cell specificity for the myelin or viral antigens.
Previous work in CD4+ T cells suggested rapid dominance of high affinity T cell clonotypes (Savage et al., 1999; Malherbe et al., 2004), yet these studies were based on pMHC II tetramer measurements. By including tetramer-negative CD4+ T cells in our analysis, a 2D affinity range >100-fold wide was present at the peak immune response for both foreign and self-antigens. This indicates heterogeneity in TCR affinity regardless of antigen specificity during the polyclonal primary immune responses and may raise issues over the accuracy that a monoclonal T cell population possessing a single affinity completely reflects the ongoing immune processes. For example, monoclonal MOG35–55-specific CD4+ T cells may differ because of limited affinity diversity from polyclonal populations of cells. In fact, the MOG35–55-specific 2D2 TCR transgenic, while a valuable model to highlight many features associated with demyelinating disease, generates a variant form of EAE (Bettelli et al., 2003, 2006; Wasserman and Evavold, 2008). Interestingly, 2D2 T cells are undetectable by the MOG-IAb tetramer (unpublished data) yet pathogenic (Bettelli et al., 2003, Wasserman and Evavold, 2008), demonstrating that low affinity MOG35–55 CD4+ T cells can induce EAE. We are in the process of carrying out experiments to test the concept that autoimmune disease outcome relates to the affinity range and diversity of the response.
Although our experiments do not directly address the effect of thymocyte negative selection or peripheral events in shaping T cell affinity as we examined expanded cell populations, the mere presence of high affinity MOG35–55 CD4+ T cells suggests that their clonal deletion was absent or incomplete. Moreover, the stable existence of high affinity self-reactive T cells refines our ideas on clonal selection and avidity maturation (Savage et al., 1999; Malherbe et al., 2004; Price et al., 2005) because they did not dominate over their low affinity counterparts at the peak of the primary immune response or on extended culture in vitro. Similarly, the affinities of the GP61–80 CD4+ T cells were broadly distributed and contained a significant population of low affinity LCMV-reactive CD4+ T cells detectable by 2D binding but not pMHC II tetramer. The presence of low affinity GP61–80 CD4+ T cells further demonstrates a lack of exclusive dominance by higher affinity T cell clones during a primary pathogen-specific response or on successive selective cycles with antigen in vitro. Thus, it is appears that breadth in affinity is maintained within a CD4+ polyclonal population, at least during the peak effector response.
To detect antigen-reactive CD4+ T cells by 2D analysis, the T cells had to recognize and proliferate to antigen encountered in vivo, regardless of low or high affinity. Moreover, we identified low and high affinity T cells within the immune-relevant tissues. In the case of the CNS, its immune privileged status would be presumed to allow primarily the myelin-reactive CD4+ T cells, which we demonstrated by 2D analysis. Further demonstrating the active role of tetramer-negative CD4+ T cells in immunity was their ability to contribute substantially to the overall cytokine responses. We found that the relative cytokine contribution of tetramer-negative CD4+ T cells was approximately equal to or greater than the high affinity responders in viral immunity. Moreover, the high and low affinity LCMV-specific CD4+ T cells had the same functional avidities despite their inherent 2D affinity differences, which is likely the result of tuning to the same level of antigen in vivo (Grossman and Paul, 1992; Slifka and Whitton, 2001). The prevalence of cytokine-positive low affinity myelin-reactive CD4+ T cells clearly outnumbered the small frequency of tetramer-positive MOG35–55 CD4+ T cells, demonstrating the importance of low affinity effectors in CNS autoimmunity.
However, cytokine analyses of CD4+ T cell effector function are limited because we and others have shown that not all antigen-specific CD4+ T cells produce cytokine (Weaver et al., 1998; Bettelli et al., 2006; Whitmire et al., 2006; Williams et al., 2008). Because tetramer and intracellular cytokine staining usually cannot be performed concurrently, they are often performed in parallel. Although we confirm in this study what others have shown, that pMHC II tetramer and cytokine responses were approximately equal in LCMV infection (Homann et al., 2001; Williams et al., 2008), we demonstrated that the tetramer-negative CD4+ T cells were major contributors to the effector cytokine response. Thus, an identical frequency of antigen-specific CD4+ T cells by cytokine and tetramer is fortuitous as opposed to being an indication that they are the same high affinity responding T cell population.
In conclusion, 2D analysis resolved the affinity of polyclonal T cell responses and revealed that low affinity CD4+ T cells participated during autoimmunity and viral infection, although they were accompanied by high affinity counterparts in both cases. Characterization of low affinity cell frequency therefore allows for increased understanding of the polyclonal T cell response and ultimately enables defining how the affinity range impacts the extent of cell-mediated immunity.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from the National Cancer Institute and housed in an Emory University Department of Animal Resources facility (Atlanta, GA) and used in accordance with an Institutional Animal Care and Use Committee–approved protocol.
Peptides.
MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) and GP61–80 (GLNGPDIYKGVYQFKSVEFD) peptides were synthesized on a Prelude peptide synthesizer (Protein Technologies, Inc.).
Cells and reagents.
All cells were cultured in RPMI 1640, supplemented with 10% FBS, 2 mM l-glutamine, 0.01 M Hepes, 100 µg/ml gentamicin (all Mediatech), and 20 µM 2-ME (Sigma-Aldrich). Polyclonal T cell lines were generated by footpad priming 2–3-mo-old male mice with 100 µg of the indicated peptide emulsified in CFA containing 1 mg/ml heat-killed Mycobacterium tuberculosis. Draining lymph nodes were harvested 10–14 d later and stimulated for 1 wk with 1 µM of the priming peptide and IL-2. Cells were CD4 purified by positive selection microbeads (Miltenyi Biotec). CD4+ T cells were restimulated weekly with irradiated syngeneic splenocytes (3,000 rad) and 1 µM peptide and IL-2. Proliferation was measured by [3H]thymidine incorporation as previously described (Sabatino et al., 2008).
Tetramer and cell surface staining.
MOG38–49-IAb (generated from a previously described construct; Sabatino et al., 2008), GP66–77-IAb, and hCLIP103–117-IAb monomers and tetramers were provided by the National Institute of Allergy and Infectious Diseases Tetramer Core Facility at Emory University. CD4+ T cells were incubated with 4 µg/ml MOG38–49-IAb (8–20 h), GP66–77-IAb tetramers (3–4 h), or hCLIP103–117-IAb in complete RPMI at 37°C. The following antibodies and stains were used for analysis: CD4 (RM4.5), CD8-α (53–6.7), B220 (RA3-6B2), CD11b (M1/70), 7-AAD (all BD), TCR-β (H57-597; eBioscience), and MHC II (M5/114.15.2; BioLegend). All flow cytometric analysis was performed on a FACSCalibur (BD), and data were analyzed using FlowJo (Tree Star, Inc.). Cell sorting on a FACSAria II was performed by the Emory University Flow Cytometry Core.
Intracellular cytokine detection.
CD4+ T cells were stimulated with IAb-expressing fibroblasts (clone FT7.1C6, (Ronchese et al., 1987) and the indicated concentrations of peptide for 5 h at 37°C in the presence of 10 µg/ml brefeldin A. T cells were stained with CD4, fixed/permeabilized using the Fix and Perm Cell Permeabilization kit (Invitrogen), and stained for intracellular IFN-γ (XMG1.2) and TNF (MP6-XT22; both BD). To calculate the percentage of maximal cytokine production, the percentage of cytokine-producing CD44+ CD4+ T cells at a given concentration of peptide stimulation was divided by the maximal percentage of cytokine-producing CD44+ CD4+ T cells that was elicited at any peptide stimulation.
Ex vivo CD4+ T cell analysis in EAE and LCMV.
EAE was induced in 6–8-wk-old female mice as previously described (Ford and Evavold, 2003; Sabatino et al., 2008). For LCMV infection, 2–3-mo-old male mice were injected i.p. with 2 × 105 pfu LCMV Armstrong (provided by R. Ahmed, Emory University; Murali-Krishna et al., 1998). At the time of analysis, all mice were euthanized and perfused with saline. At 21 d postimmunization for EAE, brains and spinal cords were harvested and pooled together from 8–10 mice per experiment. Infiltrating mononuclear cells from the CNS were isolated over Percoll (Sigma-Aldrich). 8 d post-LCMV infection, spleens were harvested and pooled together from two to four mice per experiment. All cells were CD4 purified by positive selection microbeads as described in Cells and reagents to 90–95% purity. Cells were stained with tetramer as described in Tetramer and cell surface staining and washed and stained with CD4 FITC and a dump channel cocktail of CD8-α, B220, CD11b (all PerCP), and 7-AAD. For assessing the cytokine response in LCMV, CD4+ T cells purified from the spleen at day 8 postinfection were sorted into GP66–77-IAb tetramer-positive and -negative CD44+ CD4+ T cells.
Cell preparation for micropipette adhesion frequency assay.
Human RBCs were isolated in accordance with the Institutional Review Board at the Georgia Institute of Technology and prepared as previously described (Huang et al., 2007, 2010). RBCs coated with various concentrations of Biotin-X-NHS (EMD) were coated with 0.5 mg/ml streptavidin (Thermo Fisher Scientific), followed by 1–2 µg of pMHC II monomer. The pMHC-coated RBCs were stained with anti-MHC II FITC antibody, and T cells were stained with anti-TCR FITC antibody. The site densities of IAb monomers per RBC and TCRs per T cell were derived using FITC MESF beads (Bangs Laboratories) as previously described (Huang et al., 2007, 2010) and normalized for the F/P ratios of the antibodies.
2D TCR affinity analysis.
The details of the micropipette adhesion frequency assay are described in detail elsewhere (Huang et al., 2007, 2010). In brief, a pMHC-coated RBC and T cell were placed on apposing micropipettes and brought into contact by micromanipulation for a controlled contact area (Ac) and time (t). The T cell was retracted at the end of the contact period, and the presence of adhesion (indicating TCR–pMHC ligation) was observed microscopically by elongation of the RBC membrane. This contact–retraction cycle was performed 50 times per T cell–RBC pair to calculate an adhesion frequency (Pa). The contact area was kept constant for all experiments so it would not affect the affinity comparison. For each experiment, a mean Pa was calculated based only on T cells that bound specifically to antigen. The population-averaged 2D affinity (AcKa) using the mean Pa at equilibrium (where t → ∞) was calculated using the following equation: AcKa = ln[1-Pa(∞)]/(mrml), where mr and ml reflect the receptor (TCR) and ligand (pMHC) densities, respectively.
Statistical analysis.
Statistical analyses were performed using Prism 4 (GraphPad Software, Inc.). Two-way Student’s t tests were used for all statistical comparisons, except in the comparison of the EC50 values of cytokine-producing CD4+ T cells in LCMV, where a one-way analysis of variance was used.
Online supplemental material.
Fig. S1 shows that MOG35–55 and GP61–80 CD4+ T cells do not differ in their TCR levels. Fig. S2 demonstrates that the adhesion frequencies of polyclonal MOG35–55 and GP61–80 CD4+ T cells reach steady-state within several seconds of binding. Fig. S3 shows the representative purities of tetramer-positive and -negative MOG35–55 and GP61–80 CD4+ T cells after cell sorting. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101574/DC1.
Acknowledgments
We thank A. Stout and J. Shires for preparation of the pMHC II monomers and tetramers, R. Karaffa and S. Durham for assistance with tetramer-based cell sorting experiments, and L. Edwards and M. Ford for their helpful comments.
This work was supported by National Multiple Sclerosis Society grant RG4047-A-3 and National Institutes of Health grants NS071518 (to B.D. Evavold) and AI38282 and AI060799 (to C. Zhu).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- 2D
- two-dimensional
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- GP
- glycoprotein
- LCMV
- lymphocytic choriomeningitis virus
- MOG
- myelin oligodendrocyte glycoprotein
- pMHC
- peptide–MHC
References
- Alam S.M., Gascoigne N.R. 1998. Posttranslational regulation of TCR Valpha allelic exclusion during T cell differentiation. J. Immunol. 160:3883–3890 [PubMed] [Google Scholar]
- Altman J.D., Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274:94–96 10.1126/science.274.5284.94 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Pagany M., Weiner H.L., Linington C., Sobel R.A., Kuchroo V.K. 2003. Myelin oligodendrocyte glycoprotein–specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197:1073–1081 10.1084/jem.20021603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Bischof F., Hofmann M., Schumacher T.N., Vyth-Dreese F.A., Weissert R., Schild H., Kruisbeek A.M., Melms A. 2004. Analysis of autoreactive CD4 T cells in experimental autoimmune encephalomyelitis after primary and secondary challenge using MHC class II tetramers. J. Immunol. 172:2878–2884 [DOI] [PubMed] [Google Scholar]
- Bouneaud C., Kourilsky P., Bousso P. 2000. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 13:829–840 10.1016/S1074-7613(00)00080-7 [DOI] [PubMed] [Google Scholar]
- Chesla S.E., Selvaraj P., Zhu C. 1998. Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys. J. 75:1553–1572 10.1016/S0006-3495(98)74074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F., Kozono H., White J., Marrack P., Kappler J. 1998. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 8:675–682 10.1016/S1074-7613(00)80572-5 [DOI] [PubMed] [Google Scholar]
- Falta M.T., Fontenot A.P., Rosloniec E.F., Crawford F., Roark C.L., Bill J., Marrack P., Kappler J., Kotzin B.L. 2005. Class II major histocompatibility complex-peptide tetramer staining in relation to functional avidity and T cell receptor diversity in the mouse CD4(+) T cell response to a rheumatoid arthritis-associated antigen. Arthritis Rheum. 52:1885–1896 10.1002/art.21098 [DOI] [PubMed] [Google Scholar]
- Fassò M., Anandasabapathy N., Crawford F., Kappler J., Fathman C.G., Ridgway W.M. 2000. T cell receptor (TCR)-mediated repertoire selection and loss of TCR vβ diversity during the initiation of a CD4+ T cell response in vivo. J. Exp. Med. 192:1719–1730 10.1084/jem.192.12.1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M.L., Evavold B.D. 2003. Regulation of polyclonal T cell responses by an MHC anchor-substituted variant of myelin oligodendrocyte glycoprotein 35-55. J. Immunol. 171:1247–1254 [DOI] [PubMed] [Google Scholar]
- Gebe J.A., Falk B.A., Rock K.A., Kochik S.A., Heninger A.K., Reijonen H., Kwok W.W., Nepom G.T. 2003. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur. J. Immunol. 33:1409–1417 10.1002/eji.200323871 [DOI] [PubMed] [Google Scholar]
- Gronski M.A., Boulter J.M., Moskophidis D., Nguyen L.T., Holmberg K., Elford A.R., Deenick E.K., Kim H.O., Penninger J.M., Odermatt B., et al. 2004. TCR affinity and negative regulation limit autoimmunity. Nat. Med. 10:1234–1239 10.1038/nm1114 [DOI] [PubMed] [Google Scholar]
- Grossman Z., Paul W.E. 1992. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc. Natl. Acad. Sci. USA. 89:10365–10369 10.1073/pnas.89.21.10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D., Teyton L., Oldstone M.B. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913–919 10.1038/90950 [DOI] [PubMed] [Google Scholar]
- Homann D., Lewicki H., Brooks D., Eberlein J., Mallet-Designé V., Teyton L., Oldstone M.B. 2007. Mapping and restriction of a dominant viral CD4+ T cell core epitope by both MHC class I and MHC class II. Virology. 363:113–123 10.1016/j.virol.2006.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Edwards L.J., Evavold B.D., Zhu C. 2007. Kinetics of MHC-CD8 interaction at the T cell membrane. J. Immunol. 179:7653–7662 [DOI] [PubMed] [Google Scholar]
- Huang J., Zarnitsyna V.I., Liu B., Edwards L.J., Jiang N., Evavold B.D., Zhu C. 2010. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 464:932–936 10.1038/nature08944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppa J.B., Axmann M., Mörtelmaier M.A., Lillemeier B.F., Newell E.W., Brameshuber M., Klein L.O., Schütz G.J., Davis M.M. 2010. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 463:963–967 10.1038/nature08746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh G.J., Kersh E.N., Fremont D.H., Allen P.M. 1998. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 9:817–826 10.1016/S1074-7613(00)80647-0 [DOI] [PubMed] [Google Scholar]
- Korn T., Reddy J., Gao W., Bettelli E., Awasthi A., Petersen T.R., Bäckström B.T., Sobel R.A., Wucherpfennig K.W., Strom T.B., et al. 2007. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 13:423–431 10.1038/nm1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.Y., Fairchild P.J., Smith R.M., Prowle J.R., Kioussis D., Wraith D.C. 1995. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 3:407–415 10.1016/1074-7613(95)90170-1 [DOI] [PubMed] [Google Scholar]
- Malherbe L., Hausl C., Teyton L., McHeyzer-Williams M.G. 2004. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 21:669–679 10.1016/j.immuni.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Mallone R., Kochik S.A., Reijonen H., Carson B., Ziegler S.F., Kwok W.W., Nepom G.T. 2005. Functional avidity directs T-cell fate in autoreactive CD4+ T cells. Blood. 106:2798–2805 10.1182/blood-2004-12-4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel I., Kerlero de Rosbo N., Ben-Nun A. 1995. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur. J. Immunol. 25:1951–1959 10.1002/eji.1830250723 [DOI] [PubMed] [Google Scholar]
- Mendel I., Kerlero de Rosbo N., Ben-Nun A. 1996. Delineation of the minimal encephalitogenic epitope within the immunodominant region of myelin oligodendrocyte glycoprotein: diverse V beta gene usage by T cells recognizing the core epitope encephalitogenic for T cell receptor V beta b and T cell receptor V beta a H-2b mice. Eur. J. Immunol. 26:2470–2479 10.1002/eji.1830261030 [DOI] [PubMed] [Google Scholar]
- Moon J.J., Chu H.H., Pepper M., McSorley S.J., Jameson S.C., Kedl R.M., Jenkins M.K. 2007. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 27:203–213 10.1016/j.immuni.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J., Zajac A.J., Miller J.D., Slansky J., Ahmed R. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187 10.1016/S1074-7613(00)80470-7 [DOI] [PubMed] [Google Scholar]
- Oxenius A., Bachmann M.F., Ashton-Rickardt P.G., Tonegawa S., Zinkernagel R.M., Hengartner H. 1995. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur. J. Immunol. 25:3402–3411 10.1002/eji.1830251230 [DOI] [PubMed] [Google Scholar]
- Price D.A., Brenchley J.M., Ruff L.E., Betts M.R., Hill B.J., Roederer M., Koup R.A., Migueles S.A., Gostick E., Wooldridge L., et al. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 202:1349–1361 10.1084/jem.20051357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchese F., Brown M.A., Germain R.N. 1987. Structure-function analysis of the Abm12 beta mutation using site-directed mutagenesis and DNA-mediated gene transfer. J. Immunol. 139:629–638 [PubMed] [Google Scholar]
- Sabatino J.J., Jr, Shires J., Altman J.D., Ford M.L., Evavold B.D. 2008. Loss of IFN-gamma enables the expansion of autoreactive CD4+ T cells to induce experimental autoimmune encephalomyelitis by a nonencephalitogenic myelin variant antigen. J. Immunol. 180:4451–4457 [DOI] [PubMed] [Google Scholar]
- Savage P.A., Boniface J.J., Davis M.M. 1999. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 10:485–492 10.1016/S1074-7613(00)80048-5 [DOI] [PubMed] [Google Scholar]
- Slifka M.K., Whitton J.L. 2001. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat. Immunol. 2:711–717 10.1038/90650 [DOI] [PubMed] [Google Scholar]
- Vollers S.S., Stern L.J. 2008. Class II major histocompatibility complex tetramer staining: progress, problems, and prospects. Immunology. 123:305–313 10.1111/j.1365-2567.2007.02801.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman H.A., Evavold B.D. 2008. Induction of anergy by antibody blockade of TCR in myelin oligodendrocyte glycoprotein-specific cells. J. Immunol. 180:7259–7264 [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Saparov A., Kraus L.A., Rogers W.O., Hockett R.D., Bucy R.P. 1998. Heterogeneity in the clonal T cell response. Implications for models of T cell activation and cytokine phenotype development. Immunol. Res. 17:279–302 10.1007/BF02786452 [DOI] [PubMed] [Google Scholar]
- Whitmire J.K., Benning N., Whitton J.L. 2006. Precursor frequency, nonlinear proliferation, and functional maturation of virus-specific CD4+ T cells. J. Immunol. 176:3028–3036 [DOI] [PubMed] [Google Scholar]
- Williams M.A., Ravkov E.V., Bevan M.J. 2008. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 28:533–545 10.1016/j.immuni.2008.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge L., Lissina A., Cole D.K., van den Berg H.A., Price D.A., Sewell A.K. 2009. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 126:147–164 10.1111/j.1365-2567.2008.02848.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Bevan M.J. 2006. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 25:261–270 10.1016/j.immuni.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Lee S.Y., Bevan M.J. 2009. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 458:211–214 10.1038/nature07657 [DOI] [PMC free article] [PubMed] [Google Scholar]